Abstract
BACKGROUND:
CheckMate 025 showed superior efficacy for nivolumab over everolimus in patients with advanced renal cell carcinoma (aRCC), with improved safety and tolerability. This analysis assesses long-term clinical benefits of nivolumab versus everolimus.
METHODS:
The randomized, open-label, phase 3 CheckMate 025 trial (NCT01668784) included patients with clear cell aRCC previously treated with 1–2 antiangiogenic regimens. Patients were randomized to nivolumab 3 mg/kg Q2W or everolimus 10 mg QD until progression or unacceptable toxicity. Primary endpoint: overall survival (OS). Secondary endpoints: confirmed objective response rate (ORR), progression-free survival (PFS), safety, and health-related quality of life (HRQoL).
RESULTS:
821 patients were randomized to nivolumab (n=410) or everolimus (n=411); 803 patients were treated—406 with nivolumab and 397 with everolimus. With minimum follow-up of 64 months (median 72 months), nivolumab maintained an OS benefit versus everolimus (median [95% CI] 25.8 months [22.2–29.8] vs 19.7 months [17.6–22.1]; HR, 0.73 [95% CI, 0.62–0.85]), with 5-year OS probabilities of 26% and 18%, respectively. ORR was higher with nivolumab (94 [23%] of 410 vs 17 [4%] of 411; P<.001). PFS also favored nivolumab (HR, 0.84 [95% CI, 0.72–0.99]; P=0.0331). The most common any-grade treatment-related adverse events were fatigue (34.7%) and pruritus (15.5%) with nivolumab, and fatigue (34.5%) and stomatitis (29.5%) with everolimus. HRQoL improved versus baseline with nivolumab but remained the same or deteriorated with everolimus.
CONCLUSION:
The superior efficacy of nivolumab over everolimus was maintained after extended follow-up with no new safety signals, supporting the long-term benefits of nivolumab monotherapy in patients with previously treated aRCC.
Keywords: Nivolumab, everolimus, CheckMate 025, aRCC, immune checkpoint inhibitor, previously treated
Lay summary
CheckMate 025 compared the effects of nivolumab (a novel immunotherapy) with everolimus (an older standard-of-care therapy) for the treatment of advanced kidney cancer in patients who had progressed on antiangiogenic therapy. After 5 years of study, nivolumab continues to be better than everolimus in extending the lives of patients, providing a long-lasting response to treatment and improving quality of life with a manageable safety profile. The results demonstrate that the clinical benefits with nivolumab compared with everolimus in previously treated patients with advanced kidney cancer continue in the long term.
Precis for use in the Table of Contents
The results of the 5-year follow-up analysis of the CheckMate 025 trial demonstrate that the clinical benefits are sustained with nivolumab over everolimus in previously treated patients with aRCC in the long term. OS, PFS, and ORR benefits are maintained with nivolumab versus everolimus, and more patients treated with nivolumab experience an improved HRQoL, while the safety of nivolumab is manageable and compares favorably with everolimus.
INTRODUCTION
Nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor, disrupts PD-L1-mediated signaling to restore the immune system’s antitumor defenses.1–3 Nivolumab monotherapy has previously demonstrated antitumor activity associated with improved overall survival (OS) in multiple malignancies including advanced renal cell carcinoma (aRCC).1
The phase 3 CheckMate 025 trial compared nivolumab versus everolimus, an mTOR inhibitor, in patients who had aRCC with a clear cell component and had previously been treated with antiangiogenic therapy (NCT01668784). At an interim analysis performed with a minimum follow-up of 14 months, the trial was stopped early because of demonstrated OS benefit with nivolumab over everolimus (HR, 0.73; 95% CI, 0.57– 0.93; P=.002]); a survival benefit was observed with nivolumab regardless of tumor PD-L1 expression level.4 Objective response rate (ORR) per investigator was significantly improved with nivolumab versus everolimus (P<.001), and although median progression-free survival (PFS) was similar between treatment arms, a delayed benefit with nivolumab was observed after 6 months of treatment.4 Confirmed investigator-assessed ORR (95% CI) was 21.5% (17.6–25.8) with nivolumab versus 3.9% (2.2–6.2) with everolimus.1 Beyond the observed clinical benefits, nivolumab was associated with improvements in patient-reported health-related quality of life (HRQoL) outcomes versus everolimus.5 Long-term updates critically inform the benefit/risk ratio of immunotherapeutic regimens. Here, we report an updated and expanded analysis with an extended minimum follow-up of 64 months in patients treated with nivolumab or everolimus in CheckMate 025.
METHODS
Study Design and Participants
A detailed study methodology was previously reported.4 Briefly, CheckMate 025 was a randomized, open-label, phase 3 trial conducted across 146 university- or hospital-based sites in 24 countries globally. Patients were aged ≥18 years, had histologically confirmed advanced or metastatic RCC with a predominantly clear cell component, and measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Eligible patients had previously received 1 or 2 antiangiogenic therapies and had a Karnofsky performance status ≥70.
Randomization and Masking
Patients were randomly assigned (1:1) to nivolumab or everolimus through an interactive voice response system. Randomization was done via permuted blocks within each stratum, by block and stratified by Memorial Sloan Kettering Cancer Center risk group (favorable vs intermediate vs poor), number of prior antiangiogenic therapies in the advanced or metastatic setting (1 vs 2), and geographical region (USA/Canada vs Western Europe vs rest of the world). Patients and investigators were not masked to treatment assignment because this was an open-label trial.
Study Oversight
The study was approved by an institutional review board or independent ethics committee at each center and was conducted in accordance with Good Clinical Practice guidelines, as defined by the International Conference for Harmonisation. All patients provided written informed consent based on the principles of the Declaration of Helsinki.
Procedures
Patients were randomized 1:1 to receive nivolumab (3 mg/kg intravenously every 2 weeks) or everolimus (10 mg/day orally) until disease progression, unacceptable toxicity, or withdrawal of consent. Patients were permitted to continue treatment beyond progression if a clinical benefit was identified by the investigator and the adverse event (AE) profile was acceptable. Dose reductions and escalations were permitted for everolimus but not for nivolumab, while dose delays were permitted for both treatments. Tumor assessments were performed at baseline, every 8 weeks for the first year, then every 12 weeks until disease progression or discontinuation of treatment and were evaluated by the investigator per RECIST v1.1. Crossover from everolimus to a nivolumab extension phase was allowed per a protocol amendment implemented after superiority for OS in patients receiving nivolumab was demonstrated in the primary analysis (July 2015). Patients treated with everolimus could be assessed for crossover to nivolumab if they met criteria for laboratory values and if all toxicities attributed to prior anticancer therapy, except alopecia and fatigue, had resolved to grade 1 or baseline before initiating nivolumab. A 14-day washout period for prior systemic anticancer therapy was required before the first nivolumab crossover dose.
AEs were graded per National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.6 Select AEs were defined as AEs of special clinical interest that may differ in type, frequency, or severity from AEs associated with non-immunotherapies; these may require immunosuppression for their management, and early recognition may mitigate severe toxicity. HRQoL was assessed using the Functional Assessment of Cancer Therapy Kidney Symptom Index–Disease-Related Symptoms (FKSI-DRS) scoring algorithm.7
Outcomes
This prespecified follow-up analysis included the original primary endpoint of OS, together with secondary endpoints of confirmed ORR per investigator using RECIST v1.1, PFS per investigator using RECIST v1.1, safety, and patient-reported HRQoL.4 The effects of various baseline clinical features on OS were assessed post hoc by univariable and multivariable models in an exploratory analysis of both arms.
Treatment-free interval was defined as the time between protocol therapy discontinuation and subsequent systemic anticancer treatment initiation, or the time between protocol therapy discontinuation and date last known alive in patients who never received subsequent systemic anticancer treatment.
Treatment-related AEs were reported between the first dose and 30 days after the last dose of study therapy, or at the beginning of the nivolumab extension phase, whichever came first. Treatment-related AEs that continued beyond this time point were followed to resolution or until deemed irreversible by the investigator. Treatment-related select AEs were reported between the first dose and 30 days after the last dose of study therapy for patients in the nivolumab arm and included events occurring in skin, gastrointestinal, endocrine, hepatic, pulmonary, or renal systems. Median times to onset and resolution of treatment-related select AEs were reported for patients in the nivolumab and the everolimus arms.
Statistical Analysis
The Kaplan-Meier method was used to estimate OS, PFS, duration of response, and time to resolution of select AEs.8 OS and PFS HRs for nivolumab versus everolimus were estimated using the Cox proportional hazards model9 with treatment group as a single covariate. ORRs and the corresponding 95% CIs were calculated based on the Clopper and Pearson method.10 A post hoc analysis of the effects of clinically relevant baseline features—tumor PD-L1 expression, neutrophil-to-lymphocyte ratio, median sum of reference diameters of target lesions, prior nephrectomy, and individual International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk factors—on OS was performed using univariable and multivariable models sequentially for each intent-to-treat treatment arm separately to differentiate between factors relevant to nivolumab and everolimus. Each factor was first analyzed individually in the univariable analysis. Baseline factors associated with OS at P<.1 in the univariable model were entered into a full Cox proportional hazards multivariable regression model. Backward regression was used to build a parsimonious (reduced) multivariable model that included all baseline factors associated with OS at P<.1. Descriptive statistics were used to assess treatment-related AEs, onset of select AEs, and changes from baseline in HRQoL.
RESULTS
Patients
Between October 9, 2012, and March 14, 2014, 821 patients were randomized (410 into the nivolumab arm, 411 into the everolimus arm), and 803 were treated (406 in the nivolumab arm, 397 in the everolimus arm). Baseline characteristics have been previously reported and were balanced between arms (Supporting Table 1). The minimum follow-up was 64 months (median, 72 months). As of the August 2019 database lock, 100 (24.6%) patients randomized to the nivolumab arm were still alive, versus 65 (16.4%) patients initially randomized to everolimus. Ten (2.5%) patients in the nivolumab arm and 2 (0.5%) in the everolimus arm continued to receive treatment. The primary reason for discontinuation in both arms was disease progression (78.1% in the nivolumab arm and 74.1% in the everolimus arm; Supporting Fig. 1).
Sixty-five (16.4%) patients in the everolimus arm crossed over to the nivolumab extension. Patients who were eligible to cross over to the nivolumab extension phase were a highly select group of patients who progressed on everolimus therapy and were healthy enough to begin nivolumab treatment.
Efficacy
In all randomized patients, OS benefit was maintained with nivolumab versus everolimus (HR, 0.73; 95% CI, 0.62–0.85; P<.0001; Fig. 1A). The 36-, 48-, and 60- month OS probabilities (95% CI) with nivolumab versus everolimus were 39% (34–44) versus 30% (25–34), 30% (25–34) versus 23% (19–27), and 26% (21–30) versus 18% (14–22), respectively.
Figure 1.
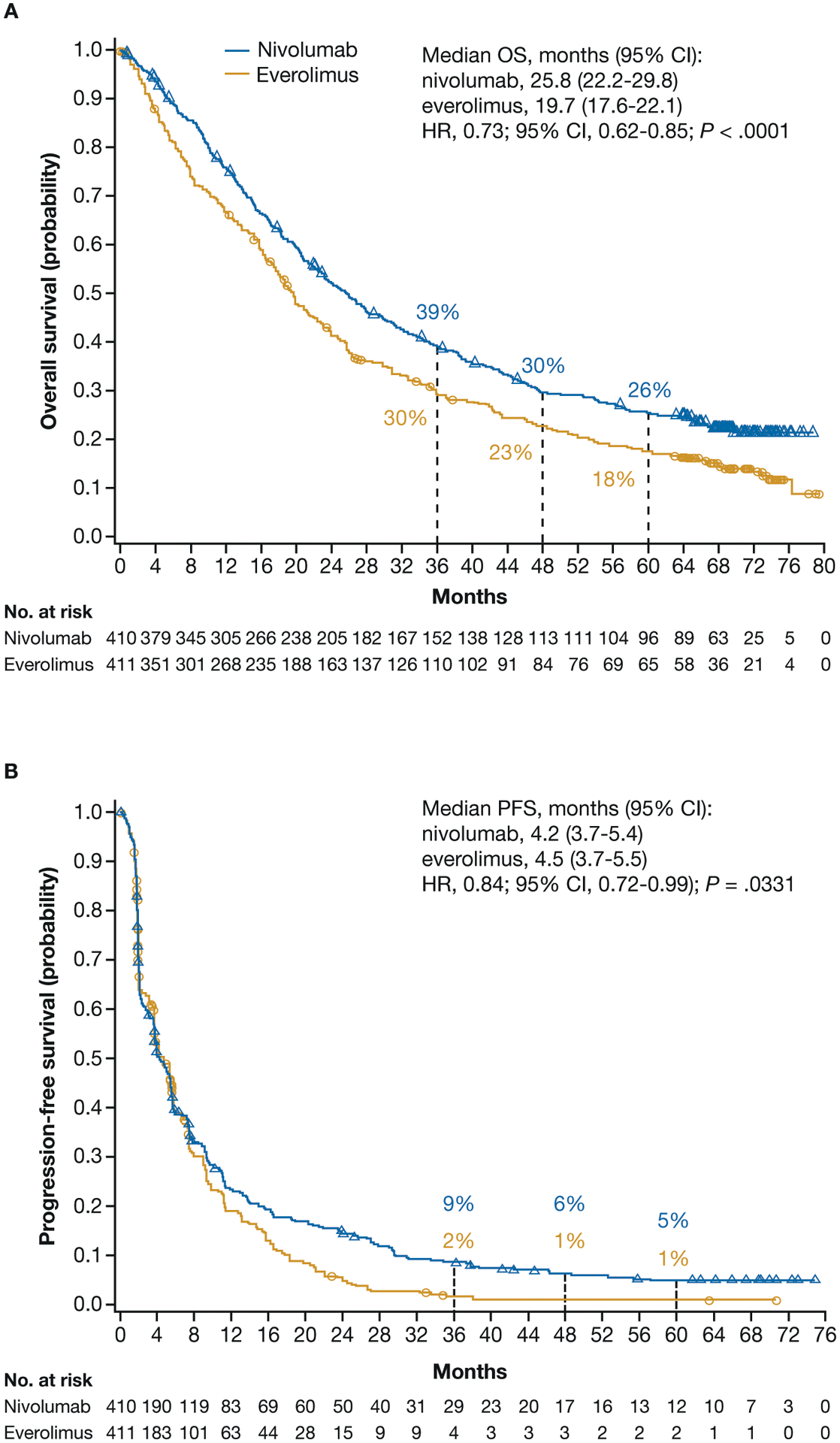
OS (A) and PFS (B) for patients treated with nivolumab and everolimus.
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
PFS with nivolumab and everolimus was similar through 6 months, after which the Kaplan-Meier curves separated, favoring nivolumab with extended follow-up (HR, 0.84; 95% CI, 0.72–0.99; P=.0331; Fig. 1B). The 36-, 48-month, and 60-month PFS probabilities (95% CI) with nivolumab versus everolimus were 9% (6–12) versus 2% (1–4), 6% (4–9) versus 1% (0–3), and 5% (3–8) versus 1% (0–3), respectively.
ORR (95% CI) was 22.9% (18.9–27.3) with nivolumab and 4.1% (2.4–6.5) with everolimus (odds ratio 6.86; 95% CI, 4.01–11.74; P<.0001; Table 1). Complete response was observed in 1.0% (n=4) of patients in the nivolumab group versus 0.5% (n=2) in the everolimus group, while partial response was seen in 22.0% (n=90) of the nivolumab-treated patients versus 3.6% (n=15) of the everolimus-treated patients.
TABLE 1.
Best Overall Response and Objective Response Rates
| Nivolumab (n=410) | Everolimus (n=411) | Crossover to nivolumab (n=65) | |
|---|---|---|---|
| Objective response rate, n (%) 95% CI | 94 (22.9) 18.9–27.3 | 17 (4.1) 2.4–6.5 | 5 (7.7) 2.5–17.0 |
| Odds ratio (95% CI) | 6.86 (4.0–11.7) | – | |
| Best overall response, n (%) | |||
| Complete response | 4 (1.0) | 2 (0.5) | 1 (1.5) |
| Partial response | 90 (22.0) | 15 (3.6) | 4 (6.2) |
| Stable disease | 140 (34.1) | 224 (54.5) | 3 (4.6) |
| Progressive disease | 142 (34.6) | 106 (25.8) | 49 (75.4) |
| Unable to determine | 34 (8.3) | 64 (15.6) | 8 (12.3) |
| Ongoing response, n/N (%) | 26/94 (27.6) | 3/17 (17.6) | 2/5 (40.0) |
CI, confidence interval.
The median time to response was 3.5 months for nivolumab and 3.7 months for everolimus. The median duration of response (95% CI) was 18.2 months (12.9–25.8) with nivolumab and 14.0 months (8.3–19.2) with everolimus (Fig. 2), with ongoing responses at the time of the database lock in 26 of 94 (27.6%) nivolumab responders versus 3 of 17 (17.6%) everolimus responders. For patients who had a complete response with nivolumab (n=4), the median time to confirmed response in complete responders was 2.2 months, and duration of response ranged from 4.5–65.1+ months. Of the 4 patients with complete responses in the nivolumab arm, 2 patients had an ongoing response. Both patients were off treatment and did not receive subsequent therapy. For patients who had a complete response with everolimus (n=2), the median time to confirmed response in complete responders was 4.6 months, with durations of 8.3 and 13.7 months. For patients in the nivolumab arm with a partial response (n=90), the median time to confirmed response in partial responders was 4.8 months, with a median duration (95% CI) of 18 months (12.9–25.1). For patients in the everolimus arm with a partial response (n=15), the median time to confirmed response in partial responders was 3.5 months, with a median duration (95% CI) of 17.9 months (6.4–24.0).
Figure 2.
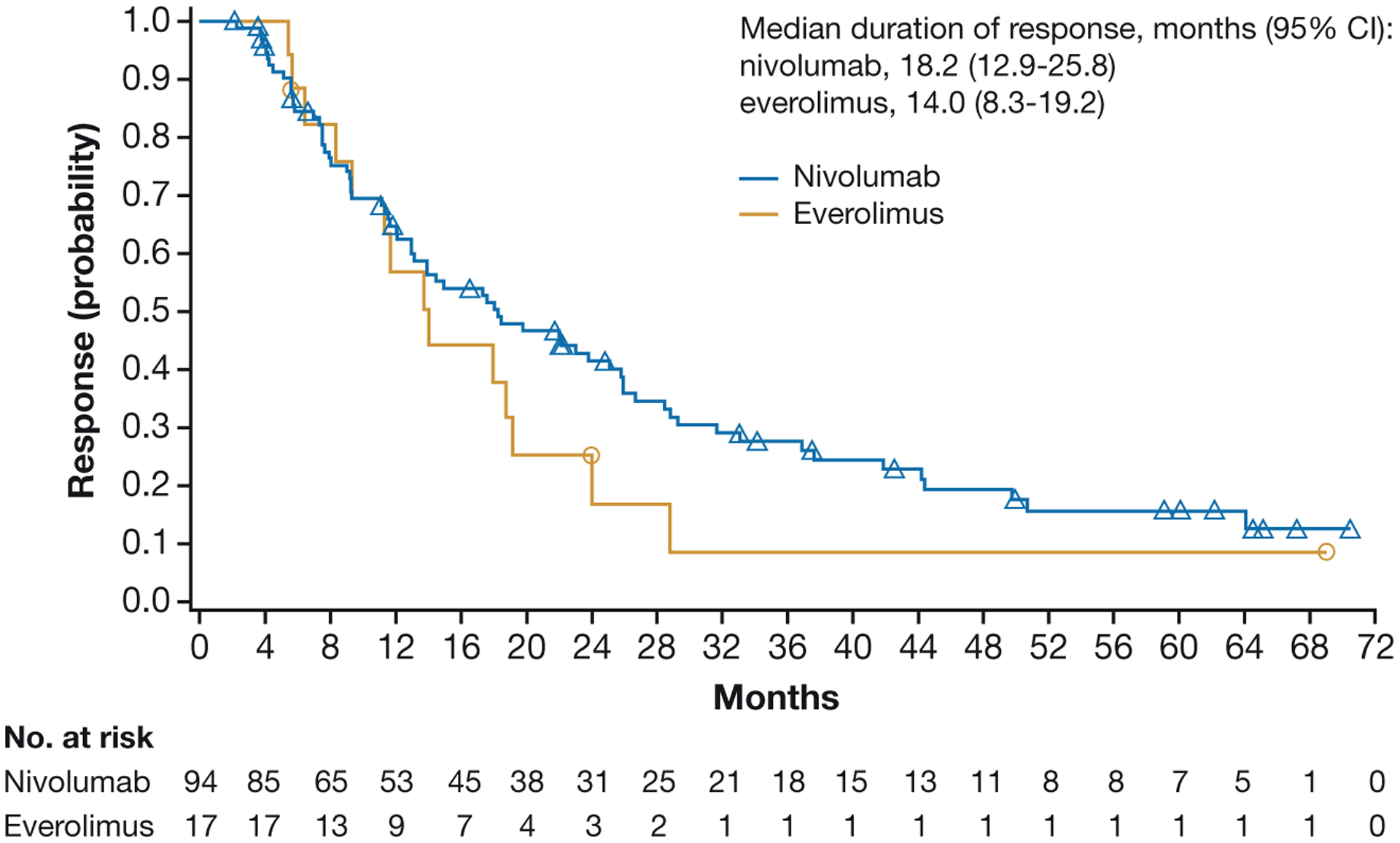
Duration of response in all randomized patients.
CI, confidence interval.
In the nivolumab arm, 59 (63%) responders received subsequent therapy versus 15 (88%) in the everolimus arm, and 8 (9%) responders in the nivolumab arm versus 1 (6%) responder in the everolimus arm remained on therapy at the time of the database lock (Fig. 3). In all responders, the median duration of treatment was 23.6 months for nivolumab and 24.4 months for everolimus. For patients who responded and were off treatment without any subsequent systemic therapy, the median (interquartile range) duration of treatment-free interval was 12.7 months (2.8–28.9) for nivolumab and 4.1 months (4.1–4.1) for everolimus.
Figure 3.
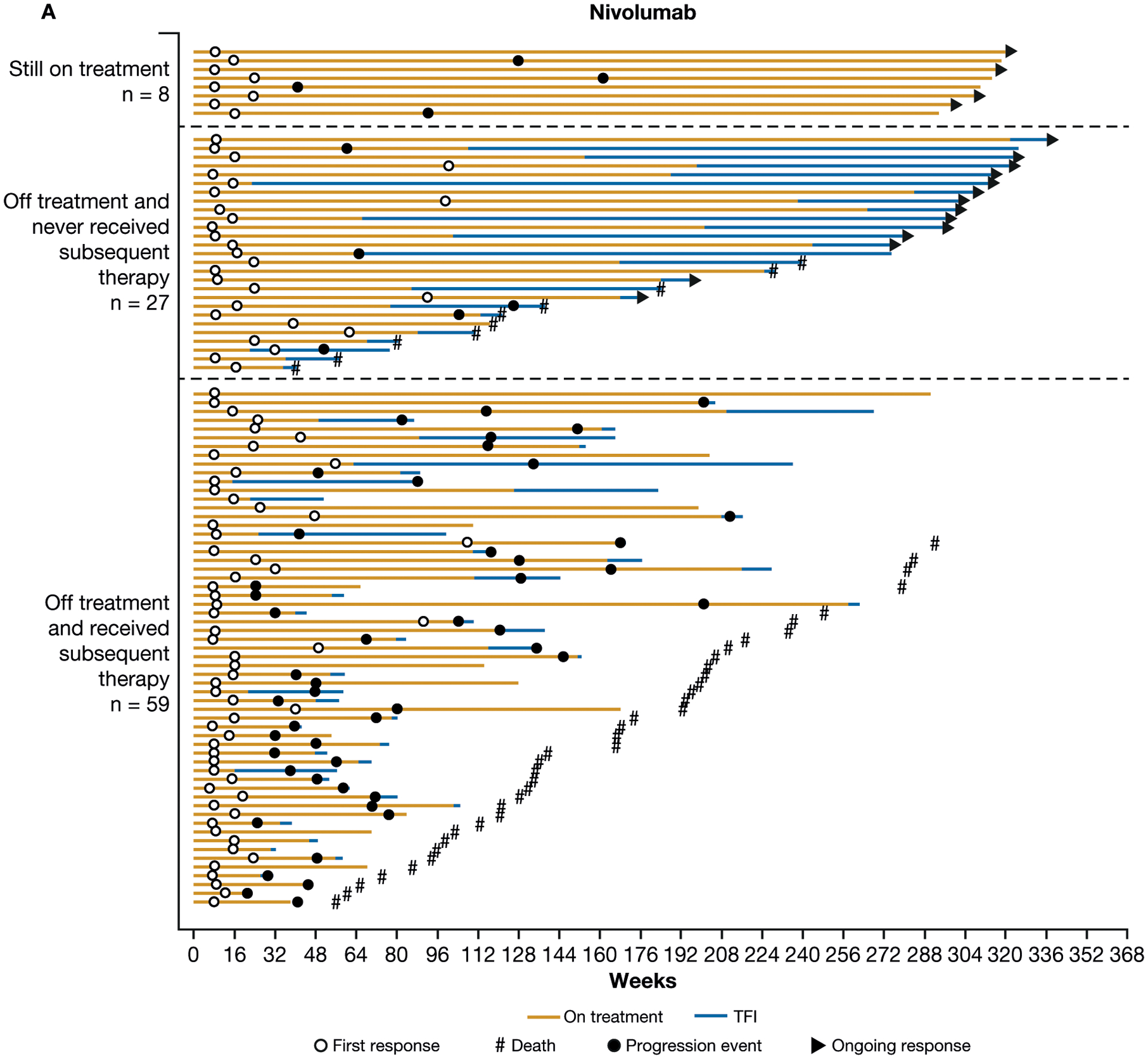
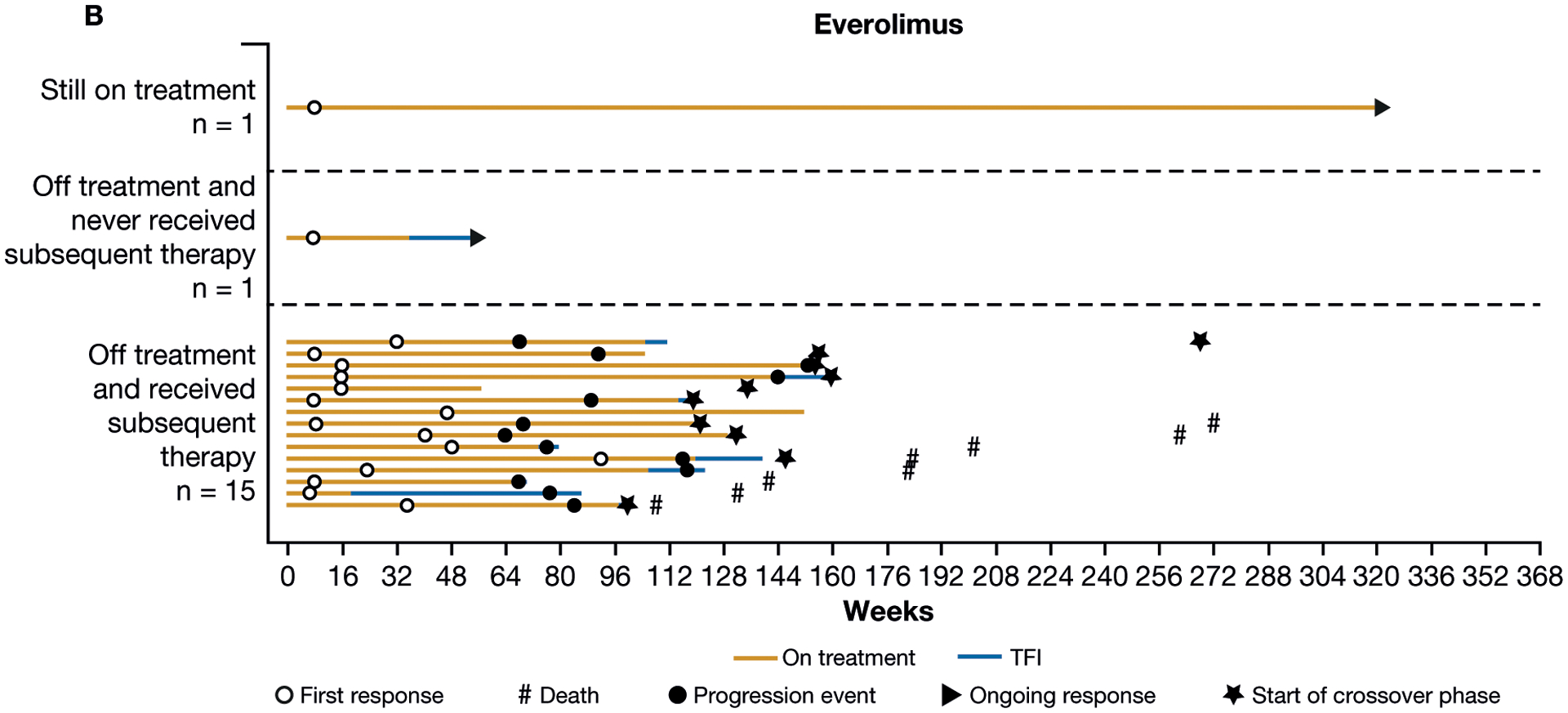
Treatment-free interval (TFI), duration of therapy, duration of response, and subsequent therapy in all patients with confirmed response to nivolumab (A) or everolimus (B).
Patients Who Crossed Over to Nivolumab
As expected, more patients who crossed over had Memorial Sloan Kettering Cancer Center and IMDC favorable-risk disease and only 1 site of metastasis, and a lower proportion had prior radiotherapy, prior pazopanib treatment, liver and bone baseline tumor sites, and tumor PD-L1 expression ≥1% (Supporting Table 1).4 Seven (10.8%) patients who crossed over from everolimus to nivolumab continued to receive treatment at the time of the 5-year analysis. The primary reasons for discontinuation in patients who crossed over from everolimus to nivolumab were disease progression (69.2%) and toxicity with nivolumab (10.8%).
Among the 65 patients who crossed over to nivolumab, the 36-, 48-, and 60-month OS probabilities from initial study randomization were 89%, 71%, and 59%, respectively (Supporting Fig. 2A). The PFS probability (95% CI) was 13% (6.0–25.0) at both 24 and 36 months post-crossover in this population (Supporting Fig. 2B). The ORR post-crossover was 7.7% (2.5–17.0), with 1.5% (n=1) of patients achieving a complete response and 6.2% (n=4) a partial response (Table 1). For patients who crossed over to nivolumab and had a confirmed response (n=5), the median time to response post-crossover was 1.9 months, with a median duration (range) of 16.5 months (7.4–35.5+) and 2 of 5 responders (40%) with ongoing response at the time of the database lock.
Impact of Baseline Clinical Features on Overall Survival: A Multivariable Model
A multivariable model was used to assess the impact of baseline clinical features on OS. Baseline factors were first analyzed individually in a univariable analysis to preclude introducing collinearity into the model (Supporting Table 2). The exploratory univariable analysis did not show baseline tumor PD-L1 expression ≥1% to be an independent prognostic factor for OS with either nivolumab or everolimus. Significant negative prognostic effects of lower hemoglobin and higher tumor burden (sum of reference diameters of target lesions) on OS were observed in the final reduced multivariable models in both the nivolumab and everolimus arms (Supplementary Table S3). Shorter time from diagnosis of metastatic disease to initiation of therapy was uniquely prognostic for shorter OS in the nivolumab arm. Higher corrected calcium, higher neutrophil/lymphocyte ratio, and presence of bone metastases (with or without soft tissue component) were uniquely prognostic for shorter OS in the everolimus arm alone (Supplementary Table S3).
Treatment Administration and Safety
In all treated patients, the overall incidence of treatment-related AEs was 80.5% (grade 3–4, 21.4%) in the nivolumab group and 88.9% (grade 3–4, 36.8%) in the everolimus group. The most common treatment-related AEs of any grade with nivolumab were fatigue (34.7%), pruritus (15.5%), nausea (15.0%), and diarrhea (13.8%; Fig. 4A). The most common treatment-related AEs of any grade with everolimus were fatigue (34.5%), stomatitis (29.5%), and anemia (24.4%; Fig. 4A). The most common grade 3–4 treatment-related AEs with nivolumab were fatigue (2.7%), anemia (2.0%), increased alanine aminotransferase (1.7%), and increased aspartate aminotransferase (1.7%). The most common grade 3–4 treatment-related AEs with everolimus were anemia (8.6%), hypertriglyceridemia (4.5%), stomatitis (4.3%), and hyperglycemia (3.8%). Treatment-related AEs of any grade leading to discontinuation occurred in 39 (9.6%) patients in the nivolumab arm and 50 (12.6%) patients in the everolimus arm. No additional treatment-related deaths were reported since the primary analysis in either arm (none in the nivolumab arm and 2 in the everolimus arm4). Among patients who crossed over from everolimus to nivolumab, the median duration of nivolumab treatment was 8.8 months (95% CI, 6.5–11.4). Treatment-related AEs of any grade occurred in 83.1% (grade 3–4, 13.8%) of patients after crossover from everolimus to nivolumab.
Figure 4.
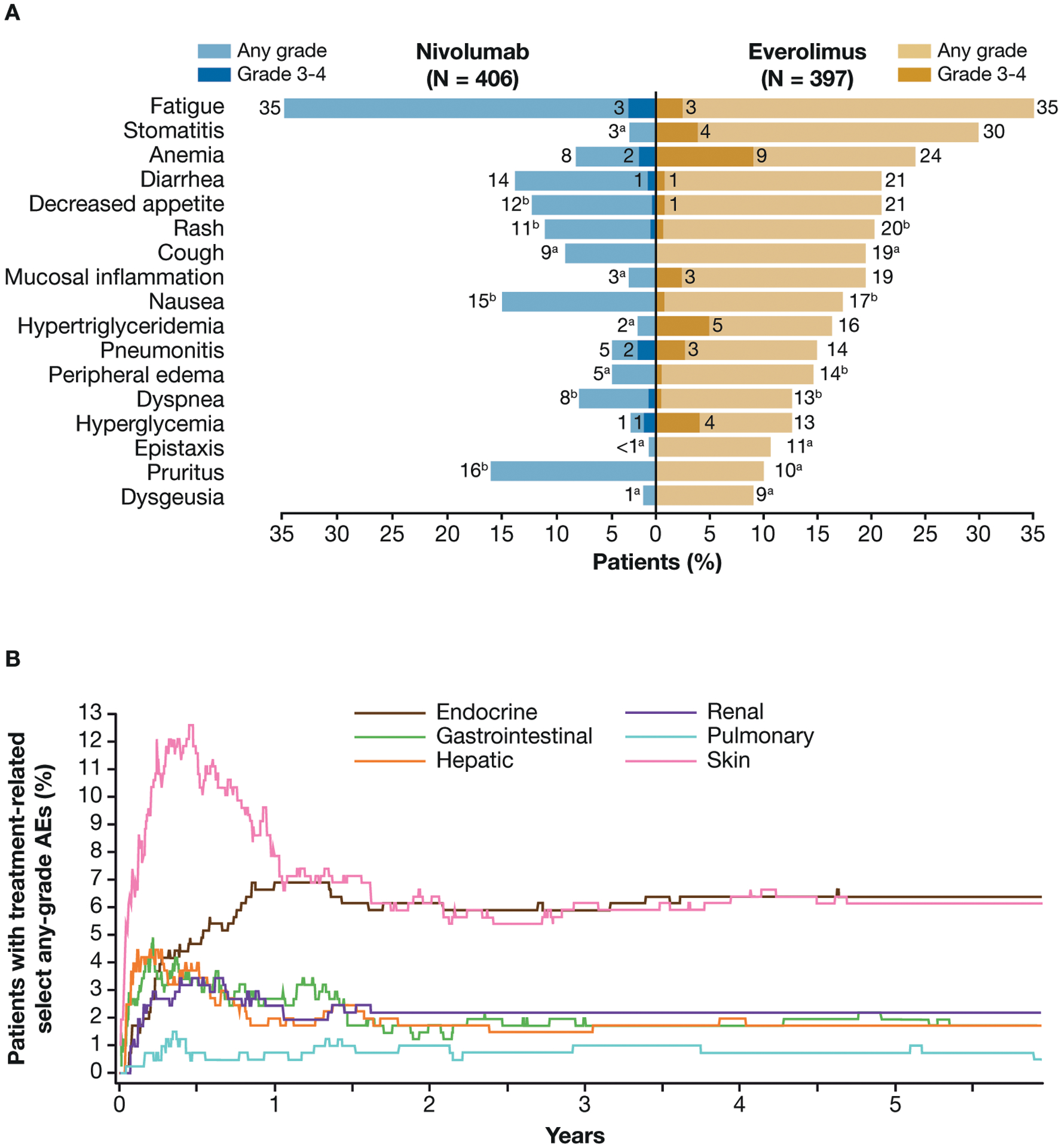
Treatment-related adverse events (AEs) reported in ≥10% of treated patients in either arm (A) and median time to onset and resolution of nivolumab-related select (immune-related) AEs of any grade (B).
aNo patient reported a grade 3–4 treatment-related AE.
b<1% of patients experienced a grade 3 or 4 treatment-related AE.
Throughout the study, any-grade treatment-related select AEs (grade 3–4) occurred among patients in the nivolumab arm as follows: skin 27.8% (1.2%), gastrointestinal 14.0% (2.2%), endocrine 11.1% (1.0%), hepatic 11.3% (3.0%), renal 6.9% (1.0%), and pulmonary 5.2% (1.5%; Supporting Table 4; data for everolimus are shown in Supporting Table 5). Tracking the most common organ classes of treatment-related select AEs over time, the incidence of most events peaked during the initial 7 months of therapy, after which the incidence declined (Fig. 4B). Some select endocrine treatment-related AEs required management with permanent hormone replacement therapy (Fig. 4B). In the nivolumab arm, 47 of 406 (11.6%) treated patients required ≥40 mg prednisone/day (or equivalent) for a median duration of 3.14 weeks for management of treatment-related select AEs.
Quality of Life
Treatment with nivolumab was associated with rapid and sustained improvement in HRQoL from baseline at each assessment point through week 104 per FKSI-DRS based on the primary analysis of CheckMate 025.5 More than 10 randomized patients with baseline plus ≥1 postbaseline HRQoL assessment had non-missing patient-reported outcome data from weeks 4 through 228 and week 236 for nivolumab and from weeks 4 through 120, and week 132 for everolimus. The average change from baseline (defined as mean change in FKSI-DRS score ≥2) was improved at weeks 56, 68, 104, 112, 116, 124, 144, 164, and 176 for patients in the nivolumab arm. Mean change from baseline remained the same or deteriorated for patients in the everolimus arm (Fig. 5).
Figure 5.
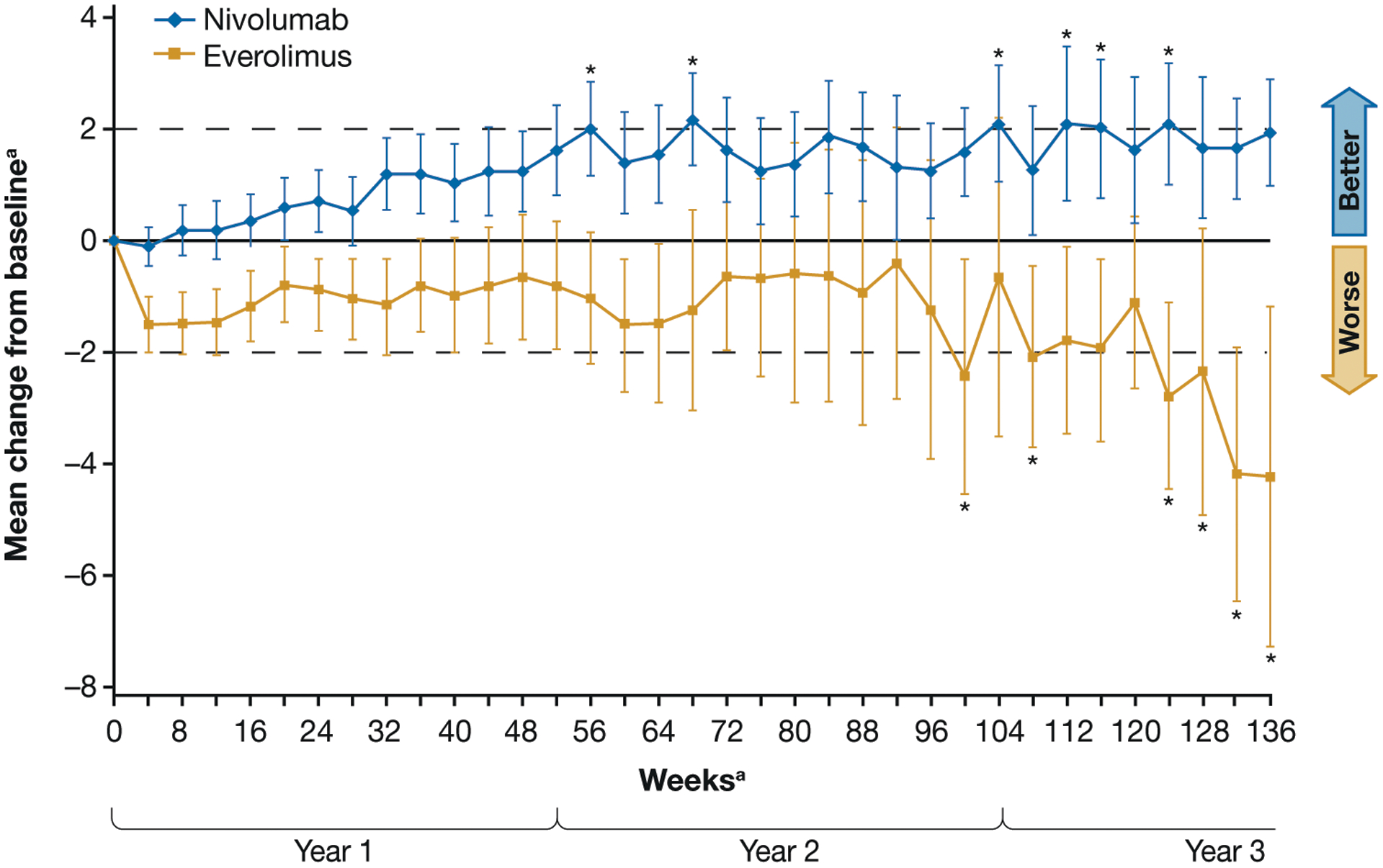
Mean changes from baseline Functional Assessment of Cancer Therapy Kidney Symptom Index–Disease-Related Symptoms (FKSI-DRS) scores.
aMean change from baseline was also clinically meaningful at weeks 144, 164, and 176 in the nivolumab arm.
bN=8 for week 232 and n<10 after week 236 in the nivolumab arm; N=9 for weeks 124, 128, and 136 in the everolimus arm, and n<10 otherwise after week 132.
*Denotes clinically meaningful improvement with nivolumab (+2) or deterioration with EVE (–2) from baseline (dashed lines).
Only time points for which data were available for ≥10 randomized patients with baseline plus ≥1 postbaseline HRQoL assessment with non-missing patient-reported outcome data per arm were included.
Time 0 indicates baseline.
Bars show 95% confidence intervals.
Subsequent Therapy
In total, 276 (67.3%) patients in the nivolumab arm and 296 (72.0%) in the everolimus arm (including those in the everolimus arm who crossed over to the nivolumab extension phase) received subsequent systemic anticancer therapy. The median (95% CI) time from last study drug dose to subsequent systemic therapy was 7.9 weeks (6.1–9.0) with nivolumab and 5.1 weeks (4.3–6.0) with everolimus. The most common subsequent systemic therapies in the nivolumab arm were everolimus (143 patients, 34.9%), axitinib (137, 33.4%), cabozantinib (58, 14.1%), and pazopanib (50, 12.2%). In the everolimus arm, the most common subsequent therapies were axitinib (169 patients, 41.1%), nivolumab (107, 26.0%, including patients who had crossed over to nivolumab), pazopanib (78, 19.0%), sorafenib (45, 10.9%), and sunitinib (46, 11.2%).
In patients who crossed over to nivolumab and received subsequent systemic therapy, the most common subsequent therapies received were axitinib (37 patients, 56.9%), pazopanib (16, 24.6%), cabozantinib (12, 18.5%), and sunitinib (14, 21.5%).
DISCUSSION
The clinical benefit of nivolumab in the treatment of patients with aRCC after antiangiogenic therapy in CheckMate 025 was demonstrated with long-term follow-up. The significant OS benefit with nivolumab over everolimus was maintained with 64 months of minimum follow-up, with 60-month OS probabilities for nivolumab versus everolimus of 26% versus 18%, respectively. In the primary analysis, median PFS was similar between arms4; however, the PFS curves separated with longer follow-up, and favored nivolumab over everolimus. The confirmed ORR was higher and more patients demonstrated ongoing response at long-term follow-up with nivolumab versus everolimus. The proportion of responders treated with nivolumab who experienced a treatment-free interval was higher than with everolimus, and a lower proportion of responders treated with nivolumab required subsequent anticancer therapy versus everolimus-treated patients, suggesting that antitumor effects persist after discontinuation of nivolumab. This pattern of longstanding response appears to be characteristic of immunotherapy-based treatment in RCC and has been previously observed with interleukin-2 therapy, and more recently in a long-term follow-up of the combination of nivolumab plus ipilimumab for previously untreated patients with aRCC in CheckMate 214.11–14
Few additional treatment-related AEs (including those leading to discontinuation) and no additional deaths were observed with longer follow-up in either arm, compared with the primary disclosure of results from this trial.15 There was a lower incidence of any-grade and high-grade treatment-related AEs with nivolumab versus everolimus, consistent with the primary analysis. Similar to previous reports in phase 1–2 trials of nivolumab monotherapy,15–17 the most common treatment-related select AEs were fatigue, pruritus, nausea, and diarrhea in this phase 3 trial. High-grade and any-grade treatment-related select AEs were uncommon with nivolumab, and most select AEs resolved and were manageable using established algorithms. For the first time we report data on the use of corticosteroids (≥40 mg prednisone daily or equivalent) to manage treatment-related select AEs occurring within 30 days of last dose. Relatively few patients required immune-modulating therapy in this setting of nivolumab monotherapy for the treatment of RCC. No new safety signals were apparent with extended follow-up versus the primary analysis of this phase 3 trial, or previous reports with the early-phase studies of nivolumab monotherapy in aRCC.4,15–17 This study included patient-reported outcomes in patients treated with nivolumab versus everolimus, and showed that nivolumab treatment resulted in sustained HRQoL benefit relative to everolimus, highlighting the favorable risk/benefit profile of nivolumab over everolimus with extended follow-up.
Patients who crossed over from everolimus to nivolumab on study were a highly selected subgroup of previously treated patients with favorable prognosis versus the overall trial population. This subgroup of crossover patients achieved a higher ORR with nivolumab, and longer OS and PFS versus patients treated with everolimus who did not cross over to nivolumab. No new safety signals were observed in patients who crossed over to nivolumab versus patients originally randomized to nivolumab treatment. These data suggest that patients with aRCC can derive clinically meaningful benefit from nivolumab treatment in the setting of later line of treatment post antiangiogenic therapy.
Although the association of baseline risk factors with poor OS outcomes trended as expected in the univariable and multivariable analysis, many did not reach statistical significance for nivolumab in the limited sample size in which this analysis was performed. The exploratory multivariable analysis showed that the association of individual risk factors with OS differed between treatment arms, and baseline tumor PD-L1 expression and most IMDC baseline risk factors were not associated with worse OS outcomes with nivolumab. Yet, lower hemoglobin, higher tumor burden (sum of reference diameters of target lesions), and shorter time from diagnosis of metastatic disease to initiation of treatment were negatively prognostic of OS with nivolumab. These results suggest that predictive or prognostic factors for OS differ for patients treated with immunotherapy versus targeted therapies, and that improved prognostic models based on underlying tumor biology dictating response to immunotherapy are needed for previously treated patients with aRCC.
The safety and efficacy of nivolumab monotherapy has been explored in metastatic RCC patient populations that were excluded from eligibility in our study, including patients with non-clear cell histology18,19, and asymptomatic brain metastases.20 These studies support the clinical benefit of nivolumab treatment in these 2 specific patient populations, which are not represented in CheckMate 025.4 The immunotherapeutic landscape of aRCC is evolving with the first approval of the combination of nivolumab plus ipilimumab in the first-line setting for patients with aRCC and intermediate or poor risk factors, followed by other immunotherapy combinations.1,21–25
In summary, this extended follow-up analysis (64 months’ minimum follow-up) reports the durability of response and survival benefit and greater probability of remaining progression-free with nivolumab versus everolimus. No new safety signals were detected with nivolumab or everolimus, and the previously observed improvement in quality of life with nivolumab was sustained. To our knowledge, this 5-year analysis of the CheckMate 025 study is the longest follow-up of a phase 3 trial of immune checkpoint inhibitor therapy reported to date in previously treated patients with aRCC.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients and their families, as well as the investigators and participating clinical study teams, for making this study possible. We also thank Elmer Berghorn for his contributions to the study; Dako, an Agilent Technologies, Inc. company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; and Bristol Myers Squibb (Princeton, NJ) and ONO Pharmaceutical Company Ltd. (Osaka, Japan). All authors contributed to and approved the manuscript; professional medical writing and editorial assistance were provided by Jenny Reinhold, PharmD, of Parexel, funded by Bristol Myers Squibb.
ROLE OF THE FUNDING SOURCE
The funders (Bristol Myers Squibb and ONO Pharmaceutical) contributed to the study design, data analysis, and data interpretation in collaboration with the authors. The funders had no role in data collection. Financial support for editorial and writing assistance was provided by the funders. A data confidentiality agreement was in place between Bristol Myers Squibb and the investigators. All authors vouch for the completeness and accuracy of the data and analyses and for the adherence of the trial to the protocol. All authors had full access to all of the data included in the study, and all authors contributed to drafting the manuscript and provided final approval to submit the manuscript. The corresponding author had full access to all of the data and final responsibility to submit for publication. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Funding:
Bristol Myers Squibb and ONO Pharmaceutical Company Ltd
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I, Immediate Family Member; Inst, Institution. Relationships may not relate to the subject matter of this manuscript.
RJM: Consulting or Advisory Role: Eisai, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer. Research Funding: Bristol Myers Squibb, Eisai, Exelixis, Novartis, Pfizer.
BE: Honoraria: Bristol Myers Squibb, Bayer, Novartis, Pfizer, Exelixis, Roche.
SG: Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Bayer, Pfizer, Exelixis, AstraZeneca, Janssen Oncology, Corvus Pharmaceuticals, Genentech/Roche, EMD Serono, Sanofi. Research Funding: Pfizer (Inst), Acceleron Pharma (Inst), Merck (Inst), Agensys (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Bayer (Inst).
HJH: Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Exelixis
Research Funding: Bristol Myers Squibb. Travel, Accommodations, Expenses: Bristol Myers Squibb, Pfizer, Exelixis.
SS: Research Funding: Bristol Myers Squibb.
SST: Consulting or Advisory Role: Bristol Myers Squibb, Calithera Biosciences, Prometheus Laboratories. Research Funding: Bristol Myers Squibb (Inst), Calithera Biosciences (Inst), Merck (Inst), Nektar Therapeutics (Inst), Peloton Therapeutics (Inst), Jounce Therapeutics (Inst), Pfizer (Inst), Genentech (Inst), Prometheus Laboratories (Inst), ARGOS Therapeutics (Inst).
JAS: Honoraria: Array, Genentech, Merck, Novartis. Consulting or Advisory Role: Array, Genentech, Merck, Novartis. Research Funding: Bristol Myers Squibb.
ERP: Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Genentech/Roche, Merck, Novartis, Pfizer, Eli Lilly Inc., Inovio, Clovis, Horizon Pharma, Exelixis, Seattle Genetics. Research Funding: Acceleron (Inst), AstraZeneca (Inst) Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Eli Lilly Inc. (Inst), Merck (Inst), Peloton (Inst), Pfizer (Inst). Patents, Royalties, Other Intellectual Property: U.S. Patent Application No. 14/588,503, pending, filed January 2, 2015. Other Relationship: Bristol Myers Squibb (fees for development of educational presentations); Merck (fees for development of educational presentations); Roche (fees for development of educational presentations); Novartis (fees for development of educational presentations).
GP: Consulting or Advisory Role: Astellas, Bristol Myers Squibb, Janssen, Novartis, Pfizer. Research Funding: Bayer.
DFM: Consulting or Advisory Role: Array BioPharma, Bristol Myers Squibb, Eisai, Exelixis, Genentech, Merck, Novartis, Pfizer. Research Funding: Prometheus (Inst).
D. Castellano: No relationship to disclose.
TKC: Research (Institutional and personal): AstraZeneca, Alexion, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, Takeda. Honoraria: AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, UptoDate, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc (Healthcare Communications Company with several brands such as OnClive, PeerView and PER), L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, New England Journal of Medicine, Lancet Oncology, Heron Therapeutics, Lilly. Consulting or Advisory Role: AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol Myers-Squibb/ER Squibb and Sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Heron Therapeutics, Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, UptoDate, NCCN, Analysis Group. Patents, royalties or other intellectual properties: International Patent Application No. PCT/US2018/12209, entitled “PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response,” filed January 3, 2018, claiming priority to U.S. Provisional Patent Application No. 62/445,094, filed January 11, 2017. International Patent Application No. PCT/US2018/058430, entitled “Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy,” filed October 31, 2018, claiming priority to U.S. Provisional Patent Application No. 62/581,175, filed November 3, 2017.
FD: Research Funding: Ipsen (Inst), Novartis (Inst), Pfizer (Inst), Analysis Group.
HG: Consulting or Advisory Role: Astellas, Bristol Myers Squibb, Novartis, Pfizer, MSD, AstraZeneca.
SO: Honoraria: Bristol Myers Squibb, Pfizer, Novartis, Eisai, Bayer, Merck & MSD. Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Novartis, Eisai, Bayer, Merck & MSD. Research Funding: Bristol Myers Squibb, Bayer.
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pfizer, Novartis, Eisai, Bayer.
MR: No relationship to disclose.
KP: Employment: Orion Pharma. Stock or Other Ownership: Faron Pharmaceuticals. Consulting or Advisory Role: MSD, Pfizer, Roche, Bristol Myers Squibb, Lilly, Ipsen. Expert Testimony: Ipsen. Travel, Accommodations, Expenses: Bristol Myers Squibb, Pfizer, Roche.
ASA: Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst).
MC: Consulting or Advisory Role: AstraZeneca, Merck, Astellas, Medivation.
JW: Honoraria: Roche, Bristol Myers Squibb, Pfizer, Novartis, MSD. Consulting or Advisory Role: Roche, Bristol Myers Squibb, Pfizer, Novartis, MSD. Speakers Bureau: Roche, Bristol Myers Squibb, Pfizer, Novartis, MSD .Travel, Accommodations, Expenses: Bristol Myers Squibb.
CC: Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Novartis, Ipsen.
SF: Research Funding: ONO Pharmaceutical Co., Ltd.
YT: Honoraria: Pfizer, Novartis, ONO Pharmaceutical Co., Ltd. Consulting or Advisory Role: ONO Pharmaceutical Co., Ltd., Novartis, Taiho. Research Funding: Takeda (Inst), Pfizer (Inst), ONO Pharmaceutical Co., Ltd. (Inst), Astellas (Inst).
TCG: Stock or Other Ownership: Bayer AG. Honoraria: Bristol Myers Squibb, Eisai, Ipsen, Merck Serono, MSD, Novartis, Roche. Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Ipsen, Merck Serono, Merck Sharp & Dohme, Novartis .Travel, Accommodations, Expenses: Bristol Myers Squibb, Ipsen, Merck Serono, MSD, Novartis.
CKK: Honoraria: Bristol Myers Squibb, Pfizer, Ipsen, Eisai. Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Ipsen, Eisai, Roche, Astellas, Janssen, EMD Serono, Merck.
FAS: Consulting or Advisory Role: Bayer, Janssen Oncology, Roche, MSD, Bristol Myers Squibb, Pfizer, Astellas Pharma, AstraZeneca. Speakers Bureau: Janssen Oncology, Astellas Pharma, Bayer, Pfizer, Bristol Myers Squibb, AstraZeneca, MSD, Roche. Research Funding: Janssen Oncology. Travel, Accommodations, Expenses: MSD, Roche, Bristol Myers Squibb.
JL: Honoraria: Bristol Myers Squibb, MSD, Pfizer, Novartis, Eisai, GlaxoSmithKline, Roche, Kymab, Secarna, Pierre Fabre, EUSA Pharma. Consulting or Advisory Role: Bristol Myers Squibb, MSD, Pfizer, Novartis, Eisai, GlaxoSmithKline, Roche, Kymab, Secarna, Pierre Fabre, EUSA Pharma. Research Funding: Bristol Myers Squibb, MSD, Pfizer, Novartis.
D. Cella: Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Pfizer, Janssen Oncology, Merck, GlaxoSmithKline. Research Funding: Bristol Myers Squibb, Novartis, Pfizer, Janssen Oncology, Merck, GlaxoSmithKline. Employment: Bristol Myers Squibb. Stock or Other Ownership: Bristol Myers Squibb.
MBM: Employment: Bristol Myers Squibb. Stock or Other Ownership: Bristol Myers Squibb.
SSS: Employment: Bristol Myers Squibb.
NMT: Research Funding: Bristol Myers Squibb, Calithera Biosciences, Pfizer. Consulting or Advisory Role: Bristol Myers Squibb, Eisai Medical Research, Exelixis, Nektar Therapeutics, Novartis, Oncorena, Pfizer.
DATA-SHARING STATEMENT
Bristol Myers Squibb’s policy on data sharing can be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html. Deidentified and anonymized datasets of clinical trial information, including patient-level data, will be shared with external researchers for proposals that are complete, for which the scientific request is valid and the data are available, consistent with safeguarding patient privacy and informed consent. Upon execution of an agreement, the deidentified and anonymized data sets can be accessed via a secured portal that provides an environment for statistical programming with R. The trial protocol and statistical analysis plan will also be available. Data will be available for 2 years from the study completion or termination of the program (November 2022).
REFERENCES
- 1.OPDIVO (nivolumab). [package insert]. Bristol Myers Squibb, Princeton, NJ, USA; 2019. [Google Scholar]
- 2.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella D, Grunwald V, Nathan P, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.AccessedDecember 15, 2019.
- 7.Cella D, Yount S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health. 2007;10(4):285–293. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 10.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 11.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(suppl 1):S55–57. [PubMed] [Google Scholar]
- 14.Tannir NM, Pal SK, Atkins MB. Second-line treatment landscape for renal cell carcinoma: a comprehensive review. Oncologist. 2018;23(5):540–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016;22(22):5461–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33(18):2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogelzang N, McFarlane J, Kochenderfer M, et al. Efficacy and safety of nivolumab in patients with non-clear cell renal cell carcinoma: results from the phase 3b/4 CheckMate 374 study. Presented at:2019 Genitourinary Cancers Symposium; February 14–16, 2019; San Francisco, CA. [Google Scholar]
- 19.Chahoud J, Msaouel P, Campbell MT, et al. Nivolumab for the treatment of patients with metastatic non-clear cell renal cell carcinoma (nccRCC): a single-institutional experience and literature meta-analysis. Oncologist. 2019. doi: 10.1634/theoncologist.2019-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarlane J, Olsen M, Molina A, et al. Safety of nivolumab in patients with clear cell or non–clear cell renal cell carcinoma: results from the phase IIIb/IV CheckMate 374 study. Presented at:16th International Kidney Cancer Symposium; November 3–4, 2017; Miami, FL. [Google Scholar]
- 21.US Food and Drug Administration. FDA approves avelumab plus axitinib for renal cell carcinoma. PublishedMay14, 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avelumab-plus-axitinib-renal-cell-carcinoma.AccessedDecember 15, 2019.
- 22.US Food and Drug Administration. FDA approves pembrolizumab plus axitinib for advanced renal cell carcinoma. PublishedApril19, 2019. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma.AccessedDecember 3, 2019.
- 23.BAVENCIO (avelumab). [package insert]. EMD Serono, Inc. and Pfizer Inc., New York, NY, USA; 2019. [Google Scholar]
- 24.KEYTRUDA (pembrolizumab) (avelumab). [package insert]. Merck & Co., Inc., Whitehouse Station, NJ, USA; 2019. [Google Scholar]
- 25.US Food and Drug Administration. FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. PublishedApril16, 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-combination-intermediate-or-poor-risk-advanced-renal-cell.AccessedDecember 15, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


