Abstract
Objective
Expectations contribute to cognitive pain modulation through opioidergically-mediated descending inhibition. Mindfulness meditation reduces pain independent of endogenous opioids, engaging unique corticothalamo-cortical mechanisms. However, it remains unknown whether expectations for pain-relief predict mindfulness-induced analgesia and if these expectations are modified by endogenous opioids.
Methods
In this secondary analysis of previously published work, 78 pain-free participants (mean age 27 ± 7 years; 50% women) were randomized to a 4-session mindfulness meditation or book-listening regimen. Expectations for intervention-induced pain-relief were assessed before and after each intervention. Pain ratings were examined after meditation or rest (control group) during noxious heat (49°C) and intravenous administration of saline-placebo or the opioid antagonist naloxone (0.15 mg/kg bolus + 0.1 mg/kg/h infusion.
Results
Mindfulness significantly lowered pain during saline and naloxone infusion. Higher expected pain-relief from mindfulness predicted lower pain intensity, r(40) = −.41, p = 0.009. The relationship between meditation-related expectations and pain intensity reductions were exhibited during naloxone, r(20) = −.76, p < .001 but not saline, r(20) = −.22, p = 0.36. Expectations for book-listening based analgesia did not significantly predict pain changes during saline, r(20) = −.37, p = .11 or naloxone, r(18) = 0.26, p = .30 in the control group.
Conclusions
These novel findings demonstrate a significant role for expectations in mindfulness-based pain-relief. However, this role was minimal during saline and stronger during opioid blockade, despite similar pain reductions. This supports growing evidence that mindfulness engages multiple mechanisms to reduce pain, suggesting that mindfulness might be an effective pain-reducing technique even for individuals with low expectations for pain-relief.
Keywords: mindfulness, expectations, naloxone, opioidergic, pain relief, meditation, placebo
Introduction
The experience of pain is modulated by a myriad of sensory, cognitive and affective factors. A wide spectrum of cognitive manipulations are postulated to reduce pain through a common final neurophysiological pathway (1). For example, analgesia produced by placebo (2–6), conditioned pain modulation (7), distraction (8), and hypnosis (9, 10) are driven by opioidergically mediated descending inhibition of pain through prefrontal (PFC) regulation of the periaqueductal gray matter. This system is characterized as the central pain modulatory physiological system (11–13).
Prior beliefs and expectations also alter behavioral and neural pain responses (14, 15). Expectations for lower pain corresponding to a frankly noxious stimulus elicit attenuated behavioral and neural pain response (i.e., the placebo effect) (16–21), while higher expectations of pain during innocuous stimulation produce higher pain responses (i.e., nocebo effects) (22–26). Manipulation of expectations modulates pain through endogenous opioidergic release (3, 4, 6, 20, 27, 28). In contrast, conditioned placebo responses on pain are not typically mediated by opioids (29). Interestingly, reappraisal-based manipulations are postulated to reduce pain independent of opioidergically-driven systems (30–33).
Mindfulness-based meditation, a cognitive practice premised on sustaining non-judgmental awareness of the present moment, a reappraisal technique, reliably reduces experimental and clinical pain (34–38). As adapted in our laboratory, mindfulness meditation participants are taught to focus their attention on their breath in a non-reactive manner. We have repeatedly demonstrated that mindfulness-based pain-relief is not mediated by endogenous opioids (33, 39, 40) and engages multiple neural mechanisms distinct from placebo analgesia to exert its pain-relieving effects (33, 41, 42). Mindfulness meditation reduces anticipation and corresponding negative pain appraisals (43). However, it remains unknown a) whether expectations play a role in mindfulness-based pain relief, and b) if endogenous opioids are involved in facilitating this postulated effect.
In the current study, a secondary analysis was conducted on our previously reported double-blinded study that demonstrated that mindfulness-based pain relief is not reversed by high-dose intravenous (IV) naloxone (39). In that study, mindfulness-based meditation during IV opioidergic antagonism (naloxone) and saline infusion produced significant reductions in pain when compared to baseline and the control groups (31, 44). Thus, it was discovered that mindfulness-based meditation does not engage endogenous opioids to reduce pain. Here, we investigated the role of expectations for mindfulness-induced pain relief during noxious heat. Further, we interrogated whether the potential relationship between expectations of mindfulness-based pain relief and actual pain relief is altered by opioid blockade.
We hypothesized that expectations would not be significantly associated with mindfulness-based pain relief. There are a number of reasons for this hypothesis. For one, mindfulness meditation is premised on sustaining non-reactive attention to the present moment and detachment from expectations (45); thus, the impact of prior expectations should be assuaged. Second, converging lines of evidence demonstrate that mindfulness-induced pain relief is associated with attenuated neural activation leading up to noxious stimulation (43, 46), providing some neural evidence for our overarching hypothesis. We also predicted that opioid blockade would not alter the effect of expectations for mindfulness-based analgesia because endogenous opioids do not mediate mindfulness-based pain relief (32, 33, 39).
Methods
Participants:
Data were collected from March 3, 2015 to June 24th, 2015. Seventy-eight participants (75 right-handed; mean age = 27 years ± 7 years; 39 males; 39 females) successfully completed all study procedures (57 were White, 8 were Asian, 7 were Black, 4 were Hispanic, 1 was Native American, and 1 self-identified as “mixed”). Study procedures were approved by the Wake Forest School of Medicine Institutional Review Board. All participants were told that the study would assess whether meditation was associated with “the release of naturally occurring opiates” and that they would “receive intravenous administration of saline or naloxone, a relatively safe drug that blocks the transmission of opioid activity.” Male and female participants were separately randomized without replacement to one of four groups (meditation + naloxone, control + naloxone, meditation + saline, or control + saline) in a double-blind manner.
Stimuli:
Medoc TSA-II (Medoc, Inc.) delivered all thermal stimuli employing a 16mm2 surface area thermal probe. To reduce habituation, the thermal probe was moved to a new stimulation site after each experimental series. All stimulus temperatures were less than or equal to 49°C and participants were free to escape the stimulator at any time by lifting their limb from a custom-made probe holder.
Psychophysical assessment of pain:
Pain intensity and unpleasantness ratings were assessed with 15cm plastic sliding visual analog scales (VAS) (47). The minimum rating (“0”) was designated as “no pain sensation” and “not at all unpleasant” whereas the maximum (“10”) was labeled as “most intense pain sensation imaginable” or “most unpleasant sensation imaginable”, respectively.
Naloxone and saline administration:
A 0.15 mg/kg bolus dose of naloxone (Naloxone HCI, Amphastar Pharmaceuticals, Inc., Rancho Cucamonga, California) or saline in 25ml normal saline was administered over 10 minutes (min) via the IV line inserted into the antecubital vein of the non-dominant arm. To ensure that naloxone would antagonize opioid receptors for entirety of the experiment, we administered a supplementary IV infusion dose of 0.1mg/kg/hour naloxone or saline immediately after the bolus infusion ceased, until the end of the experiment (~12 min). Only the study physicians, research pharmacist, and research coordinator were aware of participant-drug assignment. Participants, research nurses, and all experimenters were blinded to drug assignment.
Experimental Design
Experimental Session 1 (psychophysical training):
Participants were initially familiarized with 32, 5 second (s) duration stimuli (35 – 49°C; ventral aspect of the left forearm) and use of the VAS (48–50). Baseline (pre-intervention) psychophysical responses to noxious heat were probed by administering two heat series. Heat series [4 min and 24 s] included ten, alternating 12 s plateaus of 49°C and 35°C stimulation to the back of the right calf. VAS pain ratings were collected after each series. Prior to randomization into groups, participants were asked to rate their expectations for pain relief from each intervention: “how much do you expect that meditation will be effective in reducing your pain symptoms?” and “how much do you expect that listening to a book will be effective in reducing your pain symptoms?” using separate VAS scales (0= “not at all” – 10 = “most effective imaginable”).
After successful completion of sensory testing, participants were instructed of their respective group assignment (i.e., meditation; control). There were four groups in the present study. Participants were randomized to a mindfulness + saline, mindfulness + naloxone, book-listening control + saline, and a book-listening control + naloxone group.
Experimental session 2–5: Group intervention sessions:
Participants assigned to the mindfulness meditation intervention completed four 20-min sessions of mindfulness-based mental training on separate days. Training was premised on sustaining non-reactive attention to the breath. Participants assigned to the control group listened to The Natural History of Selborne (51) during four 20-min sessions on separate days. Participants were prohibited from talking, sleeping, or using their phones during these sessions. See (39) for complete regimen details.
Experimental Session 6: Pharmacologic infusion session:
After reporting to the Wake Forest Clinical Research Unit, participants were administered an opiate-focused urine drug screen to avoid opiate-related withdrawal symptoms. Weight, blood pressure, respiration rate and oxygen saturation were collected and monitored, respectively. Results are reported elsewhere (31). A research nurse then inserted the IV catheter into the non-dominant arm and participants placed their right calf on a custom-made thermal probe holder.
Participants were then asked to rate “how effectively do you expect mindfulness meditation and listening to a book would to reduce your pain?” using a VAS scale (0= “not at all” – 10 = “most effective imaginable”).
Rest:
Two heat series were then administered and VAS pain intensity and unpleasantness ratings were collected after each series.
Naloxone/Saline Administration:
After the first two heat series, a research nurse initiated the naloxone/placebo infusion. Participants in the meditation group were instructed to “begin meditating and continue meditating until the end of the experiment.” Control group participants were told to “close your eyes and relax until the end of the experiment.” Participants were given 10 minutes to meditate before administering the additional heat series.
Manipulation (Meditation/Control):
Two more heat series were administered during meditation or rest (i.e., control condition) and VAS pain intensity and unpleasantness ratings were collected after each series.
Data Analysis
The present analyses [SPSS 25.0 (IBM, Armonk, New York, USA)] are secondary from our previous work (39). A univariate ANOVA examined if pre-intervention pain ratings significantly varied by group.
A 4 (group) × 2 [pre (rest) vs. post (manipulation=rest/meditation)] × 2 (pain intensity vs. pain unpleasantness ratings) repeated measures (RM) ANOVA was conducted to test for the effect of the intervention and drug on heat-induced pain ratings. Post hoc assessments were performed to interpret significant interactions and pairwise comparisons.
Ratings of expected pain relief for mindfulness versus book-listening in Session 1 and in Session 6 were compared between session and between interventions using paired t-tests. A one-way ANOVA was conducted to test for baseline differences in intervention expectations by subsequent group randomization. Pearson bivariate correlations were computed between ratings of expected pain relief from both Sessions 1 and 6 and the percent change in VAS pain intensity and unpleasantness ratings from before to during the naloxone or saline infusion in Session 6. Correlations were also computed between expectation ratings and pain reductions within each group’s respective naloxone and saline conditions.
Results
Pre-intervention expectations for pain relief were higher for mindfulness than book-listening
Ratings of expected pain relief collected in Session 1 before randomization were higher for the mindfulness than for the book-listening group, t(75) = 10.97, p < 0.001; Figure 1). There were no significant group differences in Session 1 expectation ratings of pain relief corresponding to mindfulness, F(3,72) = 1.57, p = 0.20, or book-listening, F(3, 73) = 2.02, p = 0.12.
Figure 1.
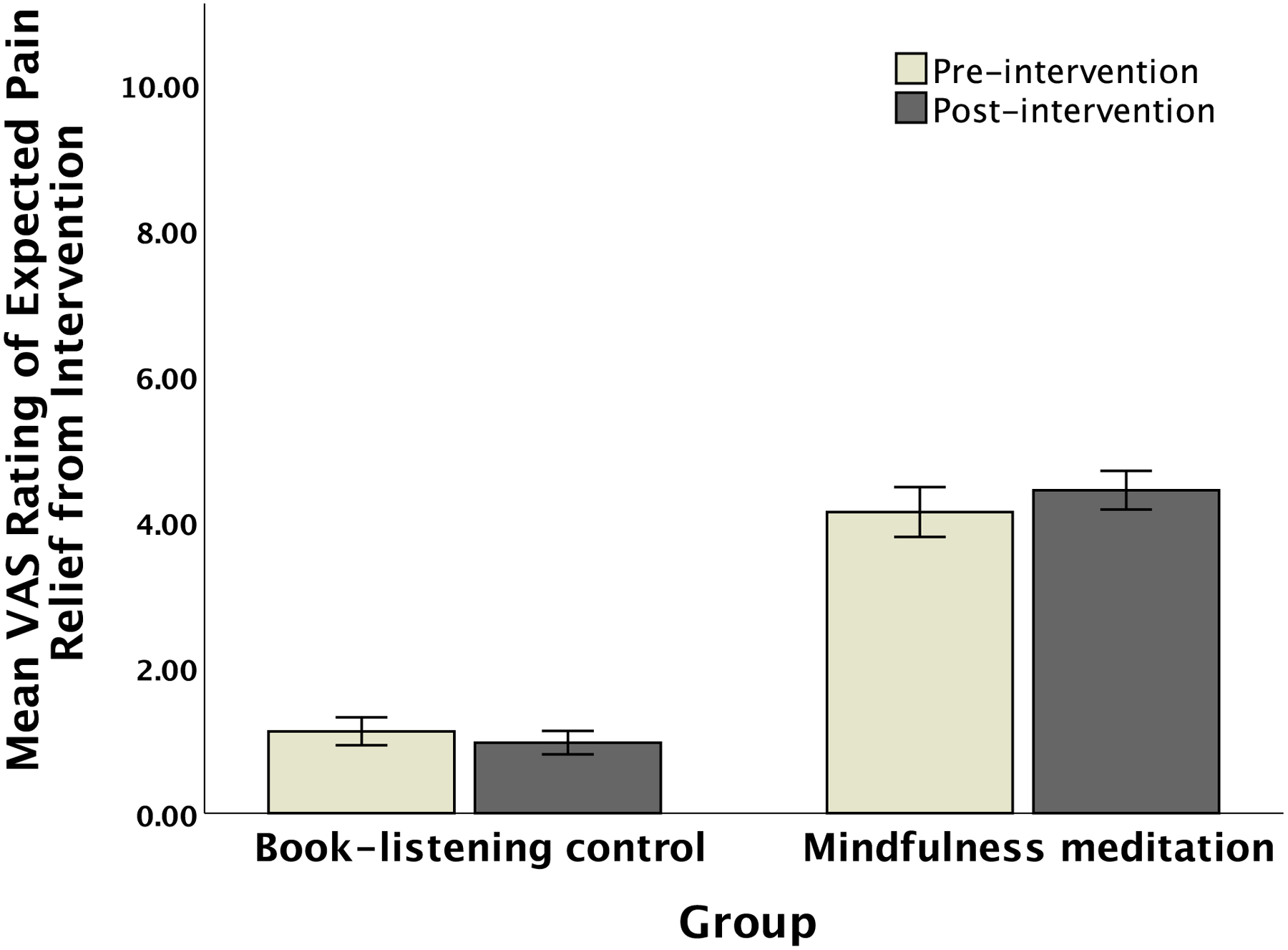
Naïve, pre-intervention (Session 1) and post-intervention (Session 6) ratings of expected pain relief from book-listening or mindfulness meditation, within each respective intervention group. Error bars depict SEM.
Book-listening based expectations for pain relief predicted higher pain in the controls
When both mindfulness meditation groups were combined, Session 1 expectations for mindfulness-based pain relief did not significantly predict mindfulness-based reductions in Session in pain intensity, r(40) = −.296, p = 0.064 or pain unpleasantness r(40) = −.107, p = 0.51. In the average response of the two book-listening control groups, higher expected book-listening pain relief was associated with higher pain intensity r(37) = .470, p = 0.003, and higher pain unpleasantness ratings, r(37) = .399, p = 0.014, in Session 6.
During opioid antagonism, pre-intervention expected pain relief predicted post-intervention mindfulness-based pain relief and book-listening associated pain increases
In the mindfulness meditation and naloxone group, specifically, higher Session 1 expectations for mindfulness-based analgesia significantly predicted mindfulness-meditation-induced reductions in pain intensity, r(20) = −.644, p = 0.002, and pain unpleasantness, r(20) = −.462, p = 0.040. Of note, in this same group, Session 1 expectations about book-listening were also predictive of mindfulness-meditation-induced reductions in pain intensity (r(20) = −.541, p = 0.014) and unpleasantness (trend, r(20) = −.401, p = 0.080). In contrast, in the mindfulness-meditation-saline group, there was no significant correlation between Session 1 mindfulness-meditation expectations and reductions in pain intensity, r(20) = 0.026, p = .91, and pain unpleasantness, r(20) = 0.217, p = .36).
The book-listening groups showed similar correlations between Session 1 expectations and pain changes experienced in Session 6 between the groups that received naloxone (pain intensity r(18) = 0.556, p = .017, pain unpleasantness , r(18) = 0.498, p = .036) and saline (pain intensity, r(20) = 0.408, p = .083, pain unpleasantness, r(20) = 0.309, p = .20.
Post-intervention expectations for pain relief were higher for mindfulness than book-listening
After the respective study interventions, ratings of expected pain relief were significantly higher for meditation-based pain relief in the meditation group than for book-listening based pain relief in the book-listening group, t(76) = 10.92, p < 0.001 (Figure 1). Completion of the meditation interventions did not significantly change expectations for pain relief in the mindfulness-meditation group, t(39) = 1.06, p = 0.30 (Figure 1). Completion of the book-listening interventions did not significantly change expectations for pain relief in the book-listening group, t(36) = 0.70, p = 0.49. Expectations for pain relief were significantly correlated between Sessions 1 and 6 for mindfulness meditation, r(40) = .588, p < 0.01 but not for book-listening control groups, r(37) = .209, p = .22.
Post-intervention expectations predicted mindfulness-based pain relief during opioid antagonism
Expected mindfulness-induced pain relief ratings after the meditation interventions predicted mindfulness-based reductions in pain intensity, r(40) = −.406, p = 0.009 and pain unpleasantness, r(40) = −.342, p = .031 ratings across both groups. In contrast, expected pain relief for the book-listening control groups did not predict ratings of pain intensity, r(38) = −.071, p = 0.67 or pain unpleasantness, r(38) = −.045, p = .79.
In the meditation + naloxone group specifically, higher expected pain relief predicted greater mindfulness-induced reductions in ratings of pain intensity, r(20) = −.76, p < .001 and pain unpleasantness, r(20) = −.55, p = .01 (Figure 2). In the meditation + saline group, expected pain relief did not predict ratings of mindfulness-based pain intensity, r(20) = −.22, p = 0.36 or pain unpleasantness, r(20) = −.23, p = .34 (Figure 2). The correlation between expectations for analgesia and pain relief reported during naloxone was significantly (p = 0.024) greater than the association between expectations for pain relief and pain relief experienced during saline infusion.
Figure 2.
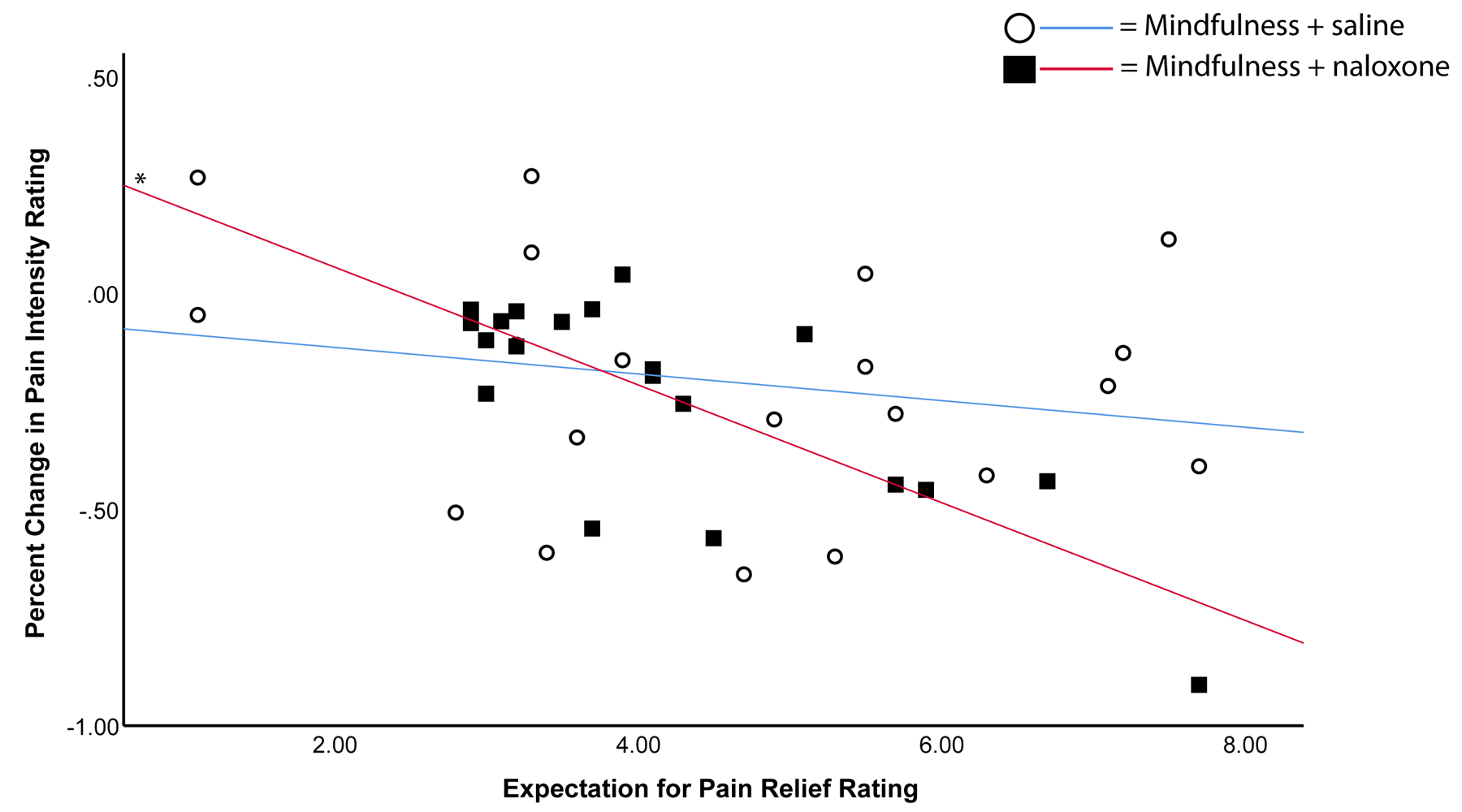
Correlations are displayed between ratings of expected pain relief due to mindfulness (“Expectation for Pain Relief Rating”) and percent changes in pain between baseline and intervention (“Percent Change in Pain Intensity Rating”). * = p < 0.01.
Expectations of pain relief for the book-listening control groups did not predict pain reductions during either saline (pain intensity r(20) = −.37, p = .11; pain unpleasantness r(20) = −.30, p = .20) or naloxone (pain intensity r(18) = 0.26, p = .30; pain unpleasantness r(20) = 0.14, p = 0.60) infusion.
Mindfulness-induced pain relief is not associated with endogenous opioids
A significant group × pre vs. post × pain type interaction, F(3, 74) = 7.01, p<.001, η2p= .22 was revealed. To interpret the significant interaction, we performed a separate RM ANOVAs on pain intensity and pain unpleasantness ratings, respectively.
Pain Intensity
There was a significant pre vs. post, F(1, 74) = 4.22, p=.04, η2p= .05 and a pre vs. post × group interaction on pain intensity ratings, F(3, 74) = 12.86, p<.001, η2p= .34. Follow-up post-hoc univariate ANOVAs and pairwise comparisons on pain intensity percent changes revealed no significant differences (p = .72) between mindfulness + naloxone (−24%) and mindfulness + saline (−21%) groups (Figure 3). The mindfulness + saline group produced significantly greater pain intensity reductions when compared to the control + saline (+21%; p = .001) and control + naloxone (+11%; p < .001). The mindfulness + naloxone group produced significantly greater pain intensity reductions than control + saline and control + naloxone (p values < .001) (Figure 3). The percent change in pain intensity ratings between the two control groups did not significantly differ (p =.38).
Figure 3.
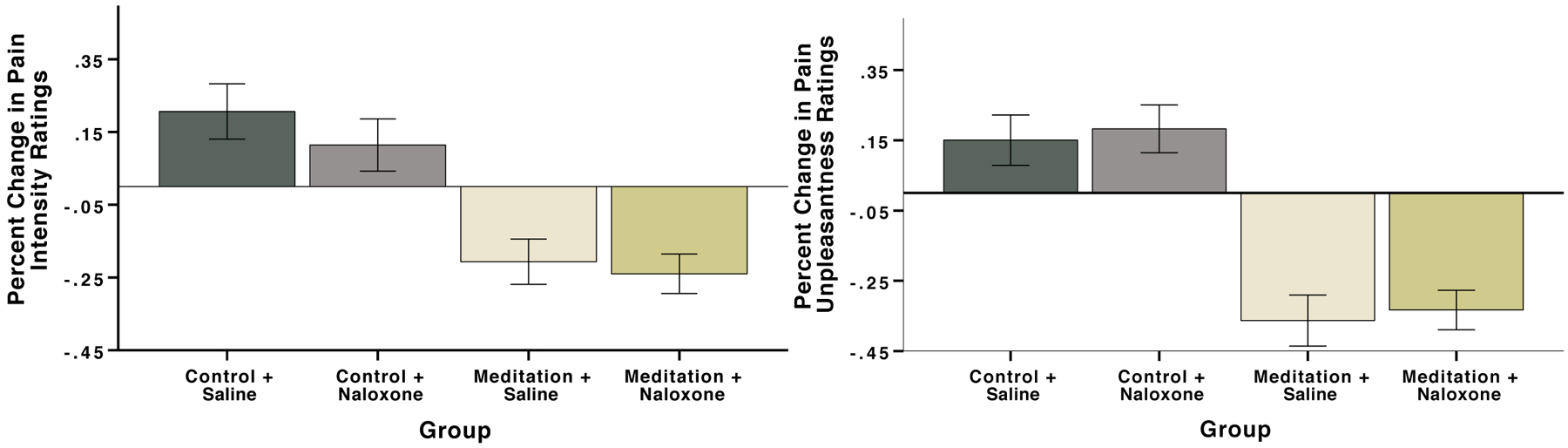
Percent change in ratings of pain intensity (A) and pain unpleasantness (B) during meditation or control book-listening and naloxone or saline infusion, compared with rest. Error bars depict SEM. There were no significant differences between the mindfulness + naloxone and mindfulness + saline group (pain intensity p = .72; pain unpleasantness p = .75). The mindfulness + saline group produced significantly greater pain relief compared to the control + saline group (pain intensity p = .001; pain unpleasantness p < .001) or control + naloxone group (pain intensity and unpleasantness both p < .001). The mindfulness + naloxone group produced significantly greater pain relief than the control + saline group or control + naloxone group (all p values < .001).
Pain Unpleasantness
There was a significant pre vs. post main effect, F(1, 74) = 14.93, p <.001, η2p= .17 and a pre vs. post × group interaction on pain unpleasantness ratings, F(3, 74) = 18.09, p<.001, η2p= .42. Post-hoc univariate ANOVAs and pairwise comparisons on the percent change in pain unpleasantness revealed no significant differences (p = .75) between mindfulness + naloxone (−33%) and mindfulness + saline (−36%) groups (Figure 3). The mindfulness + saline group produced significantly greater pain unpleasantness reductions when compared to the control + saline (+15%; p < .001) and control + naloxone (+18%; p < .001). The mindfulness + naloxone group produced significantly greater pain unpleasantness reductions than control + saline and control + naloxone (p values < .001) (Figure 3). The percent change in pain unpleasantness ratings between the two control groups did not significantly differ (p =.74).
Discussion
The current study investigated whether expectations for pain relief predicted mindfulness-induced pain reductions. Secondarily, we examined the role of endogenous opioids in the relationship between expectations and mindfulness-based pain relief. In contrast to our hypothesis, expectations for mindfulness meditation-induced pain relief did significantly predict pain reductions during mindfulness meditation. However, this correlation was significantly higher during opioid antagonism than during the control saline condition and was not statistically significant during the control saline condition alone (Figure 2). A similar pattern of correlations was found for expectations before the mindfulness meditation intervention and even for expectations of book-listening in the mindfulness group, suggesting that the role of expectations was a general role of optimism and was not based on experience with meditation.
The low and not significant correlation between expectations and mindfulness meditation-based pain relief during saline contrasts with the role of expectations in facilitating placebo (29, 52, 53) and hypnosis-induced (54, 55) analgesia. Indeed, we have previously shown that mindfulness meditation operates via distinct neural, autonomic nervous, and endogenous pain modulatory mechanisms from placebo analgesia (33, 36, 39, 42). The current finding fits with the novel, non-opioidergic pain modulatory pathway proposed to underlie the pain-relieving effects of mindfulness meditation (56). We have proposed that mindfulness meditation-based pain relief is mediated by self-regulated attention-to-breath, which may engage a PFC-thalamo-cortical pathway (31, 33, 36, 50, 57). This pathway presumably activates the GABA-ergic thalamic reticular nuclei via prefrontal projections to reduce transmission of ascending thalamocortical projections to somatosensory areas (58–60).
The low association between mindfulness-based pain relief and expectations during saline is also consistent with previous demonstrations that mindfulness reduces anticipatory pain appraisals. Mindfulness reduces anterior insular activation during the pre-stimulus onset period (46) and mindfulness-based pain relief is directly associated with lower electrophysiological anticipatory markers in the medial cingulate cortex (43). These findings suggest that mindfulness meditation may reduce expectation-driven activations in anticipation of painful stimulation, decreasing the role of expectations in the processing of acute pain.
Surprisingly, expectations did predict mindfulness-based pain relief during opioidergic antagonism. Opioids are thought to be necessary for the maintenance of expectations and their role in placebo analgesia (26, 61–63). There is precedent for a role of expectations during opioid blockade from reports of expectation-driven placebo effects being maintained during opioid blockade (64) and being unaffected by administration of an opioid analgesic drug (65). However, our result suggests that expectations are more relevant to the experience of pain during opioid blockade.
Possible explanations for the role of expectations during mindfulness-induced pain relief and opioid antagonism may reflect the mechanistic relevance of the dopaminergic and GABAergic systems. A number of brain areas are implicated in the maintenance of expectations during placebo analgesia including parts of the dopamine-rich nucleus accumbens–ventral striatum (NAc-VS), areas associated with modulating affect and value (66). Expectation-driven placebo analgesia generally increases activity in the NAc–VS (67, 68). The dopamine system is also heavily driven by gamma-aminobutyric acid (GABA) (69), an inhibitory neurotransmitter that regulates the excitability of cortical networks (70). Importantly, the release of endogenous opioids inhibits GABAergic synaptic transmission and inversely, opioid blockade increases GABAergic synaptic transmission (71). GABAergic neurons in the ventral tegmental area (VTA) are postulated to encode the value of expected reward which is then utilized by dopaminergic neurons in the VTA to compute prediction errors (i.e., the discrepancy between expected and realized rewards) (72, 73). If opioid antagonism increases GABA, this might strengthen (or induce) GABAergically mediated encoding via dopamine of expected reward, in this case pain relief (30, 74, 75), preserving the encoding of expectations for pain-relief despite the presumable present-minded focus engaged during mindfulness meditation. Although we did not test for this, we postulate that the more a participant expected and perceived meditation as rewarding in the context of positive mood and pain relief (76–78), the greater the GABA-mediated encoding of expected reward.
Before experience with the book, expectations for pain relief induced by book-listening predicted pain increases in the final session. After experience with the book, this correlation was not significant. The lack of correlation for the book-listening group may be due to a floor effect since expectations for pain relief from book-listening were quite low. Although we did not directly test for this, we postulate further that this lack of covariance may be due to a violation of expectations for book-induced analgesia after subjects experienced the “boring” and “dry” story material. Indeed, expectations for book-listening related pain relief between Session 1 and Session 6 were not significantly correlated.
In sum, we demonstrate that expectations exhibit low correlation with mindfulness meditation-based relief of acute pain under normal, opioidergic circumstances. However, we find a novel effect of endogenous opioids in modulating the role of expectations in meditation-induced pain relief; in the present study, opioid blockade strengthened the relationship between expected and experienced mindfulness-based pain relief, even for expectations rated before meditation exposure. Mindfulness may thus operate through multiple mechanisms that are shifted by the activity of opioids, potentially altering GABAergic and dopaminergic pathways. From a clinical perspective, these findings suggest that mindfulness alleviates acute pain largely independent of expectations. This is important because it suggests that under normal conditions individuals with low expectations for pain relief from mindfulness may still experience mindfulness-induced pain attenuation, an important consideration for the millions of chronic pain patients seeking a robust and reliable self-regulated pain therapy. However, further work is needed to confirm whether this finding holds true for relief of chronic pain, recurrent acute pain, and/or in a broader range of ages including older adults. That is, effects presented here may not be realized in chronic pain patients and older adults. Further, these findings demonstrate further evidence that mindfulness is mechanistically distinct from other cognitive techniques and provides mechanistic insight into the recent surge of well-controlled clinical trials demonstrating the analgesic benefits of mindfulness training on a spectrum of pain conditions (34, 37, 79–84).
Table 1.
Change in ratings (Mean and SD) of pain intensity and unpleasantness of heat administered before and during the infusion of naloxone or saline.
| Meditation + naloxone | Control + naloxone | Meditation + saline | Control + saline | |
|---|---|---|---|---|
| Pain intensity before infusion (pre) | 4.62 (1.45) | 4.11 (1.63) | 3.44 (1.78) | 4.78 (2.51) |
| Pain intensity during infusion (post) | 3.53 (1.58) N = 20 p = .000* |
4.40 (1.64) N = 18 p = .197 |
2.78 (1.64) N = 20 p = .003* |
5.33 (2.44) N = 20 p = .012* |
| Pain unpleasantness before infusion (pre) | 4.69 (1.64) | 3.88 (1.59) | 3.54 (2.11) | 5.01 (2.57) |
| Pain unpleasantness during infusion (post) | 3.11 (1.56) N = 20 p = .000* |
4.40 (1.62) N = 18 p = .073 |
2.05 (1.16) N = 20 p = .000* |
5.45 (2.54) N = 20 p = .107 |
pairwise comparison between pre and post ratings, p < 0.0125 (Bonferroni correction for four groups)
Conflicts of Interest and Source of Funding:
This work was supported by the NIH’s National Center for Complementary and Integrative Health (NCCIH) (R00-AT009466, LC), (K99/R00-AT008238; R21-AT007247; R01-AT009693; R21-AT010352, FZ), (F30-AT009165, AA-N), (K23AT008406, RW), the Mind and Life Institute, and the Wake Forest Translational Science Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no competing financial interests.
Acronyms
- VAS
visual analog scale
- IV
intravenous
- VTA
ventral tegmental area
- NAc-VS
nucleus accumbens–ventral striatum
- GABA
gamma-aminobutyric acid; prefrontal cortex (PFC)
- S
second
- Min
minute
- RM ANOVA
repeated measures analysis of variance
Footnotes
The authors declare no conflicts of interest.
References
- 1.Villemure C, Bushnell CM. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–9. [DOI] [PubMed] [Google Scholar]
- 2.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–43. [DOI] [PubMed] [Google Scholar]
- 3.Grevert P, Albert LH, Goldstein A. Partial antagonism of placebo analgesia by naloxone. Pain. 1983;16:129–43. [DOI] [PubMed] [Google Scholar]
- 4.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2:654–7. [DOI] [PubMed] [Google Scholar]
- 5.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CD, Goodin B, Kindler LL, Caudle RM, Edwards RR, Gravenstein N, Riley JL 3rd, Fillingim RB. Reduction of conditioned pain modulation in humans by naltrexone: an exploratory study of the effects of pain catastrophizing. J Behav Med. 2013;36:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Current biology : CB. 2012;22:1019–22. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson JB. Reversal of hypnosis-induced analgesia by naloxone. Lancet. 1978;2:991–2. [DOI] [PubMed] [Google Scholar]
- 10.Frid M, Singer G. Hypnotic analgesia in conditions of stress is partially reversed by naloxone. Psychopharmacology (Berl). 1979;63:211–5. [DOI] [PubMed] [Google Scholar]
- 11.Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandura A, O’Leary A, Taylor CB, Gauthier J, Gossard D. Perceived self-efficacy and pain control: opioid and nonopioid mechanisms. Journal of personality and social psychology. 1987;53:563–71. [DOI] [PubMed] [Google Scholar]
- 13.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proceedings of the National Academy of Sciences. 2005;102:12950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience. 2010;30:12964–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81:1223–39. [DOI] [PubMed] [Google Scholar]
- 19.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. [DOI] [PubMed] [Google Scholar]
- 20.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reicherts P, Gerdes AB, Pauli P, Wieser MJ. Psychological placebo and nocebo effects on pain rely on expectation and previous experience. The Journal of Pain. 2016;17:203–14. [DOI] [PubMed] [Google Scholar]
- 23.Jensen KB, Kaptchuk TJ, Chen X, Kirsch I, Ingvar M, Gollub RL, Kong J. A Neural Mechanism for Nonconscious Activation of Conditioned Placebo and Nocebo Responses. Cerebral cortex. 2015;25:3903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egorova N, Benedetti F, Gollub RL, Kong J. Between placebo and nocebo: Response to control treatment is mediated by amygdala activity and connectivity. European journal of pain. 2020;24:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeidan F, Lobanov OV, Kraft RA, Coghill RC. Brain Mechanisms Supporting Violated Expectations of Pain. Pain. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci. 1999;19:3639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–43. [DOI] [PubMed] [Google Scholar]
- 29.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. Journal of Neuroscience. 1999;19:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berna C, Leknes S, Ahmad AH, Mhuircheartaigh RN, Goodwin GM, Tracey I. Opioid-Independent and Opioid-Mediated Modes of Pain Modulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018;38:9047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeidan F, Adler-Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC, McHaffie JG, Coghill RC. Mindfulness-Meditation-Based Pain Relief Is Not Mediated by Endogenous Opioids. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:3391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May LM, Kosek P, Zeidan F, Berkman ET. Enhancement of Meditation Analgesia by Opioid Antagonist in Experienced Meditators. Psychosomatic medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells RE, Collier J, Posey G, Morgan F, Auman T, Strittameter B, Magalhaes R, Adler-Neal A, McHaffie JG, Zeidan F. Attention to breath sensations does not engage endogenous opioids to reduce pain. Pain. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherkin DC, Sherman KJ, Balderson BH, Cook AJ, Anderson ML, Hawkes RJ, Hansen KE, Turner JA. Effect of Mindfulness-Based Stress Reduction vs Cognitive Behavioral Therapy or Usual Care on Back Pain and Functional Limitations in Adults With Chronic Low Back Pain: A Randomized Clinical Trial. Jama. 2016;315:1240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gard T, Holzel BK, Sack AT, Hempel H, Vaitl D, & Ott U Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cerebral cortex. 2011;191:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC. Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:15307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain. 2008;134:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garland EL, Howard MO. Mindfulness-oriented recovery enhancement reduces pain attentional bias in chronic pain patients. Psychotherapy and psychosomatics. 2013;82:311–8. [DOI] [PubMed] [Google Scholar]
- 39.Zeidan F, Adler-Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC, McHaffie JG, Coghill RC. Mindfulness-meditation-based pain relief is not mediated by endogenous opioids. Journal of Neuroscience. 2016;36:3391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May LM, Kosek P, Zeidan F, Berkman ET. Enhancement of Meditation Analgesia by Opioid Antagonist in Experienced Meditators. Psychosom Med. 2018;80:807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC. Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. Journal of Neuroscience. 2015;35:15307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adler-Neal AL, Waugh CE, Garland EL, Shaltout HA, Diz DI, Zeidan F. The role of heart rate variability in mindfulness-based pain relief. The journal of pain : official journal of the American Pain Society. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown CA, Jones AK. Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain. 2010;150:428–38. [DOI] [PubMed] [Google Scholar]
- 44.No Zeidan F., Mindfulness Meditation-Based Analgesia Is Not Mediated by Endogenous Opioids. The American journal of medicine. 2016;129:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace BA. The Attention Revolution: Unlocking the Power of the Focused Mind. Somerville: Wisdom Publications; 2006. [Google Scholar]
- 46.Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage. 2013;64:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–26. [DOI] [PubMed] [Google Scholar]
- 48.Emerson NM, Zeidan F, Lobanov OV, Hadsel MS, Martucci KT, Quevedo AS, Starr CJ, Nahman-Averbuch H, Weissman-Fogel I, Granovsky Y, Yarnitsky D, Coghill RC. Pain sensitivity is inversely related to regional grey matter density in the brain. Pain. 2014;155:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lobanov OV, Zeidan F, McHaffie JG, Kraft RA, Coghill RC. From cue to meaning: brain mechanisms supporting the construction of expectations of pain. Pain. 2014;155:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White G. Natural History and Antiquities of Selborne. London, England: Cassell and Company; 1908. [Google Scholar]
- 52.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–56. [DOI] [PubMed] [Google Scholar]
- 53.Kirsch I, Kong J, Sadler P, Spaeth R, Cook A, Kaptchuk TJ, Gollub R. Expectancy and conditioning in placebo analgesia: separate or connected processes? Psychology of Consciousness: Theory, Research, and Practice. 2014;1:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–71. [DOI] [PubMed] [Google Scholar]
- 55.Lu DP, Lu GP, Kleinman L. Acupuncture and clinical hypnosis for facial and head and neck pain: a single crossover comparison. The American journal of clinical hypnosis. 2001;44:141–8. [DOI] [PubMed] [Google Scholar]
- 56.Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neuroscience letters. 2012;520:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeidan F, Vago DR. Mindfulness meditation-based pain relief: a mechanistic account. Annals of the New York Academy of Sciences. 2016;1373:114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guglietti CL, Daskalakis ZJ, Radhu N, Fitzgerald PB, Ritvo P. Meditation-related increases in GABAB modulated cortical inhibition. Brain stimulation. 2013;6:397–402. [DOI] [PubMed] [Google Scholar]
- 59.Nakajima M, Schmitt LI, Halassa MM. Prefrontal cortex regulates sensory filtering through a basal ganglia-to-thalamus pathway. Neuron. 2019;103:445–58. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nature neuroscience. 2002;5:1203–9. [DOI] [PubMed] [Google Scholar]
- 61.Levine J, Gordon N, Fields H. The mechanism of placebo analgesia. The Lancet. 1978;312:654–7. [DOI] [PubMed] [Google Scholar]
- 62.Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, Valeriani M. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain. 2008;139:306–14. [DOI] [PubMed] [Google Scholar]
- 63.Lobanov OV, Zeidan F, McHaffie JG, Kraft RA, Coghill RC. From cue to meaning: Brain mechanisms supporting the construction of expectations of pain. Pain. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–47. [DOI] [PubMed] [Google Scholar]
- 65.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. Journal of Neuroscience. 2012;32:8053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nature Reviews Neuroscience. 2015;16:403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh RN, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine. 2011;3:70ra14–70ra14. [DOI] [PubMed] [Google Scholar]
- 68.Lee H-F, Hsieh J-C, Lu C-L, Yeh T-C, Tu C-H, Cheng C-M, Niddam DM, Lin H-C, Lee F-Y, Chang F-Y. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153:1301–10. [DOI] [PubMed] [Google Scholar]
- 69.Scheel-Krüger J. Dopamine-GABA interactions: evidence that GABA transmits, modulates and mediates dopaminergic functions in the basal ganglia and the limbic system. Acta Neurologica Scandinavica. 1986. [PubMed] [Google Scholar]
- 70.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–5. [DOI] [PubMed] [Google Scholar]
- 71.Vaughan C, Ingram S, Connor M, Christie M. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–4. [DOI] [PubMed] [Google Scholar]
- 72.Welberg L High expectations for GABA. Nature Reviews Neuroscience. 2012;13:150–1. [DOI] [PubMed] [Google Scholar]
- 73.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. nature. 2012;482:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nees F, Becker S, Millenet S, Banaschewski T, Poustka L, Bokde A, Bromberg U, Buchel C, Conrod PJ, Desrivieres S, Frouin V, Gallinat J, Garavan H, Heinz A, Ittermann B, Martinot JL, Papadopoulos Orfanos D, Paus T, Smolka MN, Walter H, Whelan R, Schumann G, Flor H, consortium I. Brain substrates of reward processing and the mu-opioid receptor: a pathway into pain? Pain. 2017;158:212–9. [DOI] [PubMed] [Google Scholar]
- 75.Becker S, Ceko M, Louis-Foster M, Elfassy NM, Leyton M, Shir Y, Schweinhardt P. Dopamine and pain sensitivity: neither sulpiride nor acute phenylalanine and tyrosine depletion have effects on thermal pain sensations in healthy volunteers. PloS one. 2013;8:e80766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garland SN, Tamagawa R, Todd SC, Speca M, Carlson LE. Increased mindfulness is related to improved stress and mood following participation in a mindfulness-based stress reduction program in individuals with cancer. Integrative cancer therapies. 2013;12:31–40. [DOI] [PubMed] [Google Scholar]
- 77.Zeidan F, Johnson SK, Gordon NS, Goolkasian P. Effects of brief and sham mindfulness meditation on mood and cardiovascular variables. Journal of alternative and complementary medicine. 2010;16:867–73. [DOI] [PubMed] [Google Scholar]
- 78.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosomatic medicine. 2003;65:571–81. [DOI] [PubMed] [Google Scholar]
- 79.Seminowicz DA, Burrowes SA, Kearson A, Zhang J, Krimmel SR, Samawi L, Furman AJ, Keaser ML, Gould NF, Magyari T, White L, Goloubeva O, Goyal M, Peterlin BL, Haythornthwaite JA. Enhanced mindfulness based stress reduction (MBSR+) in episodic migraine: a randomized clinical trial with MRI outcomes. Pain. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morone NE, Greco CM, Moore CG, Rollman BL, Lane B, Morrow LA, Glynn NW, Weiner DK. A Mind-Body Program for Older Adults With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA internal medicine. 2016;176:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garland EL, Brintz CE, Hanley AW, Roseen EJ, Atchley RM, Gaylord SA, Faurot KR, Yaffe J, Fiander M, Keefe FJ. Mind-Body Therapies for Opioid-Treated Pain: A Systematic Review and Meta-analysis. JAMA internal medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garland EL, Atchley RM, Hanley AW, Zubieta JK, Froeliger B. Mindfulness-Oriented Recovery Enhancement remediates hedonic dysregulation in opioid users: Neural and affective evidence of target engagement. Science advances. 2019;5:eaax1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. Pain. 2011;152:361–9. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Aranda A, Feliu-Soler A, Montero-Marin J, Garcia-Campayo J, Andres-Rodriguez L, Borras X, Rozadilla-Sacanell A, Penarrubia-Maria MT, Angarita-Osorio N, McCracken LM, Luciano JV. A randomized controlled efficacy trial of mindfulness-based stress reduction compared with an active control group and usual care for fibromyalgia: the EUDAIMON study. Pain. 2019;160:2508–23. [DOI] [PubMed] [Google Scholar]


