Abstract
A 4-year-old boy presented with perianal abscess and granulomatous colitis, which led the diagnosis of Crohn’s disease. He became refractory to all available therapies and required colectomy. Targeted sequencing revealed a deleterious variant in NCF4, causing severe neutrophil dysfunction. He underwent hematopoietic stem cell transplantation (HSCT) with an excellent outcome.
Crohn’s disease, a form of inflammatory bowel disease (IBD), is a chronic relapsing and remitting condition that causes progressive tissue destruction, fistulas, and fibrosis of the gastrointestinal tract.1 Very early onset IBD (VEO-IBD) typically occurs under the age of 6, and in some cases, is caused by a single gene defect.2–4 Some subjects with VEO-IBD have atypical presentations, atypical pathological features associated with extreme phenotypes, and can be refractory to available IBD therapeutics.4–6
The literature has now reported >50 gene linked with VEO-IBD,4,7,8 of which IL-10R/IL-10, LRBA, XIAP, and nicotinamide adenosine dinucleotide phosphate (NADPH) oxidase (NCF4 and RAC2) are the most widely reported.9–16 Early cases of VEO-IBD were not accurately diagnosed owing to its underlying molecular genetic heterogeneity underlying VEO-IBD. This has resulted in poor clinical outcomes because commonly used IBD therapies failed. Recent advances in the field of genomics, immunology, and bioinformatics have allowed clinicians and scientists to make accurate molecular diagnoses in many cases of VEO-IBD. Therefore, it is imperative to use genetic testing (and functional assays if needed) to rapidly arrive at an accurate diagnosis.
Monogenetic variants in the NADPH oxidase pathway known to cause neutrophil defects in chronic granulomatosis disease (CGD) are increasingly reported.4,17–20 A delay in diagnosis can be as lethal as neutrophil defects in CGD and is associated with a 5% mortality rate.21 In patients diagnosed with IBD with a suspicion of CGD, an exact molecular diagnosis will not only guide therapy, but also prevent the use of anti-tumor necrosis factor (TNF) drugs, which increases the risk of developing serious infections.22–25 Moreover, a molecular diagnosis of CGD in VEO-IBD cases will provide the life-saving benefit of HSCT.26
Case Report
At 4 years of age, a previously healthy boy presented with a perianal abscess, constipation, and rectal bleeding. He had growth failure characterized by height at the third percentile and a body mass index of 13 with delayed bone age. A colonoscopy showed left-sided colitis characterized by deep linear ulcerations with skip lesions (Figure 1, A and B) and histology from mucosal biopsy samples confirmed chronic granulomatous inflammation. A basic immunodeficiency workup along with a phorbol myristate acetate (PMA)-stimulated dihydrorhodamine (DHR) test, a classical test for CGD, were negative. His DHR testing showed a normal stimulation index of 189 (normal range, 30–500). After failing to respond to immunomodulator therapy (6-mercaptopurine) with adequate therapeutic levels the patient was treated with anti-TNF therapy (infliximab and then switched to adalimumab). During the course of anti-TNF therapy, he developed osteomyelitis of the pubic bone, confirmed by magnetic resonance imaging, which was presumed to be an infectious complication of anti-TNF therapy. This finding required prolonged antibiotic therapy. His IBD therapy was then switched to an antiadhesion monoclonal antibody (vedolizumab) without any therapeutic benefits. Five years after his initial diagnosis, failure of multiple medical therapies, and a deceleration of his growth velocity, a subtotal colectomy and ileostomy was performed. The histology from the resected colon showed severe transmural inflammation with very large granulomas (Figure 1, C and D).
Figure 1.
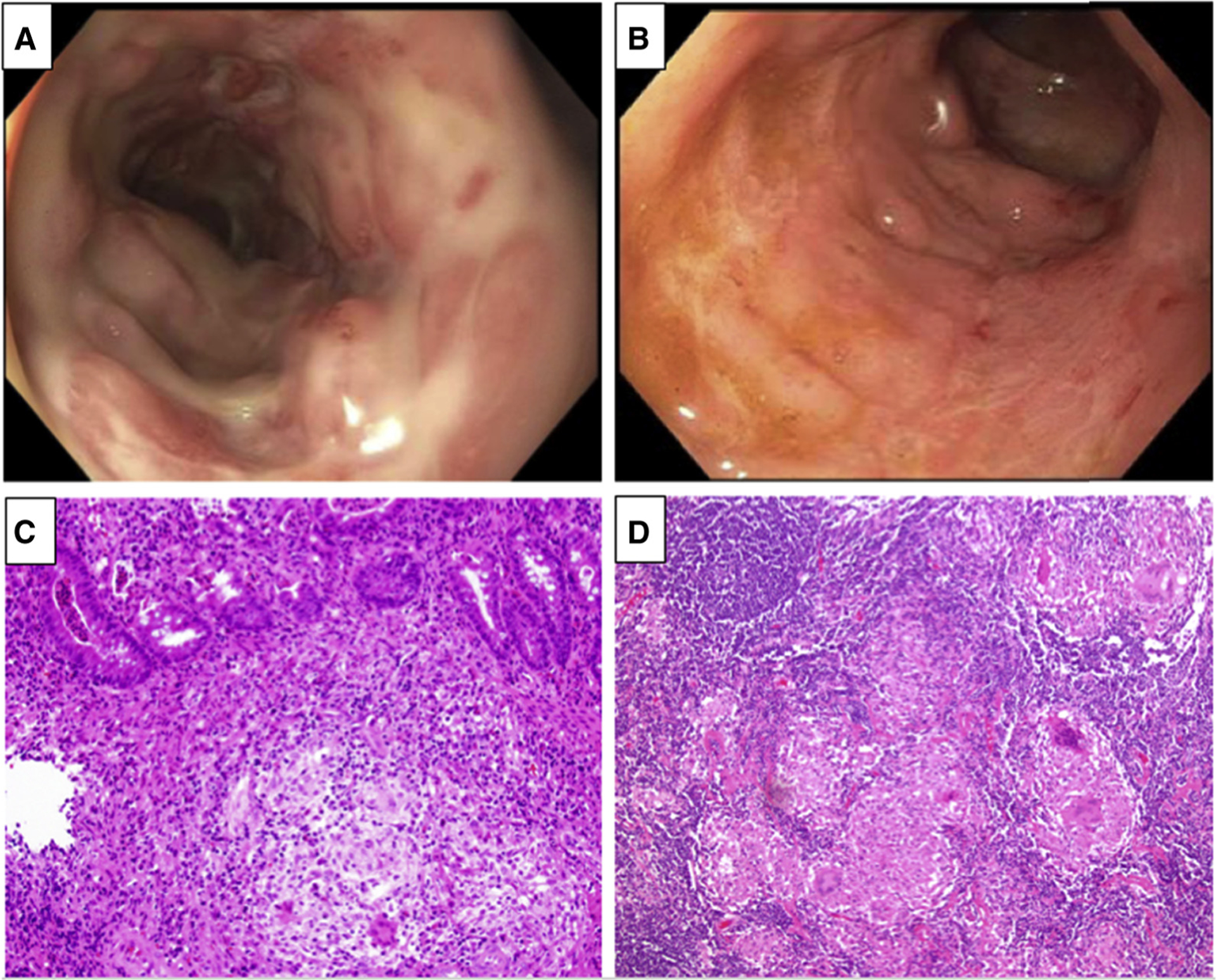
A, B, Colonoscopy images of the colonic mucosa with deep and linear ulceration with skip lesions. C, Endoscopic biopsy specimen from the right side of the colon shows discrete epithelioid non-necrotizing granulomas composed of epithelioid histiocytes and giant cells. The histiocytes are epithelioid and contain abundant eosinophilic cytoplasm with scattered multi-nucleated giant cell. The crypts show acute cystitis, crypt abscesses, crypt atrophy, and irregularity (stain: hematoxylin and eosin; original magnification × 200). D, Mesenteric lymphoid gland from the resected surgical specimen showed multiple well-formed granulomas (stain: hematoxylin and eosin; original magnification × 200).
Next-generation sequencing (NGS) of a gene panel was performed through a CLIA-certified commercial vendor. This panel tested 27 variants known to cause VEO-IBD, including NCF4, CYBB, IL10R, and XIAP among others (www.egl-eurofins.com/tests/MM160). The targeted NGS revealed a likely pathogenic and rare heterozygote variant in the NCF4 gene, represented by a substitution of guanine (G) to adenine (A) at position 179 of the coding sequence (c.179G>A). This resulted in the change of arginine at position 60 to histidine (p.Arg60His) in the protein. This variant is present in population database (rs369847561, dbSNP) and has a minor allele frequency of 0.003% in ExAC and 0.005% in gnomAD. It has been reported in Clinvar as variant of uncertain significance by Inviate and EGL Eurofins (commercial clinical testing laboratories). Although this variant has not been reported in the literature in individuals with NCF4-related disease, it is listed among variants studied for granulomatous disease (https://clinvarminer.genetics.utah. edu/variants-by-gene/NCF4). Mutations in NCF4 that code for the NADPH-oxidase complex are known to cause neutrophil dysfunction.27–29 Therefore, peripheral blood was collected from the patient to assess the functional consequence of p.Arg60His. We observed a marked decrease of NADPH oxidase activity (N-formyl-methionyl-leucyl-phenylalanine [fMLP] based assessment) in neutrophil (Figure 2, A and B) and monocyte. In addition, decreased phagocyte killing of bacteria was also noted (Figure 2, C). Taken together, these 2 findings were strongly suggestive of a defect in neutrophil function. The patient was referred to and underwent a successful allogeneic HSCT, which led to disease remission. He is doing well without any specific therapies at 6 months of follow-up. Repeat endoscopy performed showed complete mucosal healing without any residual disease.
Figure 2.

The black line shows the amount of the patient’s monocytes (A) or granulocytes (B) that displayed respiratory burst without fMLP stimulation. The red line shows the amount of patient’s cells that underwent oxidative burst after stimulation with fMLP. The blue line shows the number of control cells that respond to fMLP stimulation. A, Decreased oxidative burst in monocytes after fMLP stimulation. B, Decreased oxidative burst response in granulocytes after fMLP stimulation. C, Representative photomicrograph demonstrating patient’s neutrophils with decreased dead Staphylococcus aureus (red) and presence of still live S aureus (green) suggestive of decreased phagocytic and bactericidal activity when compared with control healthy neutrophil.
Methods
The targeted gene panel comprised of 27 genes was sequenced by the CLIA/CAP-certified Emory genetic laboratory. Briefly, the DNA library was prepared from 3 μg of genomic DNA and subjected to paired end (2 × 100) sequencing in a single lane on a HiSeq 2500 (Illumina, San Diego, California) according to the manufacturer’s methods and the commercial laboratory standards. The sample was sequenced to an average depth of 150 × to confidently call and annotate variants during the analytic process. Evaluation of neutrophil NADPH oxidase activity and reactive oxygen species production was measured in whole blood at the Cincinnati Children’s Hospital Medical Center. After physiological stimulation with fMLP (#F3506; Sigma-Aldrich, St. Louis, Missouri), neutrophils showed a marked decrease in reactive oxygen species production when compared with controls in both neutrophils and monocytes (Figure 2, A and B).18,30 Additionally, killing of Staphylococcus aureus was measured in adherent neutrophils using the acridine orange (Polysciences, Warrington, Pennsylvania), as previously published.18,30 The patient’s sample showed a marked decrease in killing when compared with controls (Figure 2, C). The abnormal oxidative function results after a physiological stimulation and defective bacterial killing suggest the NCF4 variant is likely pathogenic and results in decreased NADPH oxidase activity. To determine if this mutation (c.179G>A, rs369847561) was acquired de novo or inherited, testing of the parents and the proband by Sanger sequencing showed that c.179G>A was paternally inherited.
Discussion
Classical IBD consists of a multifactorial pathogenesis characterized by a modest or low penetrance contribution of each identified or discovered etiological factor. In contrast, VEO-IBD can be monogenic in nature, with a high effect or high penetrance contribution. Because the outcome for VEO-IBD patients can be significantly worse than classical IBD, it is critical to recognize VEO-IBD phenotypes early for a diagnosis. Genetic advances and discoveries made in classical IBD are unlikely to account for VEO-IBD genes because genome-wide association studies and high-density single nucleotide polymorphism arrays are powered to identify common variants (with a frequency of >2%−3%).31 With few exceptions, most monogenic disease variants have a low to rare frequency (<1%), which makes sequencing the best detection method; NGS technology has emerged as an attractive and cost-effective way to screen and diagnose complex diseases with monogenic behavior through custom diagnostic gene panels.32
Neutrophil dysfunction which causes Crohn’s disease-like diseases are now well-known; glycogen storage disease type 1b is a prime example.33 Neutrophils play a central role in the maintenance of intestinal homeostasis. They have naturally built-in defense mechanisms to eliminate microbes that have migrated across the mucosal epithelial layer. In addition, neutrophils help to recruit other protective immune cells during the inflammatory process to aid mucosal repair. Dysfunction of neutrophils can results in IBD.34 Recently, many genes that participate directly or indirectly in the neutrophil pathways have been implicated in IBD. The spectrum of disorders that mimic IBD is also expanding.4,35 Specifically, variants in NCF4 and RAC2 are known to cause clinical manifestation of VEO-IBD, rather than the recurrent infections seen in classical CGD.5,6 Additionally, many monogenetic variants are known to cause neutrophil dysregulation, leading to intestinal inflammation.11,21,36–38
CGD is suspected when children present with chronic bacterial and fungal infections, failure to thrive, hyperglobulinemia, anemia, lymphadenopathy, and hepatosplenomegaly. Autoimmune and autoinflammatory manifestations of CGD are increasingly recognized. Although gastrointestinal manifestations of CGD are found in 25% of patients, other phenotypes such as glomerulonephritis and pulmonary complications are common, with the lungs being the most common site of neutrophil aggregation.39 As many as 40% of patients with CGD develop Crohn’s disease-like intestinal inflammation and symptoms.40 The role of DHR in the diagnosis of CGD is also important. Similar to our case, normal PMA-stimulated DHR in patients with CGD have been reported,11 and the description of NCF4 highlighted a defect in intracellular superoxide production (not in the extracellular release) with a normal DHR.11 Also, biallelic defects in NCF4 have mainly manifested as only a gut-based disease, with no serious infections. After the identification in our patient of the c.179G>A variant in NCF4, an MLP-stimulated DHR test showed an abnormal neutrophil oxidative burst test. The fMLP is a better physiological stimulator of neutrophil oxidative burst than PMA. Although this variant is deleterious in our young patient, parental testing showed that it is inherited from the healthy father. This finding suggests that additional factors such as variable penetrance and/or expressivity could influence the effects of c.179G>A in the patient. This finding explains why genetic diseases are occasionally transmitted through unaffected parents or why healthy individuals can harbor potentially deleterious variants without suffering any obvious ill effects.
The existence of healthy homozygotes of autosomal-recessive disorders have been demonstrated.41–43 Also, in single gene disorders, although 1 gene can be primarily responsible for a disease, an independently located modifier can influence the phenotype.44 Modifier genes can affect penetrance, dominance, and expressivity.45 Therefore, there could be a modifier event with dominant effect in our patient that is not picked up by the sequencing panel, but may account for the penetrance of variant c.179G>A, which is transmitted through the unaffected father.46 The severe neutrophil defect observed in this patient suggests a potential functional impact of c.179G>A.
Colitis in CGD is refractory to most classical IBD treatments. In fact, the use of anti-TNF agents such as infliximab and adalimumab in patients with CGD has been reported to increase the incidence of infectious complications and potentially increase mortality.25,26 Decreased toxicity myeloablative conditioning followed by allogeneic HSCT is curative in CGD.14 The survival rates of patients with CGD undergoing HSCT have increased significantly in the last 15 years. In addition to CGD, other neutrophil dysfunctions caused by monogenic variants have been cured by HSCT.4 A prospective study reported 56 patients with CGD with an overall survival rate of 93%, of which 86% were cured.47 Furthermore, in patients with CGD and colitis, HSCT resulted in 90% resolution of colitis. Thus, to provide the best care possible, clinicians must be aware of IBD associated monogenic immune disorders wherein HSCT could be curative.
For children presenting with an IBD-like illness under the age of 6 years, attempts should be made to find the causal variant for VEO-IBD. The identification of monogenic etiology could help in the initiation of a better targeted therapy and even in considering HSCT as a therapeutic option if the defect is in the immune or hematopoietic compartment. Because many laboratories offer CLIA-certified high-throughput gene panel testing,48,49 it is prudent to use panel testing for a molecular diagnosis. We have identified a rare mutation in NCF4 that could contribute to an atypical clinical presentation with refractory granulomatous colitis, a feature of CGD. This case study illustrates the clinical usefulness of genetic testing and functional testing to identify underlying genetic and immune defects and offer a curative therapy.
Acknowledgments
Partially supported by National Institutes of Health (DK098231).
Glossary
- CGD
Chronic granulomatosis disease
- DHR
Dihydrorhodamine
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- HSCT
Hematopoietic stem cell transplantation
- IDB
Inflammatory bowel disease
- NADPH
Nicotinamide adenosine dinucleotide phosphate
- NGS
Next-generation sequencing
- PMA
Phorbol myristate acetate
- TNF
Tumor necrosis factor
- VEO-IBD
Very early onset IBD
Footnotes
The authors declare no conflicts of interest.
Data Statement
Data sharing statement available at www.jpeds.com.
References
- 1.Aziz DA, Moin M, Majeed A, Sadiq K, Biloo AG. Paediatric inflammatory bowel disease: clinical presentation and disease location. Pak J Med Sci 2017;33:793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianco AM, Girardelli M, Tommasini A. Genetics of inflammatory bowel disease from multifactorial to monogenic forms. World J Gastroenterol 2015;21:12296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhlig HH, Muise AM. Clinical genomics in inflammatory bowel disease. Trends Genet 2017;33:629–41. [DOI] [PubMed] [Google Scholar]
- 4.Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014;147:990–1007.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsen JR, Baldassano RN, Artis D, Sonnenberg GF. Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2015;1:462–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L, Shi T, Zhong C, Wang Y, Chang M, Liu X. IL-10 and IL-10 receptor mutations in very early onset inflammatory bowel disease. Gastroenterology Res 2017;10:65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang YH, Luo YY, Yu JD, Lou JG, Chen J. Phenotypic and genotypic characterization of inflammatory bowel disease in children under six years of age in China. World J Gastroenterol 2018;24:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrowski J, Paziewska A, Lazowska I, Ambrozkiewicz F, Goryca K, Kulecka M, et al. Genetic architecture differences between pediatric and adult-onset inflammatory bowel diseases in the Polish population. Sci Rep 2016;6:39831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammann S, Elling R, Gyrd-Hansen M, Duckers G, Bredius R, Burns SO, et al. A new functional assay for the diagnosis of X-linked inhibitor of apoptosis (XIAP) deficiency. Clin Exp Immunol 2014;176:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh RA, Villanueva J, Zhang K, Snow AL, Su HC, Madden L, et al. A rapid flow cytometric screening test for X-linked lymphoproliferative disease due to XIAP deficiency. Cytometry B Clin Cytom 2009;76: 334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matute JD, Arias AA, Wright NA, Wrobel I, Waterhouse CC, Li XJ, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood 2009;114:3309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer AN, Dimmock DP, Arca MJ, Bick DP, Verbsky JW, Worthey EA, et al. A timely arrival for genomic medicine. Genet Med 2011;13:195–6. [DOI] [PubMed] [Google Scholar]
- 13.Muise AM, Xu W, Guo CH, Walters TD, Wolters VM, Fattouh R, et al. NADPH oxidase complex and IBD candidate gene studies: identification of a rare variant in NCF2 that results in reduced binding to RAC2. Gut 2012;61:1028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seger RA, Gungor T, Belohradsky BH, Blanche S, Bordigoni P, Di Bartolomeo P, et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985–2000. Blood 2002;100: 4344–50. [DOI] [PubMed] [Google Scholar]
- 15.Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut 2013;62:1795–805. [DOI] [PubMed] [Google Scholar]
- 16.Zeissig Y, Petersen BS, Milutinovic S, Bosse E, Mayr G, Peuker K, et al. XIAP variants in male Crohn’s disease. Gut 2015;64:66–76. [DOI] [PubMed] [Google Scholar]
- 17.Andrews T, Sullivan KE. Infections in patients with inherited defects in phagocytic function. Clin Microbiol Rev 2003;16:597–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denson LA, Jurickova I, Karns R, Shaw KA, Cutler DJ, Okou DT, et al. Clinical and genomic correlates of neutrophil reactive oxygen species production in pediatric patients with Crohn’s disease. Gastroenterology 2018;154:2097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haberman Y, Tickle TL, Dexheimer PJ, Kim MO, Tang D, Karns R, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest 2014;124:3617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol 2010;125(Suppl 1):S182–94. [DOI] [PubMed] [Google Scholar]
- 21.Winkelstein JA, Marino MC, Johnston RB Jr, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–69. [DOI] [PubMed] [Google Scholar]
- 22.Deffert C, Olleros ML, Huiping Y, Herrmann FR, Zekry D, Garcia I, et al. TNF-alpha blockade in chronic granulomatous disease-induced hyper-inflammation: patient analysis and murine model. J Allergy Clin Immunol 2011;128:675–7. [DOI] [PubMed] [Google Scholar]
- 23.Galandiuk S, Davis BR. Infliximab-induced disseminated histoplasmosis in a patient with Crohn’s disease. Nat Clin Pract Gastroenterol Hepatol 2008;5:283–7. [DOI] [PubMed] [Google Scholar]
- 24.Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis 2015;60:1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzel G, Orange JS, Poliak N, Marciano BE, Heller T, Holland SM. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin Infect Dis 2010;51: 1429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold DE, Heimall JR. A review of chronic granulomatous disease. Adv Ther 2017;34:2543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giardino G, Cicalese MP, Delmonte O, Migliavacca M, Palterer B, Loffredo L, et al. NADPH oxidase deficiency: a multisystem approach. Oxid Med Cell Longev 2017;2017:4590127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keszei M, Westerberg LS. Congenital defects in neutrophil dynamics. J Immunol Res 2014;2014:303782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan BM, Zanetti KA, Robles AI, Schetter AJ, Goodman J, Hayes RB, et al. Germline variation in NCF4, an innate immunity gene, is associated with an increased risk of colorectal cancer. Int J Cancer 2014;134: 1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurickova I, Collins MH, Chalk C, Seese A, Bezold R, Lake K, et al. Paediatric Crohn disease patients with stricturing behaviour exhibit ileal granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibody production and reduced neutrophil bacterial killing and GM-CSF bioactivity. Clin Exp Immunol 2013;172:455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibeagha-Awemu EM, Peters SO, Akwanji KA, Imumorin IG, Zhao X. High density genome wide genotyping-by-sequencing and association identifies common and low frequency SNPs, and novel candidate genes influencing cow milk traits. Sci Rep 2016;6:31109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang H, Chen Y, Chen H, Ma Y, Chiang PW, Zhong J, et al. Systematic evaluation of a targeted gene capture sequencing panel for molecular diagnosis of retinitis pigmentosa. PloS One 2018;13: e0185237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froissart R, Piraud M, Boudjemline AM, Vianey-Saban C, Petit F, Hubert-Buron A, et al. Glucose-6-phosphatase deficiency. Orphanet J Rare Dis 2011;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol 2014;9:181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M’Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol 2013;6:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, De Ravin SS, Paul AR, Heller T, Ho N, Wu Datta L, et al. Genetic risk for inflammatory bowel disease is a determinant of Crohn’s disease development in chronic granulomatous disease. Inflamm Bowel Dis 2016;22:2794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muise AM, Snapper SB, Kugathasan S. The age of gene discovery in very early onset inflammatory bowel disease. Gastroenterology 2012;143:285–8. [DOI] [PubMed] [Google Scholar]
- 38.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. [DOI] [PubMed] [Google Scholar]
- 39.Song E, Jaishankar GB, Saleh H, Jithpratuck W, Sahni R, Krishnaswamy G. Chronic granulomatous disease: a review of the infectious and inflammatory complications. Clin Mol Allergy 2011;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schappi MG, Smith VV, Goldblatt D, Lindley KJ, Milla PJ. Colitis in chronic granulomatous disease. Arch Dis Child 2001;84:147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zlotogora J Penetrance and expressivity in the molecular age. Genet Med 2003;5:347–52. [DOI] [PubMed] [Google Scholar]
- 42.Beutler E The HFE Cys282Tyr mutation as a necessary but not sufficient cause of clinical hereditary hemochromatosis. Blood 2003;101: 3347–50. [DOI] [PubMed] [Google Scholar]
- 43.Beutler E, Felitti V, Gelbart T, Waalen J. Haematological effects of the C282Y HFE mutation in homozygous and heterozygous states among subjects of northern and southern European ancestry. Br J Haematol 2003;120:887–93. [DOI] [PubMed] [Google Scholar]
- 44.Cooper DN, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H. Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet 2013;132:1077–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadeau JH. Modifier genes in mice and humans. Nat Rev Genet 2001;2: 165–74. [DOI] [PubMed] [Google Scholar]
- 46.Badano JL, Katsanis N. Beyond Mendel: an evolving view of human genetic disease transmission. Nat Rev Genet 2002;3:779–89. [DOI] [PubMed] [Google Scholar]
- 47.Gungor T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet 2014;383:436–48. [DOI] [PubMed] [Google Scholar]
- 48.Payne K, Gavan SP, Wright SJ, Thompson AJ. Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat Rev Genet 2018;19:235–46. [DOI] [PubMed] [Google Scholar]
- 49.Delio M, Patel K, Maslov A, Marion RW, McDonald TV, Cadoff EM, et al. Development of a targeted multi-disorder high-throughput sequencing assay for the effective identification of disease-causing variants. PloS One 2015;10:e0133742. [DOI] [PMC free article] [PubMed] [Google Scholar]


