SUMMARY
Attention can be “covertly” directed without eye movements; yet, even during fixation, there are continuous microsaccades (MSs). In areas V4 and IT of macaques, we found that firing rates and stimulus representations were enhanced by attention but only following a MS toward the attended stimulus. The onset of neural attentional modulations was tightly coupled to the MS onset. The results reveal a major link between the effects of covert attention on cortical visual processing and the overt movement of the eyes.
In Brief
Attention can be “covertly” directed without eye movements; yet, even during fixation, there are microsaccades. We found that neural activity was enhanced by attention, but only following a microsaccade toward the stimulus, revealing a major link between covert attention and the oculomotor system.
INTRODUCTION
In primates, saccadic eye movements are critical for the visual exploration of the environment (Kagan and Hafed, 2013; Martinez-Conde et al., 2013; Otero-Millan et al., 2008). However, even during fixation, gaze position is not stable: the eyes continuously exhibit small saccadic movements. A microsaccade (MS) is a type of involuntary, binocular, and conjugate eye movement (typically <1°). MSs share largely the same underlying neural circuitry as that of larger saccades (Hafed, 2011; Hafed and Krauzlis, 2012; Hafed et al., 2009; Martinez-Conde et al., 2013; Melloni et al., 2009; Otero-Millan et al., 2008, 2011, 2013; Peel et al., 2016; Snodderly, 2016), including superior colliculi (SC) and frontal eye fields (FEFs). MSs might play an important role in restoring vision during fixation (Hafed, 2011; Martinez-Conde et al., 2006; McCamy et al., 2012), in optimal local spatial sampling during natural viewing (Otero-Millan et al., 2008; Poletti et al., 2013; Rucci et al., 2007), in oculomotor optimization of eye position on the fixation point (Tian et al., 2016, 2018), and in the spatio-temporal transformation of retinal input (Boi et al., 2017; Kuang et al., 2012).
The neurophysiological impact of MSs on the visual system is well known for the SC, FEFs, lateral geniculate nucleus (LGN), and V1. The firing rate (Bellet et al., 2017; Chen et al., 2015; Hafed, 2013; Hafed and Krauzlis, 2010; Kagan et al., 2008; Leopold and Logothetis, 1998; Martinez-Conde et al., 2000, 2009; Snodderly, 2016; Troncoso et al., 2015), burstiness (Hafed and Krauzlis, 2010; Martinez-Conde et al., 2002), and neural synchrony of cells (Bellet et al., 2017; Bosman et al., 2009; Ito et al., 2013; Lowet et al., 2016; Tian et al., 2016) are significantly modulated by MS occurrence.
A close correlation between neuronal spike activity and MS generation and deployment also extends to areas of the dorsal stream (Bair and O’Keefe, 1998; Herrington et al., 2009) and ventral stream (Leopold and Logothetis, 1998; Bosman et al., 2009). V2 and V4 firing rates and local field potentials (LFPs) show systematic modulations with MS occurrence (Leopold and Logothetis, 1998; Bosman et al., 2009). Firing rate modulation in the inferior temporal (IT) cortex by MSs have been found only weakly in one study (Leopold and Logothetis, 1998). Further, MS occurrence is tightly temporally coordinated with a low 3- to 4-Hz LFP theta rhythm (Bosman et al., 2009; Lowet et al., 2016) and alpha rhythms (Bellet et al., 2017) that are suggested to be involved in stimulus processing and attention (Lakatos et al., 2005; Schroeder and Lakatos, 2009).
Given the close relation between saccades and visual attention (Corbetta et al., 1998; Moore and Zirnsak, 2017; Moore et al., 2003; Schall, 2013), it is not surprising that most of the extrastriate visual areas whose neuronal responses are influenced by MSs also participate in the mechanisms of attentive stimulus processing. Neurons in cortical and subcortical visual areas show enhanced visual responses when attention is directed to a stimulus in the receptive field (RF), compared to when attention is directed outside the RF (Desimone and Duncan, 1995; Gregoriou et al., 2009; Zhou et al., 2016). Although in natural vision, saccadic eye movements and spatial attention are linked (“overt attention”) to the same target stimulus, most studies of spatially directed attention use a “covert” strategy, in which attention is directed toward an extrafoveal target stimulus while gaze is maintained on a fixation stimulus. There is now considerable evidence that MSs are typically made in the direction of an attended stimulus during covert attention (Engbert and Kliegl, 2003; Hafed, 2013; Hafed and Clark, 2002; Laubrock et al., 2005; Meyberg et al., 2017; Pastukhov and Braun, 2010; Rolfs et al., 2005; Yuval-Greenberg et al., 2014). Further, neurons in SC and FEFs exhibit gain enhancement if an MS is directed toward the RF location, suggesting that attention modulations might be modified around MSs (Chen et al., 2015; Hafed, 2013). However, whether MSs have a substantial influence on the attentional modulation of neuronal responses during covert attention has been unknown.
RESULTS
Locus of Attention Influences the Direction of MSs Rhythmically Occurring at 3–4 Hz
We measured multiunit activity in two awake monkeys (Macaca mulatta) recorded from laminar microelectrodes inserted in cortical areas V4 and IT during a spatial attention task (Figure 1A). The monkeys maintained fixation on a central spot while a spatial cue directed attention to one of three extra-foveal stimuli. In some sessions, the cue could occur 500–700 ms before the stimulus (cue-first sessions; Figure 1A) or after the stimulus (stim-first sessions). After 500–1,000 ms, the cued stimulus briefly (50 ms) changed color, and the monkey was rewarded for making a saccade to it. If not specified otherwise, data from stim-first sessions and cue-first sessions were combined. Stimuli were grayscale objects, all in the contralateral hemifield. Eye position and MSs were measured with an infrared eye-tracking system (Engbert and Kliegl, 2003) (Figure 1A).
Figure 1. Direction of MSs Reflect the Locus of Attention.
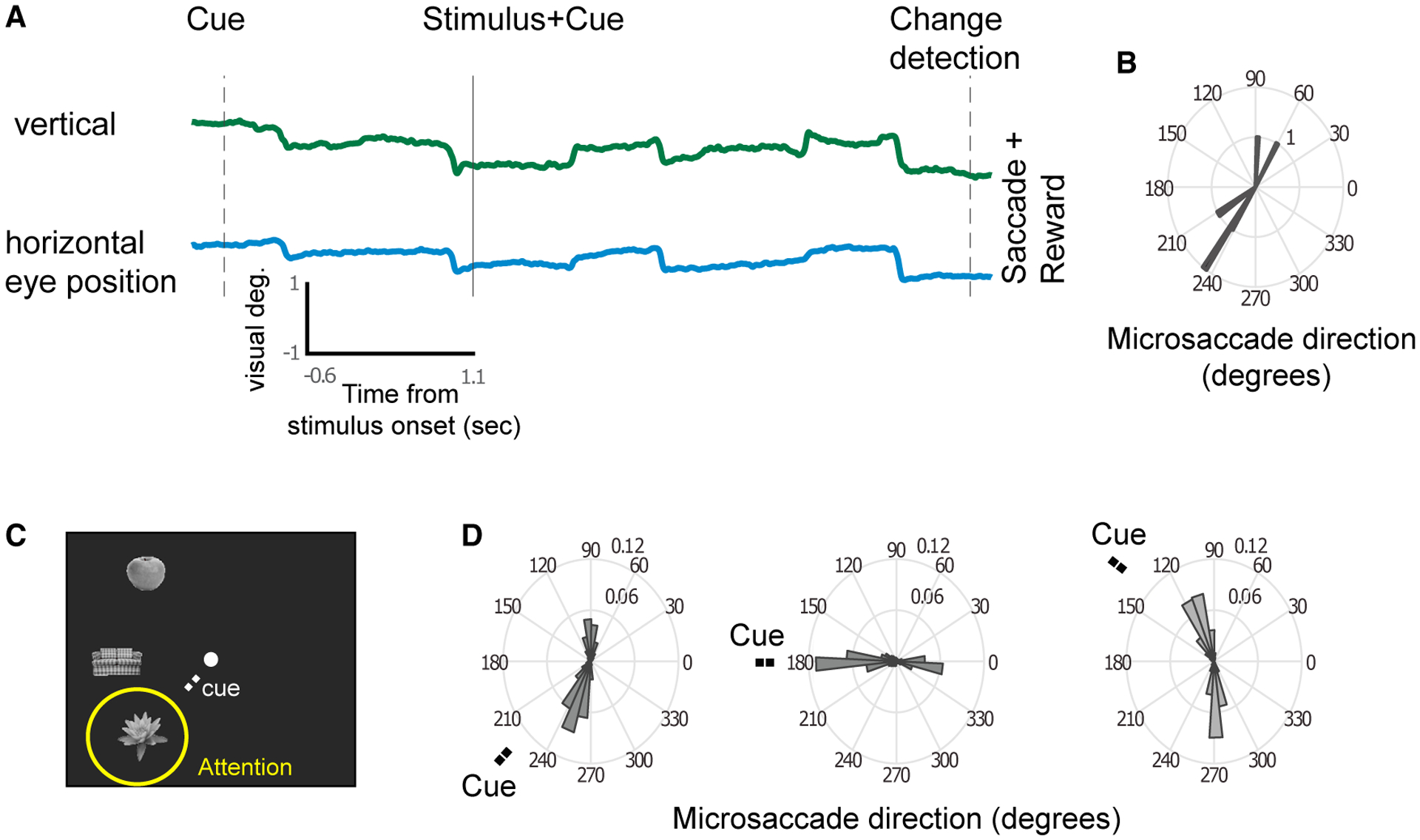
(A) Example vertical and horizontal eye traces during a trial. The rapid shifts in positions are MSs.
(B) The MS directions from the example trial.
(C) Example stimuli shown for the three stimulus locations. A cue directed attention to one of the locations.
(D) The population-averaged MS direction probability plots of MonkeyM1 for the three attention positions indicated by the “Cue” label (for monkey M2, positions were in the opposite hemifield).
MSs occurred at a median rate of 3.29 ± 0.075 Hz and showed two predominant directions for each target location, roughly in opposite directions (Figures 1A and 1B). The first MS after the cue was typically made in the direction of the stimulus that was attended (Figures 1C and 1D), and the next MS was typically directed back toward the fixation stimulus, which then was followed again by an MS directed toward the attended stimulus (and so on). The modulation of MS direction based on a cue nearby the fixation point is in line with previous literature (Hafed and Clark, 2002; Ko et al., 2010; Laubrock et al., 2005; Meyberg et al., 2017; Rolfs et al., 2005; Tian et al., 2016)
Attentional Modulation of V4 Neuronal Firing Rates Emerges only after MSs toward the Attended Stimulus
We next tested whether MS direction had an influence on V4 firing rates. V4 RFs were located in the lower left (Monkey M1) or lower right (Monkey M2) visual quadrants. In the population average firing rate histograms, responses to the RF stimulus were enhanced by attention only when a preceding MS was directed toward the attended stimulus (Figures 2A, 2C, and 2D). No such enhancement was found when MS was directed away from the attended stimulus (Figures 2B, 2E, 2F). To test this statistically, we computed an index of response modulation by attention for each cell (attention-in [Att-in] − attention-out [Att-out]/Att-in + Att-out), in which an index value of zero would indicate no effect of attention. The mean population index when the MS was directed toward the RF was 0.14 ± 0.01, which was significantly different from zero (t test, n = 253, p = 1.6−22). The mean value when the MS was directed away from the RF was 0.012 ± 0.01, which was not significantly different from zero (t test, n = 253, p = 0.1). This suggests that attention modulation is temporally coordinated with MSs directed toward the cued stimulus.
Figure 2. Neural Enhancement Occurs Only with MSs Directed towards Attention Location.
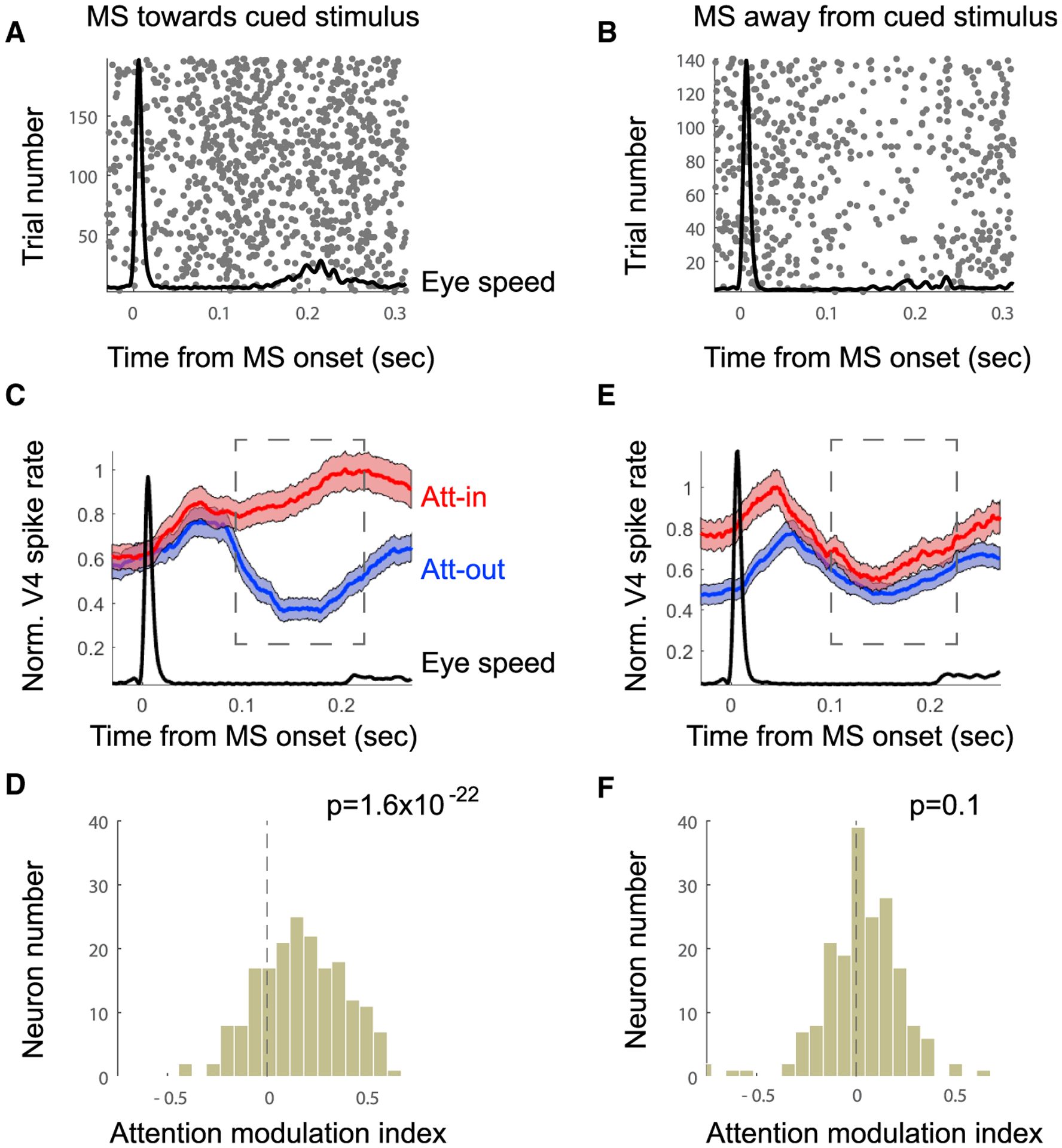
(A and B) Example spike rasters locked to onset of MS directed toward (A) or away from (B) cued stimulus (Att-in). The black line shows average eye speed.
(C and D) Population data combined across monkeys. Analysis was restricted to MSs directed toward the attended position.
(C) Normalized population-averaged V4 firing rate for attention on the RF (POS1) and outside of the RF (POS3). Shaded area indicates ±SE.
(D) Attention modulation index (Att-in − Att-out / Att-in + Att-out) (n = 253) computed 100–200 ms after MS onset.
(E and F) Same analysis as in (C) and (D), but for MSs directed away from the attention position (i.e., toward the fixation target).
We did observe positive attention modulation just before the MS onset and for a short time thereafter when the MS was directed away from the RF (Figure 2E). This is because the MS directed away from the RF was typically preceded by an MS toward the RF (probability of MS direction change computed per session and attention condition is median = 76.3 ± 1.5%; significantly different from random: t test, n = 54, p = 2−23).
Several lines of evidence suggested that the attention modulations could not be explained simply by differences in eccentricity or image shifts on the retina. First, computing the V4 firing rate modulation for different subgroups of X and Y eye positions after MSs toward or away from cued stimulus revealed no clear eye position specific effects (Figures S1A–S1C). Further, equalizing the eye position eccentricity for MSs toward and away conditions did not eliminate the effect (Figures S1D–S1G). In addition, we found that MS amplitude had little impact on the attentional modulation strength (Figures S1H–S1J).
As a more direct test of whether stimulus eccentricity or movement alone was responsible for the MS effects, we mimicked the retinal image shifts of MSs in one monkey performing a fixation task that did not involve attentional cueing. A similar three-stimulus configuration was shown, with one stimulus in the RF. We measured firing rates at 64 V4 sites, while the stimulus in the V4 RF was jerked either toward or away from the fixation point by 0.3 visual degrees (Figure 3). The movement of the RF stimulus was accompanied by corresponding motion of the two other stimuli, thereby mimicking artificially the MS retinal image shifts. These stimulus manipulations did not reproduce the MS-linked attention effects. We did observe a significant firing rate modulation as a function of jerk direction (50–150 ms after jerk onset, n = 64, p = 0.0012; 150–250 ms after jerk onset, n = 64, p = 0.0014); however, this was opposite from that expected from our results with MS direction. The modulation was also weaker (mean firing rate modulation = −2.2 spikes per second, ±0.65%) compared to firing rate modulation caused by MSs made toward versus away from the attended stimulus in the same monkey (mean firing rate modulation = 8.5 spikes per second, ±1.2%). Altogether, this suggests that attentional firing rate modulations are rather linked to extra-retinal effects (Chen et al., 2015; Hafed, 2013; Melloni et al., 2009) associated with the MS. In agreement with this interpretation, Troncoso et al. (2015) found that V1 responses differentiate between stimulus motion due to the stimulus and the same stimulus motion produced by an MS (Troncoso et al., 2015).
Figure 3. V4 Control Data with Stimulus Jerked Toward or Away from the Fixation Point.
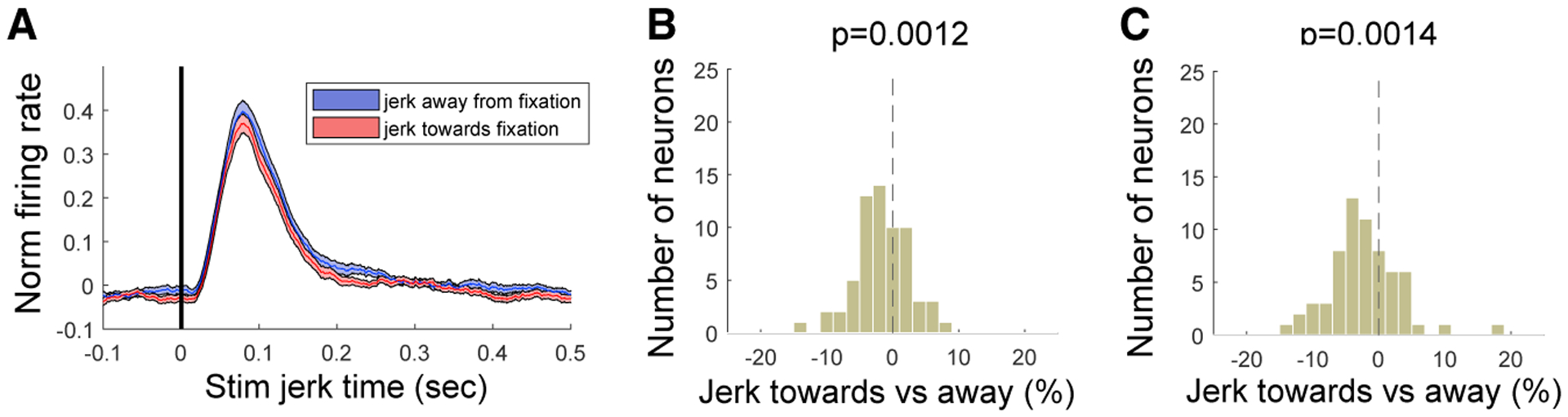
(A) V4 population averaged V4 firing rate for stimulus jerked (by 0.3°) away from fixation (blue line and shading) or toward fixation (red line and shading). Shaded area indicates ±SE. After 300 ms, the stimulus was jerked back to the original position.
(B) Firing rate modulation between jerk-toward and -away conditions (Jerktoward − Jerkaway/Jerktoward + Jerkaway) for each neuron for time windows of 50 ms to 150 ms after jerk time (transient response). The distribution did only slightly deviate from a distribution centered on zero.
(C) Same as in (B), but for time windows of 150 ms to 250 ms after the jerk time. Distribution deviated more significantly with enhanced V4 firing rate for jerk away from fixation point.
Spatial attention not only modulates V4 firing rates but also increases firing reliability, measured by the Fano factor (Cohen and Maunsell, 2009; Mitchell et al., 2007, 2009). Therefore, we tested whether attentional modulation of the Fano factor was also linked to MSs. We found that the Fano factor of V4 neurons was reduced (increased reliability) only after MSs were directed toward the attended stimulus (Figure S2). It also known that attention reduces firing rate correlations between V4 neurons for a given stimulus condition, measured by noise correlation (Cohen and Maunsell, 2009; Mitchell et al., 2009). The noise correlation value depends on the integrating counting window used (Mitchell et al., 2009). We found that the noise correlation in our dataset was reduced by attention when using integration windows of 80 ms (Figure S3). Importantly, we found that the attentional reduction of noise correlation only occurred after MSs were directed toward the attended stimulus.
Onset of Attentional Effects on Responses Is Coupled to MS Onset
The results so far showed that the attentional modulation of firing rates in V4 emerged shortly after an MS toward the cued stimulus and vanished when an MS was directed away. This suggests a linkage between the timing of the MSs and the effects of attention. When we averaged neuronal responses and eye velocity on all trials, time-locked to the attentional cue (Figure 4A), both eye velocity and attentional effects on firing rates slowly ramped up following the cue. However, MSs occurred at variable intervals following the attentional cue. When we separated trials as a function of MS timing (Figure 4B) after the cue, it revealed a tight timing relationship to the onset of attentional effects. For this analysis, we used a subset of recording sessions where the attentional cue occurred after stimulus onset (see STAR Methods). We averaged trials as a function of MS onsets with 100-ms-wide time windows centered in the earliest trials at 100 ms after the cue and in the latest trials at 400 ms after the cue. We found that the later the onset of the MS after the attentional cue, the later the onset of attentional effects on V4 firing rates (permutation test, p = 0.0042) (Figure 4B). The estimated latency of attentional effects on responses averaged across the population ranged from 140 ms for the earliest MS timing group to 402 ms for the latest MS timing group. Thus, the variability in the effects of attention in V4 is related, at least in part, to the variability in MS onset.
Figure 4. Onset of Attention Neural Effects Coupled to MS Onsets.
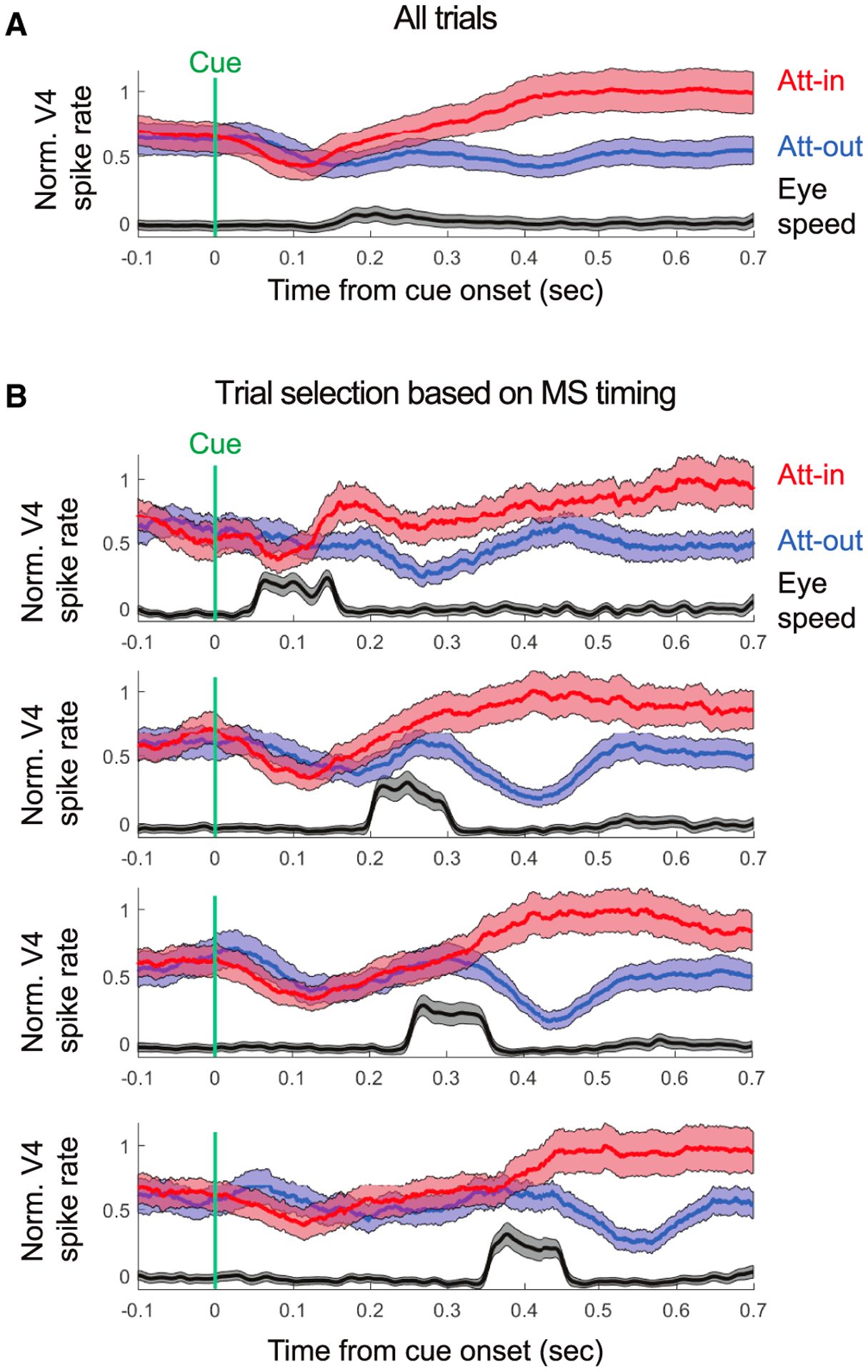
(A) Normalized V4 spike rate and the averaged eye speed as a function of cue onset for stim-first sessions. Shaded area indicates ±SE.
(B) The same as in (A), but trials were selected based on the timing of the MS occurrence toward the attended position. From top to bottom, the chosen selection time windows were: 50–150 ms, 200–300 ms, 250–350 ms, and 350–450 ms.
We found MSs within a 600-ms time window after the cue in 83% of the total number of trials. This result raised a question about the remaining 17% of the trials (“MS-free trials”) without an MS. We also found attention effects (Figure S4) after the cue in V4 in these MS-free trials (t test, n = 52; MS-free trials: p = 8.6−6; MS trials: p = 1.5−6). However, significant effects of attention on firing rates occurred just prior to the cue on these MS-free trials (t test, n = 52; MS-free trials: p = 0.016; MS trials: p = 0.1). Furthermore, on these MS-free trials, we found a significantly higher probability of MSs in the direction of the to-be-cued stimulus prior to the cue onset (t test, n = 769, p = 8−13) but not MSs away from the to-be-cued stimulus (t test, n = 4,128, p = 0.08). However, we cannot exclude that, in some trials, without a detected MS, there might have been a very small, undetected MS. Together, these results suggest that, prior to the onset of the cue on some trials, the monkey shifted attention to the to-be-cued stimulus by chance, which was accompanied by an MS toward the expected stimulus. On these trials, no further increase in attention to the cued stimulus was necessary, and no MS was made.
Decoding of Attended Stimulus Enhanced in V4 and IT only After MS toward the Stimulus
In our data, all three stimuli, the attentional cue, and the fixation stimulus were all typically located within the same large IT RF, which prevented us from defining Att-in and attention-out conditions for IT neurons in the same way that we did for V4. However, we found a significant increase of IT firing rate after MSs toward attended stimuli compared to MSs away from attended stimuli, combining all attention conditions (Figure S5).
Previous studies have shown that IT neurons become selective more for attended stimuli (Zhang et al., 2011; Zhou et al., 2016) compared to non-attended stimuli, which is in line with reports of an increased functional correlation between IT neurons and V4 neurons representing the attended stimulus (Saalmann et al., 2012; Zhou et al., 2016). A previous study using the same behavioral paradigm and stimulus configuration has shown that IT population codes for an object in clutter (distractor stimuli located in the same RF) are improved by attention directed to that object (Zhang et al., 2011). Therefore, we tested whether neural responses in V4 and in IT better discriminate 7 different visual objects as a function of attention and MS direction (Figures 5A and 5B). For V4 and IT neurons, objects were decoded at the location covering the V4 RF. We evaluated the decoding performance using an ANOVA computed in a 100-ms to 200-ms window following an MS. We found better decoding by V4 and IT neurons following an MS directed to the target stimulus (t test; V4: n = 253, p = 6.9−6; IT: n = 239, p = 6.9−4) compared to when the MS was directed away from the stimulus (t test; V4: n = 253, p = 0.24; IT: n = 239, p = 0.4; Figures 5C–5F). This suggests that IT neurons, with their RF covering the three stimuli, became most selective to stimulus information processed by V4 neurons specifically after an MS directed to the attended location.
Figure 5. Object Decoding Enhanced with MSs Directed towards Attention Location.
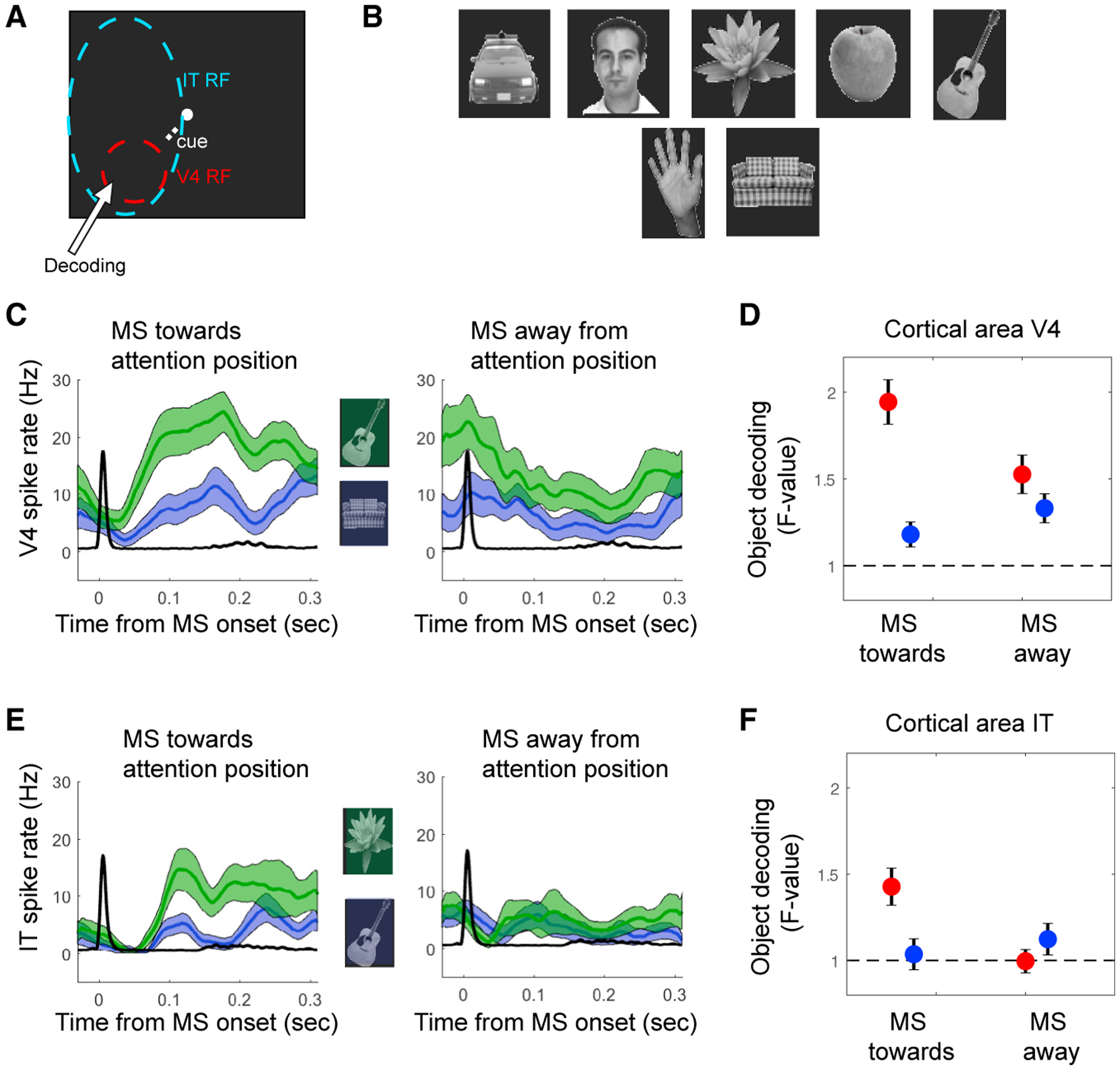
(A) Schematic outline of the V4 and IT RFs in relation to the three stimulus locations. Stimuli presented in the V4 RF were decoded.
(B) The 7 stimuli used.
(C and D) V4 neurons.
(C) Example neuron firing rates for two different stimuli (green line versus blue line) relative to the onset of MSs directed toward the attended location (left) or away from it (right).
(D) Population-averaged decoding performance based on single-cell firing rates (100–200 ms after MS onset) using ANOVA as a function of MS direction and attention position.
(E and F) The same as in (C) and (D), respectively, but for IT neurons.
DISCUSSION
An argument for the dissociation between covert attention and the oculomotor system has been that subjects can covertly shift attention while holding their gaze fixed. Indeed, most neurophysiological studies of spatial attention, including our own, have typically used a covert attention paradigm to separate the control of attention from the control of eye movements (Desimone and Duncan, 1995; Maunsell, 2015; Moore and Zirnsak, 2017). However, during visual fixation, the oculomotor system remains highly active, producing fixational eye movements, which are biased toward attended stimuli (Engbert and Kliegl, 2003; Hafed and Clark, 2002; Pastukhov and Braun, 2010; Yuval-Greenberg et al., 2014). Here, we found that the effects of attention on neuronal responses and/or object decoding in V4 and IT cortex were largely limited to a short time following an MS toward the attended stimulus. There was a tight temporal association between the timing of the MSs and the timing of attentional effects on responses. The later an MS occurred after the attentional cue, the later the effects of attention on V4 responses occurred. We hypothesize that the monkey’s attention (and eye position) involuntarily shifted back and forth from the cued stimulus to the fixation spot. Both continued fixation and attention to the cued stimulus were critical for the animals to succeed in the task.
Why was this role of MSs not observed previously in neurophysiological studies of attention? Previous studies have shown that MS direction has an influence on the neural response gain to a stimulus (Bellet et al., 2017; Chen et al., 2015; Hafed, 2013). In FEFs and the SC, neurons gave enhanced responses to stimuli presented just before an MS, and this effect was larger when the MS was directed into the hemifield containing the RF (Chen et al., 2015). To our knowledge, we were the first to directly test neural modulations separately for MSs directed toward versus away from an attended stimulus rather than pooling responses locked to all MSs, regardless of MS direction and attention spot. This manipulation revealed that the attentional enhancement of neuronal responses only occurred with an MS directed to the RF stimulus in V4. In the IT cortex, responses were enhanced with an MS toward the attended stimulus in the large IT RF. Stimulus decoding from IT responses was also best when an MS was directed to the attended stimulus. We suggest that future studies of response variability, noise correlation, up and/or down states, and so on in fixating animals should take MS direction into account. The influential concept of “covert spatial attention,” which is that extrafoveal stimuli can be attended without any movement of the eyes, may be based on a flawed assumption. Feature-based attention, when it has no spatial component, likely results from different mechanisms.
A recent study of attention in human subjects reported effects of attention on visual processing, even when the stimuli were located within the representation of the fovea (Poletti et al., 2017). These effects were found regardless of whether an MS occurred toward the attended stimulus. Although it is difficult to compare our results, because we do not have any V4 data with stimuli located within the fovea, it is worth noting that we also found effects of attention on V4 responses on trials when no MSs were made following the attentional cue. However, we found that an MS toward the to-be-cued stimulus often preceded the cue on these trials, suggesting that a new MS is not always triggered if an attentional shift and MS have already been made in the direction of an expected stimulus.
Our findings are in line with the notion of active sensing (Schroeder and Lakatos, 2009; Schroeder et al., 2010) and attentional rhythmic sampling (Bellet et al., 2017; Fiebelkorn et al., 2013; Hafed et al., 2015; Landau and Fries, 2012; VanRullen, 2013), in which attention is not sustained at a single location but rhythmically shifts among relevant objects of the visual scene. The rhythmic sampling has been linked to delta and/or theta rhythms occurring at about 3–7 Hz. MSs occur at a similar frequency and are tightly locked to delta and/or theta neural rhythms (Bosman et al., 2009). Whether MSs and delta and/or theta rhythms share the same generator remains unclear. Our results suggest that MSs represent a highly valuable marker for understanding cognitive processes and contribute significantly to visual attention.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Eric Lowet (elowet@mailfence.com).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Procedures were done according to National Institutes of Health guidelines and were approved by the Massachusetts Institute of Technology Animal Care and Use Committee. Two male monkeys (weight: 11–13 kg) were used. Monkeys were implanted under aseptic conditions with a post to fix the head and recording chambers over areas V4 and IT. Behavioral training was carried out using Cortex software.
METHOD DETAILS
Visual stimuli and task
We used a spatial attention paradigm (see details in Zhou et al., 2016). Briefly, V4 RFs in two monkeys (Macaca mulatta) were first mapped during a fixation task, with a stimulus flashed in both hemifields at 1° to 15° eccentricities. All RFs used in the recording sessions were between 5° and 7° (RF center) eccentricity located in the lower left (Monkey M1) or lower right (Monkey M2) visual quadrant. The RFs of IT neurons were large and not clearly defined using our RF mapping procedure. In the attention task, the monkeys maintained fixation on a central white spot (fixation window size in deg = 3° × 3°). The monkey attended to one of three stimuli from a set of seven monochrome objects: hand, flower, face, couch, car, guitar, and kiwi. Each stimulus was 2.3° × 2.3° in size, displayed on an LCD screen. For cue-first conditions, the attended location was cued before the stimulus was displayed. Monkeys kept their gaze on the central spot for 500 ms, after which a cue, consisting of a dim line segment, appeared near the center spot and “pointed” to a location to be attended. At 500–700 ms after cue onset, three stimuli appeared on the screen (one target, three distracters). Monkeys were rewarded with a drop of juice if they made a saccade to the target when it changed slightly in color, which occurred between 500 and 1,000 ms after stimulus onset. For stimulus-first conditions, the three stimuli appeared 500 ms after the monkey began fixating the central spot, and 500–700 ms before the appearance of the attention cue. The other parts of the task were the same as in cue-first trials. For both conditions, one of the distracters changed color before the target color change on half the trials, and the monkeys were required to keep central fixation until the target changed color.
Surgery and recording
All surgical details were described in (Zhou et al., 2016). All procedures and animal care were in accordance with the NIH guidelines. For the recording sessions, single units and multiunit spikes and local field potentials (LFPs) were acquired using an OmniPlex system (Plexon Inc, Dallas, USA). Linear probes containing 1–16 electrodes were used (U-Probe, Plexon Inc). Neural signals were filtered between 250 Hz and 8 kHz, amplified and digitized at 40 kHz to obtain spike data, and filtered between 0.7 and 170 Hz to obtain the LFP signals. Eye movements were recorded by an infrared eye-tracking system (Eye Link II, SR Research Ltd., Ontario, Canada) at a sampling rate of 500 Hz.
Firing rate analysis
The spike trains were first convolved with a rectangular window of 50 ms width. The firing rate traces were first subtracted by averaged activity in the baseline period (the last 200 ms period before the stimulus onset). Then they were normalized by the maximum rate in the Attention in conditions.
Microsaccade detection
We first smoothed horizontal and vertical eye signals (rectangular window of ± 5 ms) and differentiated the signals over time. The differentiated horizontal and vertical eye signals were then combined and further smoothed (rectangular window of ± 5 ms). We then used the detection algorithm designed by Engbert and Kliegl (Engbert and Kliegl, 2003) to obtain MS onset (relative velocity threshold = 4, minimum saccade duration = 8 ms). The quality of MS detection was verified by visual inspection of the eye traces on single trials as well as the shape of the MS interval distribution (e.g., whether there were too many intervals close to zero). We roughly estimate the effective spatial resolution (that can vary from session to session) at about 0.1 deg, meaning that MSs below 0.1 deg are difficult to distinguish from noise.
In all sessions the MS direction distribution was clearly bimodal for a given attention condition. To define a MS as toward or away from the attended stimulus, we could split the distribution in half such that one main direction was in one half and the orthogonal direction was in the other half.
Stimulus decoding
Seven different visual objects were shown for each of the three stimulus locations. To quantify how well neuronal responses in V4 and IT could be used to separate all of the stimuli, we applied an ANOVA to quantify (F-value) the separation based on the firing rate recorded on single electrode contacts. To estimate the firing rate, we averaged the mean rate for time windows locked to the MS (100–200 ms after onset). The analysis was done for MSs directed toward or away from the cued stimulus. We equalized the number of windows used for the analysis for both MS directions. The analysis was restricted to the stimulus location that covered the V4 locations for both V4 and IT neurons.
Eye position analysis
MSs directed toward or away from the cued stimulus were linked to different averaged eye positions. Hence, the stimulus had on average a different position in relation to the V4 RFs, which might influence the firing rate differences observed. To test this, we equalized the eye positions in terms of eye position eccentricity. Eye position eccentricity is computed by taken the squared distance of X and Y eye position during stimulus presentation relative to X and Y in the fixation baseline. The eye position eccentricities were computed for MSs toward and MSs away from the cued stimulus. The values were normalized relative to the mean value of the MS away population to reduce variability. We matched the eye position eccentricity distribution of MSs away from the cued stimulus with the 25th-75th distribution of MSs toward the cued stimulus. After the matching the eccentricity differences, there were no significant differences based on MS direction. For the fine grain eye position analysis, the X and Y eye positions were subtracted and normalized by the mean and variance of positions from MS away windows. Population-averaged V4 firing rate were obtained from time-windows 100–250 ms after MS either directed toward (red) or away (blue) from cued stimulus. Population data were grouped into 5 groups (20% percentile steps) dividing equally the X or Y eye position distribution.
Fano factor
For computing the Fano factor we divided the firing rate variance over the mean firing rate. This was done on spike trains that were convolved with a rectangular window of 100 ms width. For assessing the attention modulation, we computed the averaged difference of the Fano factor values of the attention toward and away conditions for time windows locked to MSs (100–200 ms after onset) for MSs directed toward or away from the cued stimulus.
Noise correlation
Noise correlation (NC) is the Pearson’s correlation of spike counts between neurons for a given stimulus condition. It represents the correlation (shared variability) not explained by stimulus variation. We computed the NC for all neuron pairs for a given V4 laminar probe. We excluded neurons which had firing rates below 1Hz to avoid unstable NC measures. For the MS-dependence of NC we used a counting time window of 80 ms. For assessing the attentional modulation as a function of MS direction we chose time windows 100–200 ms after MS onset. NC was only computed if at least 10 windows could be found for a given stimulus, MS direction and attention condition.
Attention latency and permutation test
To estimate the latency of attentional firing effects in a robust way, we divided the trials into 7 subsets according to MS timing after the cue (50–150 ms, 100–200 ms, 150–250 ms, 200–300 ms, 250–350 ms, 300–350 ms and 350–400 ms). We estimated the attentional latency for each block and then extracted the slope by linear regression analysis. The latency for each MS timing group was estimated by first computing a paired t test for each time point after the cue (ranging from 50 ms to 700 ms after the cue) between Att-in and att-out firing rates over the neural population. We then included only time points that had a t-value greater than 2.5. From the distribution of time points, we took the median of the time points within the first 20th percentile of the time point distribution (estimated values from earliest to latest MS timing group were: 142 ms, 217 ms, 253 ms, 266 ms, 308 ms, 371 ms, 402 ms).
To assess whether the observed slope was statistically significant, we randomized 10000 times the correspondence of trials to the seven MS timing blocks and extracted the slope to obtain a null slope distribution. From the null slope distribution, we determined the likelihood of the experimentally observed slope.
V4 stimuli jerk control datasets
In these control sessions, the animals were only required to fixate the fixation target through the whole trial. The same stimuli were shown as in the attention conditions, with 3 stimuli shown at three different position in the same hemifield (as in the attention sessions). The animal did not receive a cue or make a saccade to a desired target. Also, distractors and targets did not change color. Following a random time period after the onset of the stimulus array, the stimulus array was moved such that the stimulus within the V4 RF was jerked either toward or away from the fixation point. After a brief time period (300ms) the stimulus array was returned back to its original position. Thus, the whole stimulus array was shifted in unison, mimicking the effects of a MS. The distance that stimuli were jerked was always 0.3° toward or away from fixation.
QUANTIFICATION AND STATISTICAL ANALYSES
The details of all quantitative and statistical analysis performed are described in the Method Details, in the main text, or in the figure legends. Error bars and shaded regions on plots indicate either mean ± SE.
DATA AND SOFTWARE AVAILABILITY
The data presented in the manuscript are available by request.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and Algorithms | ||
| MATLAB | MathWorks | https://www.mathworks.com/products/matlab.html |
| Offline Sorter | Plexon | Offline Sorter v3.3.5 – Windows 7 (64 bit) |
| Behavioral stimulus control | Cortex | http://dally.nimh.nih.gov/index.html |
| Other | ||
| 16-contact laminar electrodes | Plexon | Plexon U-probe |
| Eye tracking | Eyelink | Eyelink 1000 |
Highlights.
Attentional modulation in V4 emerges only after microsaccades toward the stimulus
Onset of attention modulations is coupled to microsaccade onset
Object decoding is enhanced in V4 and IT after microsaccades toward the cued stimulus
Linkage between attention modulation and microsaccades is extra-retinal
ACKNOWLEDGMENTS
This study was supported by NIH grants EY017292 and EY017921. B.G. had a Science without Borders fellowship-CNPq/CAPES grant (11733/13-6). H.Z. was supported by National Key R&D Program of China grant 2017YFC1307500, National Natural Science Foundation of China grant 31671108, CAS Hundred Talent program and grant 172644KYSB20160175, and Shenzhen grants JCYJ 20151030140325151, KQTD 20140630180249366, and GJHZ 20160229200136090. We thank Dimitrios Pantazis for comments.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and can be found with this article online at https://doi.org/10.1016/j.neuron.2018.05.041.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Bair W, and O’Keefe LP (1998). The influence of fixational eye movements on the response of neurons in area MT of the macaque. Vis. Neurosci 15, 779–786. [DOI] [PubMed] [Google Scholar]
- Bellet J, Chen C-Y, and Hafed ZM (2017). Sequential hemifield gating of alpha and beta behavioral performance oscillations after microsaccades. J. Neurophysiol 118, 2789–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boi M, Poletti M, Victor JD, and Rucci M (2017). Consequences of the oculomotor cycle for the dynamics of perception. Curr. Biol 27, 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Womelsdorf T, Desimone R, and Fries P (2009). A microsaccadic rhythm modulates gamma-band synchronization and behavior. J. Neurosci 29, 9471–9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Ignashchenkova A, Thier P, and Hafed ZM (2015). Neuronal response gain enhancement prior to microsaccades. Curr. Biol 25, 2065–2074. [DOI] [PubMed] [Google Scholar]
- Cohen MR, and Maunsell JHR (2009). Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci 12, 1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, and Shulman GL (1998). A common network of functional areas for attention and eye movements. Neuron 21, 761–773. [DOI] [PubMed] [Google Scholar]
- Desimone R, and Duncan J (1995). Neural mechanisms of selective visual attention. Annu. Rev. Neurosci 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Engbert R, and Kliegl R (2003). Microsaccades uncover the orientation of covert attention. Vision Res 43, 1035–1045. [DOI] [PubMed] [Google Scholar]
- Fiebelkorn IC, Saalmann YB, and Kastner S (2013). Rhythmic sampling within and between objects despite sustained attention at a cued location. Curr. Biol 23, 2553–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, and Desimone R (2009). High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324, 1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM (2011). Mechanisms for generating and compensating for the smallest possible saccades. Eur. J. Neurosci 33, 2101–2113. [DOI] [PubMed] [Google Scholar]
- Hafed ZM (2013). Alteration of visual perception prior to microsaccades. Neuron 77, 775–786. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, and Clark JJ (2002). Microsaccades as an overt measure of covert attention shifts. Vision Res 42, 2533–2545. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, and Krauzlis RJ (2010). Microsaccadic suppression of visual bursts in the primate superior colliculus. J. Neurosci 30, 9542–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, and Krauzlis RJ (2012). Similarity of superior colliculus involvement in microsaccade and saccade generation. J. Neurophysiol 107, 1904–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, and Krauzlis RJ (2009). A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323, 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Chen C-Y, and Tian X (2015). Vision, Perception, and Attention through the Lens of Microsaccades: Mechanisms and Implications. Front. Syst. Neurosci 9, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington TM, Masse NY, Hachmeh KJ, Smith JET, Assad JA, and Cook EP (2009). The effect of microsaccades on the correlation between neural activity and behavior in middle temporal, ventral intraparietal, and lateral intraparietal areas. J. Neurosci 29, 5793–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J, Maldonado P, and Grün S (2013). Cross-frequency interaction of the eye-movement related LFP signals in V1 of freely viewing monkeys. Front. Syst. Neurosci 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan I, and Hafed ZM (2013). Active vision: microsaccades direct the eye to where it matters most. Curr. Biol 23, R712–R714. [DOI] [PubMed] [Google Scholar]
- Kagan I, Gur M, and Snodderly DM (2008). Saccades and drifts differentially modulate neuronal activity in V1: effects of retinal image motion, position, and extraretinal influences. J. Vis 8, 1–25. [DOI] [PubMed] [Google Scholar]
- Ko HK, Poletti M, and Rucci M (2010). Microsaccades precisely relocate gaze in a high visual acuity task. Nat. Neurosci 13, 1549–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X, Poletti M, Victor JD, and Rucci M (2012). Temporal encoding of spatial information during active visual fixation. Curr. Biol 22, 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, and Schroeder CE (2005). An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol 94, 1904–1911. [DOI] [PubMed] [Google Scholar]
- Landau AN, and Fries P (2012). Attention samples stimuli rhythmically. Curr. Biol 22, 1000–1004. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Engbert R, and Kliegl R (2005). Microsaccade dynamics during covert attention. Vision Res 45, 721–730. [DOI] [PubMed] [Google Scholar]
- Leopold DA, and Logothetis NK (1998). Microsaccades differentially modulate neural activity in the striate and extrastriate visual cortex. Exp. Brain Res 123, 341–345. [DOI] [PubMed] [Google Scholar]
- Lowet E, Roberts MJ, Bosman CA, Fries P, and De Weerd P (2016). Areas V1 and V2 show microsaccade-related 3–4-Hz covariation in gamma power and frequency. Eur. J. Neurosci 43, 1286–1296. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, and Hubel DH (2000). Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat. Neurosci 3, 251–258. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, and Hubel DH (2002). The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc. Natl. Acad. Sci. USA 99, 13920–13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, and Dyar TA (2006). Microsaccades counteract visual fading during fixation. Neuron 49, 297–305. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, and Hubel DH (2009). Microsaccades: a neurophysiological analysis. Trends Neurosci 32, 463–475. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Otero-Millan J, and Macknik SL (2013). The impact of microsaccades on vision: towards a unified theory of saccadic function. Nat. Rev. Neurosci 14, 83–96. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR (2015). Neuronal mechanisms of visual attention. Annu. Rev. Vis. Sci 1, 373–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCamy MB, Otero-Millan J, Macknik SL, Yang Y, Troncoso XG, Baer SM, Crook SM, and Martinez-Conde S (2012). Microsaccadic efficacy and contribution to foveal and peripheral vision. J. Neurosci 32, 9194–9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni L, Schwiedrzik CM, Rodriguez E, and Singer W (2009). (Micro) saccades, corollary activity and cortical oscillations. Trends Cogn. Sci 13, 239–245. [DOI] [PubMed] [Google Scholar]
- Meyberg S, Sinn P, Engbert R, and Sommer W (2017). Revising the link between microsaccades and the spatial cueing of voluntary attention. Vision Res 133, 47–60. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, and Reynolds JH (2007). Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55, 131–141. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, and Reynolds JH (2009). Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, and Zirnsak M (2017). Neural mechanisms of selective visual attention. Annu. Rev. Psychol 68, 47–72. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, and Fallah M (2003). Visuomotor origins of covert spatial attention. Neuron 40, 671–683. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Troncoso XG, Macknik SL, Serrano-Pedraza I, and Martinez-Conde S (2008). Saccades and microsaccades during visual fixation, exploration, and search: foundations for a common saccadic generator. J. Vis 8 (14), 1–18, 21. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Serra A, Leigh RJ, and Martinez-Conde S (2011). Triggering mechanisms in microsaccade and saccade generation: a novel proposal. Ann. N Y Acad. Sci 1233, 107–116. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Langston RE, and Martinez-Conde S (2013). An oculomotor continuum from exploration to fixation. Proc. Natl. Acad. Sci. USA 110, 6175–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastukhov A, and Braun J (2010). Rare but precious: microsaccades are highly informative about attentional allocation. Vision Res 50, 1173–1184. [DOI] [PubMed] [Google Scholar]
- Peel TR, Hafed ZM, Dash S, Lomber SG, and Corneil BD (2016). A causal role for the cortical frontal eye fields in microsaccade deployment. PLoS Biol 14, e1002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Listorti C, and Rucci M (2013). Microscopic eye movements compensate for nonhomogeneous vision within the fovea. Curr. Biol 23, 1691–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Rucci M, and Carrasco M (2017). Selective attention within the foveola. Nat. Neurosci 20, 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs M, Engbert R, and Kliegl R (2005). Crossmodal coupling of oculomotor control and spatial attention in vision and audition. Exp. Brain Res 166, 427–439. [DOI] [PubMed] [Google Scholar]
- Rucci M, Iovin R, Poletti M, and Santini F (2007). Miniature eye movements enhance fine spatial detail. Nature 447, 851–854. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, and Kastner S (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD (2013). Production, control, and visual guidance of saccadic eye movements. ISRN Neurol 2013, 752384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, and Lakatos P (2009). Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Wilson DA, Radman T, Scharfman H, and Lakatos P (2010). Dynamics of active sensing and perceptual selection. Curr. Opin. Neurobiol 20, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM (2016). A physiological perspective on fixational eye movements. Vision Res 118, 31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Yoshida M, and Hafed ZM (2016). A microsaccadic account of attentional capture and inhibition of return in Posner cueing. Front. Syst. Neurosci 10, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Yoshida M, and Hafed ZM (2018). Dynamics of fixational eye position and microsaccades during spatial cueing: the case of express microsaccades. J. Neurophysiol 119, 1962–1980. [DOI] [PubMed] [Google Scholar]
- Troncoso XG, McCamy MB, Jazi AN, Cui J, Otero-Millan J, Macknik SL, Costela FM, and Martinez-Conde S (2015). V1 neurons respond differently to object motion versus motion from eye movements. Nat. Commun 6, 8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R (2013). Visual attention: a rhythmic process? Curr. Biol 23, R1110–R1112. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Merriam EP, and Heeger DJ (2014). Spontaneous microsaccades reflect shifts in covert attention. J. Neurosci 34, 13693–13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meyers EM, Bichot NP, Serre T, Poggio TA, and Desimone R (2011). Object decoding with attention in inferior temporal cortex. Proc. Natl. Acad. Sci. USA 108, 8850–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Schafer RJ, and Desimone R (2016). Pulvinar-cortex interactions in vision and attention. Neuron 89, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in the manuscript are available by request.


