Abstract
Introduction:
The Kidney Allocation System in the United States prioritizes candidates with Estimated Post-Transplant Survival (EPTS) ≤20% to receive deceased donor kidneys with Kidney Donor Profile Index (KDPI) ≤20%.
Research Question:
We compared access to KDPI ≤ 20% kidneys for EPTS ≤ 20% candidates across the United States to determine whether geographic disparities in access to these low KDPI kidneys exist.
Design:
We identified all incident adult deceased donor kidney candidates wait-listed January 1, 2015, to March 31, 2018, using United Network for Organ Sharing data. We calculated the proportion of candidates transplanted, final EPTS, and KDPI of transplanted kidneys for candidates listed with EPTS ≤ 20% versus >20%. We compared the odds of receiving a KDPI ≤ 20% deceased donor kidney for EPTS ≤ 20% candidates across regions using logistic regression.
Results:
Among 121 069 deceased donor kidney candidates, 28.5% had listing EPTS ≤ 20%. Of these, 16.1% received deceased donor kidney transplants (candidates listed EPTS > 20%: 17.1% transplanted) and 12.3% lost EPTS ≤ 20% status. Only 49.4% of transplanted EPTS ≤ 20% candidates received a KDPI ≤ 20% kidney, and 48.3% of KDPI ≤ 20% kidneys went to recipients with EPTS > 20% at the time of transplantation. Odds of receiving a KDPI ≤ 20% kidney were highest in region 6 and lowest in region 9 (odds ratio 0.19 [0.13 to 0.28]). The ratio of KDPI ≤ 20% donors per EPTS ≤ 20% candidate and likelihood of KDPI ≤ 20% transplantation were strongly correlated (r2 = 0.84).
Discussion:
Marked geographic variation in the likelihood of receiving a KDPI ≤ 20% deceased donor kidney among transplanted EPTS ≤ 20% candidates exists and is related to differences in organ availability within allocation borders. Policy changes to improve organ sharing are needed to improve equity in access to low KDPI kidneys.
Keywords: geographic disparities, deceased donor kidney, organ allocation, health policy
Introduction and Background
Geographic disparities in access to kidney transplantation have been largely attributed to regional differences in end-stage kidney disease incidence, organ donation rates, and organ utilization practices, with organ allocation borders also playing a role.1–4 Further, comparable wait-listed kidney transplant candidates who are transplanted receive deceased donor kidneys of varying qualities, purportedly due to regional variations in the availability of organs.5 However, unlike the majority of other wait-listed candidates, those with younger age and a lower burden of comorbidities do not necessarily derive the same benefit from earlier transplantation with lower quality organs (which have shorter expected longevity).6,7
The Kidney Allocation System (KAS), implemented in late 2014, introduced the Estimated Post-Transplant Survival (EPTS) score for kidney transplant candidates to improve organ-recipient longevity matching.8 The EPTS score, calculated using candidate age, duration of dialysis, diabetes status, and history of prior organ transplantation, provides a relative assessment of a candidate’s expected posttransplant survival.9 This score is considered alongside a deceased donor’s Kidney Donor Profile Index (KDPI), a measure of relative expected allograft survival.10 Under KAS, candidates with the best 20% of EPTS scores (EPTS top 20) are prioritized to receive organs with the best 20% of KDPI scores.8
Since EPTS scores for each candidate increase as wait-list time progresses (due to increasing dialysis duration and age), candidates with low EPTS in high wait time regions may have reduced access to low KDPI organs as they age out of top 20% status and therefore lose their priority for low KDPI kidneys. If so, such candidates may not benefit from initially waiting for low KDPI organs to which they will likely lose access prior to transplantation. Additionally, allocation sequences for multi-organ transplants and highly sensitized candidates supersede top 20% allocation sequence in KAS, potentially leading to KDPI ≤ 20% kidneys being allocated to candidates with EPTS > 20% in these scenarios.11 Therefore, any regional differences in the proportion of recipients with these characteristics could affect access to KDPI ≤ 20% deceased donor kidneys for EPTS top 20 candidates. Given that the geographical disparities in kidney transplantation have persisted after the implementation of KAS, we sought to determine whether regional disparities in access to KDPI ≤ 20% kidneys for EPTS top 20 candidates are present across the United States.
Methods
Data Source and Study Population
This study used national candidate and donor data from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research files based on Organ Procurement and Transplantation Network (OPTN) data as of June 8, 2018. We identified a retrospective cohort of all incident adult (age ≥18 years) candidates listed for a kidney transplant in the United States between January 1, 2015, and March 31, 2018. We additionally identified all potential kidney donors with a donation date within the same time period, excluding those without kidney donation consent, and those with a kidney recovered for a reason other than transplant (eg, kidneys recovered for research). This research was conducted under the approval of the institutional review board of Columbia University Medical Center.
The EPTS scores were calculated for each candidate at the time of listing and at the end of follow-up (the earliest date of transplant, death, wait-list removal, or end of study follow-up on March 31, 2018), and KDPI scores were calculated for each donor. The EPTS and KDPI scores were calculated as described by the OPTN and scaled based on the mapping tables in use at the time of each event, using a May 1 transition date between tables.9,10 We used these calculated EPTS and KDPI scores to categorize candidates and kidneys by top 20% designation (EPTS or KDPI ≤ 20%, respectively) versus >20%, to reflect their categorization under KAS.
Statistical Analysis
Candidates were stratified by EPTS category at listing (listing EPTS ≤ 20% or > 20%), and outcomes of interest were deceased donor transplantation, receipt of a KDPI ≤ 20% organ, and likelihood of aging out of EPTS top 20% status if listed with EPTS ≤ 20%. Candidate characteristics of interest were compared between recipients of KDPI ≤ 20% versus KDPI > 20% kidneys using χ2 tests for categorical variables and t-tests or Kruskal-Wallis tests for continuous variables. Characteristics were presented as column percentages, mean (standard deviation), or median (interquartile range [IQR]). We compared outcomes between listing and transplant EPTS groups using χ2 tests.
We also examined geographic differences in the candidate and donor groups, as well as candidate disposition for those who were listed with EPTS ≤ 20% status and organ disposition for KDPI ≤ 20% kidneys. We compared the proportion of candidates listed with EPTS ≤ 20% status and the proportion who subsequently lost their top 20% status between OPTN regions using χ2 tests. For recipients who were originally listed with EPTS ≤ 20% status, we compared the odds of receiving a KDPI ≤ 20% organ between OPTN regions via unadjusted logistic regression, using the region with the highest likelihood of receiving a KDPI ≤ 20% organ (region 6) as the reference region.
We measured the availability of KDPI ≤ 20% kidneys for EPTS ≤ 20% candidates across OPTN regions by calculating the proportion of all potential donors with KDPI ≤ 20% relative to the number of wait-listed candidates with EPTS ≤ 20%. We additionally calculated the proportion of KDPI ≤ 20% transplanted into recipients with EPTS > 20%, including those that were used for highly sensitized recipients (calculated Panel Reactive Antibodies [cPRA] >97%) and as part of multi-organ transplants. Proportions were compared across regions using χ2 tests. Pearson correlation coefficients were calculated to assess the correlation between the proportion of candidates listed with EPTS ≤ 20%, or the number of donors with KDPI ≤ 20% per EPTS ≤ 20% candidate, and the likelihood of an EPTS ≤ 20% candidate receiving a KDPI ≤ 20% kidney at the regional level. Significance for 2-sided hypothesis tests was set at α of .05, and all analyses were performed using Stata/MP version 15.1 (StataCorp, College Station, Texas).
Results
For the 121 069 newly listed candidates wait-listed from January 2015 to March 2018 included in our analysis, 28.5% had EPTS top 20% designation at listing (Figure 1). Of note, this describes candidates’ initial EPTS score at listing and does not reflect the overall fraction of wait-listed candidates with EPTS ≤ 20% at any given moment. Rather, this proportion was greater than 20% due to increasing EPTS score over time for each wait-listed candidate. The likelihood of transplantation by the end of follow-up was similar for candidates with a listing EPTS ≤ 20% (16.1% transplanted) and listing EPTS > 20% (17.1% transplanted).
Figure 1.
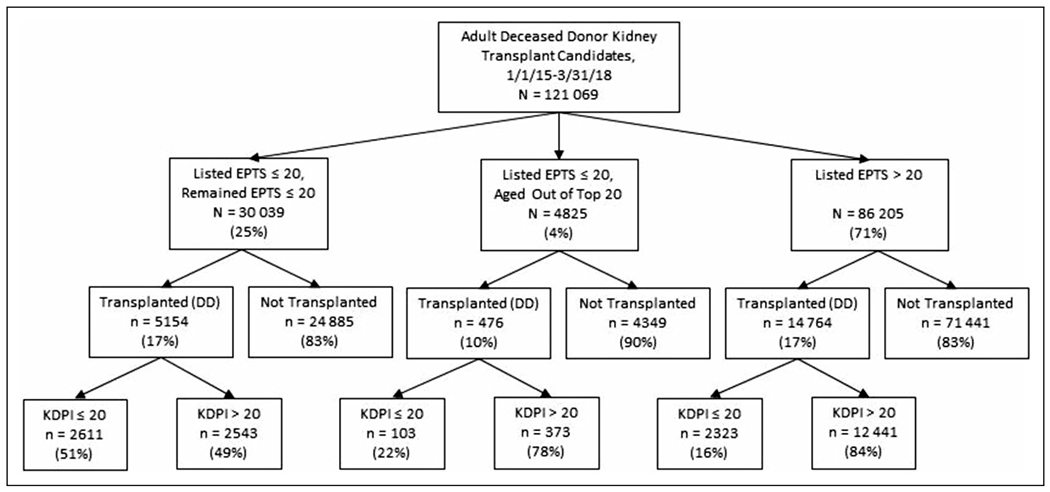
Flow diagram of outcomes for all adult deceased donor kidney transplant candidates added to the waitlist in the United States (January 1, 2015, to March 31, 2018), by EPTS listing category. DD indicates deceased donor; EPTS, Estimated Post-Transplant Survival; KDPI, Kidney Donor Profile Index.
Recipients who had a listing EPTS ≤ 20% were much more likely to receive a KDPI ≤ 20% kidney (49.4% vs 16.2% for recipients with listing EPTS > 20%, P < .001). However, the distribution of KDPI for transplanted recipients was broad for both recipients with listing EPTS ≤ 20% and those with listing EPTS > 20% (Figure 2). Younger age at transplant and male sex were associated with increased likelihood of transplantation with a KDPI ≤ 20% kidney, among recipients originally listed with EPTS ≤ 20% (Supplemental Table 1).
Figure 2.
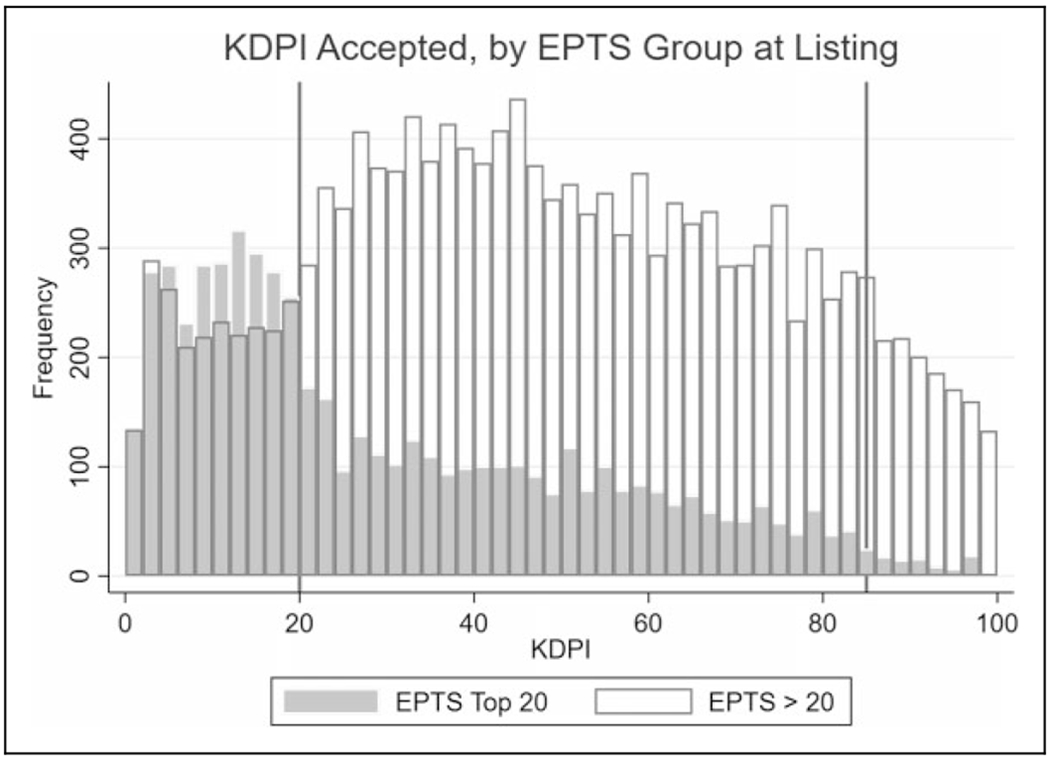
Kidney Donor Profile Index distributions for transplant recipients by Estimated Post-Transplant Survival listing category, January 1, 2015, to March 31, 2018. There was significant overlap in KDPI between EPTS ≤ 20% and EPTS > 20% recipients. Vertical lines represent KDPI 20% and 85%. EPTS indicates Estimated Post-Transplant Survival; KDPI, Kidney Donor Profile Index.
Among all 34 562 candidates who had EPTS ≤ 20% at the time of wait-listing, 4251 (12.3%) lost their top 20% status by the end of follow-up (median follow-up: 21.2 months; Table 1). Recipients who lost (aged out of) their top 20% status by the time of transplant were less likely to receive KDPI ≤ 20% organs than their counterparts who maintained EPTS top 20% status (21.5% vs 51.6%, P < .001; Figure 1). Recipients who were listed with EPTS ≤ 20% and received a KDPI ≤ 20% organ tended to wait slightly longer for transplant than those who received a KDPI > 20% organ (median time to transplant, 176 days [IQR: 367] vs 150 days [IQR: 328]; Supplemental Table 1).
Table 1.
Outcomes for Adult Deceased Donor Kidney Transplant Candidates Listed With Top 20 EPTS in the United States Between January 1, 2015, and March 31, 2018, by Region.
| Region | Candidates (n) | % Listed With EPTS ≤ 20 Statusa | % of EPTS ≤ 20 Candidates Who Lost EPTS ≤ 20 Statusa | OR of KDPI ≤ 20%, for Deceased Donor Kidney Recipients with EPTS ≤ 20% at Listing |
|
|---|---|---|---|---|---|
| OR | 95% CI | ||||
| 1 | 4985 | 29.1 | 14.8 | 0.31 | 0.19-0.48 |
| 2 | 16 576 | 26.1 | 13.8 | 0.30 | 0.21-0.42 |
| 3 | 16 908 | 28.7 | 13.6 | 0.32 | 0.23-0.44 |
| 4 | 12 841 | 28.2 | 11.7 | 0.42 | 0.30-0.60 |
| 5 | 20 780 | 28.9 | 11.9 | 0.28 | 0.20-0.39 |
| 6 | 3453 | 30.2 | 11.7 | Reference group | |
| 7 | 10 126 | 31.1 | 11.4 | 0.26 | 0.18-0.37 |
| 8 | 6521 | 28.8 | 10.2 | 0.59 | 0.41-0.85 |
| 9 | 8613 | 29.3 | 12.3 | 0.19 | 0.13-0.28 |
| 10 | 8688 | 28.9 | 11.9 | 0.44 | 0.31-0.62 |
| 11 | 11 578 | 27.7 | 11.3 | 0.39 | 0.28-0.55 |
| Overall | 121 069 | 28.5 | 12.3 | ||
Abbreviations: CI, confidence interval; EPTS, Estimated Post-Transplant Survival; KDPI, Kidney Donor Profile Index; OR, odds ratio.
χ2 P < .001 for differences between regions in that column.
We examined whether there were geographic differences in access to KDPI ≤ 20% kidneys for candidates with listing EPTS ≤ 20%. The proportion of candidates with EPTS ≤ 20% at listing ranged from 26.1% (region 2) to 31.1% (region 7; Table 1). The odds of receiving a KDPI ≤ 20% organ were significantly higher in region 6 than any other part of the country and lowest in region 9 (odds ratio vs region 6 = 0.19; 95% confidence interval: 0.13-0.28; Table 1, Supplemental Figure 1)—a variation that was not strongly associated with the varying proportion of top 20% candidates (r = 0.22, r2 = 0.05).
We therefore sought to determine whether donor pool differences explained this observation. Region 6 had the highest ratio of deceased donors with KDPI ≤ 20% per EPTS ≤ 20% candidate (0.30, representing 25.4% of all donors) and region 9 had the lowest ratio (0.09, representing 16.3% of all donors; Figure 3). The proportion of donors with KDPI ≤ 20% strongly correlated with the ratio of deceased donors with KDPI ≤ 20% per EPTS ≤ 20% candidate at the regional level (r = 0.91, r2 = 0.83). There was also a strong linear correlation (r = 0.92, r2 = 0.84) between the ratio of deceased donors with KDPI ≤ 20% per EPTS ≤ 20% candidate and the likelihood of an EPTS ≤ 20% candidate receiving a KDPI ≤ 20% kidney at the regional level (Figure 3). The likelihood of losing EPTS ≤ 20% status by transplant or end of follow-up varied as well and was lowest in region 8 (10.2%) and highest in Region 1 (14.8%; Table 1).
Figure 3.
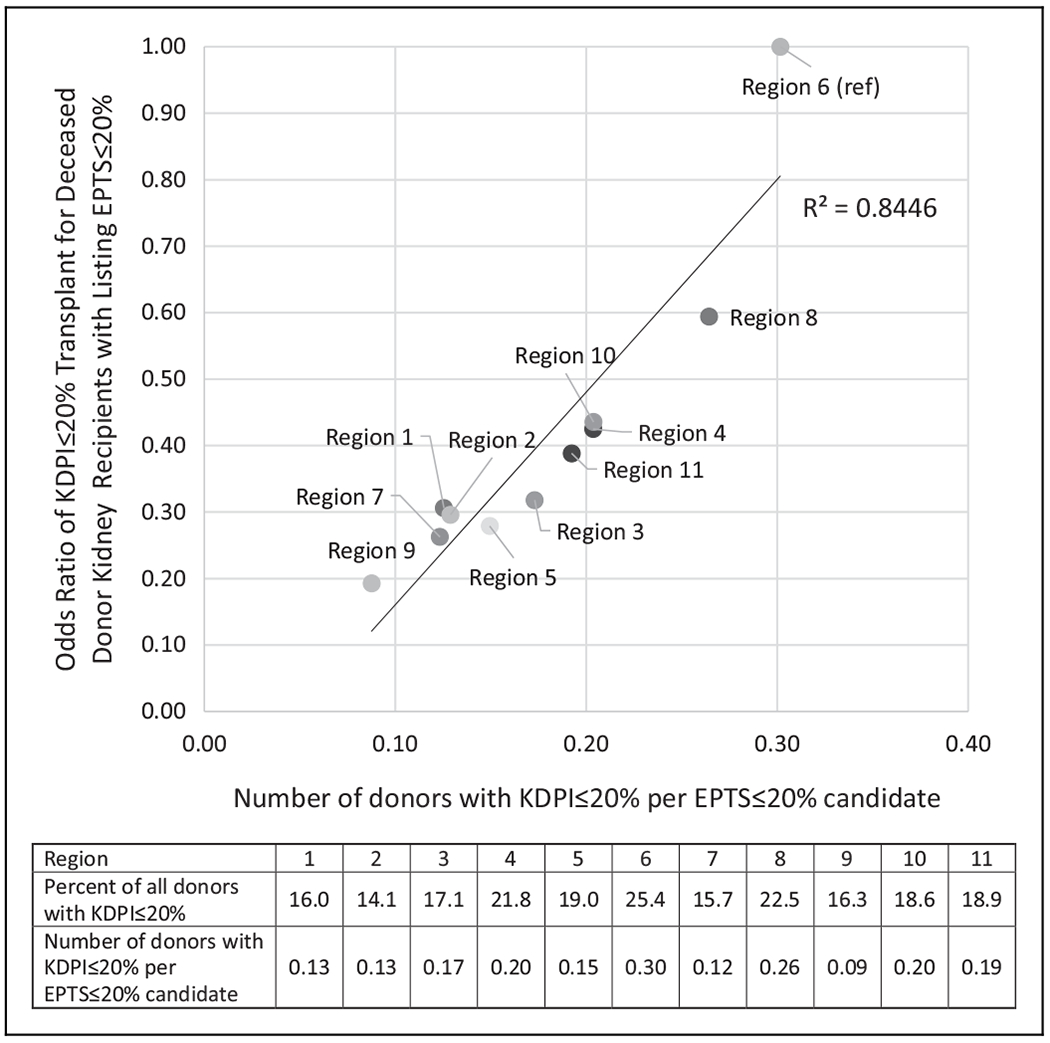
Relationship between low KDPI organ availability and likelihood of KDPI ≤ 20% transplant for Estimated Post-Transplant Survival (EPTS) top 20 candidates, by United Network for Organ Sharing region, January 1, 2015, to March 31, 2018. EPTS indicates Estimated Post-Transplant Survival; KDPI, Kidney Donor Profile Index.
Finally, we assessed the disposition of low KDPI (KDPI ≤ 20%) used for recipients with higher EPTS scores. Nearly half (48%) of low KDPI ≤ 20% kidneys were received by patients with EPTS > 20% at their time of transplant (range: 36% in region 6 to 53% in region 7; Table 2). Of these kidneys, 66% were used in multi-organ transplants (representing 32% of all KDPI ≤ 20% kidneys) and 15% went to patients with cPRA > 97%, but there were considerable regional differences in the proportion of KDPI ≤ 20% kidneys transplanted in EPTS > 20% recipients via multi-organ allocation or high PRA (Table 2).
Table 2.
Disposition of Top 20 KDPI Organs Transplanted in Recipients With EPTS > 20%, by Region, January 1, 2015, to March 31, 2018.
| Region | KDPI ≤ 20% Kidneys Transplanted (n) | n (%) for Recipients With EPTS > 20%a | Among KDPI ≤ 20% Kidneys Transplanted in EPTS > 20% Recipients |
|
|---|---|---|---|---|
| n (%) Used for cPRA > 97%a | n (%) Used for Multi-Organ Transplantsb | |||
| 1 | 117 | 54 (46%) | 11 (20%) | 28 (52%) |
| 2 | 543 | 285 (52%) | 68 (24%) | 162 (57%) |
| 3 | 766 | 399 (52%) | 40 (10%) | 282 (71%) |
| 4 | 646 | 316 (49%) | 46 (15%) | 212 (67%) |
| 5 | 772 | 387 (50%) | 62 (16%) | 263 (68%) |
| 6 | 245 | 87 (36%) | 5 (6%) | 59 (68%) |
| 7 | 387 | 204 (53%) | 31 (15%) | 147 (72%) |
| 8 | 425 | 158 (37%) | 19 (12%) | 112 (71%) |
| 9 | 215 | 98 (46%) | 9 (9%) | 55 (56%) |
| 10 | 449 | 214 (48%) | 23 (11%) | 141 (66%) |
| 11 | 581 | 283 (49%) | 53 (19%) | 174 (61%) |
| Overall | 5146 | 2485 (48%) | 367 (15%) | 1635 (66%) |
| P < .001 | P < .001 | P < .001 | ||
Abbreviations: cPRA, calculated panel-reactive antibodies; EPTS, Estimated Post-Transplant Survival; KDPI, Kidney Donor Profile Index.
P < .001 for column difference.
P = .001 for differences between regions in that column.
Discussion
Although kidney transplantation remains the treatment of choice for end-stage kidney disease, particularly for younger patients, access to transplant remains limited by an organ shortage in the United States.12–14 This shortage has a disproportionate impact on certain geographic regions and results in disparities in access to transplantation that have persisted despite a goal to eliminate location as a barrier to transplant.1–4,15,16 Here, we demonstrated that the large geographic differences that affected the general candidate pool also extended to unequal access to high-quality kidneys for candidates who were likely to benefit the most from transplant.
The finding that recipients with top 20% EPTS status at listing were 5 times more likely to receive a KDPI ≤ 20% kidney in region 6 compared to region 9 underscores just how wide geographic disparities can be for access to certain subsets of better quality organs. We noted significant differences in regional organ quality that likely explained our findings, with KDPI ≤ 20% donors comprising as little as 14.1% (region 2) and as much as 25.4% (region 6) of deceased donors. Prior work by Goldberg et al identified regional differences in consent for donation, but their results identified regions 7 and 8 as high performers, suggesting donation consent differences alone do not explain our findings.17 Variations in regional death rates also do not appear to be driving these discrepancies, which underscores the need for further study to identify the causes of differences in organ quality and availability.18
The total number of regional wait-list candidates did not explain the differences observed either; region 1, with the second fewest number of candidates, had the highest proportion of candidates who lost top 20% status or among the lowest odds of transplantation with a low KDPI kidney. Similarly, the proportion of candidates initially listed with top 20% status, which varied from 26.1% in region 2 to 31.1% in region 7, did not explain differences in access to low KDPI organs, which would suggest that disparities in access to low KDPI kidneys are primarily a function of the pool of available organs within each region. This lends greater support to studying the proposed borderless allocation system—one that takes into account geographic feasibility and medical priority, rather than strict and somewhat arbitrary regional borders.19 Such proposals are currently being considered by the OPTN and have the potential to make access to organs more equitable for candidates across the country. However, such proposals will not address the overall shortage of kidneys available for transplantation, and strategies to improve overall organ donation rates are also needed. One additional possible solution to reduce disparities in access to low KDPI kidneys could be to allow individuals with top 20% status to retain this preferential allocation status, much in the same way that candidates wait-listed below the age of 18 do. However, such a strategy would increase the number of candidates eligible for KDPI ≤ 20% kidneys without increasing the supply of these high-quality organs.
Our results reinforce previously published findings about the high proportion of low KDPI kidneys transplanted via multi-organ allocation: Two thirds of low-KDPI kidneys that went to recipients with EPTS > 20% were transplanted as part of a multi-organ transplant, thus shrinking the pool of low KDPI kidneys available to EPTS ≤ 20% recipients. The prioritization of multi-organ recipients has raised concerns about unfairly disadvantaging EPTS top 20% kidney-only candidates, especially given practice pattern variation in multi-organ listing criteria.20,21 Standardization of multi-organ listing criteria and the use of safety net kidney allocation in recipients of other organs, as has been done for simultaneous liver–kidney candidates, may help ensure that high-quality kidneys are being utilized optimally.20
We also found that candidates who were listed with EPTS ≤ 20% but had reached EPTS > 20% at transplant or end of follow-up were least likely to have been transplanted and, when transplanted, had almost the same likelihood of KDPI > 20% transplant as those who had EPTS > 20% for the entire time on the wait-list. It is likely that the lower percentage of transplanted individuals in this group primarily reflects the fact that candidates who are not transplanted spend a longer time on the waitlist and therefore have a longer at-risk period in which to age out. Analyses of the organ offers to these candidates are needed to determine whether these candidates are less likely to accept higher KDPI offers even after losing top 20% EPTS status. If this pattern is found, it would suggest either a failure to recognize that candidates have aged out or a reluctance to use higher KDPI organs for otherwise excellent candidates who are just on the other side of the 20% divide.
In the current allocation system, the implications of our findings pertain to considering higher KDPI organ offers to low EPTS recipients. Top 20% candidates in regions with lower likelihoods of low KDPI transplant should be aware that they have a higher chance of losing access to low KDPI offers and earlier acceptance of higher KDPI offers may provide a better survival advantage. Shared decision-making tools that help centers and candidates evaluate organ offers should include information about how long a candidate will continue to have preferential access to KDPI ≤ 20% kidneys. Candidates should also be actively informed when they lose this preferential status. It should be noted that estimating the duration of remaining time with top 20% designation can be challenging for patients who have not yet started dialysis, as the timing of eventual dialysis initiation is not known in advance but can have a large impact on the EPTS trajectory when it occurs.
Longer follow-up of this cohort is needed to determine whether these regional differences in access to KDPI ≤ 20% kidneys persist as a greater proportion of candidates age out of top 20% EPTS status, since the majority of included candidates were not transplanted within our short post-KAS study window. Given that there was an initial increase in high cPRA transplants after KAS implementation due to prevalent candidates with high cPRA and long wait time, and that we observed a significant proportion of KDPI ≤ 20% kidneys used for high-cPRA recipients with EPTS > 20%, ongoing evaluation of these trends is also needed to determine if they persist over time.22,23 We also acknowledge that the concepts of EPTS ≤ 20% and KDPI ≤ 20% create arbitrary dichotomies when comparing groups of candidates and organs, similar to the dichotomy created by the Expanded Criteria Donor status, which was eventually abandoned. There are not, for example, clinically meaningful differences between kidneys with KDPI 20% versus 21% when considering organ offers. However, these dichotomies are codified in the allocation system and therefore directly impact access to certain organs. It should also be noted that we found a steep drop-off after KDPI = 20% when examining the distribution of KDPI for kidneys transplanted in EPTS ≤ 20% recipients, suggesting that this dichotomy has an identifiable impact on real-world utilization decisions. The KDPI labeling effects on organ utilization have also been well described by prior studies.24
Match run data for transplanted kidneys were not included in our data set. We were therefore unable to compare the frequency and characteristics of offers for candidates with EPTS ≤ 20% versus EPTS ≤ 20%, which could differ between regions and impact the likelihood of transplantation. This information could potentially demonstrate how many offers for KDPI > 20% kidneys are declined by recipients who expected to receive offers for better quality organs. Future studies should examine match run data and assess the number, timing, and donor quality of offers for candidates starting out with EPTS ≤ 20% versus EPTS > 20% and see how they change for candidates initially listed with EPTS top 20 status after aging out. These data could also reveal any potential geographic differences in the number of EPTS ≤ 20% candidates who decline offers for KDPI ≤ 20% before they are used in candidates with EPTS > 20%.
Conclusions
We identified large geographic disparities in access to KDPI ≤ 20% organs for kidney transplant candidates with top 20% EPTS status at listing. This disparity appears to exceed that seen for overall national kidney transplantation, and the regional differences appear to be driven to a large extent by differences in the donor pool composition and by multi-organ transplant allocation. Candidates should be aware of these disparities when weighing KDPI > 20% offers, and centers should be cognizant of expected remaining EPTS ≤ 20% time for candidates when evaluating organ offers. Our findings underscore the urgent need for policy changes that address the current geographic inequity in access to deceased donor kidneys, particularly those from low KDPI donors. Allocation system changes should ensure that equal need translates to equal access to kidneys throughout the country.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. This work was supported by a Young Investigator Grant from the National Kidney Foundation (to S.A.H.). S.A.H. is also supported by the National Center for Advancing Translational Sciences (KL2 TR001874). S.M. was supported by NIH/NIDDK/NIAID/NIHMD (R01 DK114893 and U01 DK116066). The funders had no role in the study design, analysis, or manuscript preparation.
Footnotes
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Davis AE, Mehrotra S, Kilambi V, et al. The effect of the State-wide Sharing variance on geographic disparity in kidney transplantation in the United States. Clin J Am Soc Nephrol. 2014; 9(8):1449–1460. doi: 10.2215/CJN.05350513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis AE, Mehrotra S, McElroy LM, et al. The extent and predictors of waiting time geographic disparity in kidney transplantation in the United States. Transplantation. 2014;97(10): 1049–1057. doi: 10.1097/01.tp.0000438623.89310.dc. [DOI] [PubMed] [Google Scholar]
- 3.Zhou S, Massie AB, Luo X, et al. Geographic disparity in kidney transplantation under KAS. Am J Transplant. 2018;18(6): 1415–1423. doi: 10.1111/ajt.14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathur AK, Ashby VB, Sands RL, Wolfe RA. Geographic variation in end-stage renal disease incidence and access to deceased donor kidney transplantation. Am J Transplant. 2010; 10(4 Pt 2):1069–80. doi: 10.1111/j.1600-6143.2010.03043.x. [DOI] [PubMed] [Google Scholar]
- 5.Garonzik-Wang JM, James NT, Weatherspoon KC, et al. The aggressive phenotype: center-level patterns in the utilization of suboptimal kidneys. Am J Transplant. 2012;12(2):400–408. doi: 10.1111/j.1600-6143.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 6.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294(21):2726–2733. [DOI] [PubMed] [Google Scholar]
- 7.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14(10):2310–2316. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 8.Organ Procurement and Transplantation Network. Policies. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_08.Published2019. Accessed July 31, 2019.
- 9.Organ Procurement and Transplantation Network. A guide to calculating and interpreting the Estimated Post-Transplant Survival (EPTS) score used in the Kidney Allocation System (KAS). https://optn.transplant.hrsa.gov/media/1511/guide_to_calculating_interpreting_epts.pdf.Published2018. Accessed July 31, 2019.
- 10.Organ Procurement and Transplantation Network. Guide to calculating and interpreting the Kidney Donor Profile Index (KDPI). https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf.Published2018. Accessed December 4, 2018.
- 11.Organ Procurement and Transplantation Network. Policy 8: Allocation of Kidneys. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf.Published2019. Accessed July 31, 2019.
- 12.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. [DOI] [PubMed] [Google Scholar]
- 13.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11(5):917–922. [DOI] [PubMed] [Google Scholar]
- 14.Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16(6):1859–1865. [DOI] [PubMed] [Google Scholar]
- 15.Organ Procurement and Transplantation Network—HRSA. Final rule with comment period. Fed Regist. 1998;63(63):16296–338. [PubMed] [Google Scholar]
- 16.Stewart DE, Wilk AR, Toll AE, et al. Measuring and monitoring equity in access to deceased donor kidney transplantation. Am J Transplant. 2018;18(8):1924–1935. doi: 10.1111/ajt.14922. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg DS, French B, Abt PL, Gilroy RK. Increasing the number of organ transplants in the united states by optimizing donor authorization rates. Am J Transplant. 2015;15(8): 2117–2125. doi: 10.1111/ajt.13362. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Mortality Tables. https://www.cdc.gov/nchs/data/dvs/mortfinal2007_worktable23r.pdf.Accessed July, 31 2019.
- 19.Snyder JJ, Salkowski N, Wey A, Pyke J, Israni AK, Kasiske BL. Organ distribution without geographic boundaries: a possible framework for organ allocation. Am J Transplant. 2018; 18(11):2635–2640. doi: 10.1111/ajt.15115. [DOI] [PubMed] [Google Scholar]
- 20.Miles CD, Westphal S, Liapakis A, Formica R. Simultaneous liver-kidney transplantation: impact on liver transplant patients and the kidney transplant waiting list. Curr Transplant Rep. 2018;5(1):1–6. doi: 10.1007/s40472-018-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reese PP, Veatch RM, Abt PL, Amaral S. Revisiting multi-organ transplantation in the setting of scarcity. Am J Transplant. 2014;14(1):21–6. doi: 10.1111/ajt.12557. [DOI] [PubMed] [Google Scholar]
- 22.Hart A, Gustafson SK, Skeans MA, et al. OPTN/SRTR 2015 Annual data report: early effects of the new kidney allocation system. Am J Transplant. 2017;17(suppl 1):543–564. doi: 10.1111/ajt.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016;16(6):1834–1847. doi: 10.1111/ajt.13770. [DOI] [PubMed] [Google Scholar]
- 24.Stewart DE, Garcia VC, Aeder MI, Klassen DK. New insights into the alleged kidney donor profile index labeling effect on kidney utilization. Am J Transplant. 2017;17(10):2696–2704. doi: 10.1111/ajt.14379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


