Abstract
Long intergenic non-coding RNA 00657 (LINC00657) or “non-coding RNA activated by DNA damage” (NORAD) is an extremely conserved and copious long non-coding RNA (lncRNA). This transcript has pivotal role in the preservation of genome integrity. Several researches have appraised the role of NORAD in the evolution of human cancers with most of them indicating an oncogenic role for this lncRNA. Several miRNAs such as miR-199a-3p, miR-608, miR−155−5p, miR-590-3p, miR-495-3p, miR-608, miR-202-5p, miR-125a-3p, miR-144-3p, miR−202−5p, and miR-30a-5p have been recognized as targets of NORAD in different cancer cell lines. In addition, NORAD has interactions with cancer-related pathways, particularly STAT, TGF-β, Akt/mTOR, and PI3K/AKT pathway. Over-expression of NORAD has been related with poor clinical outcome of patients with diverse types of neoplasms. Collectively, NORAD is a prospective marker and target for combating cancer.
Keywords: NORAD, lncRNA, cancer, expression, carcinogenesis
Introduction
Long intergenic non-coding RNA 00657 (LINC00657) or alternatively named as “non-coding RNA activated by DNA damage” (NORAD) is an extremely conserved and copious long non-coding RNA (lncRNA; Lee et al., 2016). This transcript has a crucial role in the conservation of genome stability since its inactivation results in striking aneuploidy in formerly karyotypically normal cells (Lee et al., 2016). This function of NORAD is exerted through sequestering Pumilio RNA Binding Family Members (Lee et al., 2016). In addition, NORAD has functional interactions with an element of DNA-damage system namely RNA Binding Motif Protein X-Linked (RBMX). NORAD regulates the capacity of RBMX to construct a ribonucleoprotein complex which encompasses a number of proteins such as topoisomerase I (Munschauer et al., 2018). Depletion of NORAD results in high rate of chromosome segregation impairments, abridged replication-fork speed and changed cell-cycle movement (Munschauer et al., 2018). Due to the critical role of NORAD in the maintenance of genome stability and cell cycle progression, it is not surprising that dysregulation of this lncRNA leads to cancer. Therefore, several studies have appraised the role of this NORAD in initiation or progression of diverse types of malignancies. In the current review, we describe the role of NORAD in the evolution of human cancers based on the conducted experiments in cell lines, animal models and human subjects.
Cell Line Studies
Expression of NORAD has been down-regulated in endometrial cancer cells. Forced up-regulation of this lncRNA suppressed growth of endometrial cancer cells and enhanced their apoptosis. Such effects have been exerted through NORAD binding with the anti-apoptotic protein Far Upstream Element Binding Protein 1 (FUBP1). Interaction between NORAD and FUBP1 has been shown to decrease nuclear localization of this anti-apoptotic protein, releasing the pro-apoptotic gene promoters from FUBP1 occupation and enhancing apoptosis in these cells (Han et al., 2020). A single study in colorectal cancer cells showed down-regulation of NORAD. Forced over-expression of NORAD reduced cell viability and invasiveness of these cells while enhanced cell apoptosis. This lncRNA has increased expression of Calpain 7 (CAPN7) and suppressed activity of PI3K/AKT pathway (Lei et al., 2018). However, two other studies in colorectal cancer cells reported the role of NORAD in increasing cell viability, proliferation, migration and invasion while inhibiting apoptosis (Wang et al., 2018; Zhang et al., 2018). Other studies in diverse cancer cell lines also supported the oncogenic role of this lncRNA. For instance, in ovarian cancer cells, over-expression of NORAD has been correlated with down-regulation of miR-199a-3p. NORAD silencing could suppress proliferation, invasiveness, migratory potential, and epithelial-mesenchymal transition (EMT) of these cells. Functional studies confirmed the direct interplay between NORAD and miR-199a-3p (Xu C. et al., 2020). Besides, NORAD up-regulation has enhanced migration and invasion of hepatocellular carcinoma cells through sponging miR-202-5p, which acts as a tumor-suppressor miRNA through the TGF-β pathway (Yang et al., 2019a). The functional effect of NORAD in activation of TGF-β signaling has also verified in breast cancer cells (Zhou et al., 2019). In lung cancer cells, NORAD promotes EMT-like characteristics through activation of TGF-β signaling. In this type of cancer, importin β1 has been found to be a binding partner of NORAD. NORAD silencing has inhibited the physical interaction between importin β1 with Smad3 to some extent, thus blocking amassment of Smad complexes in the nucleus following induction with TGF−β. Therefore, NORAD facilitates the interaction between importin β1 and Smad3 to enhance nuclear amassment of Smad complexes following exposure to TGF-β (Kawasaki et al., 2018). Lentivirus-mediated silencing of NORAD in epithelial cancer cells has inhibited proliferation, reduced chemoresistance and attenuated cell cycle progression. These roles are exerted through acting as a molecular sponge for hsa-miR-155-5p (Tong et al., 2019). In cervical cancer cells, NORAD enhances expression of SIP1 to increase cell proliferation, invasiveness and EMT. These effects are due to sponging miR-590-3p (Huo et al., 2018). In neuroblastoma, in addition to enhancement of cell proliferation and invasion, NORAD increases doxorubicin resistance possibly through suppression of apoptosis and autophagy. These effects are exerted through miR-144-3p/HDAC8 axis (Wang et al., 2020). In osteosarcoma cell lines, NORAD regulates cancer cell features via acting as a molecular sponge for hsa-miR-199a-3p (Wang et al., 2019). Another study has shown that transcription of NORAD is suppressed by the YAP/TAZ-TEAD complex, a transducer of Hippo pathway. NuRD complex also facilitates transcriptional silencing of NORAD through this route. NORAD exerts effective suppressive impact on migration and invasion of neoplastic cell lines, and blockage of NORAD expression contributes in the pro-migratory and invasive impacts of the YAP pathway. Functionally, NORAD uses its numerous repeated sequences to act as a multifaceted scaffold for binding and isolating S100P, thus inhibiting S100P-associated pro-metastatic cascades (Tan et al., 2019).
Non-coding RNA activated by DNA damage has also been found to increase expression of the PI3K/AKT/mTOR pathway-related proteins. Expression of these proteins has not not considerably influenced by miR-520a-3p mimic. However, co-transfection of NORAD and miR-520 mimic has upturned the expression of these proteins. NORAD silencing has not affected expression of PI3K/AKT/mTOR pathway-associated proteins, while anti-miR-520 has enhanced expression of these proteins. Taken together, NORAD has been shown to induce the activity of PI3K/AKT/mTOR signaling through sponging miR-520 (Wan et al., 2020).
Table 1 displays summary of studies which evaluated expression of NORAD in cancer cell lines.
TABLE 1.
Summarized results of studies which evaluated expression of NORAD in cell lines (Δ: knock-down, EMT: epithelial–mesenchymal transition).
| Cancer types | Targets/regulators and signaling pathways | Cell lines | Function | Ref |
| Endometrial cancer | FUBP1 | ISK and SPEC-2 | ↑ NORAD: ↓ cell growth and ↑ apoptosis | Han et al., 2020 |
| Ovarian cancer | miR-199a-3p | SKOV3, HO8910, A2780, OVCAR-3 and IOSE80 | Δ NORAD: ↓cell proliferation, invasiveness, and migration ability | Xu C. et al., 2020 |
| miR-608/STAT3 | SKOV3, Caov3, A2780, HO-8910, OVCAR3 and HOEpiC | Δ NORAD: ↓cell viability, migration, invasiveness and ↑ apoptosis. mediating the antineoplastic impacts of physcion 8-O-b-glucopyranoside | Yang et al., 2019b | |
| Epithelial ovarian cancer | miR-155-5p | SK-OV-3, CAOV-3, CAOV-4, OVCAR-3, HEY-T30, ES-2, SW/626 and HS832.Tc | Δ NORAD: ↓ cell proliferation and chemoresistance | Tong et al., 2019 |
| Cervical cancer | miR-590-3p/SIP1 | SiHa, HeLa, ME180, C33a, CaSki and Ect1/E6E7 | Δ NORAD: ↓ cells proliferation, colony formation ability, invasion and EMT | Huo et al., 2018 |
| Breast cancer | TGF-β pathway | MCF-7, MDA-MB-231 and MCF10A | Δ NORAD: ↓ cell proliferation, migration and invasion | Zhou et al., 2019 |
| YAP/TAZ-TEAD complex and S100P | MDA-MB-231, Hs578T, T47D, ZR75 | Δ NORAD: ↑ cell migration and invasion | Tan et al., 2019 | |
| miR-323a-3p/PUM1/eIF2 | MCF-7, MDA-MB-231, MDA-MB-468, MDA-MB-453, T47D and MCF10A | Δ NORAD: ↓ cell viability, invasion and migration | Shi et al., 2021 | |
| Prostate cancer | miR-495-3p/TRIP13 | DU145, 22Rv1, LNCaP and RWPE-1 | Δ NORAD: ↑ cell apoptosis and ↓ cell proliferation, migration, and invasion | Chen et al., 2020 |
| miR-541-3p/PKM2 | 22Rv1, DU145, PC-3, C4-2B and RWPE-1 | Δ NORAD: ↓ cell proliferation, migration and invasion | Hu et al., 2021 | |
| – | LNCaP, 22Rv1, PC-3, DU145 and RWPE-1 | Δ NORAD: ↓ cell proliferation, migration and ↑ cell apoptosis | Zhou et al., 2019 | |
| miR-30a-5p/RAB11A/WNT/β-catenin pathway | PC-3, LNCap, 22RV1, DU-145 and RWPE-1 | Δ NORAD: ↓ cell proliferation, invasion and EMT | Zhang and Li, 2020 | |
| Bladder cancer | – | TSSCUP, T24, 639 V and UMUC1 | Δ NORAD: ↓ cells proliferation and colony formation ability | Li et al., 2018 |
| Renal cell carcinoma | miR-144-3p/MYCN | 86-O, A498, ACHN, OS-RC-2 and HK-2 | ↑ NORAD: ↑ cell proliferation and migratory potential | Zhao W. et al., 2020 |
| Gastric cancer | RhoA/ROCK1 Pathway | AGS, BGC-823, HGC-27, MGC-803 and GES-1 | Δ NORAD: ↑ cell apoptosis and ↓ cell proliferation and Metastatic Behavior | Yu et al., 2019 |
| miR-608/FOXO6 | MKN28, MKN45, SGC7901, SNU-16 and GES-1 | Δ NORAD: ↓ tumor growth, migration and ↑ cell apoptosis | Miao et al., 2019 | |
| miR-214/Akt/mTOR | BSG823, MKN28, BGC803, BGC823 and GSE1 | Δ NORAD: ↑ cell apoptosis and ↓ cell proliferation | Tao et al., 2019 | |
| Colorectal cancer | CAPN7 and PI3K/AKT pathway | HCT116, Caco2, Caco205, SW620, SW480 and NCM460 | ↑ NORAD: ↓ Cell Proliferation and Invasion | Lei et al., 2018 |
| – | HCT116 and SW1116 | Δ NORAD: ↓ cell viability, migration and invasion | Wang et al., 2018 | |
| miR-202-5p | SW480, HCT116 and FHC | Δ NORAD: ↓ Cell Proliferation, migration, invasion and ↑ Cellular Apoptosis | Zhang et al., 2018 | |
| miR-203a | HCT116, SW620, SW480, HT29 and NCM460 | Δ NORAD: ↓ Cell invasion | Zhao L. et al., 2020 | |
| Pancreatic cancer | miR-125a-3p/RhoA | SW1990, Capan-1, PANC-1, AsPC-1, CFPAC-1, MIAPaCa-2 and BxPC-3 | Δ NORAD: ↓cell migration and invasion | Li et al., 2017 |
| Hepatocellular carcinoma | miR-144-3p/SEPT2 | Hep3B, Huh7, BEL-7402, HCCLM3 and LO2 | Δ NORAD: ↓cell proliferation, colony formation and ↑ apoptosis | Tian et al., 2020 |
| miR-202-5p/TGF-β | SMMC-7721, Huh7, PLC/PRF/5, and Hep3B | ↑ NORAD: ↑ cell proliferation, enhanced the colony construction, cell migration and invasion | Yang et al., 2019a | |
| miR-211-5p/FOXD1/VEGF-A axis | Δ NORAD: ↓ cell proliferation, migration and angiogenesis | Sun et al., 2021 | ||
| Lung cancer | CXCR4 and CXCL12/RhoA/ROCK pathway | A549, SPC-A1, SK-MES-1 and 16HBE | Δ NORAD: ↓ Cell Proliferation, Migration and Invasion | Wu Y. et al., 2020 |
| miR-30a-5p/ADAM19 | H460, H1299, A549, and SCLC-21H and HBE | Δ NORAD: ↓cell proliferation, migration, invasion and ↑ cell apoptosis | Wu H. et al., 2020 | |
| YAP/TAZ-TEAD complex and S100P | H460, CL1-0, CL1-5,293T and 293FT | Δ NORAD: ↑ cell migration and invasion | Tan et al., 2019 | |
| Non-small cell lung cancer | miR-129-1-3p/SOX4 | H446 and A549 | Δ NORAD: resensitized to DDP (cisplatin) | Huang et al., 2020 |
| TGF- β | A549 | Δ NORAD: ↓cell migration and EMT-like morphological changes | Kawasaki et al., 2018 | |
| miR-656-3p/AKT1 | SPC-A1, H460, H1650, A549 and HBE | ↑ NORAD: ↑ cell proliferation and migration | Chen T. et al., 2019 | |
| miR-136-5p/E2F1 | A549, H1975, H1650, LK-2, H1299, H460 and HBE | Δ NORAD: ↓cell proliferation and glycolysis | Gao et al., 2019 | |
| miR-520a-3p/PI3k/Akt/mTOR Signaling pathway | A549, H1299, H460, SK-MES-1, Calu3 and HEK293T | Δ NORAD: ↓cell Proliferation, Migration and Invasion | Wan et al., 2020 | |
| miR-422a | A549, SK-MES-1, H1975, SK-LU-1 and 16HBE | ↑ NORAD: ↑cell viability, migration, invasion and EMT | Chen et al., 2021 | |
| miR-455/CDK14 | NCI-H1650 and HCC827 | Δ NORAD: ↓ proliferation ability | Wang C. et al., 2021 | |
| miR-202-5p/P-gp | A549/DPP | Δ NORAD: ↑ cisplatin sensitivity in A549/DPP cells | Shen et al., 2020 | |
| miR-363-3p/PEAK1 and ERK1/2 signaling pathway | H1975, H1299, A549, 95D, and H460, (HEK)-293 T, BEAS-2B and MRC5 | Δ NORAD: ↓ invasion and EMT | Geng et al., 2021 | |
| Papillary Thyroid carcinoma | miR-202-5p | K1, BCPAP, TPC1 and NPA187 and HT-ori3 | ↑ NORAD: ↑ cell growth, invasion, migration and EMT | Chen Y. et al., 2019 |
| Esophageal cancer | miR-26a-5p/CKS2 via MDM2/p53/Bcl2/Bax pathway | KYSE-150, ECA-109 and HEEC | Δ NORAD: ↓ cell proliferation, invasion, migration and ↑ cell apoptosis | Zhang et al., 2020 |
| Oral squamous cell carcinoma | miR-150 | Fadu, SCC-25, CAL-27, Tca8113 and Hs 680.Tg | Δ NORAD: ↓ cell proliferation | Xu F. et al., 2020 |
| Malignant melanoma | miR-205/EGLN2 | A375, WM451, SK-MEL-24, WM35 and HM | Δ NORAD: ↓ cell migration and invasion | Chen Y. et al., 2019 |
| Glioma | AKR1B1 | GSC11, M059J, U251, T98G and A735 | Δ NORAD: ↓ cell proliferation, invasion, migration and ↑ cell apoptosis | Luo et al., 2020 |
| Neuroblastoma | – | SK-N-BE, SMS-KAN, SMS-KCN, CHLA-15, CHLA-122, NBL-W, SK-N-BE, SMS-KANR, SMS-KCNR, CHLA20, CHLA-136 and NBL-WR | NORAD may be able to predict neuroblastoma outcome | Utnes et al., 2019 |
| miR-1443p/HDAC8 | SK-N-SH, IMR-32 and HUVEC | Δ NORAD: ↓ cell proliferation, migration, invasion and ↑ apoptosis, autophagy and doxorubicin resistance | Wang et al., 2020 | |
| Chromosomal instability | SH-SY5Y and SK-N-BE (Munschauer et al., 2018) | Δ NORAD: ↑ cell proliferation, migration and cell cycle arrest specially impaired sister chromatid cohesion and segregation | Yu et al., 2020 | |
| Osteosarcoma | miR-199a-3p | Saos-2, 143B, HOS, KHOS/240S, MG-63, U-2OS, SK-ES- and Hs755 | Δ NORAD: ↓ cell proliferation and invasion | Wang et al., 2019 |
| miR-410-3p | HOS/DDP | Δ NORAD: ↓ cell proliferation and ↑ sensitivity to cisplatin | Xie et al., 2020 | |
| miR-155-5p | 143B, HOS, MG63, Saos-2, U2OS, hFOB and HEK-293T | Δ NORAD: ↓ cell proliferation, migration and invasion | Wang Y. et al., 2021 |
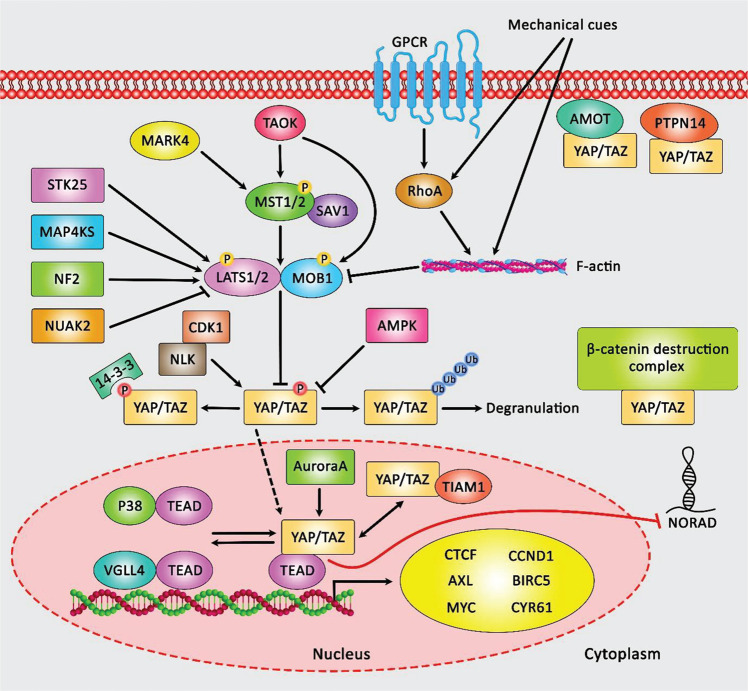
Figure 1 depicts the role of Hippo cascade transducer YAP/TAZ-TEAD complex in inhibiting the expression of lncRNA NORAD in lung and breast neoplasms, and consequent attenuation of the tumor suppressor roles of NORAD in tumor cells.
FIGURE 1.
A schematic representation of the crosstalk between Hippo signaling cascade and lncRNA NORAD in lung and breast neoplasms. YAP/TAZ is mainly modulated via the canonical Hippo cascade, MST1/2-SAV1, and LATS1/2-MOB1. LATS1/2 could in turn phosphorylate YAP/TAZ and suppress its function through either ubiquitination and proteasome-mediated degradation or 14-3-3-mediated cytoplasmic sequestration. Unphosphorylated YAP/TAZ is transferred to the nucleus, where it could interact with TEAD transcription factors and trigger the expression of various target genes. LATS1/2 could be upregulated via STK25, TAOK, NF2, and MAP4KS, while being inhibited through GPCR-RHOA-mediated F-actin function mechanical cues as well as NUAK2. In addition, expression of MST1/2 is regulated by TAOK and MARK4. Expression of YAP/TAZ is also modulated in an independent manner from LATS. Besides, PTPN14 and AMOT could interact with YAP/TAZ and sequester it in the cell membrane. Expression of YAP/TAZ is downregulated via the β-catenin demolition complex or TIAM1 through a direct interaction. Phosphorylation of YAP/TAZ is triggered by CDK1, AMPK, Aurora A, NLK, and various RTKs. In addition, p38 and VGLL4 could interact with TEAD and inhibit the function of YAP/TAZ (Yamaguchi and Taouk, 2020). Mounting evidence has collectively demonstrated that the Hippo pathway transducer YAP/TAZ-TEAD complex could play an effective role in suppressing the expression level of lncRNA NORAD in both lung and breast cancers. Its downregulation correlates with enhancement of migration, invasion as well as metastasis in tumor cells (Tan et al., 2019).
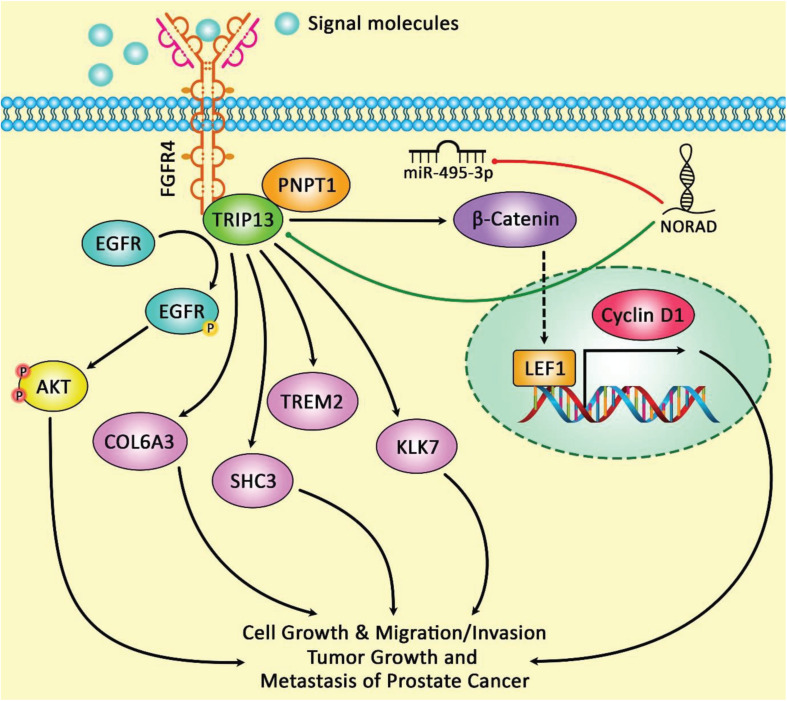
Figure 2 demonstrates the modulation of TRIP13 expression through lncRNA NORAD indicating that TRIP13 upregulation could suppress the impacts of miR-495-3p up-regulation on the proliferation, apoptosis, migratory potential, and invasiveness of prostate cancer cells.
FIGURE 2.
The schematic diagram of the role of lncRNA NORAD in the regulation of TRIP13 expression in prostate cancer. Overexpression of NORAD and TRIP13 and downregulation of miR-495-3p have been in prostate cancer cells. LncRNA NORAD could modulate TRIP13 expression through sponging miR-495-3p, and thereby enhancing cell proliferation, migration, and invasion as well as reducing cell apoptosis in tumor cells. In fact, NORAD could play an important role as a sponge for miR-495-3p in prostate cancer cells that attenuates the potent tumor suppressive activity of this miRNA in target cells (Chen et al., 2020).
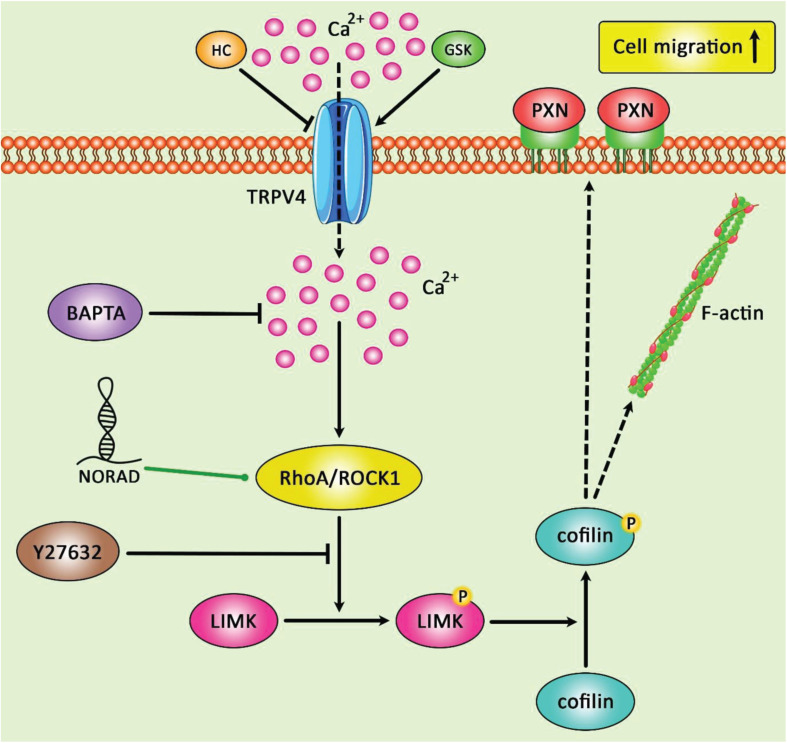
Figure 3 represents the oncogenic role of NORAD in gastric cancer progression via modulating the expression levels of RhoA/ROCK1.
FIGURE 3.
A schematic summary of the crosstalk between the RhoA/ROCK1 singling pathway and lncRNA NORAD in gastric cancer. The figure depicts the impact of RhoA/ROCK1 involved in LIMK/cofilin/TRPV4/Ca2+ pathway in gastric cancer. LncRNA NORAD could promote the expression levels of RhoA and ROCK1, and thereby enhancing cell proliferation, migration and invasiveness and reducing cell apoptosis in gastric cancer cells (Yu et al., 2019).
Human Studies
Based on the assessment of data available in The Cancer Genome Atlas (TCGA) as well as an independent cohort of patients with endometrial cancer, expression of NORAD has been decreased in endometrial cancer samples compared with normal tissue samples in association with cancer progression. Notably, has been identified as the underlying mechanism of NORAD down-regulation in these samples (Han et al., 2020). A single study in patients with colorectal cancer demonstrated down-regulation of NORAD in tumor tissues particularly in samples obtained from patients developed distant metastasis. Down-regulation of NORAD has been associated with poor patients’ outcome, advanced tumor size and TNM stage (Lei et al., 2018). Apart from these two studies, other studies have reported up-regulation of NORAD in tumoral samples compared with non-tumoral samples from the same tissue. Such over-expression has also been verified in other cohorts of patients with colorectal cancer (Wang et al., 2018; Zhang et al., 2018). Besides, expression of this lncRNA has been up-regulated in hepatocellular carcinoma (HCC) samples compared with adjacent tissues in correlation with poor overall survival (Yang et al., 2019a). Over-expression of NORAD in cervical cancer patients has been correlated with higher stage, lymph nodes and vascular involvement, and poor survival (Huo et al., 2018). Table 2 depicts the results of studies which evaluated expression of NORAD in clinical samples.
TABLE 2.
Summarized results of studies which assessed expression of NORAD in clinical samples (OS: overall survival, ANTT: adjacent non-tumoral tissue).
| Cancer types | Number of clinical samples (tissue, serum, etc.) | Expression tumor vs normal | Kaplan–Meier analysis | Univariate cox regression | Multivariate cox regression | Ref |
| Endometrial cancer (EC) | 56 EC tissues, 54 ANTTs and 20 normal endometrial tissues, TCGA data | Down | Decreased NORAD level was correlated with poor survival in patients with EC | – | – | Han et al., 2020 |
| Ovarian cancer (OC) | 86 paired of OC tissues and ANNTs | Up | – | – | – | Xu C. et al., 2020 |
| 56 paired of OC tissues and ANNTs | Up | – | – | – | Yang et al., 2019b | |
| Epithelial ovarian cancer (EOC) | 17 paired of EOC tissues and ANNTs | Up | – | – | – | Tong et al., 2019 |
| Cervical cancer (CC) | 47 paired of CC tissues and ANNTs | Up | NORAD upregulation was correlated with poor OS in CC patients | – | – | Huo et al., 2018 |
| Breast cancer (BC) | 21 BC tissues and 10 ANNTs | Up | NORAD upregulation was correlated with worse survival compared to the downregulation groups | – | – | Zhou et al., 2019 |
| 44 BC tissues (subtypes: HER2, luminal A, luminal B, basal-like and triple-negative) | Up (Differentially expressed among BC subtypes) | Higher expression of NORAD in Basal-like subtype correlated with lower disease-free survival rate | – | – | Mathias et al., 2021 | |
| 108 paired of BC tissues and ANNTs | Up | – | – | – | Shi et al., 2021 | |
| Prostate cancer (PC) | 30 paired of PC tissues and ANNTs | Up | – | – | – | Chen et al., 2020 |
| 74 paired of PC tissues and ANNTs | Up | Higher NORAD expression associated with poor survival | – | – | Hu et al., 2021 | |
| 45 paired of PC tissues and ANNTs | Up | – | – | – | Zhang and Li, 2020 | |
| Bladder cancer | 10 paired of BC tissues and ANNTs | Up | Over-expression of NORAD was significantly associated with worse OS | Tumor stage, histological grade, lymph node involvement, and NORAD expression were significantly associated with OS | NORAD over-expression was independent prognostic indicator for OS. | Li et al., 2018 |
| Renal cell carcinoma (RCC) | 36 paired of RC tissues and ANNTs | Up | – | – | – | Zhao W. et al., 2020 |
| Esophageal Squamous Cell Carcinoma (ESCC) | 106 paired of ESCC tissues and ANTTs | Up | NORAD upregulation was correlated with poor OS and disease-free survival in ESCC patients | Tumor differentiation, lymph node metastasis, UICC stage and NORAD expression were significantly associated with ESCC. | NORAD expression levels and UICC stage were independent prognostic factors in ESCC. | Wu et al., 2017 |
| Gastric cancer (GC) | 65 paired of GC tissues and ANTTs, GEO database | Up | NORAD upregulation was significantly correlated with worse OS in GC patients | – | – | Yu et al., 2019 |
| 40 paired of GC tissues and ANTTs | Up | NORAD upregulation was significantly correlated with the worse prognosis of the GC patients | – | – | Miao et al., 2019 | |
| 36 paired of GC tissues and ANTTs | Up | – | – | – | Tao et al., 2019 | |
| Colorectal cancer (CRC) | 80 paired of CC tissues and ANTTs | Down | – | – | – | Lei et al., 2018 |
| 60 paired of CRC tissues and ANTTs. Serum samples from142 CRC patients, 136 normal subjects, and 71 patients with benign disorders | Up | – | – | – | Wang et al., 2018 | |
| 47 paired of CRC tissues and ANTTs | Up | Higher expression levels of NORAD suggested poorer prognosis in CRC patients compared to lower group | – | – | Zhang et al., 2018 | |
| Serum samples from 32 CRC patients and 48 precancerous patients and 110 healthy controls | Up | – | – | – | Shaker et al., 2019 | |
| 30 paired CRC tissues and ANTTs | Up | NORAD higher expression levels associated with poor OS in CRC patients | – | – | Zhao L. et al., 2020 | |
| Pancreatic ductal adenocarcinoma (PDAC) | 33 paired PDAC tissues and ANTTs | Up | PDAC patients with higher NORAD expression had shorter OS and recurrence-free survival | – | – | Li et al., 2017 |
| Hepatocellular carcinoma (HCC) | Starbase data | Up | – | – | – | Tian et al., 2020 |
| 29 HCC tissues and their ANTTs | Up | NORAD upregulation correlated with shorter OS rate and higher recurrence rate in HCC patients | Sex, tumor size and NORAD expression were significantly associated with OS | NORAD expression was an independent indicator of OS and recurrence after surgery. | Wu Y. et al., 2020 | |
| Up | – | – | – | Sun et al., 2021 | ||
| Lung cancer (LC) | Up | – | – | – | Wu Y. et al., 2020 | |
| 31 paired of LC tissues and ANTTs | Up | – | – | – | Wu H. et al., 2020 | |
| Non-small cell lung cancer (NSCLC) | 60 paired od NSCLC tissues and ANTTs | Up | High expression levels of NORAD suggested poorer prognosis in NSCLC patients | – | – | Huang et al., 2020 |
| 24 paired of NSCLC tissues and ANTTs | Up | – | – | – | Chen T. et al., 2019 | |
| 80 paired of NSCLC tissues and ANTTs | Up | – | – | Gao et al., 2019 | ||
| 26 paired of NSCLC tissues and ANTTs | Up | – | – | – | Wan et al., 2020 | |
| 50 paired of NSCLC tissues and ANTTs | Up | – | – | – | Chen et al., 2021 | |
| Up | Higher NORAD expression levels associated with worse prognosis in NSCLC patients | – | – | Wang C. et al., 2021 | ||
| 15 paired of NSCLC tissues and ANTTs | Up | – | – | – | Geng et al., 2021 | |
| Papillary thyroid carcinoma (PTC) | 40 paired of PTC tissues ANTTs | Up | – | – | – | Chen Y. et al., 2019 |
| Oral squamous cell carcinoma (OSCC) | 32 paired of OSCC tissues ANTTs | Up | Higher expression of NORAD predicted worse prognosis in OSCC patients | – | – | Xu F. et al., 2020 |
| Malignant melanoma (MM) | 62 MM tissues and 20 normal tissues | Up | – | – | – | Chen Y. et al., 2019 |
| Neuroblastoma (NB) | 38 paired of NB tissues and normal tissues | Up | – | – | – | Wang et al., 2020 |
| 40 NB tumor specimens | Down | Lower NORAD expression correlated with poor OS and event free survival in NB patients | – | – | Yu et al., 2020 | |
| Glioblastoma (GBM) | TCGA (168 GBM tissues and 5 normal brain tissues) GTEx (105 normal brain tissues) | Up | – | – | – | Peng et al., 2020 |
| Osteosarcoma | 69 paired of tumor bone tissues and ANTTs | Up | – | – | – | Wang et al., 2019 |
| 30 paired of osteosarcoma tissues and ANTTs | Up | – | – | – | Wang Y. et al., 2021 |
Prognostic Role of NORAD in Malignancies
Apart from endometrial cancer in which up-regulation of NORAD determined good prognosis (Han et al., 2020), in other types of cancers, including cervical cancer (Huo et al., 2018), breast cancer (Zhou et al., 2019), bladder cancer (Li et al., 2018), esophageal cancer (Wu et al., 2017), gastric cancer (Yu et al., 2019), colorectal cancer (Zhang et al., 2018), pancreatic cancer (Li et al., 2017), hepatocellular carcinoma (Yang et al., 2019a), and lung cancer (Huang et al., 2020), its up-regulation was an indicator of poor survival.
Animal Studies
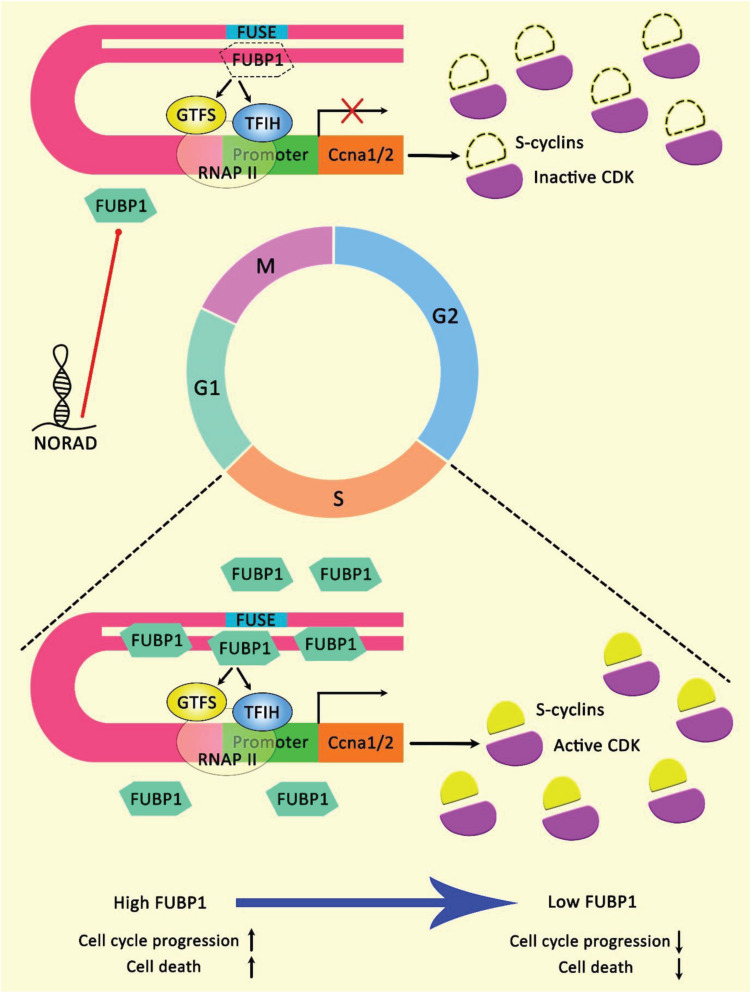
Endometrial cancer is among few cancer types in which NORAD exerts anti-oncogenic effects. Such effects have been verified in animal models since NORAD silencing has enhanced tumor growth in the xenograft model. On the other hand, over-expression of FUBP1-binding fragment of NORAD has attenuated tumor growth in this model (Han et al., 2020). Figure 4 illustrates the effect of lncRNA NORAD binding with FUBP1 in endometrial cancer cells.
FIGURE 4.
A schematic illustration of the interaction of lncRNA NORAD and FUBP1 in endometrial cancer. FUBP1 is a master transcriptional regulator of various genes via interacting with FUSE. FUBP1 protein level is upregulated in the S phase. Reducing in the expression level of FUBP1 could affect cell cycle progression, particularly in the S phase, via downregulating Ccna gene theat encodes cyclin A. Fubp1-cyclin A axis could play a crucial role in triggering various types of cancers. Heterogeneous expression patterns of Fubp1 could be seen among several cancer tissues, illustrating its multiple and sophisticated functions in cancer development (Han et al., 2020). Accumulating evidence elucidates that epigenetic inactivation of NORAD could promote cell growth and reduce apoptosis in endometrial cancer cells. NORAD/FUBP1 interaction could inhibit FUBP1 nuclear localization, and thereby downregulating the recruitment of FUBP1 on promoters of target pro-apoptotic genes, triggering apoptosis in tumor cells (Han et al., 2020).
Apart from this study, other in vivo studies have shown the role of NORAD in enhancement of tumor progression in animal models. For instance, NORAD increases the growth of neuroblastoma tumors in animal models via miR-144-3p/HDAC8 axis (Wang et al., 2020). Moreover, growth of osteosarcoma tumors in animals has been attenuated by NORAD silencing in the implanted cells (Wang et al., 2019). Further studies in malignant melanoma, cervical cancer, breast cancer and lung cancer supported oncogenic effects of NORAD in xenograft models. Table 3 recapitulates the results of studies which evaluated the role of NORAD in the development of cancer in animal models.
TABLE 3.
Outline of studies which assessed function of NORAD in animal models (Δ: knock down or deletion).
| Cancer types | Animal models | Function and comment | Ref |
| Endometrial cancer | Female BALB/c nude mice | Δ NORAD: ↑ Tumor growth | Han et al., 2020 |
| Epithelial ovarian cancer | Adult female athymic nude mice | Δ NORAD: ↓ Tumor volume | Tong et al., 2019 |
| Cervical cancer | Athymic BALB/c mice | Δ NORAD: ↓ Tumor volume and weight | Huo et al., 2018 |
| Breast cancer | Female BALB/c mice | Δ NORAD: ↓ Tumor Growth | Zhou et al., 2019 |
| male BALB/c-nu/nu nude mice | Δ NORAD: ↓ Tumor Growth | Shi et al., 2021 | |
| Prostate cancer | BALB/c-nu mice | Δ NORAD: ↓ bone metastasis | Hu et al., 2021 |
| BALB/C nude mice | Δ NORAD: ↓ Tumor volume and weight | Zhang and Li, 2020 | |
| Gastric cancer | BALB/c nude mice | Δ NORAD: ↓ Tumor volume | Tao et al., 2019 |
| Colorectal cancer | Male BALB/c nude mice | Δ NORAD: ↓ Tumor Growth | Zhang et al., 2018 |
| Hepatocellular carcinoma | Mice | Δ NORAD: ↓ Tumor Growth | Tian et al., 2020 |
| nude mice | Δ NORAD: ↓ Tumor weight | Yang et al., 2019a | |
| Lung cancer | Male athymic nude BALBC/c | Δ NORAD: ↓ Tumor Growth | Wu Y. et al., 2020 |
| Non-small cell lung cancer | Mice | Δ NORAD: ↓ Tumor weight and volume and metastasis | Wan et al., 2020 |
| Malignant melanoma | Male BALB/c-nu/nu | Δ NORAD: ↓ Tumor Growth | Chen Y. et al., 2019 |
| Neuroblastoma | Flank of mice | Δ NORAD: ↓ Tumor Growth | Wang et al., 2020 |
| Osteosarcoma | Nude female BALB/c mice | Δ NORAD: ↓ Tumor Growth | Wang et al., 2019 |
Discussion
Numerous studies have evaluated the role of NORAD in the development of cancer. With the exception of two studies in endometrial and colorectal cancer, other studies indicate the oncogenic role of this lncRNA in diverse cancer types. Several miRNAs such as miR-199a-3p, miR-608, miR−155−5p, miR-590-3p, miR-495-3p, miR-608, miR-202-5p, miR-125a-3p, miR-144-3p, miR−202−5p, and miR-30a-5p have been recognized as targets of NORAD in different cancer cell lines. In addition, NORAD has interactions with cancer-related pathways such as STAT, TGF-β, Akt/mTOR, and PI3K/AKT pathway. The role of NORAD in activation of TGF-β has been verified in different cancers, namely hepatocellular carcinoma, breast cancer and lung cancer. This function is implicated in the enhancement of EMT features and invasive properties of cancer cells. Therefore, in addition its role in the initiation of cancer possibly through influencing genomic stability, NORAD partakes in the progression of cancer through enhancement of EMT. In addition, NORAD has a role in the modulation of response of cancer cells to a number of chemotherapeutic drugs such as doxorubicin and cisplatin (Huang et al., 2020; Wang et al., 2020).
In vivo studies in xenograft models of ovarian, cervical, breast, gastric, colorectal, liver and lung cancers as well as neuroblastoma and osteosarcoma have shown the efficacy of NORAD-targeting therapeutic options in reducing tumor burden. Therefore, this lncRNA is a putative target for treatment of cancer.
The prognostic value of NORAD has been verified in diverse cancer types such as lung, liver, pancreatic, colorectal, breast and cervical cancers where over-expression of this lncRNA was correlated with poor survival. Based on the significant difference in expression of this lncRNA between cancerous and non-cancerous tissues, assessment of its expression might provide a diagnostic tool for cancer. However, the sensitivity and specificity of this marker should be assessed in diverse cancer types. Moreover, assessment of its expression in body fluid such as blood, serum and urine might help in the development of non-invasive diagnostic methods. The latter possible application of NORAD has not been assessed yet.
The data presented above indicate up-regulation of NORAD in almost all types of neoplasm. Moreover, functional studies have shown the pro-proliferative, pro-migratory, and pro-metastatic abilities of NORAD. Collectively, NORAD in an oncogenic lncRNA in most tissues and a possible target for inventions against cancer. Future investigations are required to support its application as diagnostic marker in the clinical settings.
Author Contributions
MT and SG-F wrote the draft and revised it. ND, TA, BH, and AA collected the data and designed the tables and figures. All authors read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Chen F., Liu L., Wang S. (2020). Long non-coding RNA NORAD exhaustion represses prostate cancer progression through inhibiting TRIP13 expression via competitively binding to miR-495-3p. Cancer Cell Int. 20 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Qin S., Gu Y., Pan H., Bian D. (2019). Long non-coding RNA NORAD promotes the occurrence and development of non-small cell lung cancer by adsorbing MiR-656-3p. Mol. Genet. Genomic Med. 7:e757. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen Y., Cao K., Li J., Wang A., Sun L., Tang J., et al. (2019). Overexpression of long non-coding RNA NORAD promotes invasion and migration in malignant melanoma via regulating the MIR-205-EGLN2 pathway. Cancer Med. 8 1744–1754. 10.1002/cam4.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Che Q., Xie C. (2021). NORAD regulates epithelial-mesenchymal transition of non-small cell lung cancer cells via miR-422a. Mol. Med. Rep. 23:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Weng T., Wang L., Shi B., Meng W., Wang X., et al. (2019). Long non-coding RNA NORAD promotes cell proliferation and glycolysis in non-small cell lung cancer by acting as a sponge for miR-136-5p. Mol. Med. Rep. 19 5397–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Q., Li Z., Li X., Wu Y., Chen N. (2021). LncRNA NORAD, sponging miR-363-3p, promotes invasion and EMT by upregulating PEAK1 and activating the ERK signaling pathway in NSCLC cells. J. Bioenerg. Biomembr. 53 321–332. 10.1007/s10863-021-09892-6 [DOI] [PubMed] [Google Scholar]
- Han T., Wu Y., Hu X., Chen Y., Jia W., He Q., et al. (2020). NORAD orchestrates endometrial cancer progression by sequestering FUBP1 nuclear localization to promote cell apoptosis. Cell Death Dis. 11:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.-Y., Chen J., Qin X.-H., You P., Ma J., Zhang J., et al. (2021). . Long non-coding RNA NORAD promotes the prostate cancer cell extracellular vesicle release via microRNA-541-3p-regulated PKM2 to induce bone metastasis of prostate cancer. J. Exp. Clin. Cancer Res. 40 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Xing S., Peng A., Yu Z. (2020). NORAD accelerates chemo-resistance of non-small-cell lung cancer via targeting at miR-129-1-3p/SOX4 axis. Biosci. Rep. 40:BSR20193489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo H., Tian J., Wang R., Li Y., Qu C., Wang N. (2018). Long non-coding RNA NORAD upregulate SIP1 expression to promote cell proliferation and invasion in cervical cancer. Biomed. Pharmacother. 106 1454–1460. 10.1016/j.biopha.2018.07.101 [DOI] [PubMed] [Google Scholar]
- Kawasaki N., Miwa T., Hokari S., Sakurai T., Ohmori K., Miyauchi K., et al. (2018). Long noncoding RNA NORAD regulates transforming growth factor-β signaling and epithelial-to-mesenchymal transition-like phenotype. Cancer Sci. 109 2211–2220. 10.1111/cas.13626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kopp F., Chang T.-C., Sataluri A., Chen B., Sivakumar S., et al. (2016). Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell 164 69–80. 10.1016/j.cell.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Wang Y., Wang X., Bai J. (2018). LINC00657 promotes the development of colon cancer by activating PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 22 6315–6323. [DOI] [PubMed] [Google Scholar]
- Li H., Wang X., Wen C., Huo Z., Wang W., Zhan Q., et al. (2017). Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol. Cancer 16 1–14. 10.1155/2019/1569638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li C., Chen J., Liu P., Cui Y., Zhou X., et al. (eds) (2018). “High expression of long noncoding RNA NORAD indicates a poor prognosis and promotes clinical progression and metastasis in bladder cancer,” in Urologic Oncology: Seminars and Original Investigations, (Amsterdam: Elsevier; ). [DOI] [PubMed] [Google Scholar]
- Luo L., Chen C., He H., Cai M., Ling C. (2020). Silencing of Long Non-Coding RNA (LncRNA) Non-Coding RNA Activated by DNA Damage (NORAD) Inhibits Proliferation, Invasion, Migration, and Promotes Apoptosis of Glioma Cells via Downregulating the Expression of AKR1B1. Med. Sci. Monit. 26:e922659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias C., Pedroso G. A., Pabst F. R., Lima R. S. D., Kuroda F., Cavalli I. J., et al. (2021). So alike yet so different. Differential expression of the long non-coding RNAs NORAD and HCG11 in breast cancer subtypes. Genet. Mol. Biol. 44:e20200153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z., Guo X., Tian L. (2019). The long noncoding RNA NORAD promotes the growth of gastric cancer cells by sponging miR-608. Gene 687 116–124. 10.1016/j.gene.2018.11.052 [DOI] [PubMed] [Google Scholar]
- Munschauer M., Nguyen C. T., Sirokman K., Hartigan C. R., Hogstrom L., Engreitz J. M., et al. (2018). The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature 561 132–136. 10.1038/s41586-018-0453-z [DOI] [PubMed] [Google Scholar]
- Peng Q., Li R., Li Y., Xu X., Ni W., Lin H., et al. (2020). Prediction of a competing endogenous RNA co-expression network as a prognostic marker in glioblastoma. J. Cell. Mol. Med. 24 13346–13355. 10.1111/jcmm.15957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker O. G., Ali M. A., Ahmed T. I., Zaki O. M., Ali D. Y., Hassan E. A., et al. (2019). Association between LINC00657 and miR-106a serum expression levels and susceptibility to colorectal cancer, adenomatous polyposis, and ulcerative colitis in Egyptian population. IUBMB Life 71 1322–1335. 10.1002/iub.2039 [DOI] [PubMed] [Google Scholar]
- Shen J.-G., Xu S.-N., Yin L.-G. (2020). LncRNA NORAD/miR-202-5p regulates the drug resistance of A549/DDP to cisplatin by targeting P-gp. Gen. Physiol. Biophys. 39 481–489. 10.4149/gpb_2020027 [DOI] [PubMed] [Google Scholar]
- Shi P., Zhang J., Li X., Li W., Li H., Fu P. (2021). Long non-coding RNA NORAD inhibition upregulates microRNA-323a-3p to suppress tumorigenesis and development of breast cancer through the PUM1/eIF2 axis. Cell Cycle [Epub Online ahead of print]. 10.1080/15384101.2021.1934627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D.-S., Guan C.-H., Wang W.-N., Hu Z.-T., Zhao Y.-Q., Jiang X.-M. (2021). LncRNA NORAD promotes proliferation, migration and angiogenesis of hepatocellular carcinoma cells through targeting miR-211-5p/FOXD1/VEGF-A axis. Microvasc. Res. 134:104120. 10.1016/j.mvr.2020.104120 [DOI] [PubMed] [Google Scholar]
- Tan B.-S., Yang M.-C., Singh S., Chou Y.-C., Chen H.-Y., Wang M.-Y., et al. (2019). LncRNA NORAD is repressed by the YAP pathway and suppresses lung and breast cancer metastasis by sequestering S100P. Oncogene 38 5612–5626. 10.1038/s41388-019-0812-8 [DOI] [PubMed] [Google Scholar]
- Tao W., Li Y., Zhu M., Li C., Li P. (2019). LncRNA NORAD Promotes Proliferation And Inhibits Apoptosis Of Gastric Cancer By Regulating miR-214/Akt/mTOR Axis. OncoTargets Ther. 12 8841–8851. 10.2147/ott.s216862 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tian Q., Yan X., Yang L., Liu Z., Yuan Z., Shen Z., et al. (2020). lncRNA NORAD promotes hepatocellular carcinoma progression via regulating miR-144-3p/SEPT2. Am. J. Transl. Res. 12 2257–2266. [PMC free article] [PubMed] [Google Scholar]
- Tong L., Ao Y., Zhang H., Wang K., Wang Y., Ma Q. (2019). Long noncoding RNA NORAD is upregulated in epithelial ovarian cancer and its downregulation suppressed cancer cell functions by competing with miR-155-5p. Cancer Med. 8 4782–4791. 10.1002/cam4.2350 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Utnes P., Løkke C., Flægstad T., Einvik C. (2019). Clinically relevant biomarker discovery in high-risk recurrent neuroblastoma. Cancer Inform. 18:1176935119832910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Yao Z., Chen W., Li D. (2020). The lncRNA NORAD/miR-520a-3p Facilitates Malignancy in Non-Small Cell Lung Cancer via PI3k/Akt/mTOR Signaling Pathway. OncoTargets Ther. 13 1533–1544. 10.2147/ott.s230954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Xu L., Zhang J., Cheng X., Xu Q., Wang J., et al. (2020). LncRNA NORAD accelerates the progression and doxorubicin resistance of neuroblastoma through up-regulating HDAC8 via sponging miR-144-3p. Biomed. Pharmacother. 129:110268. 10.1016/j.biopha.2020.110268 [DOI] [PubMed] [Google Scholar]
- Wang C., Wu D., He M., Guan L., Bai D., Liang B. (2021). LncRNA NORAD accelerates the progression of non-small cell lung cancer via targeting miRNA-455/CDK14 axis. Minerva Med. [Epub Online ahead of print]. 10.23736/S0026-4806.21.07194-9 [DOI] [PubMed] [Google Scholar]
- Wang L., Du L., Duan W., Yan S., Xie Y., Wang C. (2018). Overexpression of long noncoding RNA NORAD in colorectal cancer associates with tumor progression. OncoTargets Ther. 11 6757–6766. 10.2147/ott.s176354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zou J., Chen H., Zhang P., Lu Z., You Z., et al. (2019). Long noncoding RNA NORAD regulates cancer cell proliferation and migration in human osteosarcoma by endogenously competing with miR-199a-3p. IUBMB Life 71 1482–1491. 10.1002/iub.2064 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou B., Yan L., Wu J., Xing Z., Zhang S., et al. (2021). lncRNA NORAD promotes the progression of osteosarcoma via targeting of miR-155-5p. Exp. Ther. Med. 21 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Qin W., Lu S., Wang X., Zhang J., Sun T., et al. (2020). Long noncoding RNA ZFAS1 promoting small nucleolar RNA-mediated 2′-O-methylation via NOP58 recruitment in colorectal cancer. Mol. Cancer 19 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Lim Z.-F., Li Z., Gu L., Ma W., Zhou Q., et al. (2017). NORAD expression is associated with adverse prognosis in esophageal squamous cell carcinoma. Oncol. Res. Treat. 40 370–374. [DOI] [PubMed] [Google Scholar]
- Wu Y., Shen Q., Niu Y., Chen X., Liu H., Shen X. (2020). LncNORAD interference inhibits tumor growth and lung cancer cell proliferation, invasion and migration by down-regulating CXCR4 to suppress RhoA/ROCK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 24 5446–5455. [DOI] [PubMed] [Google Scholar]
- Xie X., Liu W., Duan Z., Li X., Zhang L., Yang G. (2020). LncRNA NORAD targets miR-410-3p to regulate drug resistance sensitivity of osteosarcoma. Cell. Mol. Biol. 66 143–148. 10.14715/cmb/2020.66.3.22 [DOI] [PubMed] [Google Scholar]
- Xu C., Zhu L., Sun D., Yao H., Han D. (2020). Regulatory mechanism of lncRNA NORAD on proliferation and invasion of ovarian cancer cells through miR-199a-3p. Eur. Rev. Med. Pharmacol. Sci. 24 1672–1681. [DOI] [PubMed] [Google Scholar]
- Xu F., Xu X., Hu X. (2020). LINC00657 promotes malignant progression of oral squamous cell carcinoma via regulating microRNA-150. Eur. Rev. Med. Pharmacol. Sci. 24 2482–2490. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Taouk G. M. (2020). A Potential role of YAP/TAZ in the interplay between metastasis and metabolic alterations. Front. Oncol. 10:928. 10.3389/fonc.2020.00928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Cai J. B., Peng R., Wei C. Y., Lu J. C., Gao C., et al. (2019a). The long noncoding RNA NORAD enhances the TGF-β pathway to promote hepatocellular carcinoma progression by targeting miR-202-5p. J. Cell. Physiol. 234 12051–12060. 10.1002/jcp.27869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yan Y., Chen Y., Li J., Yang J. (2019b). Involvement of NORAD/miR-608/STAT3 axis in carcinostasis effects of physcion 8-O-β-glucopyranoside on ovarian cancer cells. Artif. Cells Nanomed. Biotechnol. 47 2855–2865. 10.1080/21691401.2019.1637884 [DOI] [PubMed] [Google Scholar]
- Yu S., Peng H., Zhu Q., Wu Y., Wu F., Han C., et al. (2019). Silencing the long noncoding RNA NORAD inhibits gastric cancer cell proliferation and invasion by the RhoA/ROCK1 pathway. Eur. Rev. Med. Pharmacol. Sci. 23 3760–3770. [DOI] [PubMed] [Google Scholar]
- Yu Y., Chen F., Jin Y., Yang Y., Wang S., Zhang J., et al. (2020). Downregulated NORAD in neuroblastoma promotes cell proliferation via chromosomal instability and predicts poor prognosis. Acta Biochim. Pol. 67 595–603. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li X.-Y., Hu P., Ding Y.-S. (2018). LncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol. Res. 26 1411–1418. 10.3727/096504018x15190844870055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-M., Wang J., Liu Z.-L., Liu H., Cheng Y.-F., Wang T. (2020). LINC00657/miR-26a-5p/CKS2 ceRNA network promotes the growth of esophageal cancer cells via the MDM2/p53/Bcl2/Bax pathway. Biosci. Rep. 40:BSR20200525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li Y. (2020). Long non-coding RNA NORAD contributes to the proliferation, invasion and EMT progression of prostate cancer via the miR-30a-5p/RAB11A/WNT/β-catenin pathway. Cancer Cell Int. 20 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Liu C., Yan S., Hu G., Xiang K., Xiang H., et al. (2020). LINC00657 promotes colorectal cancer stem-like cell invasion by functioning as a miR-203a sponge. Biochem. Biophys. Res. Commun. 529 500–506. 10.1016/j.bbrc.2020.04.049 [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang L., Xu F. (2020). LncRNA NORAD stimulates proliferation and migration of renal cancer via activating the miR-144-3p/MYCN axis. Eur. Rev. Med. Pharmacol. Sci. 24 10426–10432. [DOI] [PubMed] [Google Scholar]
- Zhou K., Ou Q., Wang G., Zhang W., Hao Y., Li W. (2019). High long non-coding RNA NORAD expression predicts poor prognosis and promotes breast cancer progression by regulating TGF-β pathway. Cancer Cell Int. 19 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]