Abstract
Purpose
To analyze the origin and anatomic course of the hepatic arteries by using digital subtraction angiography (DSA) and multidetector CT in a large study sample.
Materials and Methods
This retrospective study included 5625 patients who underwent liver CT and chemoembolization between January 2005 and December 2018 (mean age, 60 years ± 11 [range, 11–99 years]; 4464 males). The CT and DSA images were reviewed to evaluate the visceral arterial anatomy for variations in the celiac axis and hepatic arteries. Aberrant right hepatic arteries (aRHAs) and aberrant left hepatic arteries (aLHAs) were defined on the basis of their origin and anatomic course. Statistical analyses were performed to evaluate the association between aRHAs and aLHAs and the association between variations in the hepatic artery and celiac axis.
Results
Right hepatic arteries were categorized as being aRHAs (if originating from the proximal to middle common hepatic artery, gastroduodenal artery, superior mesenteric artery [SMA], celiac axis, aorta, splenic artery, or left gastric artery [LGA]) or as being aLHAs (if arising from the LGA, celiac axis, aorta, or SMA). The prevalence of aRHAs (15.63%; 879 of 5625) and the prevalence aLHAs (16.32%; 918 of 5625) were similar. Patients with an aRHA were more likely to have an aLHA than those without an aRHA (29.01% vs 13.97%; P < .001), and patients with an aLHA were more likely to have an aRHA than those without an aLHA (27.78% vs 13.26%; P < .001). There was no association between the hepatic arterial variations and celiac axis variations. A hypothetical anatomic model summarizing the observed variations was created.
Conclusion
A comprehensive list of hepatic arterial variations and a three-dimensional hypothetical model for the observed variations were described.
Keywords: CT, Angiography, Liver, Anatomy, Arteries
© RSNA, 2021
Supplemental material is available for this article.
See also commentary by Sutphin and Kalva in this issue.
Keywords: CT, Angiography, Liver, Anatomy, Arteries
Summary
The origins, anatomic courses, and prevalence of aberrant right and left hepatic arteries were comprehensively evaluated by using CT and digital subtraction angiographic images in 5625 patients.
Key Points
■ In relation to the portal vein, common hepatic artery or bile duct, or fissure for ligamentum venosum, anatomic courses of the right and left hepatic arteries were different depending on their origins.
■ The prevalence of aberrant hepatic arteries overall was 27.41%, the prevalence of aberrant right hepatic arteries (aRHAs) was 15.63%, the prevalence of aberrant left hepatic arteries (aLHAs) was 16.32%, and the prevalence of having both an aRHA and an aLHA was 4.53%.
■ Patients with an aRHA were more likely to have an aLHA than those without an aRHA (29.01% vs 13.97%; P < .001), and patients with an aLHA were more likely to have an aRHA than those without an aLHA (27.78% vs 13.26%; P < .001).
Introduction
Anatomic variations of hepatic arteries are frequently observed and have a reported prevalence of 13%–48% (1–14). Information on anatomic variations in hepatic arteries of patients can be of great importance when planning hepatobiliary surgery, liver transplant and donor procedures, transarterial therapy, and other endovascular interventions (15,16). The presence of hepatic arterial variations may require changes to surgical techniques or, in the absence of appropriate preoperative evaluations, may lead to unintentional hemorrhaging or biliary complications (15–17). Interventional radiologists performing hepatic arterial embolization or chemoembolization must have knowledge of the variable anatomic patterns of hepatic arteries to diagnose and treat bleeding foci or tumors in the liver supplied by aberrant hepatic arteries (17,18).
The most frequently employed classifications for describing hepatic arterial variations in the previous literature are the Michels classification (14) and its modification by Hiatt (13). Although they are milestones in describing hepatic arterial anatomy, these classification systems and the previous studies using them have limitations. First, the anatomic course of the hepatic artery and its topographic relation to the adjacent structures are not considered. For example, the right hepatic artery (RHA) from normal or aberrant origins can show variable anatomic courses, including those crossing the common hepatic duct or common bile duct anteriorly and those crossing the portal vein posteriorly (19–23). Second, the analysis of aberrant hepatic arteries that are not categorized by using these classification systems may have been different between studies. Several studies have reported aberrant hepatic arteries of rare origins, including the aorta, celiac trunk, and splenic artery, which are not included in these classification systems (2,3,5,7,8,10,24–26). Additionally, although the hepatic artery originating from the celiac common hepatic artery (CHA) is typically considered normal (as described in Michels’ work), there have been studies reporting a hepatic artery originating from the CHA or gastroduodenal artery (GDA) as being aberrant (21,24,25,27,28). Therefore, it is important to establish appropriate criteria for aberrant hepatic arteries that consider their origins and anatomic courses. Third, although there have been several case reports, the prevalence of extremely rare hepatic arterial variations such as an aberrant RHA (aRHA) originating from the splenic artery (26,29,30) could not be evaluated, even in the previous studies that included a relatively large number (n > 1000) of patients (1,10,11). Further studies including a larger number of patients are required. Fourth, the previous studies may be limited because each only used a single method to obtain data: cadaveric analysis (12,14,25,31), operation record analysis (6,11), CT (1,4,9,10), or digital subtraction angiography (DSA) (2,7).
The purposes of our study were to analyze the origin and three-dimensional anatomic course of the hepatic arteries by using both DSA and multidetector CT in a large study sample and to define aberrant hepatic arteries. In addition, we aimed to evaluate the association between right and left hepatic arterial variations, to evaluate the association between hepatic arterial and celiac trunk variations, and to suggest a hypothetical anatomic model to summarize the observed hepatic arterial variations.
Materials and Methods
Patients
This study was approved by the institutional review board of the authors’ institution, and the requirement for informed consent was waived because of the retrospective nature of the study. To the best of our knowledge, there was no patient overlap between the present study and prior published studies. We retrospectively searched the electronic medical records of our institution and identified 6755 consecutive patients who underwent initial transarterial chemoembolization between January 2005 and December 2018. Among them, patients with a previous history of abdominal surgery (n = 1099) and those who did not undergo the appropriate preprocedural liver protocol CT with a section thickness greater than 3 mm (n = 31) were excluded. Consequently, a total of 5625 patients (mean age, 60 years ± 11 [range, 11–99 years]), comprising 4464 males (mean age, 59 years ± 11 [range, 18–99 years]) and 1161 females (mean age, 62 years ± 12 [range, 11–92 years]), were included (Fig E1 [supplement]).
Contrast-enhanced Liver CT
One of the following multidetector CT scanners was used to perform the liver protocol CT scans: a 128-channel Ingenuity scanner (Philips Healthcare); a 64-channel Brilliance 64 (Philips Healthcare) or Sensation 64 (Siemens Medical Solutions) scanner; a 16-channel Brilliance 16 (Philips Healthcare), Sensation 16 (Siemens Medical Solutions), or Aquilion 16 (Toshiba Medical Systems) scanner; an eight-channel LightSpeed Ultra scanner (GE Medical Systems); or a four-channel MX8000 scanner (Philips Healthcare).
The scanning parameters used for liver CT examinations included detector configurations of 64 × 0.625 mm, 16 × 0.75 mm, 8 × 1.25 mm, and 4 × 2.5 mm; a tube voltage setting of 120 kVp; section thicknesses of 2.5–3.2 mm; reconstruction intervals of 2.0–3.0 mm; and a matrix size of 512 × 512. After precontrast images were obtained, 1.6-mL/kg of contrast material (Bonorex 350, Central Medical Service, or Xenetix 350, Guerbet) was intravenously injected at a rate of 3–5 mL/sec by using an automatic power injector. The timing of the arterial phase scan was determined by using an automatic bolus tracking technique. It started 11–19 seconds after attenuation in the descending aorta reached 100 HU. The portal venous phase scans were initiated 20–33 seconds after arterial phase scanning, and the equilibrium phase scans were performed 180 seconds after the administration of the contrast material. The imaging protocol used in the present study was the standard protocol of the authors’ institution.
DSA Procedures
When performing transarterial chemoembolization, DSA images of the celiac axis and superior mesenteric artery (SMA) were obtained to evaluate the vascular anatomy of the patients by using one of the following angiographic units (Axiom Artis dTA/VB30, Artis Zee, and Angiostar, Siemens Medical Solutions; Allura Xper FD20 and V-3000, Philips Healthcare). For celiac axis angiography, 20–40 mL of iodinated contrast material (Pamiray 300, DongKook Pharmaceutical) was administered at a rate of 4–6 mL/sec by using a 5F angiographic catheter (RH, Cook Medical). For SMA angiography, a total of 9–20 mL of contrast material was injected at a rate of 3–6 mL/sec by using a 5F catheter. If anatomic variation of the celiac axis or hepatic artery was suspected on the basis of the preprocedural CT or celiac or SMA angiographic findings, additional selective angiography was performed for the complete evaluation of the hepatic arterial supply.
Image Analysis
Two board-certified radiologists (T.W.C. and J. W. Chung; 8 and 35 years of experience in radiology, respectively) retrospectively reviewed all preprocedural liver CT and DSA images and determined the anatomic patterns of the hepatic arteries. When they had different opinions, consensus was reached during additional image review sessions in which the two radiologists participated and conducted discussion. Initially, the presence of the anatomic variation of the celiac axis was analyzed (32). Thereafter, to determine the criteria for an aberrant hepatic artery, the origins and three-dimensional anatomic courses of the RHAs and left hepatic arteries (LHAs) were analyzed in relation to adjacent structures by using thin-section dynamic CT. For the hepatic arteries arising from arteries other than the CHA, origins were recorded. For the hepatic arteries arising from the CHA or its branch, the origins were subdivided into the proximal to middle portion (proximal two-thirds) of the CHA, the distal portion (distal one-third) of the CHA, and the GDA. In patients with celiac axis variations, the CHA itself can frequently show aberrant anatomic courses, thereby making it difficult to determine the course of the RHAs and LHAs (32). Therefore, the analysis of the anatomic course was carried out only in patients without celiac axis variations. For each RHA, the anatomic course in relation to the portal vein and common hepatic duct or common bile duct was analyzed. For each LHA, the anatomic course was evaluated to determine whether it passed through the fissure for ligamentum venosum. On the basis of the origins of the hepatic arteries and their characteristic anatomic courses, we determined which anatomic variations should be categorized as aberrant hepatic arteries or major anatomic variations and which should be classified as minor variations, not aberrant hepatic arteries. We defined aberrant hepatic arteries either as those with abnormal origin or on the basis of the frequency of abnormal anatomic courses within the portal space. Specifically, any hepatic artery arising from vessels other than the CHA were considered aberrant; hepatic arteries arising off the proximal to middle (proximal two-thirds) CHA, off the distal (distal one-third) CHA, and off the GDA were also considered aberrant if the majority of arteries for each of these segments had an abnormal preductal or retroportal course.
According to the criteria determined previously, the prevalence of aRHAs and aberrant LHAs (aLHAs) supplying the right and left lobes were analyzed. Thereafter, we evaluated whether there were associations between the presence of an aRHA and the presence of an aLHA or between hepatic arterial and celiac trunk variations. Finally, we proposed a hypothetical anatomic model to summarize the observed hepatic arterial variations.
Statistical Analysis
A comparison between groups was performed by using the Pearson χ2 test or Fisher exact test, as appropriate. All statistical analyses were performed with SPSS version 25.0 software (IBM), and P values less than .05 were considered to indicate statistically significant differences.
Results
Anatomic Variations in the Hepatic Artery
Anatomic variations of the celiac axis were identified in 12.14% (683 of 5625) of patients. In 4942 patients without celiac axis variations, the anatomic courses of the RHAs and LHAs were different depending on their origins (Table 1). The prevalence rates of a preductal course were 6.11% (193 of 3158) in RHAs arising from the proper hepatic artery and 11.78% (118 of 1002) in those from the distal CHA, whereas the prevalence was 98.43% (125 of 127) in RHAs arising from the GDA. On the basis of this result, we categorized RHAs originating from the GDA as aberrant and characterized those arising from the distal CHA as nonaberrant. All of the RHAs originating from the proximal to middle CHA showed retroportal courses and were therefore considered to be aberrant hepatic arteries. Consequently, we categorized RHAs originating from the proximal to middle CHA, GDA, SMA, celiac axis, aorta, splenic artery, and left gastric artery (LGA) as aRHAs, and we characterized LHAs arising from the LGA, celiac axis, aorta, and SMA as aLHAs (Figs 1, 2). Anatomic variations such as the RHA and LHA separately originating from the distal CHA were considered to be minor variations, as they maintain a classic anatomic course with respect to the common bile duct and portal vein (Fig 3).
Table 1:
Anatomic Course of the Right and Left Hepatic Arteries by Origin
Figure 1:
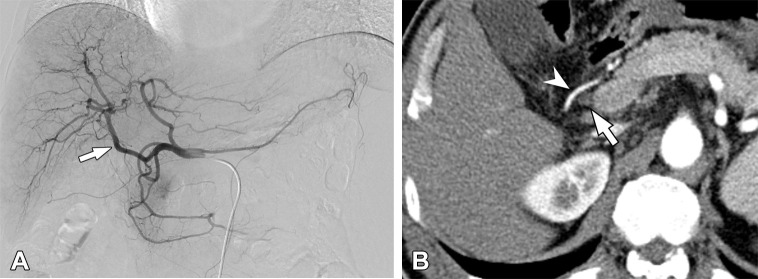
Aberrant right hepatic artery (aRHA) originating from the gastroduodenal artery (GDA) in a 59-year-old man. (A) The digital subtraction angiographic image obtained at the common hepatic artery shows the aRHA (arrow) originating from the GDA. (B) On an axial arterial phase CT image, the aRHA (arrowhead) is coursed anteriorly to the common bile duct (arrow).
Figure 2:
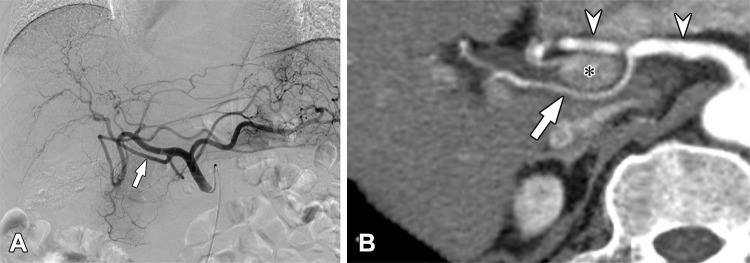
Aberrant right hepatic artery (aRHA) originating from the proximal common hepatic artery (CHA) in a 71-year-old man. (A) The digital subtraction angiographic image obtained at the celiac axis demonstrates the aRHA (arrow) arising from the proximal CHA. (B) On a curved multiplanar reconstruction image, the aRHA (arrow) arising from the CHA (arrowhead) is crossing the main portal vein (asterisk) posteriorly.
Figure 3:
Schematic diagrams of the normal hepatic artery and representative cases of minor hepatic arterial variations. (A) Normal hepatic arterial anatomy. (B) Trifurcation of the common hepatic artery (CHA) into the right and left hepatic arteries and gastroduodenal artery. (C) The right and left hepatic arteries are separately and sequentially originating from the distal CHA. Red = hepatic artery, blue = portal vein, green = bile duct.
The prevalence rates of aRHAs and aLHAs by origin are presented in Table 2, Figures 4 and 5, and Figures E2 and E3 (supplement). aRHAs and aLHAs were observed in 15.63% (879 of 5625) and 16.32% (918 of 5625) of the patients, respectively (Table 2). In 4.53% (255 of 5625) of patients, both an aRHA and an aLHA were identified (Table 3). The results of the present study recategorized according to Hiatt classification system and compared with those of previous studies are presented in Table E1 (supplement). The patients with an aRHA were more likely to have an aLHA than patients without an aRHA (29.01% [255 of 879] vs 13.97% [663 of 4746]; P < .001), whereas patients with an aLHA were more likely to have an aRHA than patients without an aLHA (27.78% [255 of 918] vs 13.26% [624 of 4707]; P < .001).
Table 2:
Celiac Axis Variations and Aberrant Hepatic Arteries by Origin
Figure 4:
Schematic diagrams of aberrant right hepatic arteries (aRHAs) of various origins: (A) from the superior mesenteric artery (SMA), (B) from the gastroduodenal artery (GDA), (C) from the celiac axis, (D) from the proximal to middle common hepatic artery (CHA), and (E) from the aorta. Red = hepatic artery, blue = portal vein, green = bile duct.
Figure 5:
Schematic diagrams of aberrant left hepatic arteries (aLHAs) from (A) the left gastric artery (LGA) and from (B) the celiac axis. Red = hepatic artery, blue = portal vein, green = bile duct.
Table 3:
Number of Patients with Right and/or Left Aberrant Hepatic Arteries by Origin
Between patients with celiac axis variations and those without, no evidence of a difference was found in the prevalence of aRHAs (14.20% [97 of 683] vs 15.82% [782 of 4942]; P = .27) or aLHAs (15.52% [106 of 683] vs 16.43% [812 of 4942]; P = .55). As shown in Table 2, the prevalence of aRHAs and aLHAs according to their origins were similar between patients with celiac axis variations and those without, except for in patients with an aRHA arising from the celiac axis (0.44% [three of 683] vs 2.06% [102 of 4942]; P = .001) and in patients with an aRHA arising from the proximal to middle CHA (2.05% [14 of 683] vs 0.83% [41 of 4942]; P = .002). The number of aRHAs, aLHAs, and co-occurring aRHAs and aLHAs found in patients without celiac axis variations are shown in Table 4.
Table 4:
Number of Patients without Celiac Axis Variations with Right and/or Left Aberrant Hepatic Arteries
Rare Variations
Extremely rare variations observed in this study included aRHAs originating from the splenic artery (n = 2) or LGA (n = 1) and aLHAs originating from the aorta (n = 1) or SMA (n = 1) (Table 2; Figs E3–E5 [supplement]). The patient with an aLHA arising from the SMA also had an aRHA arising from the SMA and an aLHA arising from the LGA (Fig E5 [supplement]). The aLHA originated from the more proximal segment of the SMA than did the aRHA, coursed anteriorly to the main portal vein, and supplied segment 3. There was no crossing among the aLHAs arising from the SMA, common bile duct, or common hepatic duct. In contrast, the aRHA arising from the SMA showed a retroportal course and supplied the right lobe.
Anatomic Model
A hypothetical three-dimensional anatomic model based on the observed cases was created to summarize the potential anatomic courses of the hepatic arteries, including their topographic relation to the bile duct and portal vein (Fig 6).
Figure 6:
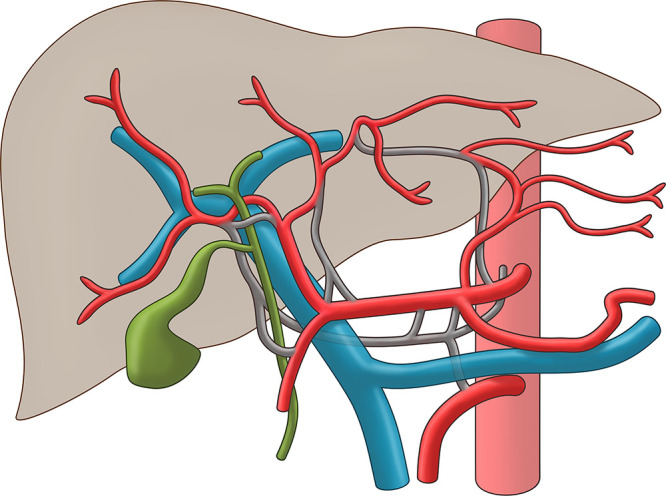
Hypothetical anatomic model summarizing the observed hepatic arterial variations. Vessels colored in red indicate normal arterial anatomy, whereas arteries colored in gray represent possible anatomic courses. Blue = portal vein, green = bile duct.
Discussion
Hepatic arterial variation encompasses a wide spectrum of anatomic variations, including variations of the CHA and individual hepatic arteries (lobar, sectional, or segmental hepatic arteries), in terms of their origins and anatomic courses. In a previous study, we described the anatomic variations of the celiac axis and CHA in 5002 patients (32). In the present study, as the next step for systematic analysis of hepatic arterial variation, we analyzed individual hepatic arterial variations. The term aberrant hepatic artery refers to a hepatic artery with an origin or anatomic course that is substantially atypical. The hepatic arteries arising from arteries other than the CHA (ie, the aorta, celiac trunk, SMA, or LGA) have been indisputably considered to be aberrant in the previous literature because of their atypical origins. In contrast, although hepatic arteries arising from the CHA or its branches can show atypical anatomic courses, there have been no clear criteria for these types of aberrant hepatic arteries. In the present study, we suggest criteria for defining aberrant hepatic arteries and evaluating their prevalence, and we propose a three-dimensional hypothetical anatomic model. On the basis of these criteria, we categorized RHAs arising from the proximal to middle CHA or from the GDA as aberrant hepatic arteries.
The aberrant hepatic arteries, especially aRHAs, can be of clinical importance when performing hepatobiliary surgeries. In liver transplantation, appropriate arterial supply to the graft is essential for its survival (33,34). Sophisticated back-table reconstruction may be required in patients with an aRHA arising from the SMA or other origins that show a retroportal course (34–36). Pérez-Saborido et al (36) reported that aRHAs from the SMAs of donors requiring back-table reconstruction were associated with longer cold ischemia time and worse overall survival. In addition, because bile ducts are supplied via hepatic arteries only, arterial compromise can cause bile duct complications such as biliary stricture (15,34). Hepatic arteries with an aberrant course can be more vulnerable to ischemic damage caused by excessive dissection during surgery (15). In the context of pancreaticoduodenectomy, iatrogenic injury of aRHAs can lead to bilioenteric anastomosis ischemia (37,38). During laparoscopic cholecystectomy, an aRHA can easily be confused with a cystic artery and can accidentally be injured during dissection if not identified correctly (39). Information on the anatomic courses of aberrant hepatic arteries in relation to the adjacent structures can help prevent inadvertent iatrogenic injury to the hepatic arteries.
In the present study, a significant association was found between the presence of aRHAs and the presence of aLHAs, and patients with an aRHA or an aLHA were approximately twice as likely to have a contralateral aberrant hepatic artery as those without. Therefore, when evaluating the anatomy of the hepatic artery in patients with an aberrant hepatic artery, the presence of an additional aberrant hepatic artery should be meticulously evaluated. Conversely, no association was found between hepatic arterial and celiac axis variations in this study, although a previous study reported a higher prevalence of celiac axis variation in patients with hepatic arterial variations than in those without (26.5% [nine of 34] vs 5.3% [four of 76]; P < .001) (40).
The aberrant hepatic arteries in this study included both replaced and accessory hepatic arteries, as defined in Michels’ study (14). Our method of analysis is in line with that suggested by Hiatt, a simpler modification of Michels classification that does not make a distinction between the accessory arteries and replaced arteries (13).
When reclassified according to the Hiatt system for comparability, the results of the present study were similar to those of previous studies with large study populations (Table E1 [supplement]). According to the Hiatt classification system, the results of our study were type 1 findings (normal) in 72.59% of patients (4083 of 5625), type 2 findings (aLHA from LGA) in 11.70% (658 of 5625), type 3 findings (aRHA from SMA) in 6.95% (391 of 5625), and type 4 findings (aLHA from LGA and aRHA from SMA) in 3.15% (177 of 5625). Anatomic variations not classified into Hiatt types 1–4 were categorized as “other” (Table E1 [supplement]). Because of the different criteria used for classification, it was difficult to compare variations included in this category among studies.
As a study with a large study population, the present study included many rare anatomic variations that were not categorized by the Michels and Hiatt classification systems. The prevalence rates of aRHAs and aLHAs originating from the celiac axis, reported in the literature as 0.33%–3.33% and 0.21%–2.00%, respectively, were observed to be 1.87% (105 of 5625) and 0.09% (five of 5625) in the present study, respectively (2,5,25,41). Although there have been previous studies reporting the prevalence rates of aRHAs and aLHAs directly arising from the aorta as being 0.08%–1.43% and 1.43%–1.67%, respectively, the prevalence rates in our study were 0.11% (six of 5625) and 0.02% (one of 5625), respectively (2,8,10,24,31,42). We found two aRHAs arising from the splenic artery, one of which was found in a patient with celiac axis variation (hepatomesenteric trunk and gastrosplenic trunk). This variation is known to be extremely rare, and only three cases have been reported in the literature (26,29,30). An aLHA arising from the SMA is another extremely rare anatomic variant identified in the present study, and we found one case in the literature (31). Finally, to the best of our knowledge, there have been no previous studies reporting aRHAs originating from the LGA.
Our study may have limitations when compared with cadaveric studies in terms of describing narrow-caliber aberrant hepatic arteries. To minimize this limitation, we included patients who underwent both CT and DSA and analyzed the resulting images comprehensively. Although DSA may be superior in depicting small-sized arteries compared with CT, CT demonstrates strength in the evaluation of the vascular territory of the artery, as well as depicting its topographic relation to the adjacent structures (1,2,4,8,10). Therefore, CT and DSA can play complementary roles in the evaluation of hepatic arterial anatomy.
In conclusion, on the basis of the review of CT and DSA images of 5625 patients, we provide comprehensive lists of hepatic arterial variations and propose a three-dimensional hypothetical anatomic model to summarize the observed hepatic arterial variations.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: T.W.C. disclosed no relevant relationships. J.W. Chung disclosed no relevant relationships. H.C.K. disclosed no relevant relationships. M.L. disclosed no relevant relationships. J.W. Choi disclosed no relevant relationships. H.J.J. disclosed no relevant relationships. S.H. disclosed no relevant relationships.
Abbreviations:
- aLHA
- aberrant LHA
- aRHA
- aberrant RHA
- CHA
- common hepatic artery
- DSA
- digital subtraction angiography
- GDA
- gastroduodenal artery
- LGA
- left gastric artery
- LHA
- left hepatic artery
- RHA
- right hepatic artery
- SMA
- superior mesenteric artery
References
- 1. Saba L , Mallarini G . Anatomic variations of arterial liver vascularization: an analysis by using MDCTA . Surg Radiol Anat 2011. ; 33 ( 7 ): 559 – 568 . [DOI] [PubMed] [Google Scholar]
- 2. Koops A , Wojciechowski B , Broering DC , Adam G , Krupski-Berdien G . Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies . Surg Radiol Anat 2004. ; 26 ( 3 ): 239 – 244 . [DOI] [PubMed] [Google Scholar]
- 3. Thangarajah A , Parthasarathy R . Celiac axis, common hepatic and hepatic artery variants as evidenced on MDCT angiography in South Indian population . J Clin Diagn Res 2016. ; 10 ( 1 ): TC01 – TC05 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gümüs H , Bükte Y , Özdemir E , et al . Variations of the celiac trunk and hepatic arteries: a study with 64-detector computed tomographic angiography . Eur Rev Med Pharmacol Sci 2013. ; 17 ( 12 ): 1636 – 1641 . [PubMed] [Google Scholar]
- 5. Abdullah SS , Mabrut JY , Garbit V , et al . Anatomical variations of the hepatic artery: study of 932 cases in liver transplantation . Surg Radiol Anat 2006. ; 28 ( 5 ): 468 – 473 . [DOI] [PubMed] [Google Scholar]
- 6. Fonseca-Neto OCLD , Lima HCS , Rabelo P , Melo PSV , Amorim AG , Lacerda CM . Anatomic variations of hepatic artery: a study in 479 liver transplantations . Arq Bras Cir Dig 2017. ; 30 ( 1 ): 35 – 37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Covey AM , Brody LA , Maluccio MA , Getrajdman GI , Brown KT . Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients . Radiology 2002. ; 224 ( 2 ): 542 – 547 . [DOI] [PubMed] [Google Scholar]
- 8. Ugurel MS , Battal B , Bozlar U , et al . Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography . Br J Radiol 2010. ; 83 ( 992 ): 661 – 667 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Cecco CN , Ferrari R , Rengo M , Paolantonio P , Vecchietti F , Laghi A . Anatomic variations of the hepatic arteries in 250 patients studied with 64-row CT angiography . Eur Radiol 2009. ; 19 ( 11 ): 2765 – 2770 . [DOI] [PubMed] [Google Scholar]
- 10. Löschner C , Nagel SN , Kausche S , Teichgräber U . Hepatic arterial supply in 1297 CT-angiographies . Rofo 2015. ; 187 ( 4 ): 276 – 282 . [DOI] [PubMed] [Google Scholar]
- 11. López-Andújar R , Moya A , Montalvá E , et al . Lessons learned from anatomic variants of the hepatic artery in 1,081 transplanted livers . Liver Transpl 2007. ; 13 ( 10 ): 1401 – 1404 . [DOI] [PubMed] [Google Scholar]
- 12. Chen H , Yano R , Emura S , Shoumura S . Anatomic variation of the celiac trunk with special reference to hepatic artery patterns . Ann Anat 2009. ; 191 ( 4 ): 399 – 407 . [DOI] [PubMed] [Google Scholar]
- 13. Hiatt JR , Gabbay J , Busuttil RW . Surgical anatomy of the hepatic arteries in 1000 cases . Ann Surg 1994. ; 220 ( 1 ): 50 – 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michels NA . Newer anatomy of the liver and its variant blood supply and collateral circulation . Am J Surg 1966. ; 112 ( 3 ): 337 – 347 . [DOI] [PubMed] [Google Scholar]
- 15. Sahani D , Mehta A , Blake M , Prasad S , Harris G , Saini S . Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery . RadioGraphics 2004. ; 24 ( 5 ): 1367 – 1380 . [DOI] [PubMed] [Google Scholar]
- 16. Noussios G , Dimitriou I , Chatzis I , Katsourakis A . The main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature . J Clin Med Res 2017. ; 9 ( 4 ): 248 – 252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cirocchi R , D'Andrea V , Amato B , et al . Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance . Surgeon 2020. ; 18 ( 2 ): 100 – 112 . [DOI] [PubMed] [Google Scholar]
- 18. Favelier S , Germain T , Genson PY , et al . Anatomy of liver arteries for interventional radiology . Diagn Interv Imaging 2015. ; 96 ( 6 ): 537 – 546 . [DOI] [PubMed] [Google Scholar]
- 19. Mugunthan N , Kannan R , Jebakani CF , Anbalagan J . Variations in the origin and course of right hepatic artery and its surgical significance . J Clin Diagn Res 2016. ; 10 ( 9 ): AC01 – AC04 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Honma S , Matsuda W , Kudo M . Right hepatic artery traveling anteriorly to the common bile duct . Anat Sci Int 2013. ; 88 ( 2 ): 93 – 96 . [DOI] [PubMed] [Google Scholar]
- 21. Dandekar U , Dandekar K , Chavan S . Right hepatic artery: a cadaver investigation and its clinical significance . Anat Res Int 2015. ; 2015 412595 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katagiri H , Sakamoto T , Okumura K , Lefor AK , Kubota T . Aberrant right hepatic artery arising from the celiac trunk: a potential pitfall during laparoscopic cholecystectomy . Asian J Endosc Surg 2016. ; 9 ( 1 ): 72 – 74 . [DOI] [PubMed] [Google Scholar]
- 23. Asif M , Anewenah LS , Reddy N , Khan FA . Replaced right and left hepatic arteries: a variation in origin and course . BMJ Case Rep 2017. ; 2017 bcr2016218345 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hardy KJ , Jones RM . Hepatic artery anatomy in relation to reconstruction in liver transplantation: some unusual variations . Aust N Z J Surg 1994. ; 64 ( 6 ): 437 – 440 . [DOI] [PubMed] [Google Scholar]
- 25. Garg S , Sahni D , Kumar H , Yadav TD , Aggarwal A , Gupta T . The segmental branching of the hepatic arteries in the liver: a cadaveric study . Anat Sci Int 2019. ; 94 ( 2 ): 216 – 223 . [DOI] [PubMed] [Google Scholar]
- 26. De Blasi V , Makkai-Popa ST , Arru L , Pessaux P , Azagra JS . Rare anatomic variation of the hepatic arterial blood supply: case report and literature review . Surg Radiol Anat 2019. ; 41 ( 3 ): 343 – 345 . [DOI] [PubMed] [Google Scholar]
- 27. Yamashita K , Hashimoto D , Itoyama R , et al . Accessory right hepatic artery branched from gastroduodenal artery . Surg Case Rep 2015. ; 1 ( 1 ): 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polguj M , Gabryniak T , Topol M . The right accessory hepatic artery; a case report and review of the literature . Surg Radiol Anat 2010. ; 32 ( 2 ): 175 – 179 . [DOI] [PubMed] [Google Scholar]
- 29. Al Zahrani Y , AlMat'hami A , Alobaidi H , Wiseman D , Mujoomdar A . Accessory right hepatic artery arising from splenic artery supplying hepatocellular carcinoma identified by computed tomography scan and conventional angiography: a rare anatomic variant . Ann Vasc Surg 2017. ; 38 : 316 . e1 – 316 . e5 . [DOI] [PubMed] [Google Scholar]
- 30. Caruso F , Dondossola D , Fornoni G , Caccamo L , Rossi G . Right hepatic artery from splenic artery: the four-leaf clover of hepatic surgery . Surg Radiol Anat 2016. ; 38 ( 7 ): 867 – 871 . [DOI] [PubMed] [Google Scholar]
- 31. Chaib E , Ribeiro MA Jr , Saad WA , Gama-Rodrigues J . The main hepatic anatomic variations for the purpose of split-liver transplantation . Transplant Proc 2005. ; 37 ( 2 ): 1063 – 1066 . [DOI] [PubMed] [Google Scholar]
- 32. Song SY , Chung JW , Yin YH , et al . Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA . Radiology 2010. ; 255 ( 1 ): 278 – 288 . [DOI] [PubMed] [Google Scholar]
- 33. Ahn CS , Lee SG , Hwang S , et al . Anatomic variation of the right hepatic artery and its reconstruction for living donor liver transplantation using right lobe graft . Transplant Proc 2005. ; 37 ( 2 ): 1067 – 1069 . [DOI] [PubMed] [Google Scholar]
- 34. Liang Y , Ye S , Shi X , et al . Experiences of microsurgical reconstruction for variant hepatic artery in living donor liver transplantation . Cell Biochem Biophys 2013. ; 65 ( 2 ): 257 – 262 . [DOI] [PubMed] [Google Scholar]
- 35. Vasconcelos-Filho JM , Magalhães PRM , Monteiro BR , et al . Frequency of anatomic variations on hepatic arteries and types of reconstruction employed: study on livers prepared for transplantation . Transplant Proc 2020. ; 52 ( 5 ): 1312 – 1313 . [DOI] [PubMed] [Google Scholar]
- 36. Pérez-Saborido B , Pacheco-Sánchez D , Barrera Rebollo A , et al . Incidence of hepatic artery variations in liver transplantation: does it really influence short- and long-term results? . Transplant Proc 2012. ; 44 ( 9 ): 2606 – 2608 . [DOI] [PubMed] [Google Scholar]
- 37. Lee JM , Lee YJ , Kim CW , Moon KM , Kim MW . Clinical implications of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy . World J Surg 2009. ; 33 ( 8 ): 1727 – 1732 . [DOI] [PubMed] [Google Scholar]
- 38. Rubio-Manzanares-Dorado M , Marín-Gómez LM , Aparicio-Sánchez D , et al . Implication of the presence of a variant hepatic artery during the Whipple procedure . Rev Esp Enferm Dig 2015. ; 107 ( 7 ): 417 – 422 . [DOI] [PubMed] [Google Scholar]
- 39. Gupta V , Jain G . Safe laparoscopic cholecystectomy: adoption of universal culture of safety in cholecystectomy . World J Gastrointest Surg 2019. ; 11 ( 2 ): 62 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arifuzzaman M , Nasim Naqvi SS , Adel H , Adil SO , Rasool M , Hussain M . Anatomical variants of celiac trunk, hepatic and renal arteries in a population of developing country using multidetector computed tomography angiography . J Ayub Med Coll Abbottabad 2017. ; 29 ( 3 ): 450 – 454 . [PubMed] [Google Scholar]
- 41. Araujo Neto SA , Franca HA , de Mello Júnior CF , et al . Anatomical variations of the celiac trunk and hepatic arterial system: an analysis using multidetector computed tomography angiography . Radiol Bras 2015. ; 48 ( 6 ): 358 – 362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaki SM , Abdelmaksoud AHK , Khaled BEA , Abdel Kader IA . Anatomical variations of hepatic artery using the multidetector computed tomography angiography . Folia Morphol (Warsz) 2020. ; 79 ( 2 ): 247 – 254 . [DOI] [PubMed] [Google Scholar]