Supplemental Digital Content is available in the text.
Keywords: blood pressure, hypertension, mice, tail, testosterone
Abstract
This study was conducted to test the hypothesis that the CYP1B1 (cytochrome P450 1B1)-testosterone metabolite 6β-hydroxytestosterone contributes to angiotensin II-induced hypertension by promoting activation of group IV cPLA2α (cytosolic phospholipase A2α) and generation of prohypertensive eicosanoids in male mice. Eight-week-old male intact or orchidectomized cPLA2α+/+/Cyp1b1+/+ and cPLA2α–/–/Cyp1b1+/+ and intact cPLA2α+/+/Cyp1b1–/– mice were infused with angiotensin II (700 ng/kg/min, subcutaneous) for 2 weeks and injected with 6β-hydroxytestosterone (15 μg/g/every third day, intraperitoneal). Systolic blood pressure was measured by tail-cuff and confirmed by radiotelemetry. Angiotensin II-induced increase in systolic blood pressure, cardiac and renal collagen deposition, and reactive oxygen species production were reduced by disruption of the cPLA2α or Cyp1b1 genes or by administration of the arachidonic acid metabolism inhibitor 5,8,11,14-eicosatetraynoic acid to cPLA2α+/+/Cyp1b1+/+ mice. 6β-hydroxytestosterone treatment restored these effects of angiotensin II in cPLA2α+/+/Cyp1b1–/– mice but not in orchidectomized cPLA2α–/–/Cyp1b1+/+ mice, which were lowered by 5,8,11,14-eicosatetraynoic acid in cPLA2α+/+/Cyp1b1–/– mice. Antagonists of prostaglandin E2-EP1/EP3 receptors and thromboxane A2-TP receptors decreased the effect of 6β-hydroxytestosterone in restoring the angiotensin II-induced increase in systolic blood pressure, cardiac and renal collagen deposition, and reactive oxygen species production in cPLA2α+/+/Cyp1b1–/– mice. These data suggest that 6β-hydroxytestosterone promotes angiotensin II-induced increase in systolic blood pressure and associated pathogenesis via cPLA2α activation and generation of eicosanoids, most likely prostaglandin E2 and thromboxane A2 that exerts prohypertensive effects by stimulating EP1/EP3 and TP receptors, respectively. Therefore, agents that selectively block these receptors could be useful in treating testosterone exacerbated angiotensin II-induced hypertension and its pathogenesis.
Hypertension is the leading cause of cardiovascular diseases and mortality.1 It has been well established that hypertension is associated with renal dysfunction and end-organ damage in various animal experimental models of hypertension, including Ang II (angiotensin II).2,3 Testosterone is known to exert cardiovascular effects and has been implicated in the development of hypertension.4–7 The expression of androgen receptor and metabolism of testosterone into various products that include 6β-hydroxytestosterone (6βOHT) are both increased in hypertrophic left ventricles from humans and spontaneously hypertensive rats and decreased in the presence of a human left ventricle–assistance device.8 Testosterone is metabolized into 6βOHT in adult rat cultured myocytes.9 Previously, we showed that the CYP1B1 (cytochrome P450 1B1)-testosterone-derived metabolite 6βOHT is required (acts as a permissive factor) for the development of Ang II-induced hypertension, production of reactive oxygen species (ROS), and cardiac and renal pathogenesis in male mice.10,11 However, the mechanism by which 6βOHT promotes the effect of Ang II to increase blood pressure (BP) is not known.
Ang II-induced hypertension is mediated by group IV cPLA2α (cytosolic phospholipase A2α) activation, via the release of arachidonic acid (AA) and subsequent generation of prohypertensive eicosanoids.12 These include AA-COX (cyclooxygenase) derived thromboxane A2 (TXA2) that acts via prostanoid receptor (TP) and prostaglandin E2 (PGE2) that acts via the EP1/EP3 receptors. Since the testosterone-CYP1B1 generated metabolite 6βOHT is required for Ang II to cause hypertension,10 we performed this study to test the hypothesis that 6βOHT promotes Ang II-induced hypertension and its pathogenesis by enhancing cPLA2α activity resulting in the generation of eicosanoids with prohypertensive effects.
Methods
The authors declare that a detailed Methods Section and all supporting data are available within the article and in the Data Supplement. Other details of analytic methods, study materials, and the data will be made available by the corresponding authors upon reasonable request.
Animal Experiments
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals under protocols approved by the University of Tennessee Health Science Center (UTHSC) Institutional Animal Care and Use Committee (IACUC). Intact or orchidectomized (Orchi) cPLA2α+/+/Cyp1b1+/+, cPLA2α−/−/Cyp1b1+/+, and intact cPLA2α+/+/Cyp1b1−/− mice (all on a C57BL/6J background) were randomly divided into various treatment groups and infused with Ang II (700 ng/kg per minute) or saline for 14 days with subcutaneously implanted osmotic pumps (Alzet, Cupertino, CA; model 1002). Systolic BP (SBP) was measured by the noninvasive tail-cuff method (Kent Scientific; model XBP 1000) and confirmed by radiotelemetry.
We examined the effects of the following genetic modifications or treatments on Ang II-induced hypertension and associated cardiac and renal pathogenesis: (1) cPLA2α gene disruption (cPLA2α−/−/Cyp1b1+/+) in intact mice; (2) treatment of cPLA2α+/+/Cyp1b1+/+ mice with the AA metabolism inhibitor ETYA (5,8,11,14-eicosatetraynoic acid, 50 mg/kg, by intraperitoneal injection every third day)12,13; (3) treatment with the CYP1B1-testosterone generated metabolite, 6βOHT (15 μg/g by intraperitoneal injection every third day) in Orchi-cPLA2α+/+/Cyp1b1+/+ and cPLA2α−/−/Cyp1b1+/+ mice and in intact cPLA2α+/+/Cyp1b1−/− mice; (4) treatment with ETYA+6βOHT in cPLA2α+/+/Cyp1b1−/− mice; and (5) treatment with 6βOHT+EP1 (SC19220),14 EP3 (L-798106),15 or TP (Terutroban)16 receptor antagonists (10 μg/g, by subcutaneous injection every second day) in intact cPLA2α+/+/Cyp1b1−/− mice.
Histological Analysis
Heart and kidney sections from various treatment groups were stained using Masson’s trichrome kit for collagen and dihydroethidium for ROS detection. We used a Pannoramic scanner (3DHISTECH, Budapest, Hungary) available in the UTHSC Core Facility for collagen detection in the heart and kidney sections as described previously.17 The dihydroethidium-stained sections were visualized using a fluorescence microscope (model IX50, Olympus America) with a dual-wavelength filter, excitation at 375 nm, and emission at 585 nm described previously.18 The images were viewed and quantified by blinded investigators using ImageJ 1.42 analysis software (NIH: http://imagej.nih.gov/ij).
Western Blot Analysis
Kidneys were lysed in TissureLyser II (Qiagen) and centrifuged. An equal amount of protein from each lysate was subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The blots were probed with primary anti-phospho (p)-cPLA2α and total (t)-cPLA2α antibodies and the corresponding secondary antibodies. The density of the bands was quantified by blinded investigators using ImageJ 1.42 software (NIH: http://imagej.nih.gov/ij).
Urinary Levels of PGE2 and TXA2 Metabolites
Twenty-four hour urine samples were collected by placing the mice in metabolic cages. PGE2 is rapidly converted in vivo to its 13, 14-dihydro-15-keto metabolite, which undergoes further degradation to yield PGA products. We measured the concentrations of PGE2 metabolites in urine using a Prostaglandin E Metabolite EIA Kit (Cayman Catalog No. 514531), as per the manufacturer’s instructions. This kit enables the conversion of 13, 14-dihydro-15-keto PGA2 and 13, 14-dihydro-15-keto PGE2 to a single, stable derivative that can be quantified. TXA2 is rapidly hydrolyzed nonenzymatically to form TXB2. We measured urinary TXB2 levels using an ELISA kit (Cayman Catalog No. 501020).
Urinary Levels of Proinflammatory Cytokines
Aliquots of urine samples containing 10X protease/phosphatase inhibitor cocktail of AEBSF (4-benzenesulfonyl fluoride hydrochloride), aprotinin, pepstatin, and leupeptin (Cell signaling, Catalog No. 5872S) in Tris base buffer (10 in 90 µL urine) were stored at −80 °C. Inflammatory cytokines IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO, IL-10, IL-12p70, and TNF-α were measured using a V-PLEX Plus Proinflammatory Panel1 (mouse) Kit (catalog No. K15048G-1; Meso scale Discovery, MSD, Rockville, MD), as per the manufacturer’s instructions. Measurement of urinary levels of these cytokines was used as a biomarker of renal inflammation. Prespecified cytokines of interest included in this panel were IL-1β, IL-6, IL-10, and TNF-α.
Statistical Analysis
The data were expressed as the mean±SEM with P<0.05 considered statistically significant. Statistical analysis was performed using Prism 6 (GraphPad Software). For the BP data, a comparison between the groups was performed using 2-way ANOVA with repeated measures. Unpaired t test was used for comparisons between 2 groups with normally distributed data. Multiple groups with normally distributed variables were compared by 1-way ANOVA followed by Tukey multiple comparisons test. In most experiments, the primary outcomes and main comparisons exceeded a power of 0.8 with the number of animals used.
Results
Ang II Increased BP in 6βOHT-Treated Orchi-cPLA2α+/+/Cyp1b1+/+ and Intact cPLA2α+/+/Cyp1b1−/− but Not in Orchi-cPLA2α−/−/Cyp1b1+/+ Mice
CYP1B1 contributes to Ang II-induced hypertension in male mice and rats,19,20 and orchidectomy reduces the ability of Ang II to increase BP.6 Ang II activates cPLA2α and releases AA from tissue phospholipids.21 AA modulates one or more of the cardiovascular effects of Ang II and has been implicated in hypertension.12 In the present study, we observed that systemic infusion of Ang II for 2 weeks increased the SBP as measured by tail-cuff and increased mean arterial pressure, SBP, and diastolic BP as measured by radiotelemetry in 6βOHT-treated Orchi-cPLA2α+/+/Cyp1b1+/+ (Figure 1A, 1D through 1F) and intact cPLA2α+/+/Cyp1b1−/− (Figure 1B, 1D through 1F), but not in Orchi-cPLA2α−/−/Cyp1b1+/+ mice (Figure 1C through 1F). Consistent with our previous report, we observed that the 2-week infusion with Ang II (subcutaneous via osmotic pump) increased the SBP as measured by tail-cuff in intact cPLA2α+/+/Cyp1b1+/+ mice (Figure S1A in the Data Supplement). This effect was decreased in cPLA2α+/+/Cyp1b1−/− mice (Figure S1B) and attenuated in cPLA2α−/−/Cyp1b1+/+ mice (Figure S1C). Ang II did not alter the heart rate (Figure S1D), pulse pressure (Figure S1E), or locomotor activity (Figure S1F) in 6βOHT-treated Orchi-cPLA2α+/+/Cyp1b1+/+, intact cPLA2α+/+/Cyp1b1−/−, and Orchi-cPLA2α−/−/Cyp1b1+/+ mice as measured by radiotelemetry. We have observed that the increase in SBP by Ang II, measured by tail-cuff and radiotelemetry at the same time of the day are comparable.18 Therefore, we measured the SBP in the remaining experiments in this study by tail-cuff.
Figure 1.
Ang II (Angiotensin II) increased the systolic blood pressure (SBP) in 6β-hydroxytestosterone (6βOHT)-treated orchidectomized (Orchi)-cPLA2α+/+/Cyp1b1+/+ and intact male cPLA2α+/+/Cyp1b1/ but not in Orchi-cPLA2α//Cyp1b1+/+ mice. Measurement of SBP by tail-cuff in Orchi-cPLA2α+/+/Cyp1b1+/+ (A), intact male cPLA2α+/+/Cyp1b1/ (B), and Orchi-cPLA2α//Cyp1b1+/+ (C) mice. Measurement of mean arterial BP (MAP, D), SBP (E), and diastolic BP (DBP, F) by radiotelemetry showed similar effects of 6βOHT treatment on the action of Ang II in the mice mentioned above. Saline was used as vehicle control for Ang II. Data are Mean±SEM (n=4-5/group). A 2-way repeated measures ANOVA followed by Tukey multiple comparisons in A through F. *P<0.05 vs. Day 0 value (the day before implantation of the osmotic pump) within the group, $P<0.05 vs 6βOHT+Saline in the corresponding groups; #P<0.05 vs Orchi-cPLA2α//Cyp1b1+/+.
To determine whether cPLA2α affects testosterone levels, we examined the effect of cPLA2α gene disruption on the effect of Ang II on plasma levels of testosterone. Plasma testosterone levels were similar in cPLA2α+/+/Cyp1b1+/+ and cPLA2α−/−/Cyp1b1+/+mice and not altered by Ang II (Figure S2).
Testosterone-CYP1B1 Generated Metabolite, 6βOHT Restored Ang-II-Induced Cardiac and Renal Fibrosis in Orchi-cPLA2α+/+/Cyp1b1+/+ and Intact cPLA2α+/+/Cyp1b1−/− but Not in Orchi-cPLA2α−/−/Cyp1b1+/+ Mice
Orchidectomy and disruption of the Cyp1b1 or cPLA2α genes minimize the effect of Ang II to cause cardiac and renal hypertrophy and fibrosis in male mice.10–12 In the present study, we observed that Ang II increased cardiac and renal fibrosis as indicated by increased collagen deposition (blue color) in 6βOHT-treated Orchi-cPLA2α+/+/Cyp1b1+/+ and intact cPLA2α+/+/Cyp1b1−/− but not in Orchi-cPLA2α−/−/Cyp1b1+/+ mice (Figure 2A through 2C). Consistent with our previous studies,10–12 Ang II produced cardiac and renal fibrosis in intact cPLA2α+/+/Cyp1b1+/+ mice (Figure S3A through S3C). These effects were decreased in cPLA2α+/+/Cyp1b1−/− mice and further attenuated in cPLA2α−/−/Cyp1b1+/+ mice (Figure S3A through S3C).
Figure 2.
Ang II (angiotensin II) increased the cardiac and renal collagen deposition detected by Masson’s Trichrome staining in 6β-hydroxytestosterone (6βOHT)-treated orchidectomized (Orchi)-cPLA2α+/+/Cyp1b1+/+ and intact male cPLA2α+/+/Cyp1b1/ but not in Orchi-cPLA2α//Cyp1b1+/+ mice. A, Representative images of heart and kidney. B, Quantitation of cardiac collagen deposition. C, Quantitation of renal collagen deposition. Saline was used as vehicle control for Ang II. Data are Mean±SEM (n=3/group). A 1-way ANOVA followed by Tukey multiple comparisons in B and C.
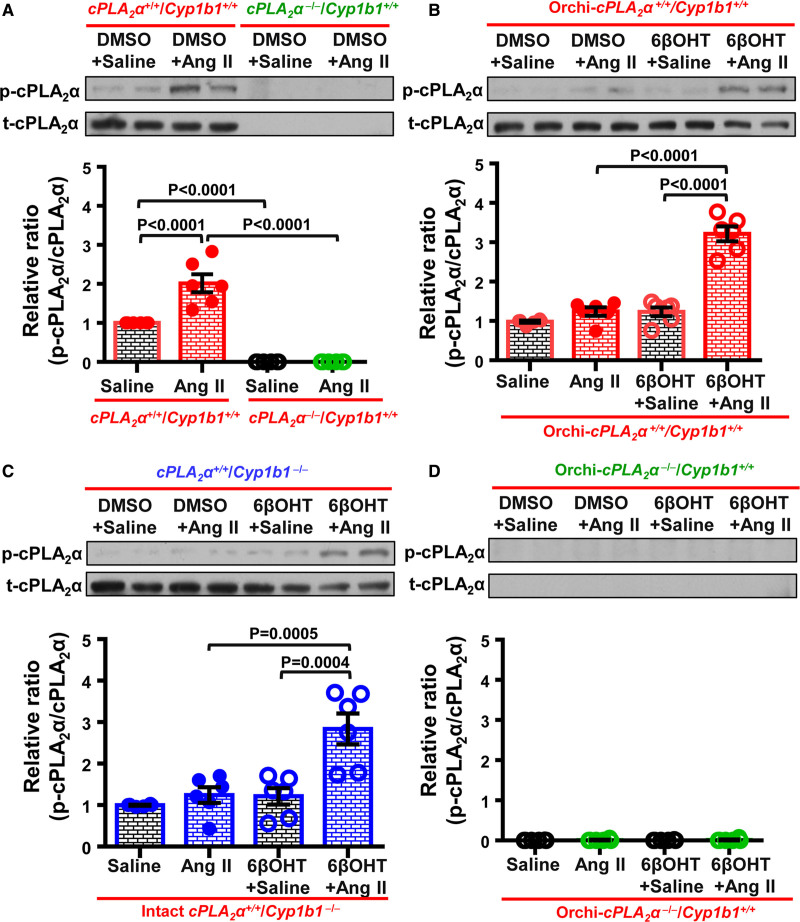
Testosterone-CYP1B1 Generated Metabolite, 6βOHT Restored Ang II-Induced Increase in Renal cPLA2α Activity in Orchi-cPLA2α+/+/Cyp1b1+/+ and Intact cPLA2α+/+/Cyp1b1−/− but Not in Orchi-cPLA2α−/−/Cyp1b1+/+ Mice
Several BP regulating hormones, including Ang II, activate cPLA2 and release AA.22–25 We observed that Ang II increased cPLA2α activity in intact cPLA2α+/+/Cyp1b1+/+ but not in cPLA2α−/−/Cyp1b1+/+ mice, as measured by an increased ratio of p-cPLA2α to t-cPLA2α expression in the kidney (Figure 3A). However, this effect was blunted in Orchi-cPLA2α+/+/Cyp1b1+/+ (Figure 3B), intact cPLA2α+/+/Cyp1b1−/− (Figure 3C), and Orchi-cPLA2α−/−/Cyp1b1+/+ mice (Figure 3D). 6βOHT treatment restored the ability of Ang II to increase cPLA2α activity in Orchi-cPLA2α+/+/Cyp1b1+/+ (Figure 3B), intact cPLA2α+/+/Cyp1b1−/− (Figure 3C) but not in Orchi-cPLA2α−/−/Cyp1b1+/+ mice (Figure 3D).
Figure 3.
Ang II (Angiotensin II) increased cPLA2α (cytosolic phospholipase A2α) activity measured by an increased ratio of phosphorylated (p) to total (t)-cPLA2α expression in the kidney of cPLA2α+/+/Cyp1b1+/+ mice, an effect which was blunted in orchidectomized (Orchi)-cPLA2α+/+/Cyp1b1+/+ and intact male cPLA2α+/+/Cyp1b1/ mice and restored by 6β-hydroxytestosterone (6βOHT) treatment. cPLA2α expression remained absent in intact or Orchi-cPLA2α//Cyp1b1+/+ mice. Representative blots and quantitation of cPLA2α expression in cPLA2α+/+/Cyp1b1+/+ and cPLA2α//Cyp1b1+/+ (A), Orchi-cPLA2α+/+/Cyp1b1+/+ (B), cPLA2α+/+/Cyp1b1/ (C), and Orchi-cPLA2α//Cyp1b1+/+ (D) mice. Saline was used as vehicle control for Ang II, and dimethyl sulfoxide (DMSO) as vehicle control for 6βOHT. Top panel: representative blots; bottom panel: quantitation. Data are Mean±SEM (n=6/group). A 1-way ANOVA followed by Tukey multiple comparisons in A through D.
Testosterone-CYP1B1 Generated Metabolite, 6βOHT Restored Ang II-Induced Increased Urinary Excretion of PGE2 and TXA2 Metabolites in Orchi-cPLA2α+/+/Cyp1b1+/+ and Intact cPLA2α+/+/Cyp1b1−/− but Not in Orchi-cPLA2α−/−/Cyp1b1+/+ Mice
On activation by Ang II, cPLA2α releases AA from tissue phospholipids that are metabolized into TXA2 and PGE2, F2α, D2, and I2 by COX enzymes.21,26 The vascular effects of PGE2 and TXA2 contribute to prohypertensive mechanisms, PGE2 via activation of the EP1/EP3 receptors and TXA2 via activation of the TP receptor.16,27 In the current study, Ang II increased the urinary excretion of PGE2 metabolites and the TXA2 metabolite TXB2 in cPLA2α+/+/Cyp1b1+/+ mice (Figure S4A and S4B). However, the Ang II-induced increase in urinary excretion of PGE2 metabolites was abrogated (Figure S4A) and the increase in TXB2 (Figure S4B) was markedly reduced in cPLA2α+/+/Cyp1b1−/− mice. We observed that the effect of Ang II to increase urinary excretion of PGE2 and TXA2 metabolites was blunted in cPLA2α−/−/Cyp1b1+/+ mice (Figure S4A and S4B), a finding similar to our previously published data.12 Testosterone-CYP1B1 metabolite 6βOHT treatment restored the effect of Ang II to increase the urinary excretion of PGE2 metabolites (Figure S4C) and TXB2 (Figure S4D) in Orchi-cPLA2α+/+/Cyp1b1+/+ and intact cPLA2α+/+/Cyp1b1−/− but not in Orchi-cPLA2α−/−/Cyp1b1+/+ mice.
AA Metabolism Inhibitor Decreased the Effect of 6βOHT to Restore Ang II-Induced Increased SBP, Cardiac and Renal Fibrosis, and Urinary Excretion of PGE2 and TXA2 Metabolites in Intact cPLA2α+/+/Cyp1b1−/− Mice
Administration of ETYA blunted the Ang II-induced increase in SBP in cPLA2α+/+/Cyp1b1+/+ mice (Figure 4A), as described previously.12 In this study, ETYA attenuated the effect of 6βOHT to restore the Ang II-induced increase in SBP in intact cPLA2α+/+/Cyp1b1−/− mice (Figure 4A). ETYA also decreased the Ang II-induced increase in cardiac and renal fibrosis (Figure 4B through 4D) as indicated by reduced collagen deposition, and reduced the urinary excretion of PGE2 metabolites (Figure 4E) and TXB2 (Figure 4F) in intact cPLA2α+/+/Cyp1b1−/− mice.
Figure 4.
Arachidonic acid inhibitor 5, 8, 11, 14-eicosatetraynoic acid (ETYA) decreased the Ang II (angiotensin II)-induced increase in systolic blood pressure (SBP), cardiac, and renal collagen deposition, and urinary excretion of prostaglandin E2 (PGE2) metabolites and thromboxane A2 (TXA2) metabolite TXB2 in cPLA2α+/+/Cyp1b1+/+ and 6β-hydroxytestosterone (6βOHT)-treated intact male cPLA2α+/+/Cyp1b1/ mice. A, SBP measured by tail-cuff. B, Representative images of heart and kidney. C, Quantitation of cardiac collagen deposition. D, Quantitation of renal collagen deposition. E, Quantitation of urinary excretion of PGE2 metabolites. F, Quantitation of urinary excretion of TXA2 metabolite TXB2. Saline was used as vehicle control for Ang II and dimethyl sulfoxide (DMSO) as vehicle control for 6βOHT and ETYA. Data are Mean±SEM (n=3-5/group). A 2-way repeated measures ANOVA followed by Tukey multiple comparisons in A and 1-way ANOVA followed by Tukey multiple comparisons in C through F. *P<0.05 vs Day 0 (Day of osmotic pump implantation for Ang II infusion); &P<0.05 vs ETYA+Saline and ^P vs DMSO+Ang II in cPLA2α+/+/Cyp1b1+/+; $P<0.05 vs 6βOHT+ETYA+Saline and @P<0.05 vs 6βOHT+Ang II in cPLA2α+/+/Cyp1b1/.
Antagonists of PGE2-EP1, EP3, and TXA2-TP Receptors Reduced the Effect of 6βOHT to Restore Ang II-Induced Increased SBP, and Cardiac and Renal Fibrosis in Intact cPLA2α+/+/Cyp1b1−/− Mice
Antagonists of PGE2-EP1 (EP1RA), EP3 (EP3RA), and TXA2 (TPRA) receptors reduced the ability of 6βOHT to restore Ang II-induced increase in SBP (Figure 5A) and cardiac and renal fibrosis (Figure 6B through 6D) in cPLA2α+/+/Cyp1b1−/− mice.
Figure 5.
Receptor antagonists of prostaglandin E2 (PGE2) EP1 (EP1RA), EP3 (EP3RA), and thromboxane A2 (TXA2) (TPRA) decreased the effect of 6β-hydroxytestosterone (6βOHT) to restore Ang II (angiotensin II)-induced increase in systolic blood pressure (SBP), cardiac and renal collagen deposition detected by Masson’s Trichrome staining in cPLA2α+/+/Cyp1b1/ mice. A, SBP measured by tail-cuff. B, Representative images of heart and kidney. C, Quantitation of cardiac collagen deposition. D, Quantitation of renal collagen deposition. Saline was used as vehicle control for Ang II. Data are Mean±SEM (n=3-5/group). A 2-way repeated measures ANOVA followed by Tukey multiple comparisons in A and 1-way ANOVA followed by Tukey multiple comparisons in C and D. *P<0.05 vs Day 0 (Day of osmotic pump implantation for Ang II infusion); aP<0.05 vs EP1RA+6βOHT+Saline, bP<0.05 vs EP1RA+6βOHT+Saline, cP<0.05 vs TPRA+6βOHT+Saline and dP<0.05 vs 6βOHT+Ang II in cPLA2α+/+/Cyp1b1/.
Figure 6.
Ang II (Angiotensin II) failed to increase the production of reactive oxygen species (ROS) measured by the quantitation of 2-hydroxyethidium fluorescence in the heart and kidney in cPLA2α+/+/Cyp1b1/ mice and restored by 6β-hydroxytestosterone (6βOHT) but decreased by the arachidonic acid metabolism inhibitor 5, 8, 11, 14-eicosatetraynoic acid (ETYA) and receptor antagonists of prostaglandin E2 (PGE2) EP1 (EP1RA), EP3 (EP3RA), and thromboxane (TX) A2 (TPRA). Effect of 6βOHT and ETYA in Ang II-infused cPLA2α+/+/Cyp1b1/ mice on cardiac (A, representative images, and B, quantitation) and renal (A, representative images, and C, quantitation) ROS production. Effect of EP1RA, EP3RA, and TPRA on 6βOHT-treated Ang II-infused cPLA2α+/+/Cyp1b1/ mice on cardiac (D, representative images, and E, quantitation) and renal (C, representative images, and F, quantitation) ROS production. Saline was used as vehicle control for Ang II, and dimethyl sulfoxide (DMSO) as vehicle control for 6βOHT, ETYA, EP1RA, EP3RA, and TPRA. Data are Mean±SEM (n=3/group). A 1-way ANOVA followed by Tukey multiple comparisons in B, C, E and F.
6βOHT Promotes Ang II-Induced Cardiac and Renal ROS Production via cPLA2α in Male Mice
Oxidative stress has been implicated in various models of hypertension, including the Ang II model.28,29 Processes contributing to these phenomena have been attributed, in part, to increased generation of ROS, particularly superoxide (·O2−) and hydrogen peroxide (H2O2),30,31 which function as important second messengers.31,32 We previously showed that Ang II-induced ROS production in male mice was abrogated by orchidectomy or by disruption of the Cyp1b1 or cPLA2α genes.10–12 In the present study, we show that treatment of intact cPLA2α+/+/Cyp1b1+/+ mice with the AA metabolism inhibitor ETYA reduced the effect of Ang II to increase cardiac and renal ROS production (Figure S5A through S5C) similar to that observed in intact cPLA2α−/−/Cyp1b1+/+ mice in response to Ang II. Moreover, the testosterone-CYP1B1 generated metabolite 6βOHT restored the effect of Ang II to increase cardiac and renal ROS production in Orchi-cPLA2α+/+/Cyp1b1+/+ but not in Orchi-cPLA2α−/−/Cyp1b1+/+ mice (Figure S5D through S5F). However, Ang II failed to increase cardiac and renal ROS production in intact cPLA2α+/+/Cyp1b1−/− mice, which were restored by 6βOHT and decreased by ETYA treatment (Figure 6A through 6C). Furthermore, treatment with EP1RA, EP3RA, and TPRA decreased the effect of 6βOHT in restoring the Ang II-induced increase in cardiac and renal ROS production in intact cPLA2α+/+/Cyp1b1−/− mice (Figure 6D through 6F).
6βOHT Promotes Ang II-Induced Excretion of Proinflammatory Cytokines via cPLA2α in Male Mice
Disruption of the cPLA2α gene protects male mice from the Ang II-induced increases in renal F4/80+ macrophages and CD3+ T lymphocytes infiltration.12 To examine the effect of 6βOHT on Ang II-induced renal inflammation, we measured the urinary excretion of proinflammatory cytokines. Ang II increased the urinary excretion of IL-1β, IL-6, and IL-12p70 (Figure S6A) in intact cPLA2α+/+/Cyp1b1+/+ mice. This effect was maintained after 6βOHT treatment in Orchi-cPLA2α+/+/Cyp1b1+/+ and intact cPLA2α+/+/Cyp1b1−/− but not in Orchi-cPLA2α−/−/Cyp1b1+/+ mice. Ang II also increased the urinary excretion of TNF-α (Figure S6A) and KC/GRO (Figure S6B) in intact cPLA2α+/+/Cyp1b1+/+ and 6βOHT-treated Orchi-cPLA2α+/+/Cyp1b1+/+, intact cPLA2α+/+/Cyp1b1−/−, and in Orchi-cPLA2α−/−/Cyp1b1+/+ mice. Among other cytokines, Ang II increased IFN-γ only in intact cPLA2α+/+/Cyp1b1−/− and IL-4 only in Orchi-cPLA2α+/+/Cyp1b1+/+ mice (Figure S6B).
Discussion
The present study provides the following novel information: (1) The CYP1B1-testosterone generated metabolite 6βOHT promoted Ang II-induced hypertension, cardiac and renal fibrosis, ROS production, and increased urinary levels of cytokines IL-1β, IL-6, and IL-12p70 by enhancing cPLA2α activity and generation of AA-COX metabolites PGE2 and TXA2 in male mice, (2) PGE2 through the EP1/EP3 receptors, and TXA2 via TP receptors contribute to the effects of 6βOHT to promote Ang II-induced increase in BP and associated cardiac and renal fibrosis and ROS production in male mice.
Previously, we reported that cPLA2α gene disruption prevents Ang II-induced hypertension, cardiac and renal fibrosis, and ROS production in male mice.12 Moreover, we showed that Ang II-induced hypertension and associated pathogenesis are also decreased by disruption of the Cyp1b1 gene, similar to the effect of orchidectomy, and restored by 6βOHT treatment.10,11 In the present study, we demonstrated that disruption of the cPLA2α gene does not alter the plasma testosterone levels in response to Ang II. This observation, together with our previous finding that Ang II stimulation of 6βOHT production was abolished by disruption of Cyp1b1 gene,10 suggests that 6βOHT must act upstream of cPLA2α. Supporting this view was our finding that the effect of 6βOHT to restore the Ang II-induced increase in SBP measured by tail-cuff, or mean arterial pressure, SBP and diastolic BP measured by radiotelemetry, and cardiac and renal fibrosis as indicated by collagen deposition, and ROS production was ameliorated in Orchi-cPLA2α+/+/Cyp1b1+/+ and intact cPLA2α+/+/Cyp1b1−/− but not Orchi-cPLA2α−/−/Cyp1b1+/+ mice. From these observations, it follows that the effect of 6βOHT to promote Ang II-induced increases in BP, cardiac and renal fibrosis, and ROS production are dependent on cPLA2α. Ang II activates cPLA2α, which selectively catalyzes the release of AA from tissue phospholipids.23 Our finding that the Ang II-induced increase in cPLA2α activity indicated by its phosphorylation in the kidney of intact cPLA2α+/+/Cyp1b1+/+ mice was abolished in Orchi-cPLA2α−/−/Cyp1b1+/+, Orchi-cPLA2α+/+/Cyp1b1+/+, and intact cPLA2α+/+/Cyp1b1−/− mice, suggests that one or more CYP1B1-testosterone derived metabolite, most likely 6βOHT, is required for cPLA2α activation by Ang II. Supporting this hypothesis was our demonstration that 6βOHT treatment restored the effect of Ang II to cause cPLA2α phosphorylation without altering its expression in the kidneys of Orchi-cPLA2α+/+/Cyp1b1+/+ and intact cPLA2α+/+/Cyp1b1−/−, but not in cPLA2α−/−/Cyp1b1+/+ mice. The mechanism by which 6βOHT promotes Ang II-induced cPLA2α activation is not known. It is possible that 6βOHT acts on genomic androgen receptor or GPRC6A (nongenomic G protein-coupled receptor C6A) via independent pathways or by crosstalk between GPRC6A and nuclear androgen receptor to enhance the activity of one or more signaling molecules, including the cellular calcium levels and ERK1/2 (extracellular signal-regulated kinase) activity33 that are involved in cPLA2α activation.34 Future studies will explore this mechanism.
Our findings that (1) Ang II increased the urinary excretion of PGE2 measured as its metabolites, and TXB2, the stable metabolite of TXA2, in cPLA2α+/+/Cyp1b1+/+ mice and (2) Ang II increased the urinary excretion of PGE2 metabolites and TXB2 in 6βOHT-treated Orchi-cPLA2α+/+/Cyp1b1+/+ and intact cPLA2α+/+/Cyp1b1−/−, but not Orchi-cPLA2α−/−/Cyp1b1+/+ mice, suggests that AA released by cPLA2α via COX-generated metabolite(s) mediate the action of 6βOHT to promote Ang II-induced hypertension, cardiac and renal fibrosis, and ROS production. However, the fact that the effect of Ang II on urinary TXB2 level like on the SBP, in the intact cPLA2α+/+/Cyp1b1−/− mice was reduced but not abolished suggests that testosterone and/or its other metabolites contribute to these effects of Ang II. Supporting this conclusion was our observation that AA metabolism inhibitor ETYA12 decreased the effect of 6βOHT to restore the Ang II-induced increase in BP, and cardiac and renal fibrosis, ROS production, and the urinary output of PGE2 metabolites and TXB2 in intact cPLA2α+/+/Cyp1b1−/− mice. Ang II-induced cardiac hypertrophy and fibrosis and renal injury have been attributed to the increase in BP.35–38 Therefore, restoration by 6βOHT of Ang II-induced cardiac and renal fibrosis could also result from an increase in BP in intact cPLA2α+/+/Cyp1b1−/− mice. Ang II infusion in intact cPLA2α+/+/Cyp1b1−/− mice in the absence of 6βOHT increased SBP by 10 to 20 mmHg but did not cause cardiac and renal fibrosis suggesting that this small increase in SBP was most likely insufficient to produce these effects. Mechanical stretch can also increase cPLA2 activity and production of eicosanoids39 and ROS.40 Therefore, hypertension-induced stretch in these mice might enhance cPLA2α activation and eicosanoids production that contributes to the effect of 6βOHT on restoring cardiac and renal fibrosis associated with Ang II-induced hypertension. However, the prohypertensive eicosanoids that contribute to Ang II-induced hypertension also include lipoxygenase generated 12S-hydroxyeicosatetraenoic acid, and CYP450 A1 derived 20-hydroxyeicosatetraenoic acid.41,42 Therefore, the contribution of these eicosanoids to the observed effects of 6βOHT on restoring cardiac and renal fibrosis associated with Ang II-induced hypertension cannot be excluded, and it is a subject of current investigation.
COX-AA-derived metabolites PGE2 activate EP1/EP3 receptors to produce vasoconstriction and activate EP2 and EP4 receptors to produce vasodilation43 and signaling of TXA2 through TP receptor results in vasoconstriction.44 The PGE2 through EP1 and/or EP3,14,15 and TXA2 via TP45,46 receptors, contributes to the vasoconstrictor and hypertensive effects of Ang II.14,15,47 PGE2/EP3 signaling pathway has also been implicated in Nω-nitro-L-arginine methyl ester hydrochloride/high salt–induced hypertension48 and impaired vasodilation and hypertension in the high salt diet-fed S-P467L mice with decreased peroxisome proliferator-activated receptor-gamma activity.49 In the present study, the selective antagonists of EP1 (SC19220),50 EP3 (L-798106),15 and TXA2-TP (Terutroban)51 receptors inhibited the effect of 6βOHT to restore the Ang II-induced increase in SBP, cardiac and renal fibrosis, and ROS production in the intact cPLA2α+/+/Cyp1b1−/− mice. These observations suggest that PGE2 and TXA2 contribute to these effects of 6βOHT via activation of PGE2-EP1 and EP3, and TP receptors, respectively. Since EP1, EP3, and TP receptor antagonists did not alter the basal BP in 6βOHT-treated mice in the absence of Ang II, it appears that the amount of PGE2 and TXA2 formed by the low basal activity of cPLA2α and AA release is insufficient to increase BP. Also, it is possible that PGE2, via its effects on the EP2 and EP4 receptors that decrease the vascular tone,43 masks the vasoconstrictor effect of PGE2 that is mediated via EP1 and EP3 and TXA2 through TP receptors.
The mechanism by which PGE2 via EP1 and EP3 receptors, and TXA2 via TP receptor participate in the effect of 6βOHT to promote Ang II-induced increase in SBP could involve an increase in vascular tone caused by the increase in cell calcium facilitated by these eicosanoids14,44,52,53 and the role of calcium sensitization via Rho-kinase activation52,53 in this process remains to be determined. Ang II increases the generation of ROS and isolevuglandin protein adducts in dendritic cells, the proliferation of T cells, and increased levels of cytokines that contribute to its hypertensive effect and renal fibrosis.54 In the Nω-nitro-L-arginine methyl ester hydrochloride/high salt-induced model of hypertension, PGE2 via EP3 receptors results in ROS production, dendritic cell activation, and accumulation of isolevoglandin protein adducts in spleen cells, production of proinflammatory cytokines, and renal fibrosis.48 PGE2 also acts directly on dendritic cells via the EP1 receptor to increase isolevuglandin adducted proteins.48 PGE2-EP3 receptors have also been implicated in the Ang II-induced increase in cardiac expression of NOX2 (NADPH oxidase), production of proinflammatory cytokines, cardiac dysfunction, and hypertrophy.47
We reported previously that Cyp1b1 gene disruption in male mice inhibits Ang II-induced infiltration of renal CD4+ T cells and was restored by 6βOHT treatment.11 Therefore, it is possible that PGE2 and TXA2 via EP1 and EP3 and TP receptors, respectively, contribute to the effect of 6βOHT to promote Ang II-induced hypertension, cardiac and renal fibrosis by increasing ROS production, the activity of immune cells, and the generation of proinflammatory cytokines. Supporting this view were our data that Ang II-induced increased urinary excretion of proinflammatory cytokines IL-1β, IL-6, and IL-12p70 was reduced in 6βOHT-treated Orchi-cPLA2α−/−/Cyp1b1+/+ but not in 6βOHT-treated Orchi-cPLA2α++/Cyp1b1+/+ and intact cPLA2α+/+/Cyp1b1−/− mice. Surprisingly, the Ang II-induced increase in excretion of TNF-α was similar in intact cPLA2α++/Cyp1b1+/+ and 6βOHT-treated Orchi-cPLA2α++/Cyp1b1+/+, intact cPLA2α+/+/Cyp1b1−/− and Orchi-cPLA2α−/−/Cyp1b1+/+ mice, suggesting that the effect of 6βOHT in the promotion of Ang II-induced excretion of TNF-α is independent of cPLA2α activity. Therefore, it seems that TNF-α does not contribute to the effect of 6βOHT in promoting Ang II-induced hypertension and associated pathogenesis. Further studies are required to elucidate the significance and the mechanism by which 6βOHT promotes Ang II-induced renal excretion of TNF-α independent of cPLA2α.
In conclusion, 6βOHT, the metabolite of testosterone produced by CYP1B1, contributes to Ang II-induced hypertension, cardiac and renal fibrosis, production of ROS, and the cytokines IL-1β, IL-6, and IL-12p70 by promoting cPLA2α activity and generating the AA-COX metabolites PGE2 and TXA2 in male mice (Graphical Abstract; Figure S7). PGE2 and TXA2 via EP1/EP3 and TP receptors, respectively, contribute to the effects of 6βOHT to promote Ang II-induced increase in BP and associated cardiac and renal fibrosis, ROS, and cytokine production in male mice. Therefore, selective inhibitors of EP1, EP3, and TXA2 receptors and CYP1B1 activity would be useful for treating Ang II and testosterone-dependent hypertension and its pathogenesis.
Perspectives
We have shown that Ang II-induced hypertension and associated pathogenesis are mediated by prohypertensive eicosanoids produced by activation of cPLA2α in male mice.12 Moreover, testosterone-CYP1B1 generated metabolite 6βOHT also contributes to Ang II-induced hypertension and its pathogenesis.10,11 This study provides novel insight into the mechanism of interactions of cPLA2α and testosterone metabolite 6βOHT. We show that 6βOHT acts upstream of cPLA2α and by enhancing its activity in response to Ang II and production of PGE2 and TXA2, which via EP1 and EP3 and TXA2 receptors, respectively promotes Ang II-induced hypertension and associated pathogenesis. However, the site of interaction of cPLA2α and CYP1B1 is not known. In view of our recent work that testosterone-CYP1B1 generated 6βOHT in the paraventricular nucleus by increasing sympathetic activity contributes to Ang II-induced hypertension,18 it would be important to determine the interaction of cPLA2α and CYP1B1-generated 6βOHT in the paraventricular nucleus in Ang II-induced hypertension and cardiovascular and renal pathogenesis.
Acknowledgments
We thank Dr Kyle Johnson Moore, Director, Office of Scientific Writing, University of Tennessee Health Science Center for her editorial assistance.
Sources of Funding
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute grants R01HL-19134–44, R01HL-079109-09, and UTHSC CORNET Award (K.U. Malik). J.V. Bonventre was supported by National Institutes of Health, National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK) Grants R37DK039773 and R01DK072381. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
Disclosures
J.V. Bonventre is cofounder and holds equity in Goldfinch Bio. He is co-inventor on KIM-1 and kidney organoid patents assigned to Mass General Brigham. He is consultant and owns equity in Coegin Pharma. J.V. Bonventre’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare International, Boston, MA in accordance with their conflict of interest policies.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 6βOHT
- 6β-hydroxytestosterone
- AA
- arachidonic acid
- Ang II
- angiotensin II
- BP
- blood pressure
- COX
- cyclooxygenase
- cPLA2α
- cytosolic phospholipase A2α
- CYP1B1
- cytochrome P450 1B1
- ERK1/2
- (extracellular signal-regulated kinase)
- PGE2
- prostaglandin E2
- ROS
- reactive oxygen species
- SBP
- systolic blood pressure
For Sources of Funding and Disclosures, see page 1065.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.17927.
Contributor Information
Chi Young Song, Email: csong1@uthsc.edu.
Shubha R. Dutta, Email: sdutta4@uthsc.edu.
Ajeeth Pingili, Email: ajeethreddy@gmail.com.
Ji Soo Shin, Email: jshin6@uthsc.edu.
Frank J. Gonzalez, Email: gonzalef@mail.nih.gov.
Joseph V. Bonventre, Email: JBONVENTRE@BWH.HARVARD.EDU.
Novelty and Significance
What Is New?
6β-hydroxytestosterone (6βOHT), a metabolite of testosterone formed by CYP1B1 (cytochrome P450 1B1), contributes to Ang II (angiotensin II)-induced hypertension, cardiac and renal fibrosis, and reactive oxygen species (ROS) production by promoting cPLA2α (cytosolic phospholipase A2α) activation, and production of the arachidonic acid metabolites prostaglandins (PG) E2 and thromboxane (TXA2) in male mice.
PGE2 by stimulating the EP1/EP3 receptors and TXA2 via TP receptor contributes to the 6βOHT effect in promoting Ang II-induced hypertension and cardiac and renal fibrosis and producing reactive oxygen species.
What Is Relevant?
Selective inhibitors of CYP1B1 that prevent the generation of 6βOHT from testosterone or EP1, EP3, and TP receptor antagonists that minimize the effects of 6βOHT could be useful in treating Ang II-dependent hypertension in hyperandrogenism or hypogonadal males on testosterone replacement therapy.
Summary
The testosterone-CYP1B1-generated metabolite 6βOHT contributes to the development of Ang II-induced hypertension, cardiac and renal fibrosis, reactive oxygen species production, and renal proinflammatory cytokine production in male mice, most likely via enhanced cPLA2α activity. 6βOHT promotes Ang II-induced activation of cPLA2α, leading to increased production of PGE2 and TXA2. Therefore, PGE2 via EP1 and EP3, and TXA2 via TP receptors, respectively contribute to the effect of 6βOHT to promote Ang II-induced hypertension and associated pathogenesis in male mice.
References
- 1.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6 [DOI] [PubMed] [Google Scholar]
- 2.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sánchez-López E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21:16–20. doi: 10.1093/ndt/gfi265 [DOI] [PubMed] [Google Scholar]
- 4.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199 [DOI] [PubMed] [Google Scholar]
- 5.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension. 1999;344pt 2920–923. doi: 10.1161/01.hyp.34.4.920 [DOI] [PubMed] [Google Scholar]
- 6.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004 [DOI] [PubMed] [Google Scholar]
- 7.Papamitsou T, Barlagiannis D, Papaliagkas V, Kotanidou E, Dermentzopoulou-Theodoridou M. Testosterone-induced hypertrophy, fibrosis and apoptosis of cardiac cells–an ultrastructural and immunohistochemical study. Med Sci Monit. 2011;17:BR266–BR273. doi: 10.12659/msm.881930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thum T, Borlak J. Testosterone, cytochrome P450, and cardiac hypertrophy. FASEB J. 2002;16:1537–1549. doi: 10.1096/fj.02-0138com [DOI] [PubMed] [Google Scholar]
- 9.Thum T, Borlak J. Cytochrome P450 mono-oxygenase gene expression and protein activity in cultures of adult cardiomyocytes of the rat. Br J Pharmacol. 2000;130:1745–1752. doi: 10.1038/sj.bjp.0703465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pingili AK, Kara M, Khan NS, Estes AM, Lin Z, Li W, Gonzalez FJ, Malik KU. 6β-hydroxytestosterone, a cytochrome P450 1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension and its pathogenesis in male mice. Hypertension. 2015;65:1279–1287. doi: 10.1161/HYPERTENSIONAHA.115.05396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pingili AK, Thirunavukkarasu S, Kara M, Brand DD, Katsurada A, Majid DS, Navar LG, Gonzalez FJ, Malik KU. 6β-Hydroxytestosterone, a Cytochrome P450 1B1-Testosterone-metabolite, mediates Angiotensin II-induced renal dysfunction in male mice. Hypertension. 2016;67:916–926. doi: 10.1161/HYPERTENSIONAHA.115.06936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan NS, Song CY, Jennings BL, Estes AM, Fang XR, Bonventre JV, Malik KU. Cytosolic phospholipase A2α is critical for angiotensin II-induced hypertension and associated cardiovascular pathophysiology. Hypertension. 2015;65:784–792. doi: 10.1161/HYPERTENSIONAHA.114.04803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun FF, McGuire JC, Morton DR, Pike JE, Sprecher H, Kunau WH. Inhibition of platelet arachidonic acid 12-lipoxygenase by acetylenic acid compounds. Prostaglandins. 1981;21:333–343. doi: 10.1016/0090-6980(81)90151-9 [DOI] [PubMed] [Google Scholar]
- 14.Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Miao Y, Zhang Y, Dou D, Liu L, Tian X, Yang G, Pu D, Zhang X, Kang J, et al. Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arterioscler Thromb Vasc Biol. 2012;32:3024–3032. doi: 10.1161/ATVBAHA.112.254052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43:364–369. doi: 10.1161/01.HYP.0000112225.27560.24 [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee K, Pingili AK, Singh P, Dhodi AN, Dutta SR, Gonzalez FJ, Malik KU. Testosterone Metabolite metabolite 6β-Hydroxytestosterone Contributes contributes to Angiotensin II-Induced induced Abdominal abdominal Aortic aortic Aneurysms aneurysms in Apoe-/- Male male Micemice. J Am Heart Assoc. 2021;10:e018536. doi: 10.1161/JAHA.120.018536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh P, Dutta SR, Song CY, Oh S, Gonzalez FJ, Malik KU. Brain Testosterone-CYP1B1 (Cytochrome P450 1B1) Generated generated Metabolite metabolite 6β-Hydroxytestosterone Promotes promotes Neurogenic neurogenic Hypertension hypertension and Inflammationinflammation. Hypertension. 2020;76:1006–1018. doi: 10.1161/HYPERTENSIONAHA.120.15567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennings BL, Sahan-Firat S, Estes AM, Das K, Farjana N, Fang XR, Gonzalez FJ, Malik KU. Cytochrome P450 1B1 contributes to angiotensin II-induced hypertension and associated pathophysiology. Hypertension. 2010;56:667–674. doi: 10.1161/HYPERTENSIONAHA.110.154518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings BL, Anderson LJ, Estes AM, Yaghini FA, Fang XR, Porter J, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome P450 1B1 contributes to renal dysfunction and damage caused by angiotensin II in mice. Hypertension. 2012;59:348–354. doi: 10.1161/HYPERTENSIONAHA.111.183301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao GN, Lassègue B, Alexander RW, Griendling KK. Angiotensin II stimulates phosphorylation of high-molecular-mass cytosolic phospholipase A2 in vascular smooth-muscle cells. Biochem J. 1994;299:197–201. doi: 10.1042/bj2990197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonventre JV, Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest. 1988;82:168–176. doi: 10.1172/JCI113566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther. 1998;284:388–398. [PubMed] [Google Scholar]
- 24.Trevisi L, Bova S, Cargnelli G, Ceolotto G, Luciani S. Endothelin-1-induced arachidonic acid release by cytosolic phospholipase A2 activation in rat vascular smooth muscle via extracellular signal-regulated kinases pathway. Biochem Pharmacol. 2002;64:425–431. doi: 10.1016/s0006-2952(02)01066-3 [DOI] [PubMed] [Google Scholar]
- 25.Muthalif MM, Benter IF, Uddin MR, Malik KU. Calcium/calmodulin-dependent protein kinase IIalpha mediates activation of mitogen-activated protein kinase and cytosolic phospholipase A2 in norepinephrine-induced arachidonic acid release in rabbit aortic smooth muscle cells. J Biol Chem. 1996;271:30149–30157. doi: 10.1074/jbc.271.47.30149 [DOI] [PubMed] [Google Scholar]
- 26.McGiff JC. Prostaglandins, prostacyclin, and thromboxanes. Annu Rev Pharmacol Toxicol. 1981;21:479–509. doi: 10.1146/annurev.pa.21.040181.002403 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Guan Y, Schneider A, Brandon S, Breyer RM, Breyer MD. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension. 2000;35:1129–1134. doi: 10.1161/01.hyp.35.5.1129 [DOI] [PubMed] [Google Scholar]
- 28.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d [DOI] [PubMed] [Google Scholar]
- 29.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2003;35:851–859. doi: 10.1016/s0022-2828(03)00145-7 [DOI] [PubMed] [Google Scholar]
- 31.Wenzel S, Taimor G, Piper HM, Schlüter KD. Redox-sensitive intermediates mediate angiotensin II-induced p38 MAP kinase activation, AP-1 binding activity, and TGF-beta expression in adult ventricular cardiomyocytes. FASEB J. 2001;15:2291–2293. doi: 10.1096/fj.00-0827fje [DOI] [PubMed] [Google Scholar]
- 32.Touyz RM, Cruzado M, Tabet F, Yao G, Salomon S, Schiffrin EL. Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: role of receptor tyrosine kinase transactivation. Can J Physiol Pharmacol. 2003;81:159–167. doi: 10.1139/y02-164 [DOI] [PubMed] [Google Scholar]
- 33.Benten WP, Lieberherr M, Sekeris CE, Wunderlich F. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett. 1997;407:211–214. doi: 10.1016/s0014-5793(97)00346-3 [DOI] [PubMed] [Google Scholar]
- 34.Leslie CC. Properties and regulation of cytosolic phospholipase A2. J Biol Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709 [DOI] [PubMed] [Google Scholar]
- 35.Harada K, Komuro I, Shiojima I, Hayashi D, Kudoh S, Mizuno T, Kijima K, Matsubara H, Sugaya T, Murakami K, et al. Pressure overload induces cardiac hypertrophy in angiotensin II type 1A receptor knockout mice. Circulation. 1998;97:1952–1959. doi: 10.1161/01.cir.97.19.1952 [DOI] [PubMed] [Google Scholar]
- 36.Jacobi J, Schlaich MP, Delles C, Schobel HP, Schmieder RE. Angiotensin II stimulates left ventricular hypertrophy in hypertensive patients independently of blood pressure. Am J Hypertens. 1999;12:418–422. [PubMed] [Google Scholar]
- 37.Sparks MA, Rianto F, Diaz E, Revoori R, Hoang T, Bouknight L, Stegbauer J, Vivekanandan-Giri A, Ruiz P, Pennathur S, et al. Direct actions of AT1 (Type 1 Angiotensin) receptors in cardiomyocytes do not contribute to cardiac hypertrophy. Hypertension. 2021;77:393–404. doi: 10.1161/HYPERTENSIONAHA.119.14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori T, Cowley AW., Jr.Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension. 2004;43:752–759. doi: 10.1161/01.HYP.0000120971.49659.6a [DOI] [PubMed] [Google Scholar]
- 39.Alexander LD, Alagarsamy S, Douglas JG. Cyclic stretch-induced cPLA2 mediates ERK 1/2 signaling in rabbit proximal tubule cells. Kidney Int. 2004;65:551–563. doi: 10.1111/j.1523-1755.2004.00405.x [DOI] [PubMed] [Google Scholar]
- 40.Ward CW, Prosser BL, Lederer WJ. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid Redox Signal. 2014;20:929–936. doi: 10.1089/ars.2013.5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001 [DOI] [PubMed] [Google Scholar]
- 42.Yiu SS, Zhao X, Inscho EW, Imig JD. 12-Hydroxyeicosatetraenoic acid participates in angiotensin II afferent arteriolar vasoconstriction by activating L-type calcium channels. J Lipid Res. 2003;44:2391–2399. doi: 10.1194/jlr.M300183-JLR200 [DOI] [PubMed] [Google Scholar]
- 43.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol. 2000;279:F12–F23. doi: 10.1152/ajprenal.2000.279.1.F12 [DOI] [PubMed] [Google Scholar]
- 44.Yamada K, Kubo K, Shuto K, Nakamizo N. Inhibition of thromboxane A2-induced vasocontraction by KF4939, a new anti-platelet agent, in rabbit mesenteric and dog coronary arteries. Jpn J Pharmacol. 1984;36:283–290. doi: 10.1254/jjp.36.283 [DOI] [PubMed] [Google Scholar]
- 45.Mistry M, Nasjletti A. Role of TXA2 in the pathogenesis of severe angiotensin II-salt hypertension. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:207–210. [PubMed] [Google Scholar]
- 46.Wilcox CS, Welch WJ, Snellen H. Thromboxane mediates renal hemodynamic response to infused angiotensin II. Kidney Int. 1991;40:1090–1097. doi: 10.1038/ki.1991.319 [DOI] [PubMed] [Google Scholar]
- 47.Bryson TD, Pandrangi TS, Khan SZ, Xu J, Pavlov TS, Ortiz PA, Peterson E, Harding P. The deleterious role of the prostaglandin E2 EP3 receptor in angiotensin II hypertension. Am J Physiol Heart Circ Physiol. 2020;318:H867–H882. doi: 10.1152/ajpheart.00538.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao L, Itani HA, do Carmo LS, Carver LS, Breyer RM, Harrison DG. Central EP3 (E Prostanoid 3) Receptors receptors Mediate mediate Saltsalt-Sensitive sensitive Hypertension hypertension and Immune immune Activationactivation. Hypertension. 2019;74:1507–1515. doi: 10.1161/HYPERTENSIONAHA.119.13850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Fang S, Lu KT, Wackman K, Schwartzman ML, Dikalov SI, Grobe JL, Sigmund CD. EP3 (E-Prostanoid 3) Receptor receptor Mediates mediates Impaired impaired Vasodilation vasodilation in a Mouse mouse Model model of Saltsalt-Sensitive sensitive Hypertensionhypertension. Hypertension. 2021;77:1399–1411. doi: 10.1161/HYPERTENSIONAHA.120.16518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sebeková K, Eifert T, Klassen A, Heidland A, Amann K. Renal effects of S18886 (Terutroban), a TP receptor antagonist, in an experimental model of type 2 diabetes. Diabetes. 2007;56:968–974. doi: 10.2337/db06-1136 [DOI] [PubMed] [Google Scholar]
- 51.Funk CD, Furci L, FitzGerald GA, Grygorczyk R, Rochette C, Bayne MA, Abramovitz M, Adam M, Metters KM. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J Biol Chem. 1993;268:26767–26772. [PubMed] [Google Scholar]
- 52.Kraemer MP, Choi H, Reese J, Lamb FS, Breyer RM. Regulation of arterial reactivity by concurrent signaling through the E-prostanoid receptor 3 and angiotensin receptor 1. Vascul Pharmacol. 2016;84:47–54. doi: 10.1016/j.vph.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005;389:763–774. doi: 10.1042/BJ20050237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.