Abstract
Aerobic exercise and physical activity (PA) are known to benefit cognition in adulthood. However, a typical older adult spends most of the day sedentary or in light PA, behaviors that are typically poorly captured by questionnaires. To better understand the associations between time spent in different intensities of lifestyle PA and cognition, we measured average time spent daily in sedentariness, light, and moderate to vigorous PA using hip-worn sensors (Actigraph accelerometers). We studied baseline data from 228 cognitively normal adults (age 60–80) who took part in a clinical trial (NCT01472744). Fluid (processing speed, memory, and reasoning) and crystallized abilities (vocabulary knowledge) were assessed with the Virginia Cognitive Aging Battery. Adjusting for age, sex, and several modifiable socioeconomic, physical and functional health factors, time spent daily in moderate to vigorous PA was positively related with fluid abilities (perceptual speed and reasoning). Furthermore, we found that those spending more time sedentary performed better on vocabulary knowledge and reasoning tasks. In contrast, time spent in light PA was not related to either fluid or crystallized abilities. Our results add to the previous literature by providing the first sensor-based evidence that crystallized and fluid abilities in older age may be associated with engagement in different intensities of daily activity. Moreover, our findings suggest that the behavior of moderate to vigorous PA is at least as important in relation to cognition as the desirable long-term physiological effects of higher-intensity PA and exercise.
Keywords: sedentariness, physical activity, cognition, cardiorespiratory fitness, aging
Normative adult cognitive development is bidirectional (Baltes, 1987): it includes age-related declines in general fluid abilities and gains in crystallized abilities (Baltes et al., 1999; Cattell, 1971; Wolman & Stricker, 1982). Fluid abilities, which include reasoning and problem solving and are associated with processing speed, executive functioning, and memory (Baltes, 1993; Salthouse et al., 2003; Schretlen et al., 2000) deteriorate in older age (Hedden & Gabrieli, 2004). On the other hand, crystallized abilities, often represented by verbal knowledge, accumulate as a result of life experience and are preserved or improve until older age (Horn, 1982; Horn & Cattell, 1967; Horn & Hofer, 1992; Singh-Manoux et al., 2012).
Importantly, the cognitive aging process is malleable within and variable across persons (Hertzog et al., 2009), partly due to lifestyle choices people make throughout their lives (Lindenberger, 2014). For example, physical activity (PA), intellectual, and social engagement may help maximize cognitive function and mitigate losses (Hertzog et al., 2009). On one hand, PA, in particular, exercise, is related to better fluid abilities in older age such as speed, executive function and working memory (Hillman et al., 2008; Kramer et al., 1999; Voss, Erickson, et al., 2013). This is thought to occur via a variety of physiological and neuronal mechanisms, such as upregulation of neurotrophic factors, cardiorespiratory fitness, and decreased risk of hypertension (Bherer et al., 2013; Voss et al., 2014). On the other hand, most social and cognitive activities known as proxies of cognitive reserve associated with better cognitive function in older age are sedentary (Clare et al., 2017; Stern, 2009; Stern et al., 1994, 2018), such as structured education (Guerra-Carrillo et al., 2017), cognitively stimulating occupational work (Bonsang et al., 2012; Rohwedder & Willis, 2010), and cognitively stimulating leisure activities such as reading, solving riddles or playing games (Hertzog et al., 2008). Yet, excessive sedentariness has negative impacts on general health (Tremblay et al., 2010) and evidence is emerging for its negative associations with cognitive and brain health (Burzynska et al., 2014; Falck et al., 2017; Voss et al., 2014). Furthermore, there is increasing recognition for the general (Varma, Tan, et al., 2014) and brain health benefits of light PA (specifically, low-intensity walking) in older age (Varma, Chuang, et al., 2014). Therefore, the aim of the current study was to examine the still poorly understood associations between daily engagement in all levels of lifestyle PA, including sedentary behaviors, light, moderate, and vigorous PA, and cognition in cognitively healthy older adults.
Most evidence relating PA to cognitive benefits in older age focuses on participation in exercise or moderate to vigorous PA. Exercise is defined as planned, structured, and repetitive PA with an objective of the improvement of physical fitness (Caspersen et al., 1985). This includes, for example, running, swimming, or participation in fitness classes (Bherer et al., 2013; Erickson & Kramer, 2009; Hillman et al., 2008). Moderate to vigorous PA is a broader term and includes both exercise as well as other high-intensity everyday life activities, such as walking or biking for transportation, climbing stairs, or strenuous housework. Moderate and vigorous PA involves elevated energy expenditure (3–6 and 6+ metabolic equivalents, respectively), and, therefore, is the most effective PA intensity to increase cardiorespiratory fitness (Helgerud et al., 2007). Exercise-induced increases in cardiorespiratory fitness have been proposed as a crucial factor underlying exercise-related benefits in fluid abilities (Clark et al., 1992; Colcombe et al., 2003, 2004; Colcombe & Kramer, 2003; Erickson et al., 2011; Hillman et al., 2008; Kramer et al., 1999; Pase et al., 2016; Raz & Daugherty, 2017; Voss, Erickson, et al., 2013; Voss, Vivar, et al., 2013). However, for most of the day, older adults engage in sedentary behaviors and light PA (Craft et al., 2012; Dunstan et al., 2012). Sedentary behavior is defined by a seated or reclined body posture and low energy expenditure (≤1.5 metabolic equivalents) and light PA includes low-intensity activities such as low-intensity walking, housework, or gardening (1.5–3 metabolic equivalents (Copeland et al., 2015; M. G. Davis et al., 2011; Rhodes et al., 2012). Therefore, given that most adults do not meet the minimum American College of Sports Medicine recommendations of engaging in moderate or vigorous PA, understanding how sedentary behavior and light PA relate to cognitive function is key for developing recommendations and interventions that will complement those focused on moderate to vigorous PA.
However, our understanding of the relationships between lifestyle PA and cognition is limited because the great majority of studies have relied on self-reports, daily activity logs or questionnaires, which have limited validity and reliability for measuring time spent in sedentary behavior and light PA or the time and intensity of typically short bouts of moderate to vigorous PA (Dogra et al., 2017; Rzewnicki et al., 2003; Sallis & Saelens, 2000; Shephard, 2003). Sensor-based tools, including accelerometers, provide more accurate estimates of sedentary behavior and light PA, which are hardest to capture with self-reports (De Carvalho Bastone et al., 2014; España-Romero et al., 2014; Gardiner et al., 2011; Gennuso et al., 2015; Healy et al., 2008; Jefferis et al., 2016; Pfister et al., 2017; Van Cauwenberg et al., 2014; Visser & Koster, 2013). For example, self-reported sedentariness (5.3 h/day) was shown to be significantly underestimated as compared with sensor-based sedentary behavior (9.4 h/day) in older populations (Heesch et al., 2018).
To date, few studies have investigated sensor-assessed PA in relation to cognition (gauging predominantly fluid abilities) in older adults. Among these, most studies focused on total PA (e.g., mean counts or steps/day) and found mostly positive associations with executive function (Barnes et al., 2008; Zeitzer et al., 2018), memory (Buchman et al., 2008; Hayes et al., 2015; Wilbur et al., 2012), greater maintenance of processing speed (Buchman et al., 2012), as well as general cognition (composite scores or short clinical assessments) (Barnes et al., 2008; Buchman et al., 2008), and reduced incidence of dementia. However, several studies reported no relationship between total PA and fluid abilities (memory, attention, speed) and crystallized abilities (verbal fluency) and general cognition (Brown et al., 2012; Halloway et al., 2017). To our knowledge, only ten studies have used sensors to measure levels of PA intensity in relation to cognition in cognitively normal older adults (Supplementary material I). Several studies reported positive associations between moderate to vigorous PA and fluid abilities such as visuospatial ability (Brown et al., 2012), memory (Wilbur et al., 2012; Zhu et al., 2017), executive function (Kerr et al., 2013; Zhu et al., 2017), general cognitive status (Stubbs et al., 2017), or crystallized ability measured as verbal fluency (Brown et al., 2012), but almost as many studies have reported non-significant associations with fluid abilities after adjusting for typical covariates such as age, sex, education, or BMI (Halloway et al., 2017, 2019; Iso-Markku et al., 2018; Johnson et al., 2016). Regarding sensor-measured light PA, several studies reported a positive association with fluid abilities including executive function (Johnson et al., 2016), perceptual speed (Kerr et al., 2013), semantic memory (Wilbur et al., 2012), general cognition (Iso-Markku et al., 2018), and cognitive status maintenance (Stubbs et al., 2017), but others found no associations (Halloway et al., 2017; Kerr et al., 2013; Zhu et al., 2017). Finally, more time spent sedentary correlated with poorer general cognition (Iso-Markku et al., 2018; Ku et al., 2017) and memory (Hayes et al., 2015), but others reported no associations with fluid abilities (Johnson et al., 2016; Zhu et al., 2017).
In sum, studies using sensors to differentiate levels of PA intensity in relation to cognition have yielded promising, yet inconsistent findings, which likely stem from differences in PA measurement and limited cognitive assessments (e.g., single tasks, short clinical assessments of cognitive status), insufficient to measure latent constructs or to contrast fluid and crystallized abilities (Supplementary material I). In addition, only age and sex have been consistently used as covariates, which is well justified by the age differences in cognition (Park et al., 2002), and age and sex differences in lifestyle PA (Bauman et al., 2012; Chodzko-Zajko et al., 2009; Hagströmer et al., 2007; Hansen et al., 2012; Hedden & Gabrieli, 2004; Janssen et al., 2016; Peters et al., 2010; Troiano et al., 2008). However, other modifiable health and socioeconomic factors need to be considered in exploring PA-cognition associations. For example, cardiorespiratory fitness is the desirable long-term benefit of engaging in moderate to vigorous PA, whereas excessive sedentariness and lack of PA are comorbid with cardiovascular and metabolic disorders such as hypertension, diabetes, and obesity (Ekelund et al., 2016; Ford et al., 1991), all of which are risk factors for cognitive decline in older age (Spiro & Brady, 2012). Furthermore, higher education and income have been associated with greater maintenance of cognitive function (Albert et al., 1995; Clare et al., 2017; Inouye et al., 1993; Stern, 2009; Stern et al., 1994) and PA engagement in older age (August & Sorkin, 2011; Birmingham et al., 1999; Bolen et al., 2000; Humphreys & Ruseski, 2006; Kari et al., 2015; Meltzer & Jena, 2010). Employment status among older adults is also important: transition to retirement is associated with declines in both PA (Feng et al., 2016) and cognitive functioning (Clouston & Denier, 2017). Finally, functional and physical health may influence the patterns of lifestyle PA (Gorman et al., 2014; Trost et al., 2002).
The current study was designed to revisit and extend previous findings linking lifestyle PA with cognition, using well-validated measures of fluid and crystallized cognitive abilities (Salthouse, 2004, 2010; Salthouse et al., 2004; Salthouse & Ferrer-Caja, 2003), sensor-measured lifestyle PA, and a comprehensive set of covariates, including, among others, cardiorespiratory fitness measured with gold-standard graded exercise test, cardiovascular risk history, blood pressure, income and education, and measures of functional fitness. We hypothesized that adults spending more time in moderate to vigorous PA would have better fluid abilities. At the same time, we predicted spending more time in sedentary behaviors would be negatively associated with fluid abilities but positively with crystallized abilities. We had less information to make a strong prediction regarding light PA, and so analyses for light PA are considered exploratory.
Method
Participants
The current study was conducted using the baseline data from a 6-month randomized controlled exercise trial (clinical study identifier NCT01472744). The study was approved by the University of Illinois institutional review board, written informed consent was obtained from all participants, and the study was performed in accordance with the 1964 Declaration of Helsinki. Healthy, low active older adults were recruited in Champaign county. Of the 1,119 participants recruited, 247 (n = 169 women) met inclusion criteria for the initial clinical trial, agreed to enroll in the study, and underwent a series of demographic, health, PA, neuroimaging, cognitive, and cardiorespiratory data collection at baseline. For more details on this clinical trial, its primary outcomes and neuroimaging data, refer to our earlier work (Baniqued et al., 2018; Burzynska et al., 2017; Ehlers et al., 2017; Fanning et al., 2017; Voss et al., 2019). Participants eligible to be enrolled in the initial clinical trial met the following criteria: (1) were between the ages of 60 and 80 years old, (2) were free from psychiatric and neurological illness and had no history of stroke, transient ischemic attack, or head trauma, (3) scored ≥ 23 on the Mini-Mental State Exam and >21 on a Telephone Interview of Cognitive Status questionnaire, (4) scored < 10 on the geriatric depression scale (GDS-15), (5) scored ≥ 75% right-handedness on the Edinburgh Handedness Questionnaire (a criterion related to functional MRI analyses), (6) demonstrated normal or corrected-to-normal vision of at least 20/40 and no color blindness, (7) were screened for safe participation in an MRI environment (e.g., no metallic implants that could interfere with the magnetic field or cause injury and no claustrophobia), and (8) reported to have participated in no more than two bouts of moderate exercise per week within the past 6-months. This last criterion was designed to target typically low-active older adults who do not exercise regularly and do not exceed the minimum recommendations of 150 moderate PA per week and, therefore, would benefit most from the aerobic exercise intervention. As previously reported (Voss et al., 2019), 33 participants of 247 reported taking anti-depressant medications; the majority (79%) of these participants were taking some form of a selective serotonin reuptake inhibitor and most others (12%) were taking drugs that block reuptake of norepinephrine and dopamine (e.g., bupropion). Finally, in addition to the general inclusion criteria, we applied a stricter cutoff regarding cognitive status (Mini-Mental State Exam score ≥ 26) for the analyses presented here to exclude the few participants with possible mild cognitive impairment. Table 1 defines our final sample with respect to age, sex, race, and ethnicity composition.
Table 1.
Sample characteristics
| N | Minimum | Maximum | M | SD | |
|---|---|---|---|---|---|
| Age | 228 | 60 | 78 | 65.3 | 4.5 |
| Sex (female) | 155 | 68% | |||
| Race & Ethnicity | |||||
| White | 196 | 86% | |||
| Black | 26 | 11.4% | |||
| Asian | 6 | 2.6% | |||
| Non-Hispanic | 224 | 98.2% | |||
| Hispanic | 3 | 1.3% | |||
| Socioeconomic factors | |||||
| Household annual income: | |||||
| <40,000 | 54 | 23.7% | |||
| >40,000 | 134 | 58.8% | |||
| Not known | 40 | 17.5% | |||
| Years of education | 234 | 12 | 26 | 15.9 | 3.0 |
| Employment status: | |||||
| Full time >35 h/week | 51 | 22% | |||
| Part time <35h/week | 30 | 13% | |||
| Retired, part time | 33 | 15% | |||
| Retired, not working | 107 | 47% | |||
| Unemployed or other | 7 | 3 % | |||
| Comfort using a computer* | 228 | 1 | 5 | 3.5 | 1.2 |
| Adult education** | 228 | 0 | 3 | .4 | .7 |
| Cardiometabolic health | |||||
| VO2 peak [ml/kg/min] | 226 | 6.5 | 34.2 | 19.7 | 4.7 |
| Mean arterial pressure | 226 | 75 | 119 | 97 | 8 |
| Cardiovascular History score | 228 | 0 | 9 | 3.7 | 2.0 |
| Body mass index | 226 | 15 | 51 | 31 | 6 |
| Diabetes (positive) | 32 | 14% | |||
| Functional health | |||||
| Self-rated health*** | 227 | 1 | 4 | 2 (median) | .76 |
| Physical health score | 228 | 22 | 63 | 47 | 9 |
| Functional fitness**** | 227 | −1.12 | 1.04 | −.0017 | .36 |
| Cognition ***** | |||||
| Perceptual Speed | |||||
| Digit symbol | 227 | 15 | 110 | 65.4 | 14.5 |
| Pattern comparison | 228 | 8 | 26 | 15 | 2.9 |
| Letter comparison | 228 | 4.5 | 18 | 9.6 | 2.0 |
| Memory | |||||
| Word recall | 228 | 19 | 65 | 44 | 8.8 |
| Logical memory | 228 | 18 | 63 | 43.7 | 8.6 |
| Paired associates (correct items averaged across 12 word pairs) | 223 | 0 | 1 | 0.34 | 0.25 |
| Reasoning | |||||
| Shipley abstract | 228 | 3 | 20 | 12 | 3.3 |
| Form board total | 195 | 0 | 21 | 5.7 | 3.7 |
| Letter set total | 196 | 4 | 15 | 11.1 | 2.7 |
| Matrix reasoning total | 197 | 1 | 16 | 8.1 | 3.0 |
| Paper folding total | 198 | 1 | 11 | 5.4 | 2.5 |
| Spatial relations | 198 | 0 | 20 | 8.0 | 4.6 |
| Vocabulary | |||||
| WIAS vocabulary total | 228 | 9 | 61 | 46.1 | 10.3 |
| Picture vocabulary total | 228 | 4 | 29 | 20.0 | 4.8 |
| Synonym total | 197 | 0 | 10 | 6.9 | 2.7 |
| Antonym total | 198 | 0 | 10 | 6.0 | 3.1 |
| Mini Mental Status Exam | 228 | 26 | 30 | 28.7 | 1.2 |
| Physical activity | |||||
| Sedentary Time (min/day) | 228 | 295 | 810 | 537 | 83 |
| Light PA (min/day) | 228 | 147 | 528 | 276 | 69 |
| Moderate to vigorous PA (min/day) | 228 | 4.7 | 139 | 40****** | 37****** |
| Wear time (days) | 228 | 3 | 17 | 7 | 1 |
| Average Counts (counts/day) | 228 | 49035 | 553117 | 181121 | 80040 |
Note.
1: low to 5: proficient;
0: never participated, 1: 1–2 classes, 2: 3–5 classes, 3: > 5 classes;
1: Excellent, 2: Very Good, 3: Good, 4: Fair, 5: Poor;
Descriptive statistics for the nine tasks from the functional fitness test are presented in the Supplementary material II;
Units refer to correct responses unless specified otherwise;
Due to skewness, median and interquartile range are presented instead of mean and SD.
Actigraphy
To reconcile previous findings with respect to measures of lifestyle PA, we used an ActiGraph device used in eight out of ten of the aforementioned studies using linking sensor-based PA measures to cognition (see Supplementary material I). Each participant was instructed to wear the accelerometer (Model GT1M or GT3X; ActiGraph, Pensacola, FL) on the non-dominant hip during waking hours for 7 consecutive days and record the time that they wore the device each day on a log. A valid measurement day consisted of at least 10 hours of valid wear-time, with a valid hour defined as no more than 60 consecutive minutes of zero counts with one-minute sampling epochs. Only data for individuals with a minimum of 3 valid days of wear time were included in analyses (Peterson et al., 2010; Troiano et al., 2008). These data were downloaded as activity counts, which represent raw accelerations that have been summed over a specific epoch length (e.g., 60s), and these counts vary based on frequency and intensity of the recorded acceleration (Fanning et al., 2017). Next, these data were processed using cut points designed specifically for older adults (Copeland & Esliger, 2009) such that 50 or fewer counts per minute corresponded with sedentary behavior, 51–1,040 counts per minute corresponded to light PA, and 1,041 counts or greater represented moderate to vigorous PA. All 234 participants had at least 3 days of valid PA data recording with on average 6.98±1.4 valid days of measurement (range 3–7). 90% of the sample (210 participants) had 6 or more valid days required to reliably measure sedentary behavior (Hart et al., 2011). Only 0.4% of the sample (9 participants) had less than 5 valid days of wear. The primary accelerometer-related variables were average daily time spent in sedentary behavior, light, and moderate to vigorous PA (Table 1). Wear time (total valid days) was used to examine the associations of wear time with sedentary behavior, light, and moderate to vigorous PA (Supplementary material III). Average daily counts (total counts/number of valid days) was used to report bivariate correlations for comparison with the existing literature (Supplementary material III). There were two outliers (>3SD) in moderate to vigorous PA and one in light PA and sedentary behavior, which were winsorized (i.e., assigned the highest number in the sample <3 SD). Our sample size was defined as the 228 out of 247 participants who had complete Actigraphy data.
Cognition: fluid and crystallized abilities
We administered a well-validated Virginia Cognitive Aging Project cognitive battery (Salthouse, 2004, 2010; Salthouse et al., 2004; Salthouse & Ferrer-Caja, 2003) consisting of 16 tasks that measure latent constructs of fluid abilities (reasoning, perceptual speed, episodic memory), and crystalized ability (vocabulary knowledge). The computer-based tasks were programmed in E-prime version 1.1 (Psychology Software Tools, Pittsburgh, PA) and administered on computers with 17″ cathode ray tube monitors. For more details on individual tasks and analysis see (Burzynska et al., 2015). First, to confirm validity of task structure as presented in (Salthouse, 2004), we performed factor analysis using principal component analysis with varimax rotation, with missing values replaced by sample mean. Next, we calculated standardized scores (z-scores) and winsorized values that were >3SD (two for digit symbol, letter comparison and WAIS vocabulary, three for pattern comparison, and one for form boards and picture vocabulary). Then, the z-scores were averaged according to the task groupings specified in Table 1, resulting in four component scores representing vocabulary knowledge, perceptual speed, memory and reasoning. Due to technical issues with saving computerized tasks, 30 participants had data for only one task within a construct of reasoning, therefore, the z-score of this task was used for the construct to maximize sample size. Note that the advantage of the current analyses was to measure latent constructs of perceptual speed, memory, reasoning, and vocabulary knowledge. For this reason, we did not include the two tasks measuring executive function (Task Switching) and working memory (Spatial Working Memory).
Modifiable socioeconomic and health factors
Socioeconomic factors included years of education and annual household income, which was dichotomized as <$40,000 and >40,000 to define low and high income in Illinois. Current cognitive activities were measured as participation in adult education (0: never participated, 1: took overall 1–2 classes, 2: took 3–5 classes, 3: took > 5 classes), employment status (dichotomized into those fully retired or unemployed 50%, n=114, and working full- or part-time), and comfort using a computer (as a proxy of time and skill, coded as 1: excellent, 2: very good, 3: good, 4: fair, 5: poor; Table 1).
We assessed cardiometabolic health with cardiorespiratory fitness, cardiovascular history score, body mass index, diabetes diagnosis, and mean arterial pressure (Table 2), namely, the main cardiometabolic diseases or risk factors comorbid with excessive sedentariness and lack of PA (Ekelund et al., 2016; Ford et al., 1991) and also linked to decreased cognitive performance in older age (Assuncao et al., 2018). Cardiorespiratory fitness was assessed as peak oxygen uptake (VO2peak) during graded maximal exercise testing on a motor-driven treadmill. Participants received consent from their personal physician prior to cardiorespiratory fitness testing. Before the treadmill test, we collected resting systolic and diastolic blood pressure, body weight, and height. The protocol involves walking at a self-selected pace with incremental grade increases of 2–3% every two min. Measurements of oxygen uptake, heart rate and blood pressure were constantly monitored. VO2 peak was measured from expired air samples taken at 30-s intervals until a peak oxygen uptake was attained; test termination was determined by symptom limitation, volitional exhaustion, and/or attainment of VO2 peak as per American College of Sports Medicine guidelines (acsm.org). Due to technical problems cardiorespiratory fitness data were not collected from 2 participants, resulting in n = 226 for VO2 peak. Body mass index was calculated as kg body mass/m2 of body height. Mean arterial pressure was calculated as (systolic blood pressure +(2 × diastolic blood pressure))/3 as a measure of systemic vascular resistance and general perfusion.
Table 2.
Backward elimination regression: secondary predictors of cognition
| Speed | Vocab. | Memory | Reasoning | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Std.β | t | p | VIF | Std.β | t | p | VIF | Std.β | t | p | VIF | Std.β | t | p | VIF | |
| Income | ||||||||||||||||
| Education | .344 | 5.0 | <.000 | 1.0 | .145 | 2.0 | .047 | 1.1 | ||||||||
| Employment | −.159 | −2.2 | .026 | 1.1 | ||||||||||||
| Computer | .300 | 4.3 | <.001 | 1.1 | .208 | 2.9 | .004 | 1.1 | .276 | 3.9 | <.001 | 1.1 | ||||
| Adult Edu. | .163 | 2.4 | .018 | 1.0 | ||||||||||||
| Mean arterial pressure | .138 | 2.0 | .045 | 1.0 | ||||||||||||
| Cardiovascular History | ||||||||||||||||
| VO2peak | .143 | 2.0 | .048 | 1.1 | ||||||||||||
| Body mass index | ||||||||||||||||
| Diabetes | ||||||||||||||||
| Health | −.183 | −2.5 | .012 | 1.1 | ||||||||||||
| Physical health | ||||||||||||||||
| Functional fitness |
Note. VIF: variance inflation factor
Self-rated health was measured on scale 1 to 5. 15% of participants described their health as excellent, 45% as very good, 36 % as good, and 4% as fair. Physical health status was also measured with the physical component score of the 12-Item Short Form Health Survey (Ware et al., 1996), with higher scores indicating better health status. The functional fitness test (Rikli & Jones, 2013) included time to walk stairs up and down, chair stand test, chair sit and reach test, arm curl test, back scratch, 8ft up and go, left leg, right leg, and dominant leg stand time (Supplementary material II). Functional fitness composite score was calculated as a sum of z-scores of all individual tests. Bivariate correlations between variables from Table 1 are presented in Supplementary material III.
Statistical analyses
All analyses were carried out using SPSS v. 26. Our aim was to test whether time spent in different intensities of lifestyle PA (i.e., sedentary behavior, light, and moderate to vigorous PA) was associated with cognitive ability across several domains. In testing these associations, we adjusted for multiple demographic, socioeconomic, and health variables. The demographic covariates included age and sex. Next, to identify a parsimonious set of predictors of each cognitive construct and to maximize degrees of freedom in our moderately-sized sample, we used backward elimination regression on the socioeconomic and health variables from Table 1. Days of accelerometer wear were excluded as they were not related to measures of lifestyle PA or cognition (Supplementary material IV). In brief, backward elimination starts with a global linear regression model with all variables included, and removes nonsignificant predictors from the model one-by-one until all are significant with a p-value criterion of 0.1 (Heinze et al., 2018; Heinze & Dunkler, 2017). Then, we carried out four multiple regression models (one for each cognitive construct as a dependent variable), with the sedentary time, light and moderate to vigorous PA, age, sex, and the selected socioeconomic and health factors as independent variables. The alpha levels for overall model fit were adjusted using Bonferroni correction (0.05/4=0.0125). The assumptions of linearity, normality of distributed errors, and uncorrelated errors were tested and fulfilled by all regression models.
Results
First, using backward elimination regression, we identified the following predictors of cognitive abilities that should be considered as covariates in the main analyses. These were the comfort at a computer, employment status, and mean arterial pressure for perceptual speed, education and adult education for vocabulary knowledge, comfort at a computer and perceived health for memory, and education, comfort at a computer, and cardiorespiratory fitness for reasoning (Table 2).
Next, multiple linear regression analysis adjusting for age, sex, education, adult education, light, and moderate to vigorous PA revealed a positive association between sedentary behavior and vocabulary knowledge (R2=.235, Fchange(7/220) = 9.7, p < 0.001, Table 3), of a small effect size (Figure 1). Education and adult education were also positively associated with vocabulary knowledge, with moderate and small effect sizes (Cohen J., 1988), respectively.
Table 3.
Associations with vocabulary knowledge
| IV | Std. β | SE | t | p | f2 | LB | UP | VIF |
|---|---|---|---|---|---|---|---|---|
| Age | −.006 | .012 | −.1 | .915 | <.02 | −.024 | .022 | 1.0 |
| Sex | .091 | .120 | 1.4 | .157 | <.02 | −.066 | .406 | 1.2 |
| Education | .364 | .018 | 5.9 | <.001 | .16 | .072 | .144 | 1.1 |
| Adult Education | .144 | .074 | 2.4 | .018 | .026 | .031 | .323 | 1.0 |
| Sedentary behavior | .170 | .001 | 2.3 | .023 | .024 | .000 | .003 | 1.6 |
| Light PA | −.136 | .001 | −1.8 | .075 | <.02 | −.004 | .000 | 1.7 |
| Moderate to vigorous PA | .072 | .002 | .99 | .325 | <.02 | −.002 | .006 | 1.5 |
VIF: variance inflation factor. LB and UP: lower and upper bounds of 95% confidence intervals.
Figure 1.
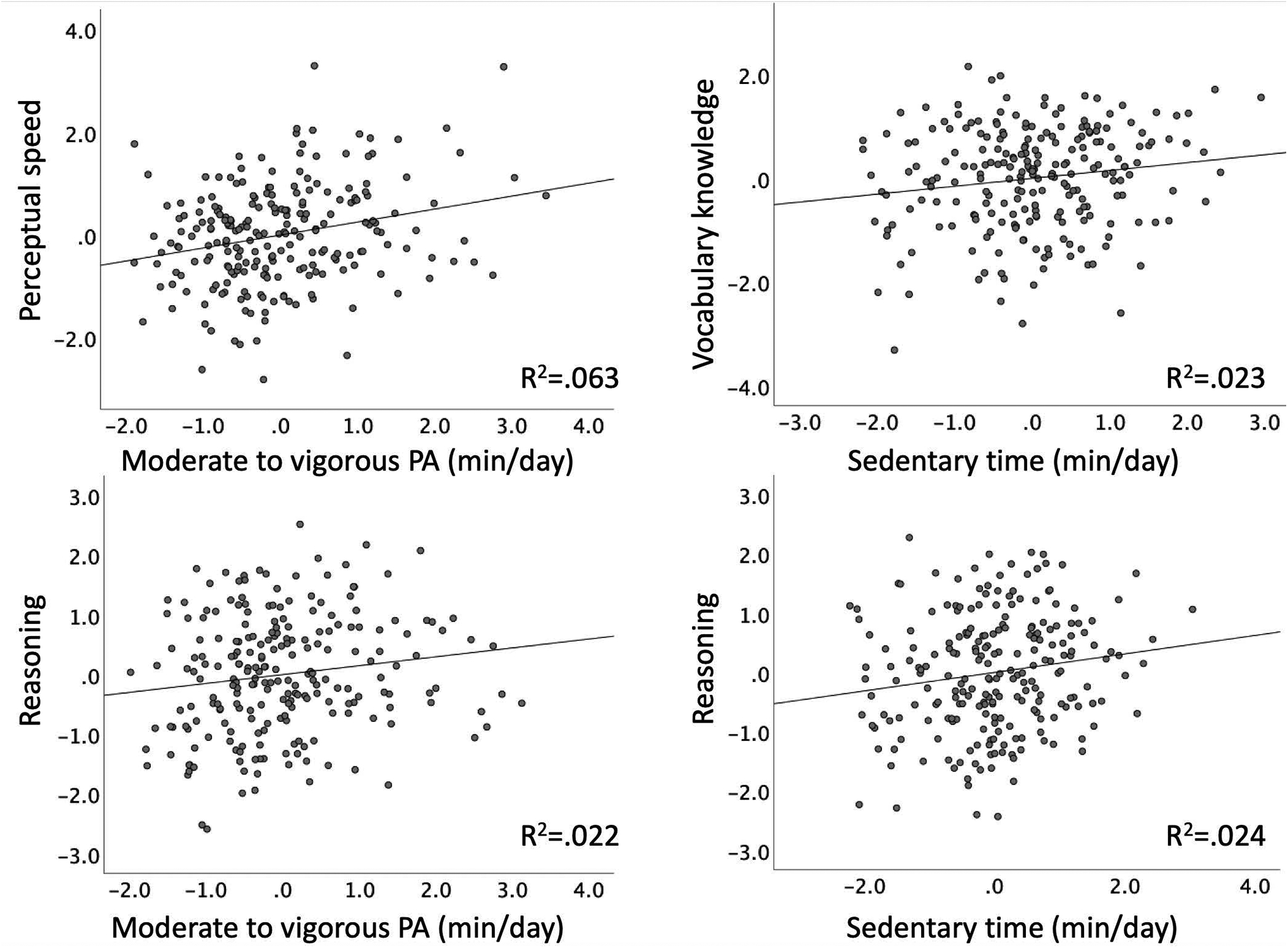
Partial regression plots showing associations of sensor-measured lifestyle PA and cognition shown in Tables 3–5. The residuals of both predictor variables (lifestyle PA) and dependent (cognition) were obtained by regressing the variable against the remaining independent variables in each model. To calculate the residuals for perceptual speed and moderate to vigorous PA (top left), age, sex, employment status, comfort at a computer, mean arterial pressure, and light PA were partialled out (Table 4); vocabulary knowledge and sedentary time residuals (top right) were created by partialling out age, sex, education, adult education, light and moderate to vigorous PA (Table 3); reasoning and moderate to vigorous PA residuals (bottom left) were calculated by regressing out age, sex, education, comfort at a computer, cardiorespiratory fitness, sedentary behavior and light PA (Table 5); reasoning and sedentary time residuals (bottom right) were created by regressing out age, sex, education, comfort at a computer, cardiorespiratory fitness, light, and moderate to vigorous PA (Table 5).
Moderate to vigorous PA was positively associated with perceptual speed in a fully adjusted model (R2=.243, Fchange(8/217) = 8.7, p < 0.001, Table 4, Figure 1). Being younger, female, employed, having greater comfort at a computer and greater mean arterial pressure was also associated with faster perceptual speed, with small effect sizes.
Table 4.
Associations with perceptual speed
| IV | Std. β | SE | t | p | f2 | LB | UP | VIF |
|---|---|---|---|---|---|---|---|---|
| Age | −.129 | .011 | −2.2 | .033 | <.02 | −.045 | .002 | 1.0 |
| Sex | .169 | .111 | 2.7 | .008 | .033 | .079 | .518 | 1.1 |
| Employment status | −.166 | .034 | −2.6 | .009 | .032 | −.159 | −.023 | 1.1 |
| Comfort at a computer | .205 | .043 | 3.3 | .001 | .050 | .057 | .227 | 1.1 |
| Mean arterial pressure | .150 | .006 | 2.5 | .013 | .033 | .003 | .027 | 1.0 |
| Sedentary behavior | −.009 | .001 | −.1 | .900 | <.02 | −.002 | .001 | 1.6 |
| Light PA | −.020 | .001 | −.3 | .799 | <.02 | −.002 | .002 | 1.7 |
| Moderate to vigorous PA | .280 | .002 | 3.8 | <.001 | .067 | .004 | .012 | 1.5 |
VIF: variance inflation factor.
Both time in sedentary behavior and moderate to vigorous PA were positively associated with reasoning in a fully adjusted model (R2=.236, Fchange(8/217) = 8.4, p < 0.001, Table 5, Figure 1). In addition, comfort at a computer and cardiorespiratory fitness showed also a positive association; all effect sizes were small. The effect of sex was statistically significant but the effect size was negligible.
Table 5.
Associations with reasoning
| IV | Std. β | SE | t | p | f2 | 95% LB | 95% UB | VIF |
|---|---|---|---|---|---|---|---|---|
| Age | −.104 | .011 | −1.7 | .088 | <.02 | −.040 | .003 | 1.0 |
| Sex | .137 | .118 | 2.0 | .047 | .018 | .004 | .470 | 1.3 |
| Education | .112 | .017 | 1.8 | .079 | <.02 | −.004 | .065 | 1.1 |
| Comfort at a computer | .274 | .042 | 4.4 | <.001 | .089 | .102 | .269 | 1.1 |
| Cardiorespiratory fitness | .162 | .013 | 2.1 | .036 | .025 | .002 | .054 | 1.7 |
| Sedentary behavior | .174 | .001 | 2.3 | .021 | .025 | .000 | .003 | 1.6 |
| Light PA | −.025 | .001 | −.3 | .746 | <.02 | −.002 | .002 | 1.7 |
| Moderate to vigorous PA | .177 | .002 | 2.2 | .028 | .024 | .001 | .009 | 1.8 |
VIF: variance inflation factor.
Finally, the overall model predicting memory was also significant (R2=.207, Fchange(7/219) = 8.1, p < 0.001), but no lifestyle PA significantly contributed to the model. Female sex and comfort at a computer were associated with better memory, with small effect sizes (Table 6). The effect of perceived health was statistically significant but of negligible effect size.
Table 6.
Associations with memory
| IV | Std. β | SE | t | p | f2 | LB | UB | VIF |
|---|---|---|---|---|---|---|---|---|
| Age | −.102 | .011 | −1.7 | .097 | <.02 | −.041 | .003 | 1.0 |
| Sex | .333 | .116 | 5.1 | <.001 | .13 | .361 | .818 | 1.2 |
| Comfort at a computer | .200 | .043 | 3.2 | .002 | .05 | .053 | .222 | 1.1 |
| Perceived health | −.137 | .068 | −2.2 | .030 | .018 | −.283 | −.015 | 1.1 |
| Sedentary behavior | .088 | .001 | 1.2 | .245 | <.02 | −.001 | .002 | 1.6 |
| Light PA | −.139 | .001 | −1.8 | .075 | <.02 | −.004 | .000 | 1.7 |
| Moderate to vigorous PA | .098 | .002 | 1.3 | .193 | <.02 | −.001 | .007 | 1.6 |
VIF: variance inflation factor.
Discussion
We investigated the relationships between time spent in different intensities of sensor-measured lifestyle PA and cognition in healthy older adults. Our main finding was that time in both moderate to vigorous PA and sedentary time were positively associated with cognition, after adjusting for multiple demographic, socioeconomic, and health covariates. Furthermore, there was a dissociation between PA intensity and cognitive performance. Namely, we found that older adults who spent more time in sedentary behavior performed better on vocabulary knowledge and reasoning tasks, whereas spending more time in moderate to vigorous PA correlated with better perceptual speed and reasoning. We discuss our findings in the light of models of cognitive aging and the existing literature. Then, we discuss implications of our results for future research and lifestyle guidelines.
Positive associations of sedentary behavior with crystallized and fluid abilities
We found that sedentary time was positively associated with vocabulary knowledge, even when adjusting for education and adult education, the well-known correlates of crystallized abilities (Ackerman, 1996). This result is consistent with our hypothesis related to the fact that most cognitive activities related to acquiring knowledge and expanding vocabulary are sedentary (Seider et al., 2016), but novel in demonstrating such relationships using sensor-measured sedentary behavior. Contrary to our hypothesis, we found a positive association between sedentary time and reasoning ability, in addition to positive correlations of reasoning with moderate to vigorous PA and cardiorespiratory fitness (discussed below).
It is tempting to speculate that the time spent sedentary was related to engaging in cognitively stimulating sedentary activities. For example, earlier studies linked computer activities, reading, playing games, craft activities, viewing educational TV or DVDs with better verbal memory and performance on Trail Making Test-B, global cognitive function, reduced subjective cognitive complaints, and decreased odds of mild cognitive impairment (Barnes et al., 2013; Geda et al., 2011; Kesse-Guyot et al., 2012; Nemoto et al., 2018). However, a bidirectional or third variable relationship is equally plausible in our cross-sectional dataset. For example, there is a well-established association between fluid abilities and educational attainment (Deary et al., 2007), whereas educational attainment and socioeconomic status is associated with more time spent reading (Smith, 1990), using a computer (Chu et al., 2009), but less time watching TV (Molina et al., 2016). In this vein, adults with higher socioeconomic status may partake in more cognitively enriching leisure activities or those with higher educational and occupational attainment may continue to engage in more cognitively stimulating sedentary activities post-retirement. Thus, future studies should combine sensor-based measures of sedentary time with 24-hour recalls (Matthews et al., 2018) and real-time assessments such as the Experience Sampling Method (Csikszentmihalyi & Larson, 2014) or Ecological Momentary Assessment (Shiffman et al., 2008) to assess the nature and context (e.g., educational content and cognitive challenge) of both leisure and occupational sedentary activities with less recall bias and greater ecological validity than standard questionnaires. For instance, watching TV, in general, is considered less cognitively stimulating and of lower energy expenditure than reading, typing, playing piano or playing board games (Ainsworth et al., 2011; Shields & Tremblay, 2008). Conversely, focused in-class training of reasoning strategies and problem solving resulted in immediate and long-term improvement of fluid ability in older adults (the ACTIVE trial, (Rebok et al., 2014). After key characteristics of sedentary activities are identified, the time-ordered or causal relationships will need to be established in observational longitudinal or experimental studies, such as by comparing vocabulary knowledge acquisition and long-term retention of selected sedentary activities (Grabe et al., 2009).
Finally, we did not find the hypothesized negative association between sedentary time and cognitive function. Importantly, the few existing studies that reported such negative associations with sensor-measured sedentariness used only short dementia screening assessments (Iso-Markku et al., 2018; Ku et al., 2017) or identified such relationships with fluid abilities only in post-hoc analyses (Hayes et al., 2015). Thus, future studies combining blood metabolic profiles with biomarkers of brain health (Voss et al., 2014) are needed to reconcile the negative metabolic effects of excessive sedentariness (Dunstan et al., 2012; Tremblay et al., 2010) with the positive associations with fluid and crystallized abilities. Of note, the lack of association of sedentary time with memory and perceptual speed is consistent with the two aforementioned studies that reported null correlations with processing speed (Johnson et al., 2016) and memory (Zhu et al. 2017).
Positive associations of moderate to vigorous PA with fluid abilities
As we hypothesized, we found positive associations between daily time in moderate to vigorous PA and fluid abilities, specifically, perceptual speed and reasoning. Those associations, although of small effect sizes, are important, because declines in processing speed, reasoning, and executive function are the hallmarks of cognitive aging (Finkel et al., 2007; Salthouse, 2010). Note that reasoning can be considered a higher-order executive function as it relies on working memory, cognitive flexibility and inhibitory control (Diamond, 2013). In line with literature, perceptual speed and reasoning showed the most negative correlation with age among the four cognitive constructs in our sample (see bivariate correlations in Supplementary material III). Our findings are an important extension of limited and inconsistent evidence linking sensor-based moderate to vigorous PA to fluid abilities, including executive function, reasoning, and perceptual speed, as outlined in the introduction. Specifically, one previous study linked peak daily PA intensity to reasoning assessed with a single task of visuospatial ability (Brown et al., 2012), whereas moderate to vigorous PA correlated with Trail Making Test-B performance assessing executive function in one study (Johnson et al., 2016) but not in another (Kerr et al., 2013). The three studies that measured perceptual speed reported non-significant correlations with moderate to vigorous PA (Halloway et al., 2017; Johnson et al., 2016; Wilbur et al., 2012).
Although our cross-sectional results cannot establish causality, several possible explanations need to be considered for future research. Processing speed largely relies on the integrity of myelinated axons within the brain’s white matter (Fields, 2008). In our earlier work, we have shown that white matter microstructure declines with age (Burzynska et al., 2009) and with fluid cognitive abilities (Burzynska et al., 2017; Madden et al., 2012). We also demonstrated that older adults spending more time in moderate to vigorous PA had lower volume of white matter lesions (Burzynska et al., 2014) and lesser 6-month decline in the prefrontal white matter (Burzynska et al., 2017). Therefore, preservation of white matter integrity could be one of the mechanisms linking moderate to vigorous PA with processing speed and reasoning, consistent with the “cortical disconnection” (Bartzokis, 2004) and the “processing speed” theories of cognitive aging (Li et al., 2004; Salthouse, 1996).
Unfortunately, we do not have sufficient information on the type of moderate to vigorous PA that our participants engaged in, which could give more insights into the observed associations. For instance, interceptive sports such as tennis may help train perceptual speed (Voss et al., 2010); however, one of the inclusion criteria was participating in two or less bouts of moderate exercise (and no vigorous exercise) per week within the past 6-months, making such explanation unlikely. Thus, future studies should supplement actigraphy with assessments of the type and context to identify the types of moderate or vigorous PA that best correlate with perceptual speed and reasoning among sedentary older adults, similar as we suggested in the previous section on sedentary activities. It is also important to acknowledge an equally possible bidirectional association, in which older adults with better fluid abilities are more comfortable in engaging in higher-intensity PA. For example, poorer performance on processing speed is the most consistent predictor of future falls and injuries (J. C. Davis et al., 2017).
Furthermore, we confirmed our predictions regarding the associations of cardiometabolic health with fluid abilities. Namely, we found that cardiorespiratory fitness was positively associated with reasoning, which agrees with and extends earlier research on executive function including control, speed, and visuospatial abilities (Colcombe & Kramer, 2003; Hillman et al., 2008). Perceptual speed was positively associated with mean arterial pressure, an index of systemic vascular resistance and blood perfusion. Although indirectly, this result converges with a recent report linking greater global cerebral perfusion with lesser declines in processing speed (Staffaroni et al., 2019).
Our data showed that cardiorespiratory fitness was not a significant predictor of perceptual speed and memory. Furthermore, the moderate-to-vigorous PA was positively associated with reasoning, after accounting for the significant positive association of cardiorespiratory fitness with reasoning. Similarly, moderate-to-vigorous PA was positively associated with perceptual speed, after accounting for the significant positive association of mean arterial pressure with speed. This suggests that engaging in moderate to vigorous PA, a behavior, is as important in relation with cognition as the long-term physiological benefits of moderate to vigorous PA, such as cardiorespiratory fitness and vascular health. This partial dissociation between moderate to vigorous PA and cardiorespiratory fitness for processing speed and reasoning is consistent with the fact that up to 50% of cardiorespiratory fitness may be determined genetically (Bouchard et al., 2011), and, therefore, may not be related to PA behavior. This finding also agrees with our earlier report on a nonagenarian track-and-field athlete who engaged in above average moderate to vigorous PA and outperformed her peers on processing speed, despite relatively low cardiorespiratory fitness (Burzynska et al., 2016). Thus, our findings motivate future research aimed at teasing apart the differential cognitive effects of engaging in structured aerobic exercise, increasing time spent in moderate to vigorous PA, and gains in cardiorespiratory fitness. Individual differences in baseline characteristics and genetics, contamination of randomized controlled trials by outside PA engagement (Ehlers et al., 2016), and mixed support for cognitive benefits of exercise interventions (Young et al., 2015) support the need for future research examining change in moderate to vigorous PA behavior and cardiorespiratory fitness separately. The results of an ongoing clinical trial comparing the effects of aerobic walking 150min/week with 225min/week on cognitive and brain health bear a great promise in elucidating the dose-response relationships between moderate to vigorous PA and cognition (Erickson et al., 2019). As of today, the recommended minimum engagement in moderate to vigorous PA for benefits in fluid abilities remains to be defined.
Contrary to our prediction, moderate to vigorous PA was not associated with episodic memory. However, we should highlight here that the existing evidence linking sensor-measured moderate to vigorous PA to memory is inconsistent and based only on several tasks of semantic memory and word list recall (Halloway et al., 2017; Wilbur et al., 2012; Zhu et al., 2017). Therefore, our non-significant results with regard to memory are not contradicting previous findings, but warrant further investigations with a broader array of tasks gauging different memory sub-processes.
Finally, with regard to secondary predictors of fluid abilities identified in our analyses, employment status is consistent with continued employment around retirement age being related to better cognition in older age (Bonsang et al., 2012; Rohwedder & Willis, 2010) and computer use has been linked with better general cognitive function across the adulthood (Tun & Lachman, 2010; Wu et al., 2019), specifically in older samples (Wu et al., 2019).
No associations of light PA with cognition
Our exploratory analyses suggested no significant associations of light PA with cognition. Thus, our null result converges with several previous studies reporting no relationships between sensor-measured light PA and performance on fluid abilities including episodic memory (Wilbur et al. 2012), processing speed, executive function, visual, and verbal memory (Kerr et al., 2017; Halloway et al., 2017; Hayes et al., 2015; Zhu et al., 2017). Moreover, the previously reported significant associations of sensor-measured light PA to cognition pertain to single tasks of fluid abilities, for example, semantic memory (Wilbur et al. 2012), processing speed (Kerr et al., 2013), executive function (Johnson et al., 2016), or short screenings for cognitive status (Iso-Markku et al. 2018) and self-rated cognition (Stubbs et al., 2017). In addition, although researchers have demonstrated that replacing sedentary time with light PA may improve a number of cardiometabolic outcomes (Buman et al., 2014), our earlier study found no benefits of increasing time in light PA over 6 months on fluid abilities measured with task switching and spatial working memory (Fanning et al., 2016). Although benefits of low-intensity walking have been reported for general, mental, and structural brain health (Varma, Chuang, et al., 2014; Varma, Tan, et al., 2014), it is possible that light PA is less beneficial for cognition than initially predicted. Yet, longitudinal studies will be needed to establish such temporal or casual associations (or lack thereof). As noted above, complimenting sensor-based data with real-time measurements or 24-hour recalls may help identify light activities that obscure the possibly existing relationships.
Limitations
Our cross-sectional study cannot establish causal associations between lifestyle activities and cognition, nor does it enable any time-ordered interpretation of the reported correlations. However, such correlational studies are critical for identifying associations that can then be further explored in observational and experimental longitudinal studies to establish time order or causality, respectively.
There is an ongoing debate on the validity and reliability of measuring sedentary behavior and PA with different types of sensors (Heesch et al., 2018). Thus, our results should be replicated using other devices that use different placements (wrist, thigh or ankle), sampling methods, intensity thresholds, and are worn continuously over the measurement period to capture a wider range of aerobic activities such as swimming, cycling, and resistance training (Wullems et al., 2017). In particular, different PA intensity cut points may need to be explored within the group of older adults, as differences in fitness level, stride length, and other factors, may contribute to some classification error at low to moderate ranges.
Ideally, real-time measurements of the type of the activity, its subjective intensity and cognitive stimulation using Experience Sampling Method (Csikszentmihalyi & Larson, 2014), Ecological Momentary Assessment (Shiffman et al., 2008) or 24-hour recalls (Matthews et al., 2018) should complement the sensor-based measurements. In addition, future studies should include a more detailed battery of processing speed and reaction time tasks, memory and executive function sub-domains, and tasks testing general knowledge beyond vocabulary and language skills.
We acknowledge that our sample was not well balanced with respect to race and ethnicity, and thus we were unable to examine differences in lifestyle PA-cognition associations between racial and ethnic groups. Future research should explore these associations in larger and more diverse samples, particularly given cultural differences that may exist towards health behaviors (August & Sorkin, 2011; Dave et al., 2015). Similarly, our results may have limited generalizability to the general older population due to relatively strict inclusion criteria. Our sample was possibly healthier, fitter, and of higher socioeconomic status, and, therefore, more homogenous than the general older population. Thus, our findings need to be tested in more diverse samples with regard to health, cognitive, and socioeconomic status. In addition, broader age ranges, including middle age and the oldest-old (the fastest growing segment of the population) are needed to probe developmental trends.
It is also important to note that the effect sizes of the associations between lifestyle PA and cognition were small, which does not mean they are not consequential. In other words, the observed correlations of lifestyle PA sampled over one week can be seemingly small, but they can be practically significant across adulthood as lifestyle behaviors repeat over time (Funder & Ozer, 2019). This needs to be tested in larger and independent samples, and in longitudinal designs.
Also, the fact that we did not observe a relationship between comfort at using a computer and vocabulary knowledge or sedentary time may be because this question was initially designed to ensure comfort when solving computer-based tasks, and not to measure time spent working at a computer or objective proficiency.
Conclusions
The value of our approach lies in quantifying daily time in different intensities of lifestyle PA using sensors and assessing crystallized and fluid abilities as separate cognitive constructs. This unique study design provided the first evidence that crystallized and fluid abilities may have distinct lifestyle correlates. The positive associations of sensor-measured moderate to vigorous PA with fluid abilities (perceptual speed and reasoning) extend the existing evidence linking exercise and cardiorespiratory fitness with cognitive and brain health. Namely, our results suggest that, in correlations with cognition, time spent in the behavior of moderate to vigorous PA is at least as important as the desirable long-term physiological benefits of exercise, such as cardiorespiratory fitness or vascular health. This may have an important practical application in helping identify those at risk of decline in fluid abilities by measuring their lifestyle levels of moderate to vigorous PA. Given the accessibility and popularity of PA trackers and cognitive assessments on phones and tablets, it is possible that such data already exist. Furthermore, we contributed new findings of positive associations between sensor-based sedentary behavior, vocabulary knowledge, and reasoning. These findings may have important implications for developing suggestions on how to increase cognitive stimulation during sedentary time. Given the disproportionate time older adults spent sitting each day, our results could inform lifestyle intervention and prevention programs on how to make the best of sedentary activities, including their context and optimal daily ratio to moderate to vigorous PA. For example, training specific fluid abilities (Rebok et al., 2014) or improving crystallized abilities (Stern et al., 2018) could help offset losses in other domains, postponing overall cognitive impairment (Horn, 1970).
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Aging at the National Institutes of Health (R37 AG025667) and funding from Abbott Nutrition through the Center for Nutrition, Learning, and Memory at the University of Illinois. We would also like to thank Anya Knecht, Susan Houseworth, Nancy Dodge, Holly Tracy, and all of the Lifelong Brain and Cognition and Exercise Psychology Laboratory graduate students and staff for their help in participant recruitment and data collection.
Footnotes
The authors declare no competing financial interests.
Contributor Information
Agnieszka Z. Burzynska, Department of Human Development and Family Studies/Molecular, Cellular and Integrative Neurosciences, Colorado State University, Fort Collins, CO, USA
Michelle W. Voss, Department of Psychological and Brain Sciences, University of Iowa, Iowa City, IA, USA
Jason Fanning, Department of Internal Medicine & Health and Exercise Sciences, Wake Forest University, Winston-Salem, MI, USA.
Elizabeth A. Salerno, Cancer Prevention Fellowship Program, Division of Cancer Epidemiology & Genetics, National Cancer Institute, Bethesda, MD
Neha P. Gothe, Department of Kinesiology and Community Health, University of Illinois, IL, USA
Edward McAuley, Department of Kinesiology and Community Health, University of Illinois, IL, USA.
Arthur F. Kramer, Beckman Institute for Advanced Science and Technology at the University of Illinois, IL, USA, Departments of Psychology and Mechanical & Industrial Engineering, Northeastern University, Boston, MA, USA
References
- Ackerman PL (1996). A theory of adult intellectual development: Process, personality, interests, and knowledge. Intelligence, 22(2), 227–257. 10.1016/S0160-2896(96)90016-1 [DOI] [Google Scholar]
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, & Leon AS (2011). 2011 compendium of physical activities: A second update of codes and MET values. Medicine and Science in Sports and Exercise, 43(8), 1575–1581. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, & Rowe JW (1995). Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychology and Aging, 10(4), 578–589. 10.1037/0882-7974.10.4.578 [DOI] [PubMed] [Google Scholar]
- Assuncao N, Sudo FK, Drummond C, De Felice FG, & Mattos P (2018). Metabolic syndrome and cognitive decline in the elderly: A systematic review. PLoS ONE, 13(3), e0194990. 10.1371/journal.pone.0194990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- August KJ, & Sorkin DH (2011). Racial/ethnic disparities in exercise and dietary behaviors of middle-aged and older adults. Journal of General Internal Medicine, 26(3), 245–250. 10.1007/s11606-010-1514-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB (1987). Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Developmental Psychology, 23(5), 611–626. 10.1037/0012-1649.23.5.611 [DOI] [Google Scholar]
- Baltes PB (1993). The aging mind: potential and limits. The Gerontologist, 33(5), 580–594. http://www.ncbi.nlm.nih.gov/pubmed/8225002 [DOI] [PubMed] [Google Scholar]
- Baltes PB, Staudinger UM, & Lindenberger U (1999). Lifespan psychology: theory and application to intellectual functioning. Annual Review of Psychology, 50, 471–507. 10.1146/annurev.psych.50.1.471 [DOI] [PubMed] [Google Scholar]
- Baniqued PL, Gallen CL, Voss MW, Burzynska AZ, Wong CN, Cooke GE, Duffy K, Fanning J, Ehlers DK, Salerno EA, Aguiñaga S, McAuley E, Kramer AF, & D’Esposito M (2018). Brain network modularity predicts exercise-related executive function gains in older adults. Frontiers in Aging Neuroscience, 9, 426. 10.3389/fnagi.2017.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, & Yaffe K (2008). Cognition in older women: The importance of daytime movement. Journal of the American Geriatrics Society, 56(9), 1658–1664. 10.1111/j.1532-5415.2008.01841.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, & Yaffe K (2013). The Mental Activity and eXercise (MAX) trial. JAMA Internal Medicine, 173(9), 797–804. 10.1001/jamainternmed.2013.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G (2004). Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of Aging, 25(1), 5–18; author reply 49–62. http://www.ncbi.nlm.nih.gov/pubmed/14675724 [DOI] [PubMed] [Google Scholar]
- Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJF, Martin BW, Alkandari JR, Andersen LB, Blair SN, Brownson RC, Bull FC, Craig CL, Ekelund U, Goenka S, Guthold R, Hallal PC, Haskell WL, Heath GW, Inoue S, … Sarmiento OL (2012). Correlates of physical activity: Why are some people physically active and others not? The Lancet, 380(9838), 258–271. 10.1016/S0140-6736(12)60735-1 [DOI] [PubMed] [Google Scholar]
- Bherer L, Erickson KI, & Liu-Ambrose T (2013). A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of Aging Research, 2013, 657508. 10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham CL, Muller JL, Palepu A, Spinelli JJ, & Anis AH (1999). (USE AGAIN) Correlates of adult’s participation in physical activity: review and update. Medicine and Science in Sports and Exercise, 160(4), 483–488. 10.1249/01.MSS.0000038974.76900.92 [DOI] [Google Scholar]
- Bolen JC, Rhodes L, Powell-Griner EE, Bland SD, & Holtzman D (2000). State-specific prevalence of selected health behaviors, by race and ethnicity--Behavioral sisk factor surveillance system, 1997. MMWR. CDC Surveillance Summaries : Morbidity and Mortality Weekly Report. CDC Surveillance Summaries, 49(2), 1–60. [PubMed] [Google Scholar]
- Bonsang E, Adam S, & Perelman S (2012). Does retirement affect cognitive functioning? Journal of Health Economics, 31(3), 490–501. 10.1016/j.jhealeco.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, & Rankinen T (2011). Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. Journal of Applied Physiology, 110(5), 1160–1170. 10.1152/japplphysiol.00973.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BM, Peiffer JJ, Sohrabi HR, Mondal A, Gupta VB, Rainey-Smith SR, Taddei K, Burnham S, Ellis KA, Szoeke C, Masters CL, Ames D, Rowe CC, & Martins RN (2012). Intense physical activity is associated with cognitive performance in the elderly. Translational Psychiatry, 2(11), e191–e191. 10.1038/tp.2012.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, & Bennett DA (2012). Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology, 78(17), 1323–1329. 10.1212/WNL.0b013e3182535d35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, & Bennett DA (2008). Total daily activity is associated with cognition in older persons. American Journal of Geriatric Psychiatry, 16(8), 697–701. 10.1097/JGP.0b013e31817945f6 [DOI] [PubMed] [Google Scholar]
- Buman MP, Winkler EAH, Kurka JM, Hekler EB, Baldwin CM, Owen N, Ainsworth BE, Healy GN, & Gardiner PA (2014). Reallocating time to sleep, sedentary behaviors, or active behaviors: Associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. American Journal of Epidemiology, 179(3), 323–334. 10.1093/aje/kwt292 [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Jiao Y, Knecht AM, Fanning J, Awick EA, Chen T, Gothe N, Voss MW, McAuley E, & Kramer AF (2017). White matter integrity declined over 6-months, but dance intervention improved integrity of the Fornix of older adults. Frontiers in Aging Neuroscience, 9, 59. 10.3389/fnagi.2017.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li S-C, Lindenberger U, & Heekeren HR (2009). Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. NeuroImage, 49(3), 2104–2112. 10.1016/j.neuroimage.2009.09.041 [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Wong CN, Chaddock-Heyman L, Olson EA, Gothe NP, Knecht A, Voss MW, McAuley E, & Kramer AF (2016). White matter integrity, hippocampal volume, and cognitive performance of a world-famous nonagenarian track-and-field athlete. Neurocase, 22(2), 135–144. 10.1080/13554794.2015.1074709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Wong CN, Voss MW, Cooke GE, McAuley E, & Kramer AF (2015). White matter integrity supports BOLD signal variability and cognitive performance in the aging human brain. PloS ONE, 10(4), e0120315. 10.1371/journal.pone.0120315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska, Chaddock-Heyman L, Voss MV, Wong CN, Gothe NP, Olson EA, Knecht A, Lewis A, Monti JMJ, Cooke GE, Wojcicki TRT, Fanning J, Chung HDH, Awick E, McAuley A, Kramer AFAAF, Voss MW, Wong CN, Gothe NP, … Kramer AFAAF (2014). Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS ONE, 9(9), e107413. 10.1371/journal.pone.0107413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, & Christenson GM (1985). Physical activity, exercise and physical fitness definitions for health-related research. Public Health Reports, 100(2), 126–131. [PMC free article] [PubMed] [Google Scholar]
- Cattell RB (1971). Abilities: Their structure, growth, and action (1st ed.). Houghton Mifflin. [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, & Skinner JS (2009). Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise, 41(7), 150–30. 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- Chu A, Huber J, Mastel-Smith B, & Cesario S (2009). “Partnering with seniors for better health”: Computer use and internet health information retrieval among older adults in a low socioeconomic community. Journal of the Medical Library Association, 97(1), 12–20. 10.3163/1536-5050.97.1.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare L, Wu YT, Teale JC, MacLeod C, Matthews F, Brayne C, & Woods B (2017). Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLoS Medicine, 14(3), e1002259. 10.1371/journal.pmed.1002259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DE, Hurst PR, Myers DB, & Spears GF (1992). Collagen concentrations in dissected tissue compartments of rat uterus on Days 6, 7 and 8 of pregnancy. Reproduction, 94(1), 169–175. 10.1530/jrf.0.0940169 [DOI] [PubMed] [Google Scholar]
- Clouston SAP, & Denier N (2017). Mental retirement and health selection: Analyses from the U.S. health and retirement study. Social Science and Medicine, 178, 78–86. 10.1016/j.socscimed.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioural science (2nd Edition). In Statistical Power Anaylsis for the Behavioral Sciences. [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, & Kramer AF (2003). Aerobic fitness reduces brain tissue loss in aging humans. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 58(2), 176–180. http://www.ncbi.nlm.nih.gov/pubmed/12586857 [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, & Kramer AF (2003). Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science, 14(2), 125–130. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, & Elavsky S (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 3316–3321. 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland JL, Clarke J, & Dogra S (2015). Objectively measured and self-reported sedentary time in older Canadians. Preventive Medicine Reports, 2, 90–95. 10.1016/j.pmedr.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland JL, & Esliger DW (2009). Accelerometer assessment of physical activity in active, healthy older adults. Journal of Aging and Physical Activity, 17(1), 17–30. 10.1123/japa.17.1.17 [DOI] [PubMed] [Google Scholar]
- Craft LL, Zderic TW, Gapstur SM, Vaniterson EH, Thomas DM, Siddique J, & Hamilton MT (2012). Evidence that women meeting physical activity guidelines do not sit less: an observational inclinometry study. The International Journal of Behavioral Nutrition and Physical Activity, 9, 122. 10.1186/1479-5868-9-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikszentmihalyi M, & Larson R (2014). Validity and reliability of the experience-sampling method. In Flow and the Foundations of Positive Psychology: The Collected Works of Mihaly Csikszentmihalyi. 10.1007/978-94-017-9088-8_3 [DOI] [Google Scholar]
- Dave SS, Craft LL, Mehta P, Naval S, Kumar S, & Kandula NR (2015). Life stage influences on U.S. South Asian women’s physical activity. American Journal of Health Promotion, 29(3), e100–e108. 10.4278/ajhp.130415-QUAL-175 [DOI] [PubMed] [Google Scholar]
- Davis JC, Best JR, Khan KM, Dian L, Lord S, Delbaere K, Hsu CL, Cheung W, Chan W, & Liu-Ambrose T (2017). Slow processing speed predicts falls in older adults with a falls history: 1-year prospective cohort study. Journal of the American Geriatrics Society, 65(5), 916–923. 10.1111/jgs.14830 [DOI] [PubMed] [Google Scholar]
- Davis MG, Fox KR, Hillsdon M, Coulson JC, Sharp DJ, Stathi A, & Thompson JL (2011). Getting out and about in older adults: The nature of daily trips and their association with objectively assessed physical activity. International Journal of Behavioral Nutrition and Physical Activity, 8, 116. 10.1186/1479-5868-8-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho Bastone A, De Souza Moreira B, Vieira RA, Kirkwood RN, Dias JMD, & Dias RC (2014). Validation of the human activity profile questionnaire as a measure of physical activity levels in older community-dwelling women. Journal of Aging and Physical Activity, 22(3), 348–356. 10.1123/JAPA.2013-0006 [DOI] [PubMed] [Google Scholar]
- Deary IJ, Strand S, Smith P, & Fernandes C (2007). Intelligence and educational achievement. Intelligence, 35(1), 13–21. 10.1016/j.intell.2006.02.001 [DOI] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S, Ashe MC, Biddle SJH, Brown WJ, Buman MP, Chastin S, Gardiner PA, Inoue S, Jefferis BJ, Oka K, Owen N, Sardinha LB, Skelton DA, Sugiyama T, & Copeland JL (2017). Sedentary time in older men and women: An international consensus statement and research priorities. British Journal of Sports Medicine, 51, 1526–1532. 10.1136/bjsports-2016-097209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan DW, Howard B, Healy GN, & Owen N (2012). Too much sitting - A health hazard. Diabetes Research and Clinical Practice, 97(3), 368–376. 10.1016/j.diabres.2012.05.020 [DOI] [PubMed] [Google Scholar]
- Ehlers DK, Daugherty AM, Burzynska AZ, Fanning J, Awick EA, Chaddock-Heyman L, Kramer AF, & McAuley E (2017). Regional brain volumes moderate, but do not mediate, the effects of group-based exercise training on reductions in loneliness in older adults. Frontiers in Aging Neuroscience, 9, 110. 10.3389/fnagi.2017.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers DK, Fanning J, Awick EA, Kramer AF, & McAuley E (2016). Contamination by an active control condition in a randomized exercise trial. PLoS ONE, 11(10), e0164246. 10.1371/journal.pone.0164246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM, Ding D, Heath G, Hallal PC, Kohl HW, Pratt M, Reis R, Sallis J, Aadahl M, Blot WJ, Chey T, Deka A, … Yi-Park S (2016). Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. The Lancet, 388(10051), 1302–1310. 10.1016/S0140-6736(16)30370-1 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Grove GA, Burns JM, Hillman CH, Kramer AF, McAuley E, Vidoni ED, Becker JT, Butters MA, Gray K, Huang H, Jakicic JM, Kamboh MI, Kang C, Klunk WE, Lee P, Marsland AL, Mettenburg J, Rogers RJ, … Wollam ME (2019). Investigating gains in neurocognition in an intervention trial of exercise (IGNITE): Protocol. Contemporary Clinical Trials, 85, 105832. 10.1016/j.cct.2019.105832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, & Kramer AF (2009). Aerobic exercise effects on cognitive and neural plasticity in older adults. British Journal of Sports Medicine, 43(1), 22–24. 10.1136/bjsm.2008.052498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, & Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- España-Romero V, Golubic R, Martin KR, Hardy R, Ekelund U, Kuh D, Wareham NJ, Cooper R, & Brage S (2014). Comparison of the EPIC physical activity questionnaire with combined heart rate and movement sensing in a nationally representative sample of older British adults. PLoS ONE, 9(2), e87085. 10.1371/journal.pone.0087085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck RS, Davis JC, & Liu-Ambrose T (2017). What is the association between sedentary behaviour and cognitive function? A systematic review. British Journal of Sports Medicine, 51(10), 800–811. 10.1136/bjsports-2015-095551 [DOI] [PubMed] [Google Scholar]
- Fanning J, Porter G, Awick EA, Ehlers DK, Roberts SA, Cooke G, Burzynska AZ, Voss MW, Kramer AF, & McAuley E (2017). Replacing sedentary time with sleep, light, or moderate-to-vigorous physical activity: effects on self-regulation and executive functioning. Journal of Behavioral Medicine, 40(2), 332–342. 10.1007/s10865-016-9788-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Croteau K, Kolt GS, & Astell-Burt T (2016). Does retirement mean more physical activity? A longitudinal study. BMC Public Health, 16, 605. 10.1186/s12889-016-3253-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD (2008). Oligodendrocytes changing the rules: Action potentials in glia and oligodendrocytes controlling action potentials. Neuroscientist, 14(6), 540–543. 10.1177/1073858408320294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, & Pedersen NL (2007). Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging, 22(3), 558–568. 10.1037/0882-7974.22.3.558 [DOI] [PubMed] [Google Scholar]
- Ford ES, Merritt RK, Heath GW, Powell KE, Washburn RA, Kriska A, & Haile G (1991). Physical activity behaviors in lower and higher socioeconomic status populations. American Journal of Epidemiology, 133(12), 1246–1256. 10.1093/oxfordjournals.aje.a115836 [DOI] [PubMed] [Google Scholar]
- Funder DC, & Ozer DJ (2019). Evaluating effect size in psychological research: Sense and nonsense. Advances in Methods and Practices in Psychological Science, 2(1), 156–168. 10.1177/2515245919847202 [DOI] [Google Scholar]
- Gardiner PA, Clark BK, Healy GN, Eakin EG, Winkler EAH, & Owen N (2011). Measuring older adults’ sedentary time: Reliability, validity, and responsiveness. Medicine and Science in Sports and Exercise, 43(11), 2127–2133. 10.1249/MSS.0b013e31821b94f7 [DOI] [PubMed] [Google Scholar]
- Geda YE, Topazian HM, Lewis RA, Roberts RO, Knopman DS, Pankratz VS, Christianson TJHC, Boeve BF, Tangalos EG, Ivnik RJ, & Petersen RC (2011). Engaging in cognitive activities, aging, and mild cognitive impairment: A population-based study. Journal of Neuropsychiatry and Clinical Neurosciences, 23(2), 149–154. 10.1176/jnp.23.2.jnp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso KP, Matthews CE, & Colbert LH (2015). Reliability and validity of 2 self-report measures to assess sedentary behavior in older adults. Journal of Physical Activity and Health, 12(5), 727–732. 10.1123/jpah.2013-0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman E, Hanson HM, Yang PH, Khan KM, Liu-Ambrose T, & Ashe MC (2014). Accelerometry analysis of physical activity and sedentary behavior in older adults: A systematic review and data analysis. European Review of Aging and Physical Activity, 11(1), 35–49. 10.1007/s11556-013-0132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabe ME, Kamhawi R, & Yegiyan N (2009). Informing citizens: How people with different levels of education process television, newspaper, and web news. Journal of Broadcasting and Electronic Media, 53(1), 90–111. 10.1080/08838150802643860 [DOI] [Google Scholar]
- Guerra-Carrillo B, Katovich K, & Bunge SA (2017). Does higher education hone cognitive functioning and learning efficacy? Findings from a large and diverse sample. PLoS ONE, 12(8), e0182276. 10.1371/journal.pone.0182276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagströmer M, Oja P, & Sjöström M (2007). Physical activity and inactivity in an adult population assessed by accelerometry. Medicine and Science in Sports and Exercise, 39(9), 1502–1508. 10.1249/mss.0b013e3180a76de5 [DOI] [PubMed] [Google Scholar]
- Halloway S, Arfanakis K, Wilbur JE, Schoeny ME, & Pressler SJ (2019). Accelerometer physical activity is associated with greater gray matter volumes in older adults without dementia or mild cognitive impairment. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 74(7), 1142–1151. 10.1093/geronb/gby010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloway S, Wilbur JE, Schoeny ME, & Barnes LL (2017). The relation between physical activity and cognitive change in older Latinos. Biological Research for Nursing, 19(5), 538–548. 10.1177/1099800417715115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BH, Kolle E, Dyrstad SM, Holme I, & Anderssen SA (2012). Accelerometer-determined physical activity in adults and older people. Medicine and Science in Sports and Exercise, 44(2), 266–272. 10.1249/MSS.0b013e31822cb354 [DOI] [PubMed] [Google Scholar]
- Hart TL, McClain JJ, & Tudor-Locke C (2011). Controlled and free-living evaluation of objective measures of sedentary and active behaviors. Journal of Physical Activity & Health, 8(6), 848–857. http://www.ncbi.nlm.nih.gov/pubmed/21832301 [DOI] [PubMed] [Google Scholar]
- Hayes SM, Alosco ML, Hayes JP, Cadden M, Peterson KM, Allsup K, Forman DE, Sperling RA, & Verfaellie M (2015). Physical activity is positively associated with episodic memory in aging. Journal of the International Neuropsychological Society, 21(10), 780–790. 10.1017/S1355617715000910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, & Owen N (2008). Objectively measured sedentary time, physical activity, and metabolic risk. Cardiovascular & Metabolic Risk, 31(2), 369–371. 10.2337/dc07 [DOI] [PubMed] [Google Scholar]
- Hedden T, & Gabrieli JDE (2004). Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews. Neuroscience, 5(2), 87–96. 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- Heesch KC, Hill RL, Aguilar-Farias N, Van Uffelen JGZ, & Pavey T (2018). Validity of objective methods for measuring sedentary behaviour in older adults: a systematic review. International Journal of Behavioral Nutrition and Physical Activity, 15(1), 119. 10.1186/s12966-018-0749-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze G, & Dunkler D (2017). Five myths about variable selection. Transplant International, 30(1), 6–10. 10.1111/tri.12895 [DOI] [PubMed] [Google Scholar]
- Heinze G, Wallisch C, & Dunkler D (2018). Variable selection – A review and recommendations for the practicing statistician. Biometrical Journal, 60(3), 431–449. 10.1002/bimj.201700067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, & Hoff J (2007). Aerobic high-intensity intervals improve more than moderate training. Medicine and Science in Sports and Exercise, 39(4), 665–671. 10.1249/mss.0b013e3180304570 [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, & Lindenberger U (2008). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest, 9(1), 1–65. 10.1111/j.1539-6053.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, & Lindenberger U (2009). Fit body, fit mind. (Cover story). Scientific American Mind, 20(4), 24–31. [Google Scholar]
- Hillman CH, Erickson KI, & Kramer AF (2008). Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience, 9(1), 58–65. 10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- Horn JL (1970). Organization of data on life-span development of human abilities. In Life-Span Developmental Psychology (pp. 423–466). 10.1016/b978-0-12-293850-4.50022-4 [DOI] [Google Scholar]
- Horn JL (1982). The theory of fluid and crystallized intelligence in relation to concepts of cognitive psychology and aging in adulthood. In Aging and Cognitive Processes (pp. 237–278). Springer US. 10.1007/978-1-4684-4178-9_14 [DOI] [Google Scholar]
- Horn JL, & Cattell RB (1967). Age differences in fluid and crystallized intelligence. Acta Psychologica, 26, 107–129. 10.1016/0001-6918(67)90011-X [DOI] [PubMed] [Google Scholar]
- Horn JL, & Hofer SM (1992). Major abilities and development in the adult period. In Intellectual development. (pp. 44–99). [Google Scholar]
- Humphreys BR, & Ruseski JE (2006). Economic determinants of participation in physical activity and sport. International Association of Sports Economists, Working Papers, 0613. [Google Scholar]
- Inouye SK, Albert MS, Mohs R, Sun K, & Berkman LF (1993). Cognitive performance in a high-functioning community-dwelling elderly population. Journals of Gerontology, 48(4), 146–151. 10.1093/geronj/48.4.M146 [DOI] [PubMed] [Google Scholar]
- Iso-Markku P, Waller K, Vuoksimaa E, Vähä-Ypyä H, Lindgren N, Heikkilä K, Sievänen H, Rinne J, Kaprio J, & Kujala UM (2018). Objectively measured physical activity profile and cognition in Finnish elderly twins. Alzheimer’s and Dementia: Translational Research and Clinical Interventions, 4, 263–271. 10.1016/j.trci.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Clarke J, & Canada S (2016). Physical activity of Canadian adults:Accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. January 2016. [PubMed]
- Jefferis BJ, Sartini C, Ash S, Lennon LT, Wannamethee SG, & Whincup PH (2016). Validity of questionnaire-based assessment of sedentary behaviour and physical activity in a population-based cohort of older men; comparisons with objectively measured physical activity data. International Journal of Behavioral Nutrition and Physical Activity, 13, 14. 10.1186/s12966-016-0338-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LG, Butson ML, Polman RC, Raj IS, Borkoles E, Scott D, Aitken D, & Jones G (2016). Light physical activity is positively associated with cognitive performance in older community dwelling adults. Journal of Science and Medicine in Sport, 19(11), 877–882. 10.1016/j.jsams.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Kari JT, Pehkonen J, Hirvensalo M, Yang X, Hutri-Kähönen N, Raitakari OT, & Tammelin TH (2015). Income and physical activity among adults: Evidence from self-reported and pedometer-based physical activity measurements. PLoS ONE, 10(8), e0135651. 10.1371/journal.pone.0135651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J, Marshall SJ, Patterson RE, Marinac CR, Natarajan L, Rosenberg D, Wasilenko K, & Crist K (2013). Objectively measured physical activity is related to cognitive function in older adults. Journal of the American Geriatrics Society, 49(1), 47–53. 10.1111/jgs.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesse-Guyot E, Charreire H, Andreeva VA, Touvier M, Hercberg S, Galan P, & Oppert JM (2012). Cross-sectional and longitudinal associations of different sedentary behaviors with cognitive performance in older adults. PLoS ONE, 7(10), e47831. 10.1371/journal.pone.0047831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, & Colcombe A (1999). Ageing, fitness and neurocognitive function. Nature, 400(6743), 418–419. 10.1038/22682 [DOI] [PubMed] [Google Scholar]
- Ku PW, Liu Y. Te, Lo MK, Chen LJ, & Stubbs B (2017). Higher levels of objectively measured sedentary behavior is associated with worse cognitive ability: Two-year follow-up study in community-dwelling older adults. Experimental Gerontology, 99, 110–114. 10.1016/j.exger.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, Hommel B, Aschersleben G, Prinz W, & Baltes PB (2004). Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science, 15(3), 155–163. http://www.ncbi.nlm.nih.gov/pubmed/15016286 [DOI] [PubMed] [Google Scholar]
- Lindenberger U (2014). Human cognitive aging: Corriger lafortune? Science, 346(6209), 572–578. 10.1126/science.1254403 [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N.-K. kuei, & Song AW (2012). Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1822(3), 386–400. 10.1016/j.bbadis.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Keadle SK, Moore SC, Schoeller DS, Carroll RJ, Troiano RP, & Sampson JN (2018). Measurement of active and sedentary behavior in context of large epidemiologic studies. Medicine and Science in Sports and Exercise, 50(2), 266. 10.1249/MSS.0000000000001428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer DO, & Jena AB (2010). The economics of intense exercise. Journal of Health Economics, 29(3), 347–352. 10.1016/j.jhealeco.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JA, Campaña JC, & Ortega R (2016). What do you prefer for a relaxing time at home: reading, watching TV or listening to the radio? Applied Economics Letters, 23(18), 1278–1284. 10.1080/13504851.2016.1150943 [DOI] [Google Scholar]
- Nemoto Y, Sato S, Takahashi M, Takeda N, Matsushita M, Kitabatake Y, Maruo K, & Arao T (2018). The association of single and combined factors of sedentary behavior and physical activity with subjective cognitive complaints among community-dwelling older adults: Cross-sectional study. PLoS ONE, 13(4), e0195384. 10.1371/journal.pone.0195384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, & Smith PK (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17(2), 299–320. http://www.ncbi.nlm.nih.gov/pubmed/12061414 [PubMed] [Google Scholar]
- Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, Decarli C, Vasan RS, & Seshadri S (2016). Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: The Framingham third generation cohort study. Hypertension, 67(3), 513–519. 10.1161/HYPERTENSIONAHA.115.06610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TM, Moore SC, Xiang YB, Yang G, Shu XO, Ekelund U, Ji BT, Tan YT, Liu DK, Schatzkin A, Zheng W, Chow WH, Matthews CE, & Leitzmann MF (2010). Accelerometer-measured physical activity in Chinese adults. American Journal of Preventive Medicine, 38(6), 583–591. 10.1016/j.amepre.2010.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MJ, Morey MC, Giuliani C, Pieper CF, Evenson KR, Mercer V, Visser M, Brach JS, Kritchevsky SB, Goodpaster BH, Rubin S, Satterfield S, & Simonsick EM (2010). Walking in old age and development of metabolic syndrome: The health, aging, and body composition study. Metabolic Syndrome and Related Disorders, 8(4), 317–322. 10.1089/met.2009.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister T, Matthews CE, Wang Q, Kopciuk KA, Courneya K, & Friedenreich C (2017). Comparison of two accelerometers for measuring physical activity and sedentary behaviour. BMJ Open Sport and Exercise Medicine, 3(1), e000227. 10.1136/bmjsem-2017-000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, & Daugherty AM (2017). Pathways to brain aging and their modifiers: Free-Radical-Induced Energetic and Neural Decline in Senescence (FRIENDS) model - A mini-review. Gerontology, 64(1), 49–57. 10.1159/000479508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, Marsiske M, Morris JN, Tennstedt SL, Unverzagt FW, & Willis SL (2014). Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62(1), 16–24. 10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RE, Mark RS, & Temmel CP (2012). Adult sedentary behavior: A systematic review. American Journal of Preventive Medicine, 42(3), e3–28. 10.1016/j.amepre.2011.10.020 [DOI] [PubMed] [Google Scholar]
- Rikli RE, & Jones CJ (2013). Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist, 53(2), 255–267. 10.1093/geront/gns071 [DOI] [PubMed] [Google Scholar]
- Rohwedder S, & Willis RJ (2010). Mental retirement. Journal of Economic Perspectives, 24(1), 119–138. 10.1257/jep.24.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzewnicki R, Auweele Y. Vanden, & De Bourdeaudhuij I (2003). Addressing overreporting on the International Physical Activity Questionnaire (IPAQ) telephone survey with a population sample. Public Health Nutrition, 6(3), 299–305. 10.1079/phn2002427 [DOI] [PubMed] [Google Scholar]
- Sallis JF, & Saelens BE (2000). Assessment of physical activity by self-report: Status, limitations, and future directions. Research Quarterly for Exercise and Sport, 71(2), 1–14. 10.1080/02701367.2000.11082780 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103(3), 403–428. 10.1037/0033-295X.103.3.403 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2004). Localizing age-related individual differences in a hierarchical structure. Intelligence, 32(6). 10.1016/j.intell.2004.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2010). Selective review of cognitive aging. Journal of the International Neuropsychological Society, 16(5), 754–760. 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, & Berish DE (2003). Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General, 132(4), 566–594. 10.1037/0096-3445.132.4.566 [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Berish DE, & Siedlecki KL (2004). Construct validity and age sensitivity of prospective memory. Memory and Cognition, 32, 1133–1148. 10.3758/BF03196887 [DOI] [PubMed] [Google Scholar]
- Salthouse TA, & Ferrer-Caja E (2003). What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging, 18(1), 91–110. http://www.ncbi.nlm.nih.gov/pubmed/12641315 [DOI] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, & Barta P (2000). Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. Journal of the International Neuropsychological Society, 6(1), 52–61. 10.1017/S1355617700611062 [DOI] [PubMed] [Google Scholar]
- Seider TR, Fieo RA, O’Shea A, Porges EC, Woods AJ, & Cohen RA (2016). Cognitively engaging activity is associated with greater cortical and subcortical volumes. Frontiers in Aging Neuroscience, 8, 94. 10.3389/fnagi.2016.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RJ (2003). Limits to the measurement of habitual physical activity by questionnaires. British Journal of Sports Medicine, 37(3), 197–206. 10.1136/bjsm.37.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M, & Tremblay MS (2008). Screen time among Canadian adults: a profile. Health Reports, 19(2), 31–43. [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4, 1–32. 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, & Dugravot A (2012). Timing of onset of cognitive decline: Results from Whitehall II prospective cohort study. BMJ, 344, d7622. 10.1136/bmj.d7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MC (1990). Reading habits and attitudes of adults at different levels of education and occupation. Reading Research and Instruction, 30(1), 50–58. 10.1080/19388079009558033 [DOI] [Google Scholar]
- Spiro AI, & Brady CB (2012). Integrating health into cognitive aging research and theory: Quo vadis? In Handbook of Cognitive Aging: Interdisciplinary Perspectives (pp. 260–283). 10.4135/9781412976589.n16 [DOI] [Google Scholar]
- Staffaroni AM, Cobigo Y, Elahi FM, Casaletto KB, Walters SM, Wolf A, Lindbergh CA, Rosen HJ, & Kramer JH (2019). A longitudinal characterization of perfusion in the aging brain and associations with cognition and neural structure. Human Brain Mapping, 40(12), 3522–3533. 10.1002/hbm.24613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, Vuoksimaa E, Arenaza Urquiljo EM, Bartrés-Faz D, … Vuoksimaa E (2018). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s and Dementia, S1552–5260(18), 33491–33495. 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, & Mayeux R (1994). Influence of education and occupation on the incidence of AD. Jama, 271(13), 1004–1010. [PubMed] [Google Scholar]
- Stubbs B, Chen LJ, Chang CY, Sun WJ, & Ku PW (2017). Accelerometer-assessed light physical activity is protective of future cognitive ability: A longitudinal study among community dwelling older adults. Experimental Gerontology, 91, 104–109. 10.1016/j.exger.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Tremblay MS, Colley RC, Saunders TJ, Healy GN, & Owen N (2010). Physiological and health implications of a sedentary lifestyle. Applied Physiology, Nutrition and Metabolism, 35(6), 725–740. 10.1139/H10-079 [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, & McDowell M (2008). Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise, 40(1), 181–188. 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- Trost SG, Owen N, Bauman AE, Sallis JF, & Brown W (2002). Correlates of adults’ participation in physical activity: Review and update. Medicine and Science in Sports and Exercise, 34(12), 1996–2001. 10.1097/00005768-200212000-00020 [DOI] [PubMed] [Google Scholar]
- Tun PA, & Lachman ME (2010). The association between computer use and cognition across adulthood: Use it so you won’t lose it? Psychology and Aging, 25(3), 560–568. 10.1037/a0019543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberg J, Van Holle V, De Bourdeaudhuij I, Owen N, & Deforche B (2014). Older adults’ reporting of specific sedentary behaviors: Validity and reliability. BMC Public Health, 14, 734. 10.1186/1471-2458-14-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Chuang Y-F, Harris GC, Tan EJ, & Carlson MC (2014). Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus, 25(5), 605–615. 10.1002/hipo.22397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Tan EJ, Wang T, Xue QL, Fried LP, Seplaki CL, King AC, Seeman TE, Rebok GW, & Carlson MC (2014). Low-intensity walking activity is associated with better health. Journal of Applied Gerontology, 33(7), 870–887. 10.1177/0733464813512896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, & Koster A (2013). Development of a questionnaire to assess sedentary time in older persons - A comparative study using accelerometry. BMC Geriatrics, 13, 80. 10.1186/1471-2318-13-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Carr LJ, Clark R, & Weng T (2014). Revenge of the “sit” II: Does lifestyle impact neuronal and cognitive health through distinct mechanisms associated with sedentary behavior and physical activity? Mental Health and Physical Activity, 84(4), 699–715. 10.1016/j.mhpa.2014.01.001 [DOI] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wójcicki TR, Mailey EL, Olson EA, Gothe N, Vieira-Potter VJ, Martin SA, Pence BD, Cook MD, Woods JA, McAuley E, & Kramer AF (2013). Neurobiological markers of exercise-related brain plasticity in older adults. Brain, Behavior, and Immunity, 28, 90–99. 10.1016/j.bbi.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Kramer AF, Basak C, Prakash RS, & Roberts B (2010). Are expert athletes “expert” in the cognitive laboratory? A meta-analytic review of cognition and sport expertise. Applied Cognitive Psychology, 24(6), 812–826. 10.1002/acp.1588 [DOI] [Google Scholar]
- Voss MW, Sutterer M, Weng TB, Burzynska AZ, Fanning J, Salerno E, Gothe NP, Ehlers DK, McAuley E, & Kramer AF (2019). Nutritional supplementation boosts aerobic exercise effects on functional brain systems. Journal of Applied Physiology, 126(1), 77–87. 10.1152/japplphysiol.00917.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, & van Praag H (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences, 17(10), 525–544. 10.1016/j.tics.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, & Keller SD (1996). A 12-Item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34(3), 220–233. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- Wilbur J, Marquez DX, Fogg L, Wilson RS, Staffileno BA, Hoyem RL, Morris MC, Bustamante EE, & Manning AF (2012). The relationship between physical activity and cognition in older latinos. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 19(5), 538–548. 10.1093/geronb/gbr137 [DOI] [PubMed] [Google Scholar]
- Wolman BB, & Stricker G (1982). Handbook of developmental psychology. Prentice-Hall. [Google Scholar]
- Wu Y-H, Lewis M, & Rigaud A-S (2019). Cognitive function and digital device use in older adults attending a memory clinic. Gerontology and Geriatric Medicine, 5, 2333721419844886. 10.1177/2333721419844886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullems JA, Verschueren SMP, Degens H, Morse CI, & Onambélé GL (2017). Performance of thigh-mounted triaxial accelerometer algorithms in objective quantification of sedentary behaviour and physical activity in older adults. PLoS ONE, 12(11), e0188215. 10.1371/journal.pone.0188215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Angevaren M, Rusted J, & Tabet N (2015). Aerobic exercise to improve cognitive function in older people without known cognitive impairment. The Cochrane Database of Systematic Reviews, 4, CD005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Blackwell T, Hoffman AR, Cummings S, Ancoli-Israel S, & Stone K (2018). Daily patterns of accelerometer activity predict changes in sleep, cognition, and mortality in older men. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 73(5), 682–687. 10.1093/gerona/glw250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Wadley VG, Howard VJ, Hutto B, Blair SN, & Hooker SP (2017). Objectively measured physical activity and cognitive function in older adults. Medicine and Science in Sports and Exercise, 49(1), 47–53. 10.1249/MSS.0000000000001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


