Abstract
BACKGROUND
The incidence of sudden cardiac death and sudden death caused by arrhythmia, as determined by autopsy, in persons with human immunodeficiency virus (HIV) infection has not been clearly established.
METHODS
Between February 1, 2011, and September 16, 2016, we prospectively identified all new deaths due to out-of-hospital cardiac arrest among persons 18 to 90 years of age, with or without known HIV infection, for comprehensive autopsy and toxicologic and histologic testing. We compared the rates of sudden cardiac death and sudden death caused by arrhythmia between groups.
RESULTS
Of 109 deaths from out-of-hospital cardiac arrest among 610 unexpected deaths in HIV-positive persons, 48 met World Health Organization criteria for presumed sudden cardiac death; of those, fewer than half (22) had an arrhythmic cause. A total of 505 presumed sudden cardiac deaths occurred between February 1, 2011, and March 1, 2014, in persons without known HIV infection. Observed incidence rates of presumed sudden cardiac death were 53.3 deaths per 100,000 person-years among persons with known HIV infection and 23.7 deaths per 100,000 person-years among persons without known HIV infection (incidence rate ratio, 2.25; 95% confidence interval [CI], 1.37 to 3.70). Observed incidence rates of sudden death caused by arrhythmia were 25.0 and 13.3 deaths per 100,000 person-years, respectively (incidence rate ratio, 1.87; 95% CI, 0.93 to 3.78). Among all presumed sudden cardiac deaths, death due to occult drug overdose was more common in persons with known HIV infection than in persons without known HIV infection (34% vs. 13%). Persons who were HIV-positive had higher histologic levels of interstitial myocardial fibrosis than persons without known HIV infection.
CONCLUSIONS
In this postmortem study, the rates of presumed sudden cardiac death and myocardial fibrosis were higher among HIV-positive persons than among those without known HIV infection. One third of apparent sudden cardiac deaths in HIV-positive persons were due to occult drug overdose. (Supported by the National Heart, Lung, and Blood Institute.)
Human immunodeficiency virus (HIV) infection is associated with increased risk of acute myocardial infarction,1 heart failure,2 and ischemic stroke.3 In 2012, we reported that in San Francisco County, California, the rate of sudden cardiac death, as defined by the World Health Organization (WHO), was 4.5 times as high among HIV-positive persons as among persons without known HIV infection,4 but postmortem data were not available.
We recently reported the results of the Postmortem Systematic Investigation of Sudden Cardiac Death (POST SCD) study, a prospective, countywide postmortem study performed with the use of medical examiner data to ascertain the precise incidence and underlying cause of all deaths due to out-of-hospital cardiac arrest and deaths presumed to be sudden cardiac deaths (as defined by the WHO) in San Francisco County. We found that just over half (55.8%) were sudden deaths from arrhythmia, as determined by autopsy.5 In the current study (HIV POST SCD), we sought to determine the underlying causes of all presumed sudden cardiac deaths and the precise incidence of sudden death caused by arrhythmia in HIV-positive persons and to compare these data with those in the countywide population without known HIV infection. Using postmortem myocardial tissue specimens, we also evaluated the extent of fibrosis to characterize the underlying histologic substrate for sudden death caused by arrhythmia among HIV-positive persons.
METHODS
INCLUSION CRITERIA AND SELECTION OF CASES FOR POSTMORTEM INVESTIGATION
All deaths due to out-of-hospital cardiac arrest occurring in San Francisco County between February 1, 2011, and September 21, 2016, in HIV-positive persons 18 to 90 years of age were included.6 The study was approved by the University of California, San Francisco, institutional review board, with additional approval by institutional review boards at all 10 hospitals (excluding children’s hospitals) and 3 emergency medical services (EMS) agencies in San Francisco County to obtain medical records. Informed consent was obtained from family members for postmortem examinations.
We defined deaths due to out-of-hospital cardiac arrest as deaths for which the EMS primary impression (the assessment by emergency responders of the cause) was cardiac arrest,7 including deaths occurring in the field or hospital emergency department if the event was witnessed or resuscitation was performed and unwitnessed deaths without resuscitation if the person was last seen alive and symptom-free within 24 hours before death.8 Persons who were resuscitated and survived to hospital admission were considered to be survivors of cardiac arrest and were excluded from our study.9 HIV status was ascertained by means of countywide medical record review, with a single positive serologic test for HIV antibodies required to establish the diagnosis.
We excluded from our study deaths of persons with severe noncardiac chronic or terminal illness (e.g., metastatic cancer); patients receiving dialysis; hospice residents; persons with identifiable noncardiac causes of death at presentation, including evidence of drug use noted at the scene of death, life-threatening trauma, homicide, or suicide; and persons who had been hospitalized within 30 days before cardiac arrest for a noncardiac illness or surgical procedure. Details of inclusion and exclusion criteria are provided in the protocol, available with the full text of this article at NEJM.org.
POSTMORTEM INVESTIGATION AND ADJUDICATION OF CAUSES OF DEATH
For all deaths due to out-of-hospital cardiac arrest as defined above, we reviewed all premortem medical records, investigator reports (including scene investigations and witness and family interviews), EMS records, prescriptions, and postmortem findings. In addition, a complete external and internal postmortem examination was performed for all deaths due to out-of-hospital cardiac arrest, excluding those for which the family did not provide consent. All postmortem examinations were performed by a single board-certified forensic pathologist (the second author), who examined all cavities, organs, and tissues, and included detailed heart and brain examinations and vitreous chemical analyses.10 (Details of autopsy procedures are provided in the Supplementary Appendix, available at NEJM.org.) Toxicology screening was performed for all persons 75 years of age or younger and for persons older than 75 years of age for whom there was no obvious cause of death (e.g., acute myocardial infarction, intracranial hemorrhage, or pulmonary embolus) on gross examination at autopsy. For patients with permanent pacemakers or implantable cardioverter–defibrillators (ICDs), device interrogation was performed.11
The primary objective of the postmortem investigation was to adjudicate a single final cause of death for each case. In addition, we sought to identify deaths that met WHO criteria for presumed sudden cardiac death (sudden unexpected death within 1 hour after symptom onset if the death was witnessed, or within 24 hours after the person was observed to be alive and symptom-free if the death was unwitnessed).8 We also sought to identify persons who died suddenly from arrhythmia, as determined by autopsy (sometimes termed “autopsy-defined sudden arrhythmic death”), who might have been able to be rescued with an ICD. These included persons who had no obvious nonarrhythmic cause of death such as acute cerebrovascular accident, viscus perforation, tamponade, pulmonary embolism, occult drug overdose or lethal levels of substances (as shown on a toxicology screening test), or acute heart failure with pulmonary edema. We followed standard forensic toxicology protocol in determining occult overdose as a cause of death, requiring findings of lethal serum levels of a drug in the absence of other pathological findings that could explain sudden death.12,13
To determine total mortality in the county and identify potential missing cases, we retrieved and reviewed all county death certificates quarterly for location, cause of death stated on the death certificate, and circumstances of death and cross-checked death certificate data with cases obtained through medical examiner surveillance.
HISTOLOGIC ANALYSES FOR MYOCARDIAL FIBROSIS
Samples of myocardium for histologic screening were obtained from five standard locations: the interventricular septum, posterobasal wall, lateral wall, mid-anterior left ventricular free wall, and right ventricular free wall. An extra section of the high septum was also obtained for histologic examination of the conduction system. Histologic sections were stained with hematoxylin and eosin and with Masson’s trichrome and independently examined by two pathologists (the second and fifth authors). Fibrosis scores were quantified by means of digital image analysis.
STATISTICAL ANALYSIS
We used U.S. Census data to estimate person-years at risk, both overall and by age, sex, and race or ethnic group. Specifically, for person-years at risk in the reference population of persons without evidence of HIV infection, we summed the census population numbers for 2011, 2012, and 2013 for San Francisco residents 18 to 90 years of age, subtracted one twelfth the numbers for 2011 and added one sixth the numbers for 2014 to account for the period of the study from February 1, 2011, to March 1, 2014, and subtracted the estimated person-years at risk among HIV-positive persons in this period. Person-years at risk among HIV-positive residents of the county were estimated as the product of the average HIV-positive population size, obtained from the San Francisco HIV Epidemiology Annual Report,14 and the length of follow-up (in months) from February 1, 2011, to September 21, 2016.
We estimated incidence rates for presumed sudden cardiac death and sudden death from arrhythmia by calculating the ratio of the numbers of events to the numbers of person-years at risk. We compared incidence rates according to HIV status, both overall and within subgroups according to age, sex, and race or ethnicity, using unadjusted Poisson regression models with robust standard errors, with numbers of deaths as the outcome and the log of person-years at risk as an offset. Analyses were performed with the use of Stata software, version 15.1 (Stata-Corp). Summary statistics are reported as point estimates and 95% confidence intervals without P values. The widths of the confidence intervals have not been adjusted for multiplicity; thus, inferences drawn from these intervals may not be reproducible.
We estimated differences in fibrosis scores by HIV status using linear models for log-transformed scores, adjusting for age, sex, and presence or absence of congestive heart failure, coronary artery disease, and cardiomyopathy. Coefficient estimates were back-transformed to obtain between-group percentage differences. Calculations of person-years at risk, incidence rates, and linear models are provided in the Supplementary Appendix.
RESULTS
STUDY POPULATION AND PRESUMED SUDDEN CARDIAC DEATHS
Between February 1, 2011, and September 21, 2016, a total of 1379 deaths among HIV-positive persons 18 to 90 years of age occurred in San Francisco County. Of these deaths, 610 were unexpected deaths reported to the medical examiner. After reviews of EMS records and scene investigations, 109 of the 610 unexpected deaths were determined to be due to out-of hospital cardiac arrest and were referred for protocol postmortem investigation (Fig. 1). The family of one person declined to consent to an autopsy; therefore, 108 persons (99%) underwent autopsy.
Figure 1. Identification of HIV-Positive Persons with Presumed Sudden Cardiac Death.
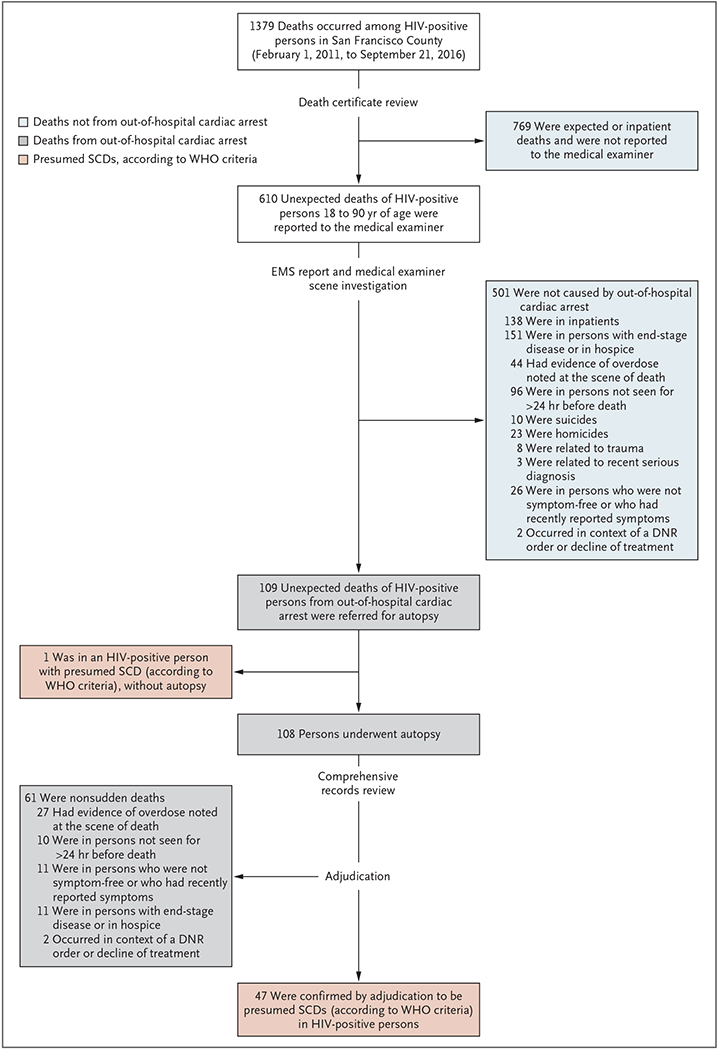
Cases of presumed sudden cardiac death (SCD) among persons with known human immunodeficiency virus (HIV) infection were identified through active medical examiner surveillance of all out-of-hospital cardiac arrests among all out-of-hospital deaths in San Francisco County from February 1, 2011, through September 21, 2016. Out-of-hospital cardiac arrest was defined as an event for which the primary impression of emergency medical services (EMS) responders was cardiac arrest. Presumed sudden cardiac death was defined according to World Health Organization (WHO) criteria. Persons who were not symptom-free when last seen alive or who had recently reported symptoms were not considered to meet criteria for presumed sudden cardiac death. DNR denotes do not resuscitate.
Adjudication of EMS reports and other medical records showed that 61 of the 109 deaths did not meet WHO criteria for presumed sudden cardiac death (i.e., they were not sudden or unexpected). Thus, we identified 48 presumed sudden cardiac deaths among HIV-positive persons (47 who underwent autopsy and 1 for whom autopsy was declined) over 68 months, for a countywide incidence of 54.5 per 100,000 person-years, accounting for 3.5% of the total mortality among HIV-positive persons (48 of 1379 deaths). Of the 48 presumed sudden cardiac deaths, 12 (25%) were witnessed.
The mean age of the 47 HIV-positive persons with presumed sudden cardiac death who underwent autopsy was 54.6 years; 94% were men and 77% were White (Table 1). The persons who were HIV-positive were younger and more likely to be male and White than the 505 persons in the reference group with presumed sudden cardiac death but without known HIV infection (as reported in the POST SCD study).5
Table 1.
Demographic Characteristics of the Study and Reference Populations.*
| Characteristic | Sudden Cardiac Deaths, HIV-Positive Persons† (N = 47) |
Nonsudden Deaths from Out-of-Hospital Cardiac Arrest, HIV-Positive Persons† (N = 61) |
Sudden Cardiac Deaths, Reference Group‡ (N = 505) |
Adult Population of San Francisco County, 2014§ (N = 717,884) |
Persons with HIV in San Francisco County, 2014¶ (N = 15,979) |
|---|---|---|---|---|---|
| Age — yr | |||||
| Mean | 54.57±10.27 | 50.81±11.62 | 63.04±14.48 | 45.43±17.76 | 51.16±11.34 |
| Range | 23–75 | 21–77 | 18–92 | 18–90 | 18–80 |
| Male sex — no. (%) | 44 (94) | 49 (80) | 344 (68) | 364,799 (51) | 14,722 (92) |
| Race or ethnic group — no. (%)‖ | |||||
| Asian | 0 | 2 (3) | 110 (22) | 236,902 (33) | 904 (6) |
| Black | 9 (19) | 19 (31) | 75 (15) | 43,073 (6) | 2014 (13) |
| Hispanic | 2 (4) | 6 (10) | 38 (8) | 107,682 (15) | 2894 (18) |
| White | 36 (77) | 32 (52) | 267 (53) | 294,332 (41) | 9708 (61) |
| Other | 0 | 1 (2) | 15 (3) | 35,894 (5) | 459 (3) |
| Unknown | — | 1 (2) | — | — | — |
Plus–minus values are means ±SD.
Included are deaths among persons with known human immunodeficiency virus (HIV) infection that occurred from February 1, 2011, to September 21, 2016.
Included are sudden cardiac deaths among persons without known HIV infection that occurred from February 1, 2011, to March 1, 2014. In 28 cases, HIV status was presumed to be negative owing to missing medical records; however, the age, sex, and race or ethnic group of the 28 persons were known and are reported. The categories of data that are missing for the 28 persons are shown in Table S1.
Data are from American Community Survey.15
Data are from the San Francisco Department of Public Health Population Health Division.16
Race or ethnic group was determined by review of medical records.
CAUSES OF SUDDEN DEATH, AS DETERMINED BY AUTOPSY
Among the 47 HIV-positive persons with presumed sudden cardiac death who had postmortem data, sudden deaths from arrhythmia, as determined by autopsy, accounted for 47% (Table 2, and Fig. S1 in the Supplementary Appendix), noncardiac causes accounted for 51%, and one death (2%) was due to a cardiac nonarrhythmic cause (acute myocardial infarction with wall rupture). The most common overall cause of presumed sudden cardiac death among HIV-positive persons was occult drug overdose, which accounted for one third of cases (16 of 48 deaths); for all 16 of these deaths, the primary EMS impression was cardiac arrest without evidence or suspicion of drug use at the scene. Lethal levels of methamphetamines, alcohol, and opiates were the most common causes. Other leading causes were coronary artery disease (23%), cardiomyopathy (11%), hypertrophy (6%), and renal failure (6%).
Table 2.
Causes of Presumed Sudden Cardiac Deaths, as Determined by Autopsy.*
| Cause | HIV-Positive (N = 47) |
Reference (N = 505) |
|---|---|---|
| number (percent) | ||
| Cardiac, arrhythmic cause | 22 (47) | 284 (56) |
| Acute coronary artery disease | 2 (4) | 51 (10) |
| Chronic coronary artery disease | 9 (19) | 111 (22) |
| Cardiomyopathy | 5 (11) | 50 (10) |
| Hypertrophy | 3 (6) | 43 (9) |
| Primary electrical disease | 2 (4) | 7 (1) |
| Mitral valve prolapse | 1 (2) | 2 (0.4) |
| Other | 0 | 20 (4) |
| Cardiac, nonarrhythmic cause | 1 (2) | 22 (5) |
| Acute MI with wall rupture | 1 (2) | 12 (2) |
| Acute MI with pump failure | 0 | 4 (1) |
| Chronic heart failure | 0 | 5 (1) |
| Pericarditis with bacterial pericardial effusion and tamponade† | 0 | 1 (0.2) |
| Noncardiac | 24 (51) | 199 (39) |
| Aortic dissection | 0 | 14 (3) |
| Aspiration or asphyxia | 0 | 5 (1) |
| Occult overdose | 16 (34) | 64 (13) |
| Gastrointestinal cause | 1 (2) | 14 (3) |
| Hypoglycemia, hyperglycemia, or DKA | 1 (2) | 9 (2) |
| Infection | 2 (4) | 21 (4) |
| Intracranial hemorrhage | 1 (2) | 18 (4) |
| Other neurologic | 0 | 11 (2) |
| Pulmonary embolism | 0 | 19 (4) |
| Renal failure | 3 (6) | 5 (1) |
| Other noncardiac | 0 | 19 (4) |
DKA denotes diabetic ketoacidosis and MI myocardial infarction.
This event occurred in a 66-year-old White female smoker who was found unresponsive 10 hours after she was last seen (at that time without symptoms). The autopsy revealed pericarditis with bacterial pericardial effusion and tamponade, severe pulmonary edema, pleural adhesions and effusions, and acute inflammation of the left ventricular septum.
The proportion of presumed sudden cardiac deaths attributed to arrhythmic causes among HIV-positive persons was similar to that in the reference group without known HIV infection (47% of the 47 presumed sudden cardiac deaths among HIV-positive persons and 56% of the 505 presumed sudden cardiac deaths in the reference group) (Table 2), including similar proportions attributed to coronary artery disease, hypertrophy, and cardiomyopathy. However, occult overdoses were more than twice as common among HIV-positive persons as among persons in the reference group.
INCIDENCE RATES IN PERSONS WITH HIV VS. PERSONS WITHOUT KNOWN HIV INFECTION
The observed incidence rate for presumed sudden cardiac death among HIV-positive persons was 53.3 deaths per 100,000 person-years, as compared with 23.7 deaths per 100,000 person-years in the reference group (incidence rate ratio, 2.25; 95% confidence interval [CI], 1.37 to 3.70). The observed incidence rates for sudden death from arrhythmia, as determined by autopsy, were 25.0 deaths per 100,000 person-years among HIV-positive persons and 13.3 deaths per 100,000 person-years in the reference group (incidence rate ratio, 1.87; 95% CI, 0.93 to 3.78) (Fig. 2). The incidence of presumed sudden cardiac death was higher among HIV-positive women than among women in the reference group (incidence rate ratio, 3.93; 95% CI, 1.07 to 14.44), but it should be noted that all three cases of sudden cardiac death in HIV-positive women were due to noncardiac causes (two occult overdoses and one intracranial hemorrhage). The incidence of presumed sudden cardiac death was higher among HIV-positive Whites than among Whites in the reference group (incidence rate ratio, 2.29; 95% CI, 1.12 to 4.69) but was not found to be increased among Black or Hispanic persons who were HIV-positive.
Figure 2. Incidence Rates for HIV-Positive Persons and the Reference Population.
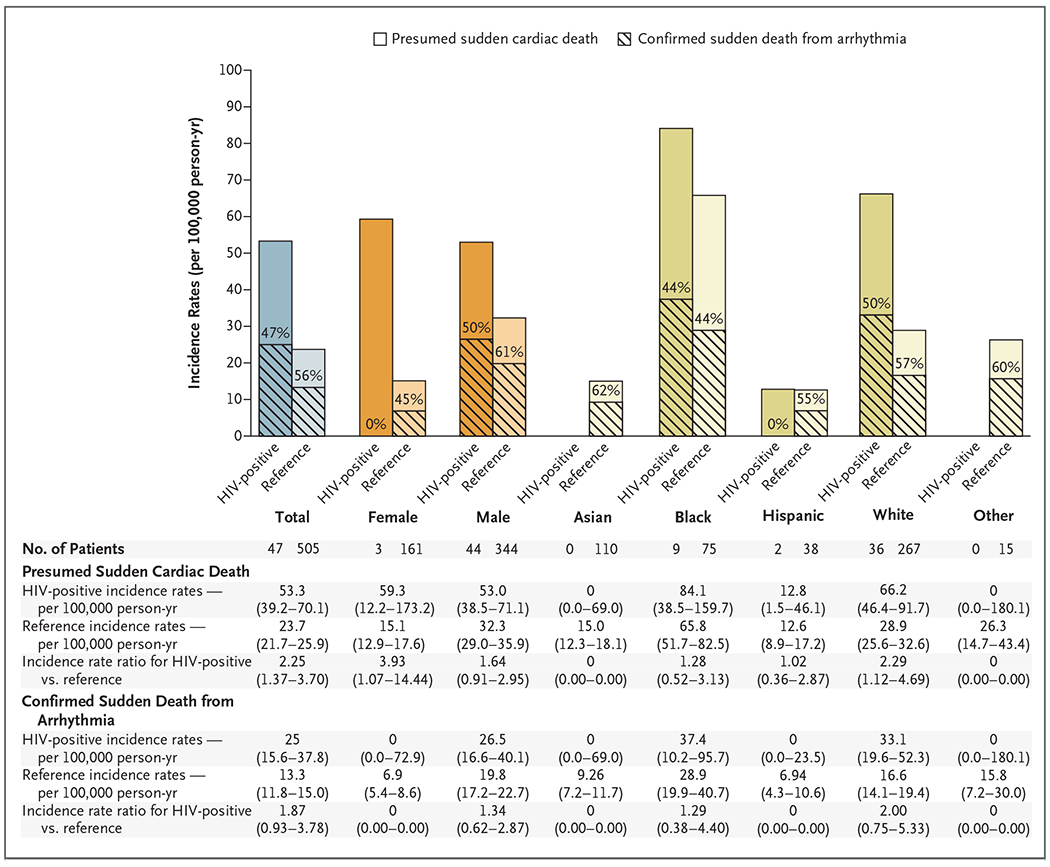
Observed incidence rates per 100,000 person-years are shown for persons with presumed sudden cardiac death and sudden death from arrhythmia, as determined by autopsy, in San Francisco County from February 1, 2011, to September 21, 2016, for HIV-positive persons and from February 1, 2011, to March 1, 2014, for persons in the reference population. The percentages within the bars are the percentages of persons with presumed sudden cardiac death whose deaths were from arrhythmia. Total, sex-specific, and race or ethnic group–specific incidence rate ratios for presumed sudden cardiac death and sudden death from arrhythmia are shown. Confidence intervals for incidence rates and incident rate ratios are 95% confidence intervals, except those for HIV-positive incidence rates for presumed sudden cardiac deaths among Asian and Other populations and confirmed sudden death from arrhythmia among Female, Asian, Hispanic, and Other population groups, which are one-sided 97.5% confidence intervals.
EFFECT OF MEDICAL HISTORY
The mean CD4 count in HIV-positive persons with presumed sudden cardiac death was 475 cells per cubic millimeter, and 79% of those persons were receiving antiretroviral therapy (Table S1). Among persons with presumed sudden cardiac death, those who were HIV-positive were more likely than persons in the reference group to have a psychiatric diagnosis (57% vs. 27%) and were also more likely to have a history of alcohol or drug use. The prevalence of previous myocardial infarction, hypertension, diabetes, heart failure, dyslipidemia, and chronic renal insufficiency was similar in the two groups. Additional details regarding all 47 HIV-positive cases are provided in Table S2.
COMPARISON OF CARDIAC PATHOLOGICAL AND HISTOLOGIC FEATURES
Measurements of the left ventricle in persons with presumed sudden cardiac death were similar in the HIV-positive group and the reference group. Persons in the reference group were more likely to have evidence of myocardial infarction (Table S3).
Tissue samples for quantitative histologic analysis were obtained from 24 HIV-positive persons and 164 persons in the reference group. The total burden of myocardial fibrosis (expressed as mean percent fibrosis) was higher in HIV-positive persons (Fig. 3). This difference in myocardial fibrosis burden in HIV-positive persons as compared with the reference group was found in cases of presumed sudden cardiac death (mean percent fibrosis, 12.5% vs. 8.7%), sudden death from arrhythmia (13.8% vs. 9.7%), and death from nonarrhythmic causes (11.3% vs. 7.0%). The burden of interstitial fibrosis, but not replacement fibrosis, was higher among HIV-positive persons.
Figure 3. Quantitative Histologic Analysis of Cardiac Fibrosis.
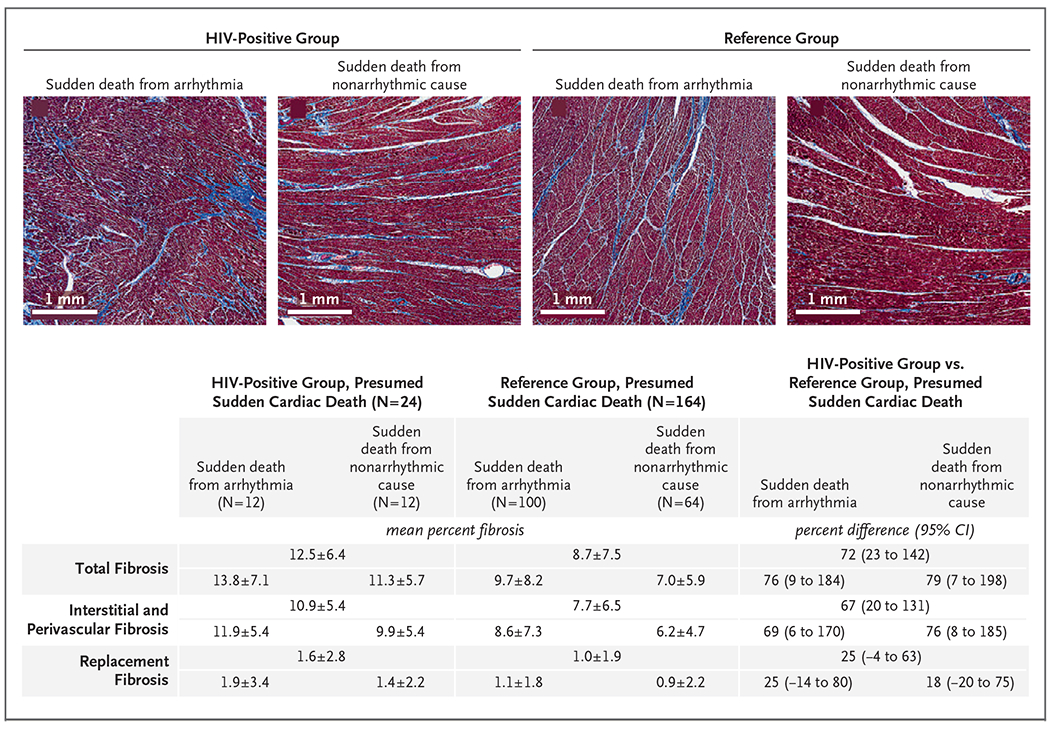
Shown are trichrome-stained sections of the left ventricle in persons with HIV infection and persons in the reference group (persons without known HIV infection) with sudden death from arrhythmia and sudden death from nonarrhythmic causes. Cases analyzed are those for which consent was obtained from the family of the deceased person. Cases with histologic data as compared with all cases of presumed sudden cardiac death (with respect to demographics, premortem conditions, and final causes of death) are shown in Table S4. For each set of fibrosis data, overall mean (±SD) and percent difference values are shown according to group (HIV-positive group or reference group) and according to cause of presumed sudden cardiac death (arrhythmic or nonarrhythmic), as determined by autopsy. Total fibrosis data include interstitial, perivascular, and replacement fibrosis from the standardized right ventricular free wall; and septal, anterior, inferior, and lateral left ventricular free-wall areas. Samples stained with Masson’s trichrome were assigned fibrosis scores quantified by means of digital image analysis with the use of the Aperio ImageScope (Leica Biosystems) Positive Pixel Count algorithm calibrated for hue and color saturation thresholds of Masson’s trichrome staining. Scores are reported as a percentage of total slide tissue area from all available sections. Differences in fibrosis scores by HIV status were estimated with the use of linear models for log-transformed scores, with adjustment for age, sex, and presence or absence of congestive heart failure, coronary artery disease, and cardiomyopathy. Coefficient estimates were back-transformed to obtain between-group percentage differences.
DISCUSSION
The HIV POST SCD study was a prospective postmortem evaluation of all incident deaths attributed to out-of-hospital cardiac arrest among HIV-positive persons from February 1, 2011, through September 21, 2016, in San Francisco County. We compared these data with those from the previously reported POST SCD study5 of out-of-hospital cardiac arrests in persons without known HIV infection; autopsy adjudication was used in both studies. In both groups, approximately half the cases of presumed sudden cardiac death were sudden death from arrhythmic causes. However, occult drug overdoses were more than twice as common in the HIV-positive group as in the reference group of persons without known HIV infection. Observed incidence rates of presumed sudden cardiac death were higher among HIV-positive persons. Moreover, among persons with presumed sudden cardiac death and sudden death from arrhythmia, as determined by autopsy, the burden of total and interstitial myocardial fibrosis was higher among HIV-positive persons than among persons in the reference group. We did not find a greater incidence of sudden death from arrhythmia among HIV-positive persons than among persons in the reference group.
In the general population, coronary artery disease has been presumed to be responsible for the majority of sudden cardiac deaths defined by conventional criteria (i.e., without postmortem validation). However, in POST SCD, we reported that as shown by autopsy, coronary artery disease accounted for only one third of all presumed sudden cardiac deaths.5 In the present study, only 23% of presumed sudden cardiac deaths were due to coronary artery disease. Thus, although incidences of acute myocardial infarction and other structural heart disease may be elevated in the context of HIV infection, they do not appear to underlie the higher incidence of sudden deaths that we observed among HIV-positive patients.
In our 2012 study, we reported a mean incidence of sudden cardiac death in HIV-positive persons that was more than 4.5 times as high as that in the general population.4 However, it is likely that some sudden cardiac deaths in that study, and indeed in all studies adjudicating sudden cardiac deaths without autopsy, were actually noncardiac deaths, since approximately half of sudden deaths,5 regardless of HIV status, were found to be noncardiac on postmortem investigation. Thus, our study shows the importance of rigorous phenotyping of sudden cardiac death to establish the underlying mechanism. Indeed, we found that the most common cause of presumed sudden cardiac death in HIV-positive persons was actually drug overdose, which highlights the role of noncardiac causes of sudden death in this context.
We found a higher incidence of interstitial cardiac fibrosis among HIV-positive persons than among persons without known HIV infection. Magnetic resonance imaging of the hearts of persons who are HIV-positive (but asymptomatic) shows a higher incidence of myocardial fibrosis, as assessed by late gadolinium enhancement,17 and decreased cardiac function. Late gadolinium enhancement is also higher in persons with nonischemic dilated cardiomyopathy and in survivors of cardiac arrest and is an independent prognostic factor for all-cause mortality beyond ejection fraction.18 Our finding provides histologic confirmation of these radiologic studies and links myocardial fibrosis to sudden death from arrhythmia in persons with HIV infection.
Chronic inflammation and immune activation are present in patients with HIV infection, including those receiving antiretroviral therapy, and inflammatory and coagulation markers are strongly predictive of death,19,20 of illness unrelated to acquired immunodeficiency syndrome,21 and of cardiovascular events.22 Inflammation and immune activation may underlie the increased cardiac fibrosis we observed in the case of sudden deaths in persons with HIV infection. Although myocardial infarction, hypertension, heart failure, and valvular disease are common causes of cardiac fibrosis,23 the proportions of persons with these conditions were similar in cases of sudden death in the group with known HIV infection and the group without known HIV infection, which suggests that other HIV-specific factors contribute to myocardial fibrosis. Furthermore, in persons who were HIV-positive, we observed a greater incidence of interstitial fibrosis, rather than the replacement fibrosis commonly attributed to coronary artery disease. Fibrosis of other organs, including lymph nodes,24,25 adipose tissue,26 and liver,27–29 has been described in the context of HIV.
Our study has some important limitations. Although this case series includes all deaths that occurred countywide during the study period, all cases come from a single setting with a distinctive population and disease characteristics that may not apply in other settings. The number of sudden cardiac deaths was small enough that our estimates of incidence rates are not highly precise (as indicated by the wide confidence intervals). As a consequence, we were not able to show a higher incidence rate for sudden death from arrhythmia among HIV-positive persons, although the point estimate for the incidence rate ratio (1.87) suggests the possibility of such an effect. In addition, our findings apply to only the 3.5% of all deaths that met the criteria for presumed sudden cardiac death.
In this prospective countywide postmortem investigation of all deaths attributed to out-of hospital cardiac arrest, we found a higher incidence of presumed sudden cardiac death among persons with known HIV infection than among persons without known HIV infection. Interstitial myocardial fibrosis was also more extensive in HIV-positive persons. One third of apparent sudden cardiac deaths among HIV-positive persons were due to occult drug overdose.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute.
We thank the families of all the victims of sudden death in San Francisco County; Nikolas Lemos, Ph.D., for expert review of all toxicologic analyses; Annie Bedigian, Joanne Probert, Shiktij Dave, and Justin Cheng for assistance with preparing figures and tables; and all forensic investigators in the Office of the Chief Medical Examiner and Emergency Medical Services personnel in San Francisco County.
Footnotes
REFERENCES
- 1.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang C-CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort study. JAMA Cardiol 2017;2:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012;60:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012;59:1891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng ZH, Olgin JE, Vittinghoff E, et al. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation 2018;137:2689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim AS, Moffatt E, Ursell PC, Devinsky O, Olgin J, Tseng ZH. Sudden neurologic death masquerading as out-of-hospital sudden cardiac death. Neurology 2016;87:1669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNally B, Robb R, Mehta M, et al. Out-of-hospital cardiac arrest surveillance — Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005–December 31, 2010. MMWR Surveill Summ 2011;60(8):1–19. [PubMed] [Google Scholar]
- 8.Sudden cardiac death: report of a WHO scientific group. World Health Organ Tech Rep Ser Report 1985;726:5–25. [PubMed] [Google Scholar]
- 9.Ricceri S, Salazar JW, Vu AA, Vittinghoff E, Moffatt E, Tseng ZH. Factors predisposing to survival after resuscitation for sudden cardiac arrest. J Am Coll Cardiol 2021;77:2353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinhaus DA, Vittinghoff E, Moffatt E, Hart AP, Ursell P, Tseng ZH. Characteristics of sudden arrhythmic death in a diverse, urban community. Am Heart J 2012;163:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng ZH, Hayward RM, Clark NM, et al. Sudden death in patients with cardiac implantable electronic devices. JAMA Intern Med 2015;175:1342–50. [DOI] [PubMed] [Google Scholar]
- 12.Skopp G Postmortem toxicology. Forensic Sci Med Pathol 2010;6:314–25. [DOI] [PubMed] [Google Scholar]
- 13.Schulz M, Iwersen-Bergmann S, Andresen H, Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit Care 2012;16:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HIV epidemiology annual report. San Francisco: Department of Public Health, 2014. [Google Scholar]
- 15.American Community Survey. Data Access and Dissemination Systems (DADS) Washington, DC: U.S. Census Bureau, 2014. [Google Scholar]
- 16.San Francisco Department of Public Health Population Health Division. HIV epidemiology annual report 2016. (https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/Annual-Report-2016-20170831.pdf).
- 17.Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013;128:814–22. [DOI] [PubMed] [Google Scholar]
- 18.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 19.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So-Armah KA, Tate JP, Chang C-CH, et al. Do biomarkers of inflammation, monocyte activation, and altered coagulation explain excess mortality between HIV infected and uninfected people? J Acquir Immune Defic Syndr 2016;72:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grund B, Baker JV, Deeks SG, et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One 2016;11(5):e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014;3(3):e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schacker TW, Reilly C, Beilman GJ, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS 2005;19:2169–71. [DOI] [PubMed] [Google Scholar]
- 25.Estes JD, Wietgrefe S, Schacker T, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis 2007;195:551–61. [DOI] [PubMed] [Google Scholar]
- 26.Utay NS, Kitch DW, Yeh E, et al. Telmisartan therapy does not improve lymph node or adipose tissue fibrosis more than continued antiretroviral therapy alone. J Infect Dis 2018;217:1770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saracino A, Cozzi-Lepri A, Shanyinde M, et al. HIV-1 co-receptor tropism and liver fibrosis in HIV-infected patients. PLoS One 2018;13(1):e0190302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V III. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis 2010;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kooij KW, Wit FWNM, van Zoest RA, et al. Liver fibrosis in HIV-infected individuals on long-term antiretroviral therapy: associated with immune activation, immunodeficiency and prior use of didanosine. AIDS 2016;30:1771–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


