Supplemental Digital Content is available in the text.
Keywords: hypertension, mice, placenta, pregnancy, trophoblast
Abstract
One driving factor for developing preeclampsia—a pregnancy disorder, often associated with poor spiral artery (SpA)-remodeling and fetal growth restriction—is the anti-angiogenic sFLT1 (soluble fms-like tyrosine kinase-1), which is found to be highly upregulated in preeclampsia patients. The sFLT1-mediated endothelial dysfunction is a common theory for the manifestation of maternal preeclampsia symptoms. However, the influence of sFLT1 on SpA-remodeling and the link between placental and maternal preeclampsia symptoms is less understood. To dissect the hsFLT1 (human sFLT1) effects on maternal and/or fetoplacental physiology in preeclampsia, sFLT1-transgenic mice with systemic hsFLT1 overexpression from midgestation onwards were used. SpA-remodeling was analyzed on histological and molecular level in placental/mesometrial triangle tissues. Maternal kidney and aorta morphology was investigated, combined with blood pressure measurements via telemetry. hsFLT1 overexpression resulted in maternal hypertension, aortic wall thickening, and elastin breakdown. Furthermore, maternal kidneys showed glomerular endotheliosis, podocyte damage, and proteinuria. preeclampsia symptoms were combined with fetal growth restriction already at the end of the second trimester and SpA-remodeling was strongly impaired as shown by persisted vascular smooth muscle cells. This phenotype was associated with shallow trophoblast invasion, delayed presence of uterine natural killer cells, and altered lymphatic angiogenesis. Overall, this study showed that circulating maternal hsFLT1 is sufficient to induce typical maternal preeclampsia-like symptoms in mice and impair the SpA-remodeling independent from the fetoplacental compartment, revealing new insights into the interaction between the placental and maternal contribution of preeclampsia.
Preeclampsia is a hypertensive pregnancy disorder not only increasing the risk of perinatal morbidity and mortality in mothers and fetuses but also affecting long-term health.1,2 Preeclampsia occurs after 20 weeks of gestation in 2% to 8% of pregnancies and is subdivided into early-onset (<34 weeks) and late-onset preeclampsia (>34 weeks),3 both associated with an accumulation of the anti-angiogenic biomarker sFLT1 (soluble fms-like tyrosine kinase-1) in maternal serum with ongoing pregnancy.4 Preeclampsia is associated with an increased frequency of preterm births, fetal growth restriction (FGR), and adverse neonatal/offspring developmental consequences,5 additionally causing high socioeconomic costs.6 Apart from removal of the placenta by delivery, no routine therapy exists to cure preeclampsia. During preeclampsia, sFLT1 acts as a decoy receptor for VEGF (vascular endothelial growth factor) receptors, by reducing free circulating levels of the proangiogenic factors of the VEGF family, including the PLGF (placental growth factor). Thus, sFLT1 is thought to be a key player in preeclampsia pathology and a main cause of maternal hypertension and proteinuria.7,8 Importantly, the sFLT1/PLGF-ratio is used to predict preeclampsia as it is highly elevated in preeclampsia patients already five weeks before first symptoms appear.9–12
Although proof of direct detrimental sFLT1 effects are still rare, experimental murine studies indicate an influence on placental development.4,13–16 A major process in successful placentation is the spiral artery (SpA)-remodeling,17,18 which occurs during early to midgestation in humans19 and mice.20 Thereby, SpAs undergo radical changes of their cellular and extracellular components, resulting in vasodilation.17 SpA-remodeling is mostly characterized by apoptosis of maternal endothelial cells and vascular smooth muscle cells (VSMCs), followed by invasion of specialized fetal trophoblast cells (TCs),19 combined with the presence of uterine natural killer (uNK) cells surrounding the SpAs.21,22 In preeclampsia pregnancies, SpA-remodeling is poor, combined with shallow trophoblast invasion.23–26 Thus, reduced vasodilation during preeclampsia affects placental perfusion, promoting uteroplacental hypoxia.7,27
Because existing sFLT1-related preeclampsia models were developed by injection of replication-deficient hsFLT1 (human sFLT1) lentiviruses or adenoviruses, they exhibit other immunologic changes additionally to sFLT1-overexpression.4,13–15 To overcome these challenges, we recently introduced doxycycline-inducible, hsFLT1-transgenic mice (hsFLT1/rtTA [reverse tetracycline-controlled trans-activator]) to receive a stable, reproducible, systemic hsFLT1 overexpression.16
The strongest negative effects on placental differentiation were previously described when hsFLT1 is expressed both, in the dam and the fetus. Upon combined hsFLT1 overexpression, a significant influence on placental labyrinthine formation has been shown, together with strong FGR, leading to nonviable fetuses. In contrast, exclusive maternal hsFLT1 overexpression revealed indirect effects defined by a reduced placental labyrinthine size, nevertheless inducing also significant reduction in fetal body weight, leading to viable fetuses.16
Although we learned a lot about molecular mechanisms of sFLT1-related endothelial dysfunction,4,28 impact of elevated hsFLT1 on the maternal physiology and the SpA-remodeling are widely unknown. Particularly, the contribution of maternal hsFLT1 overexpression in comparison to additional fetoplacental hsFLT1 overexpression to the complexity of maternal and placental characteristics of preeclampsia have not been studied so far. Considering the previously proven impact of hsFLT1 on fetoplacental vascularization, we hypothesized that circulating maternal hsFLT1 adversely affects maternal vascular adaptation during pregnancy, especially the SpA-remodeling, promoting hypertension.
Materials and Methods
The authors declare that all supporting data are available within the article and its online-only Data Supplement. The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics Statement
Animal experiments were approved and performed according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) and University Hospital Essen guidelines and local government approval by the State Agency for Nature, Environment and Consumer Protection, North Rhine-Westphalia (LANUV [Landesamt für Natur, Umwelt und Verbraucherschutz]), or in accordance with the local ethics committees in Berlin and the German laws for animal protection (G1265/12, G1644/17, and G0364/17).
Animals
The hsFLT1/rtTA model and mating is detailed described in Vogtmann et al.16 Double-transgenic hsFLT1/rtTA dams received since 10.5 days postconception (dpc) either 2 mg/mL doxycycline and 30 mg/mL sucrose per drinking water and expressed hsFLT1, named preeclampsia or received sucrose only and did not express hsFLT1, named control. Single-transgenic hsFLT1 dams (lacking the rtTA allele) treated with doxycycline do also not express hsFLT1 and served as a control for doxycycline side effects, named doxycycline control. For fetoplacental analyses, the preeclampsia group was further subdivided according to the fetal rtTA genotype into preeclampsia hom (homozygous for hsFLT1 and rtTA) and preeclampsia het (homozygous for hsFLT1 and heterozygous for rtTA), with additional fetoplacental hsFLT1 expression or preeclampsia wild type (wt), lacking the rtTA allele, with exclusive maternal hsFLT1 expression. Litters of the following dams were analyzed at 14.5 (control: n=6, doxycycline control: n=4, preeclampsia: n=6) or 18.5 dpc (control: n=11, doxycycline control: n=3, preeclampsia: n=11). Detailed procedures of further methods are included in the supplements.14,16,29–32
Statistics
D’Agostino-Pearson omnibus K2 test and Shapiro-Wilk test could not prove gaussian distribution. Statistics was performed with Kruskal-Wallis and Dunn multiple comparison test or Mann Whitney U test. Data are presented as box plot, with median, interquartile range±minimum to maximum; or mean±SEM. Sample size (n) is listed under each graph, respectively. Probability value (P value) of ≤0.05 was indicated with *, **P<0.01, ***P<0.001, and ****P<0.0001. Data were analyzed with GraphPad Prism (5.01; GraphPad, La Jolla, CA).
Results
Systemic hsFLT1 Mimics Preeclampsia in Pregnant Mice
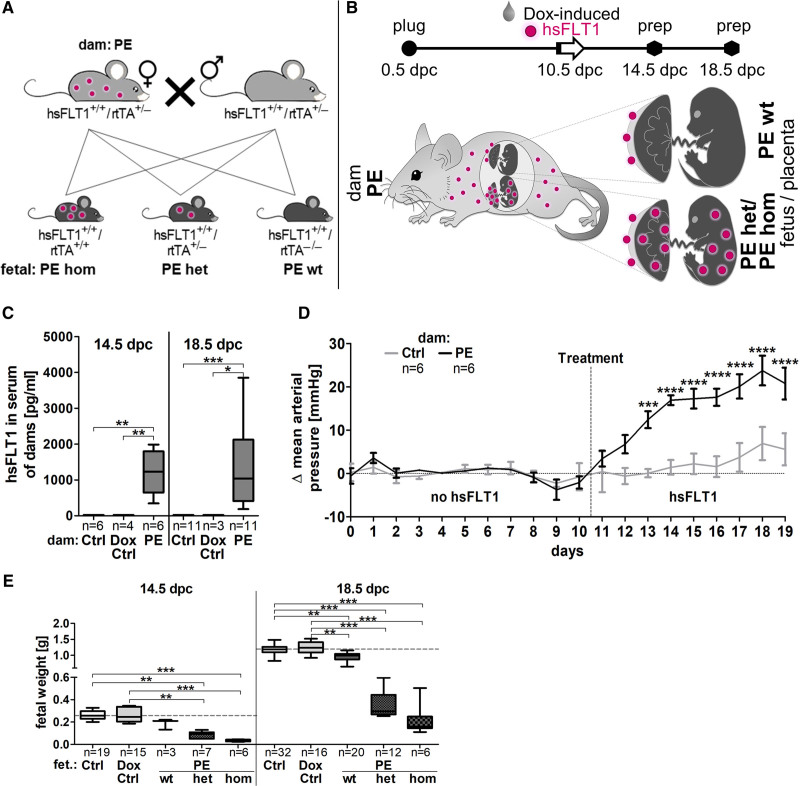
In transgenic hsFLT1/rtTA mice, hsFLT1 was systemically induced in midpregnancy (10.5 dpc) in dams and fetuses (preeclampsia het and hom) or in dams only (preeclampsia wt) depending on the fetal rtTA genotype (Figure 1A, also see16). Thereby, preeclampsia dams carried both types of fetuses in parallel during one pregnancy; fetuses either having both transgenic alleles hsFLT1 and rtTA and express hsFLT1 also by themselves (preeclampsia het/hom) or fetuses lacking the rtTA allele and did not express hsFLT1 (preeclampsia wt). Accordingly, for maternal analyzes, no distinction was possible between maternal and additional fetoplacental hsFLT1 overexpression (dams: preeclampsia), whereas this discrimination was done for placental/fetal analyzes (fetus/placenta: preeclampsia subdivided into preeclampsia wt, het, and hom). The origin of hsFLT1 expression in preeclampsia wt compared with preeclampsia het/hom group is illustrated in Figure 1B. Sample preparation was performed after physiological completion of SpA-remodeling (14.5 dpc) or at the end of the pregnancy (18.5 dpc). Highly retarded preeclampsia hom fetuses/placentas were used for histological assessment of SpA-remodeling in whole implantation sites, but not for molecular analyses due to small mesometrial triangle (MT) tissue amount.
Figure 1.
Systemic sFLT1 (soluble fms-like tyrosine kinase-1) overexpression mimics preeclampsia in mice. A, hsFLT1 (human sFLT1)/rtTA (reverse tetracycline-controlled trans-activator) mice (hsFLT1+/+ and rtTA+/−) were mated. hsFLT1+/+/rtTA+/+ or hsFLT1+/+/rtTA+/−fetuses/placentas (preeclampsia homozygous for hsFLT1 and homozygous for rtTA/homozygous for hsFLT1 and heterozygous for rtTA) expressed hsFLT1, whereas hsFLT1+/+/rtTA−/−fetuses (PE wt) did not. For fetal/spiral artery analyses the maternal PE group was further subdivided into preeclampsia homozygous for hsFLT1 and homozygous for rtTA/homozygous for hsFLT1 and heterozygous for rtTA, homozygous for hsFLT1 and wild type for rtTA. B, At 10.5 days postconception (dpc) dams were treated either with doxycycline (Dox) and sucrose (PE/Dox control [Ctrl]) or with sucrose only (Ctrl). Human sFLT1 (hsFLT1) origin in PE wt (maternal) compared with PE het/hom (maternal and fetoplacental) is illustrated. C, hsFLT1 is exclusively present in serum of induced dams (PE). D, Mean arterial pressure increases upon hsFLT1 induction at 10.5 dpc until 18.5 dpc (PE), compared with Ctrl, E, combined with reduced fetal weight gain from 14.5 until 18.5 dpc. Data are presented as box plot with median, interquartile range±upper/lower extreme, or as mean±SEM; sample size n is listed under each graph respectively; Kruskal-Wallis combined with Dunn multiple comparisons test/Mann Whitney U test; *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
Transgenic hsFLT1/rtTA dams express high hsFLT1 levels already after 4 days of induction with doxycycline, exhibiting median serum hsFLT1 of 1228 pg/mL at 14.5 dpc, comparable to 1040 pg/mL at 18.5 dpc (Figure 1C). The food and doxycycline intake and weight gain during pregnancy were not different between dams (Figure S1A through S1C in the Data Supplement). After hsFLT1 induction, maternal mean arterial pressure, systolic, and diastolic blood pressure were increased (preeclampsia) and from 13.5 dpc until the end of pregnancy significantly higher compared with the noninduced dams (control; Figure 1D and Figure S1D and S1E).
hsFLT1 overexpression led to slight maternal aortic lumen reduction and wall thickening, resulting in significantly reduced aortic lumen/total area compared with both controls (control/doxycycline control), which was combined with an increase in elastin breaks and a reduction of elastin layers in aortas of preeclampsia dams (Figure 2A through 2C and Figure S1F through S1H). Moreover, preeclampsia dams showed cytokine serum profiles differing from those of control dams (control/doxycycline control), which include downregulation of adiponectin and upregulation of FGF acid and myeloperoxidase (Figure S1I and S1J).
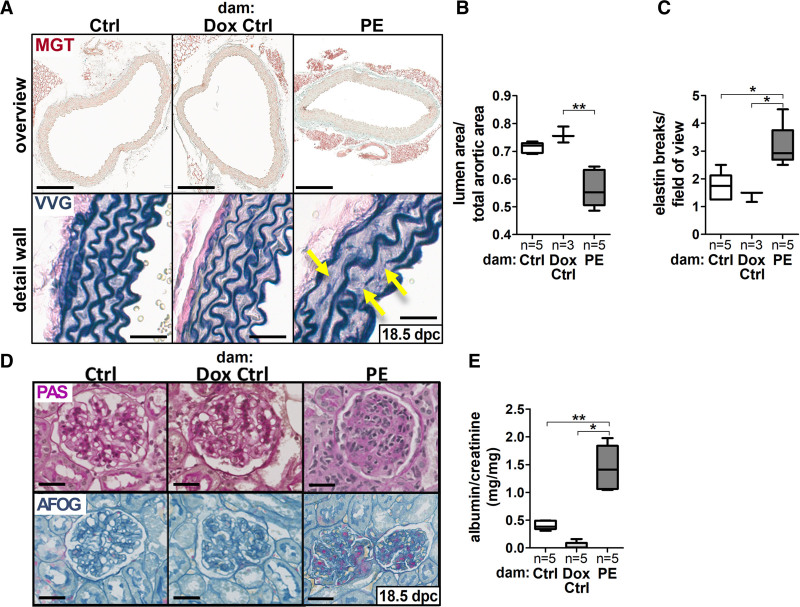
Figure 2.
sFLT1 (soluble fms-like tyrosine kinase-1) induces secondary organ failure in maternal kidney and aorta. A, Masson Goldner trichrome (MGT; scale bar: 200 µm) and Verhoeff Van Gieson (VVG; scale bar: 50 µm) stained maternal aortas (18.5 days postconception [dpc]) exhibited [B] a reduced aortic lumen/wall ratio and [C] an increased elastin breakdown (yellow arrows in A) upon hsFLT1 (human sFLT1) overexpression (preeclampsia [PE]) compared with both controls (Ctrl/doxycycline [Dox] Ctrl). D, Periodic acid-Schiff reaction (PAS) and acid fuchsin-orange G (AFOG) stain of maternal kidneys (18.5 dpc) indicating glomerular endotheliosis upon hsFLT1 (PE); scale bar = 80 µm. E, Albumin/creatinine (mg/mg) ratio (18.5 dpc), was increased in PE dams compared with Ctrl. Data are presented as box plot with median, interquartile range±upper/lower extreme; sample size n is listed under each graph respectively; Kruskal-Wallis combined with Dunn multiple comparisons test; *P<0.05 and **P<0.01.
Histological kidney analysis of preeclampsia dams showed endothelial cell swelling and occlusion of capillary lumen visualized by periodic acid-Schiff (PAS) reaction, as well as more fibrosis in acid fuchsin-orange G staining compared with both controls (Figure 2D), resembling the phenotype of glomerular endotheliosis, as seen in kidney biopsies of preeclampsia patients.33,34 Consistently, upon hsFLT1 expression in kidneys, immunostainings of Cd31 (endothelial cell marker), nephrin (podocytes slit diaphragm protein), and WT-1 (podocyte cell marker) was reduced compared with controls (Figure S2A and S2B). These changes indicate damage to the glomerular filtration barrier, which becomes clinically evident as an increased albumin to creatinine ratio (Figure 2E).
Additionally, fetal body weight was measured at 14.5 dpc and 18.5 dpc to assess the FGR onset point, indicating FGR already at 14.5 dpc combined with reduced weight gain from 14.5 to 18.5 dpc upon exclusive maternal hsFLT1 overexpression (preeclampsia wt) and limited weight gain upon maternal and fetoplacental hsFLT1 overexpression (preeclampsia het and hom) compared with both controls (control/doxycycline control; Figure 1E). Thus, most severe sFLT1 effect on fetal weight was observed, when hsFLT1 is both maternally and fetoplacentally expressed (preeclampsia het and hom). The placental weight and the litter size did not change at both time points but lead to a significantly reduced placental efficiency upon hsFLT1 overexpression (Figure S3C through S3E).
Systemic hsFLT1 Impairs SpA-Remodeling
Insufficient SpA-remodeling is associated with decreased placental perfusion, preeclampsia, and FGR.18 With our experimental approach, we can discriminate between the effects of hsFLT1 from the maternal circulation without hsFLT1 expression in the placenta and fetus (preeclampsia wt) and the combined effects of hsFLT1 overexpression in the mother and fetoplacental compartment (preeclampsia het/preeclampsia hom) on SpA-remodeling. Importantly, hsFLT1 expression was limited to preeclampsia het placentas and preeclampsia wt and preeclampsia het MTs at 14.5/18.5 dpc, with absent hsFLT1 mRNA in preeclampsia wt and control placentas and control MTs (control/doxycycline control; Figure S3A and S3B).
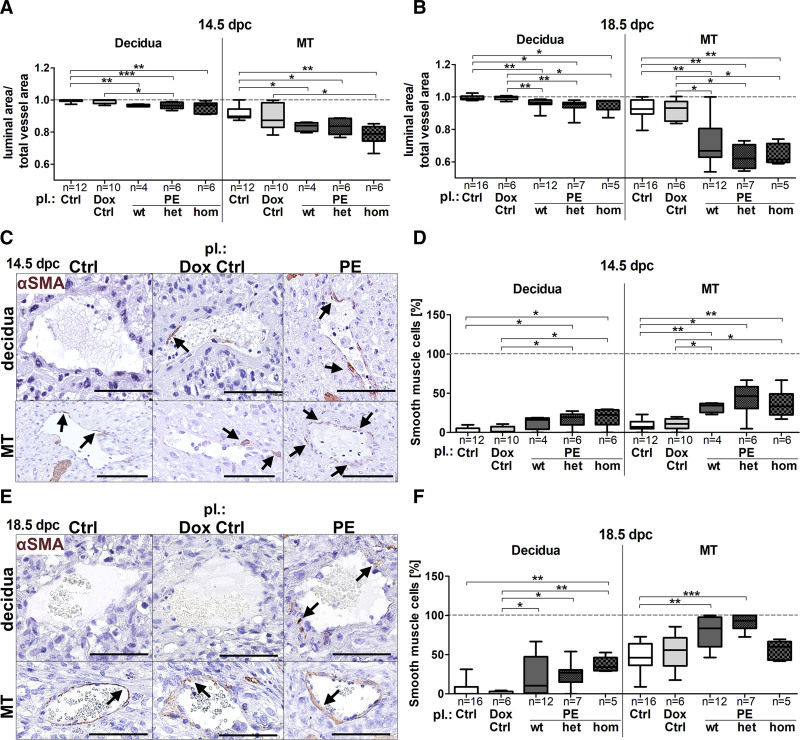
To investigate SpA vasodilation, the ratio of the luminal/total vessel area was assessed of SpAs in the decidua and the MT compartment (location: Figure S4A, ratio: Figure S4B). At 14.5 dpc luminal/total vessel area ratio was significantly decreased upon hsFLT1 overexpression compared with both controls in both compartments (Figure 3A), with no recovery until 18.5 dpc (Figure 3B), indicating remained VSMC-layers. Verification was performed by immunohistochemical staining with anti-αSMA (α-smooth muscle actin; Figure 3C and 3E), revealing an increase in persisted VSMCs upon both types of hsFLT1 overexpression (preeclampsia wt and preeclampsia het/hom) compared with controls at 14.5 dpc and 18.5 dpc (Figure 3D and 3F).
Figure 3.
sFLT1 (soluble fms-like tyrosine kinase-1) inhibits vascular smooth muscle cell degradation during spiral artery remodeling. A, At 14.5 days postconception (dpc; B) and 18.5 dpc luminal/total vessel area ratio was decreased upon hsFLT1 (human sFLT1) overexpression (preeclampsia homozygous for hsFLT1 and wild type for rtTA, homozygous for hsFLT1 and heterozygous for rtTA, homozygous for hsFLT1 and homozygous for rtTA) compared with controls (Ctrl/doxycycline [Dox] Ctrl) in decidua and mesometrial triangle (MT). C, Anti-αSMA (α-smooth muscle actin) stained spiral artery in both compartments at 14.5 dpc and (E) at 18.5 dpc; scale bar: 100 µm; arrows = αSMA-positive cells. D, Quantification of αSMA-positive cells revealed persisted vascular smooth muscle cells upon hsFLT1 induction (PE wt, het, and hom) compared with Ctrl (Ctrl/Dox Ctrl), in the MT at 14.5 dpc, (F) and 18.5 dpc. Data are presented as box plot with median, interquartile range±upper/lower extreme; sample size n is listed under each graph respectively; Kruskal-Wallis combined with Dunn multiple comparisons test; *P<0.05, **P<0.01 and ***P<0.001.
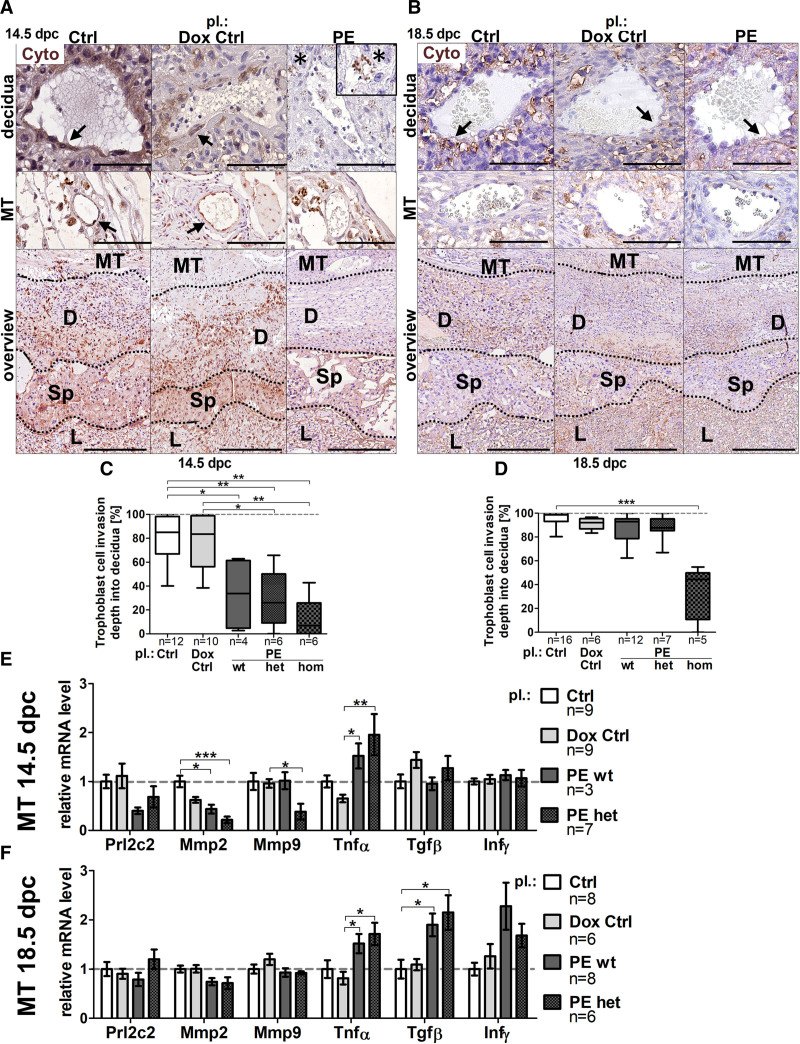
Trophoblast invasion is a key mechanism in SpA-remodeling and is impaired in human preeclampsia.35 Hence, hsFLT1 effects on TC invasiveness during SpA-remodeling was investigated by immunohistochemical staining with anti-pan Cyto (cytokeratin; Figure 4A and 4B). Quantification of invasion depth of Cyto-positive cells into the decidua revealed reduced TC-invasion upon hsFLT1 overexpression (preeclampsia wt, het, and hom) compared with controls (control/doxycycline control) at 14.5 dpc, (Figure 4C) with recovery at 18.5 dpc for preeclampsia wt and het (Figure 4D), but no recovery in preeclampsia hom. mRNA expression analysis of prolactin family 2, subfamily c, member 2 (Prl2c2) as a marker for peri/endovascular TCs, matrix metalloproteinases (Mmp2/Mmp9) for elastolysis during TC-invasion, tumor necrosis factor α (Tnfα), transforming growth factor β (Tgfβ) and interferon γ (Infγ) as negative regulating cytokines during TC-invasion was performed in MT tissue (Figure 4E/F). Markers associated with improved TC-invasion Prl2c2, Mmp2, and Mmp9 were lower expressed upon hsFLT1 overexpression (preeclampsia wt and het) compared with both controls at 14.5 dpc (Figure 4E) with increase until 18.5 dpc (Figure 4F), whereas markers associated with inhibition of TC-invasion Tnfα, Tgfβ and Infγ were unaffected at 14.5 dpc (Figure 4E) but upregulated at 18.5 dpc (Figure 4F).
Figure 4.
sFLT1 (soluble fms-like tyrosine kinase-1) impairs decidual trophoblast invasion during spiral artery remodeling. A, Anti-Cyto (cytokeratin) stained spiral arteries (SpAs) in decidua/mesometrial triangle (MT) and overview between spongiotrophoblast and decidua at 14.5 days postconception (dpc) and (B) at 18.5 dpc; scale bar: 200 µm decidua/MT, 100 µm overview; arrows denote Cyto-positive cells surrounding SpAs; asterisks denote round-shaped Cyto-positive cells not surrounding SpAs at 14.5 dpc. C, Quantification of Cyto-positive cell-migration revealed reduced trophoblast cell invasion upon hsFLT1 (human sFLT1) overexpression (preeclampsia homozygous for hsFLT1 and wild type for rtTA, homozygous for hsFLT1 and heterozygous for rtTA, and homozygous for hsFLT1 and homozygous for rtTA) compared with controls (Ctrl/doxycycline [Dox] Ctrl) at 14.5 dpc, [D] with recovery at 18.5 dpc in PE wt and het but not PE hom placentas. E/F, mRNA levels of prolactin family 2, subfamily c, member 2 (Prl2c2), matrix metalloproteinases (Mmp2 and Mmp9), were lower in PE wt and het compared with Ctrl at 14.5 dpc (E), improved until 18.5 dpc (F), whereas tumor necrosis factor α (Tnfα), transforming growth factor β (Tgfβ), and interferon γ (Infγ) were upregulated at 14.5 dpc (E) and 18.5 dpc (F). Data are presented as box plot with median, interquartile range±upper/lower extreme, or as mean±SEM; sample size n is listed under each graph respectively; Kruskal-Wallis combined with Dunn multiple comparisons test; *P<0.05, **P<0.01, and ***P<0.001. D indicates decidua; L, labyrinth, Sp, spongiotrophoblast.
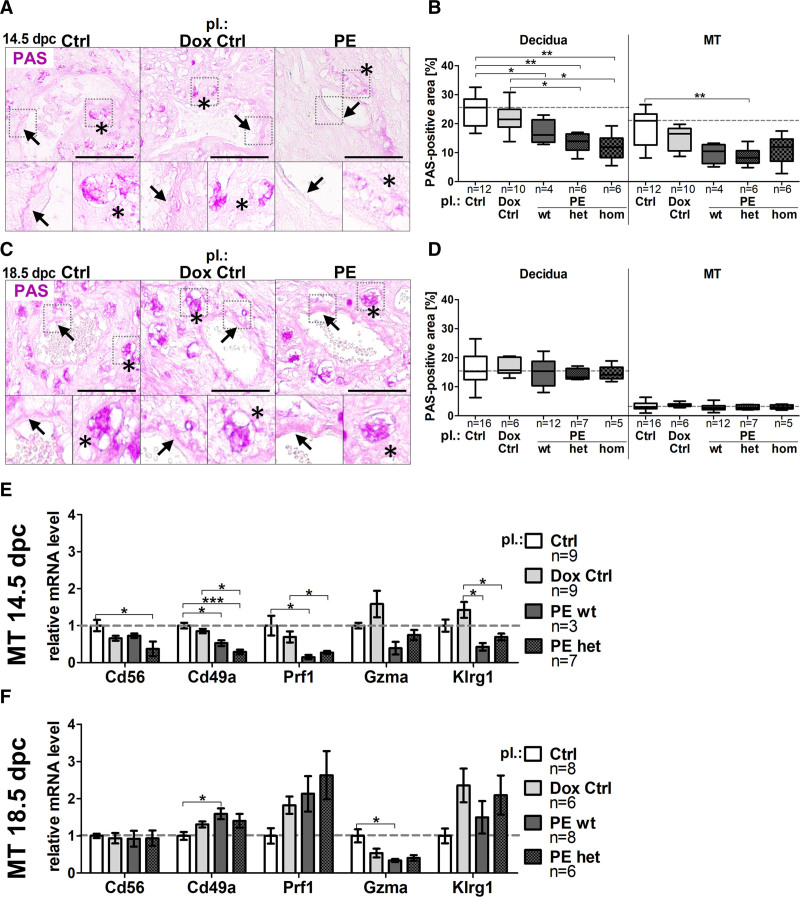
uNK cells are thought to promote SpA-remodeling21,22; thus, regulation of uNKs was expected, and subsequently glycoprotein-rich uNKs were visualized by PAS reaction (Figure 5A and 5C, asterisks). Upon hsFLT1 overexpression PAS-positive area of total decidua and MT was reduced compared with controls at 14.5 dpc (Figure 5B), compensated at 18.5 dpc (Figure 5D). At 14.5 dpc, PAS-positive fibrinoid deposition was only present in controls (control/doxycycline control) but less in preeclampsia (wt, het, hom; Figure 5A, arrows). In contrast, at 18.5 dpc fibrinoid deposition was increased in preeclampsia placentas, compared with both control groups (Figure 5C arrows). mRNA levels of uNK cell markers, neural cell adhesion molecule (Cd56), Integrin α-1 (Cd49a), perforin 1 (Prf1), granzyme A (Gzma), Killer cell lectin-like receptor subfamily G member 1 (Klrg1) in MT tissue were mostly downregulation at 14.5 dpc (Figure 5E) and an upregulation at 18.5 dpc in preeclampsia wt and het compared with controls (Figure 5F).
Figure 5.
sFLT1 (soluble fms-like tyrosine kinase-1) leads to temporally misregulated presence of uterine natural killer cell markers during spiral artery remodeling. A, Periodic acid-Schiff (PAS) reactivity on spiral arteries in decidua at 14.5 days postconception (dpc) and [C] 18.5 dpc; scale bar: 100 µm; arrows denote fibrinoid deposition; asterisks denote uterine natural killer cells. B, Upon hsFLT1 (human sFLT1; preeclampsia homozygous for hsFLT1 and wild type for rtTA, homozygous for hsFLT1 and heterozygous for rtTA, and homozygous for hsFLT1 and homozygous for rtTA) PAS-positive area of decidua and mesometrial triangle (MT) was reduced compared with controls (Ctrl/doxycycline [Dox] Ctrl) at 14.5 dpc, (D) compensated at 18.5 dpc. E/F, mRNA analysis of uterine natural killer cell marker genes neural cell adhesion molecule (Cd56), Integrin α-1 (Cd49a), perforin 1 (Prf1), granzyme A (Gzma), Killer cell lectin-like receptor subfamily G member 1 (Klrg1) in MT tissue display a downregulation at 14.5 dpc (E) and an upregulation at 18.5 dpc (F). Data are presented as box plot with median, interquartile range±upper/lower extreme, or as mean±SEM; sample size n is listed under each graph respectively; Kruskal-Wallis combined with Dunn multiple comparisons test; *P<0.05, **P<0.01 and ***P<0.001.
Overall, the negative impact of hsFLT1 expression on SpA lumen adaption, VSMC apoptosis, TC-invasion, and uNK presence seems to be independent from the source of hsFLT1 overexpression. Thus, exclusive maternal (preeclampsia wt) or combined maternal and fetoplacental (preeclampsia het/hom) hsFLT1 overexpression led to similar alterations of SpA phenotypes and marker gene expression profiles.
Systemic hsFLT1 Inhibits Mesometrial (Lymph-)Angiogenesis
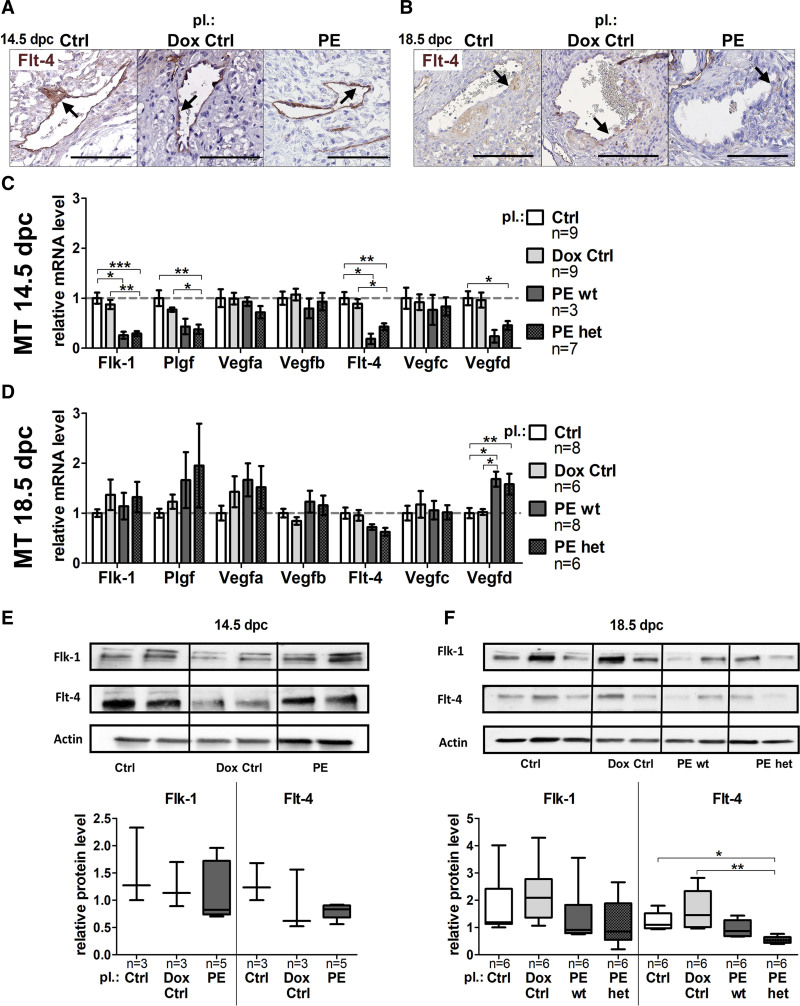
During remodeling, SpAs have noticeable similarities to lymphatic vessels, including thin coverage with VSMCs and dilating vessel lumen.36 The Vegf-signaling pathway regulates angiogenesis and lymph-angiogenesis in several tissues. Therefore, analysis of the Vegf-signaling molecules fetal liver kinase-1 (Flk-1), placental growth factor (Plgf) and vascular endothelial growth factor (Vegfa, Vegfb) as angiogenesis and Fms-related tyrosine kinase 4 (Flt-4), Vegfc, Vegfd as lymph-angiogenesis markers were performed to unravel the link between hsFLT1 upregulation and lymphatic mimicry. Immunohistochemical staining with anti–Flt-4 of SpAs revealed Flt-4 positive SpAs at both time points (Figure 6A and 6B). At 14.5 dpc, Flk-1 and its binding partner Plgf were significantly downregulated on transcriptional level upon hsFLT1 overexpression (preeclampsia wt and preeclampsia het), whereas the binding partners Vegfa and Vegfb were not affected (Figure 6C). At 18.5 dpc, downregulation of Flk-1, Plgf, Vegfa, and Vegfb was compensated or upregulated compared with controls (Figure 6D). Upon hsFLT1, overexpression (preeclampsia wt and preeclampsia het) reduced mRNA levels were additionally seen for Flt-4 and its binding partner Vegfd at 14.5 dpc (Figure 6C), with no recovery of Flt-4, but upregulation of Vegfd at 18.5 dpc (Figure 6D). Vegfc was not affected. Tendency of reduced Flk-1 and Flt-4 expression in MT tissue was verified on protein level, at 14.5 dpc (Figure 6E) but especially at 18.5 dpc (Figure 6F). All (lymph-)angiogenesis marker genes did not show significant differences if hsFLT1 is maternally (preeclampsia wt) or additionally fetoplacentally (preeclampsia het) expressed.
Figure 6.
sFLT1 (soluble fms-like tyrosine kinase-1) leads to reduced lymphatic mimicry during spiral artery remodeling. A, Anti–Flt-4–stained spiral arteries in the decidua at 14.5 days postconception (dpc) and (B) at 18.5 dpc; scale bar: 100 µm. C, At 14.5 dpc the fetal liver kinase-1 (Flk-1) and the placental growth factor (Plgf) were downregulated in preeclampsia homozygous for hsFLT1 and wild type for rtTA and preeclampsia homozygous for hsFLT1 and heterozygous for rtTA, whereas the vascular endothelial growth factors (Vegfa, Vegfb) were not affected. D, At 18.5 dpc Flk-1, Plgf, Vegfa, and Vegfb downregulation was compensated or overcompensated. C, mRNA level of Fms-related tyrosine kinase 4 (Flt-4) and Vegfd was reduced at 14.5 dpc, (D) with no recovery of Flt-4, but upregulation of Vegfd at 18.5 dpc. Vegfc was not affected. E/F, Flk-1 and Flt-4 protein level were decreased in mesometrial triangle (MT) tissue at 14.5 dpc and 18.5 dpc measured via Western Blot. Data are presented as box plots with median, interquartile range±upper/lower extreme, or as mean±SEM; sample size n is listed under each graph respectively; Kruskal-Wallis combined with Dunn multiple comparisons test. Dox indicates doxycycline. *P<0.05, **P<0.01 and ***P<0.001.
Moreover, (immuno-)histological images of the same decidual SpA at 18.5 dpc of the preeclampsia group show many Cd31-positive, Flt-4–positive, and cytokeratin-positive cells and only a few αSMA-positive cells in the PAS-positive fibrinoid deposition surrounding the SpA, which indicates a shift towards apoptotic endothelial cells and TCs stored in the fibrinoid deposition and not VSMCs (Figure S4C).
Systemic hsFLT1 Induces HIFs
The implantation site is known to be hypoxic in normal early pregnancy,37 nevertheless, prolonged hypoxic conditions are linked to placental dysfunction and adverse pregnancy outcomes.38 Because systemic hsFLT1 overexpression impairs SpA-remodeling in our model, placental hypoxic conditions were expected and mRNA analysis of hypoxia-inducible factor (Hif1α, Hif2α) and prolyl hydroxylases (Phd1, Phd2) was done at 14.5 and 18.5 dpc (Figure S4D and S4E) in placental tissue. At 14.5 dpc the mRNA level of Hif1α and Hif2α were unaffected between groups, while Phd1 and Phd2 mRNA were downregulated upon hsFLT1 overexpression (preeclampsia wt and preeclampsia het; Figure S4D). Contrary, at 18.5 dpc Hif1α and Hif2α mRNA levels were upregulated upon maternal hsFLT1 overexpression (preeclampsia wt), combined with strong increase of Phd1 and Phd2 mRNA upon maternal hsFLT1 overexpression (preeclampsia wt) and maternal and fetal hsFLT1 overexpression (preeclampsia het) compared with controls (Figure S4E). However, if HIF-proteins might be stabilized on protein level has to be elucidated.
Discussion
Due to the experimental design and by using the sFLT1-transgenic preeclampsia model, it is possible to discriminate between exclusive maternal and combined maternal and fetoplacental hsFLT1 overexpression and its contribution to alterations in SpA-remodeling during pregnancy. Although doxycycline itself shows slight effects on SpA-remodeling, as already seen by Verlohren et al,39 the current study shows that inhibition of maternal vascular adaptation was clearly induced by hsFLT1; nevertheless, independent of the hsFLT1 source. Consequentially, we gather that even exclusive maternal hsFLT1 overexpression is sufficient to impair SpA-remodeling independent from the fetoplacental compartment, with only one process stronger inhibited under combined maternal and fetoplacental hsFLT1, namely the TC-invasion. During SpA-remodeling, TC-invasion is actively regulated by fetal and not maternal parts of the implantation site and mainly depends on migratory properties of fetal TCs. Accordingly, adverse impact of combined maternal and fetoplacental hsFLT1 expression on fetal TC-migration is associated with a negative impact on fetal growth, leading to nonviable fetuses. Contrary, other processes during SpA-remodeling, like loss of maternal VSMCs, presence of maternal uNKs, and expression of lymphatic and angiogenic markers in maternal endothelial cells, seem to be unaffected by additional fetoplacental hsFLT1 expression. In these processes, the presence of hsFLT1 in maternal tissues and circulation appeared to be decisive and not additional fetoplacental hsFLT1 expression. First trials using ultrasound analyses by us revealed impaired uteroplacental vascularization with notching in the uterine artery and reversed end-diastolic velocity in the umbilical artery upon hsFLT1 overexpression, like human preeclampsia characteristics. This indicates further consequences of the impaired SpA-remodeling in our mouse model.
In human preeclampsia, it is assumed that sFLT1 is placentally derived, since Cerdeira et al40 describe a significant sFLT1 gradient between uterine and peripheral veins in preeclampsia patients, with highest sFLT1 levels in placenta and lowest in periphery. Nevertheless, only serum samples on protein level were analyzed and not tissue samples on mRNA level to determine the real origin of sFLT1 overexpression. Hence, sFLT1—as a soluble factor—could also accumulate at implantation site independent of the original sFLT1-expressing cells, overall resulting in a gradual serial presence of sFLT1. Reduced SpA-remodeling in human preeclampsia can be observed at 11th week of pregnancy, by using Doppler sonography.41 At this time point, PLGF is already reduced, but sFLT1 is not yet elevated in peripheral serum of preeclampsia patients.42 Additionally, SpA-remodeling can be improved by Aspirin intake before 16th week of pregnancy, which did not show any advantage starting later in pregnancy.43 This underlines that SpA-remodeling takes part in the first half of human pregnancy, but no more in a relevant way later. Thus, in humans, reduced SpA-remodeling can be observed before validation of sFLT1 accumulation in peripheral serum samples is possible. This fact neither confirms nor disproves the influence of sFLT1 on human SpA-remodeling because sFLT1 could already be upregulated/accumulated at the implantation site, not yet detected in serum. Although sFLT1 impact on SpA-remodeling in human preeclampsia needs further clarification, we hypothesize that the observed sFLT1 effects on SpA-remodeling in our model are independent from the original source of expression but rather depends on sFLT1 presence in maternal tissues.
However, the observed results in SpA-remodeling are in line with our hypotheses that sFLT1 overexpression leads to hypertension during pregnancy—as a second trigger—via negatively influencing maternal vasculature like the aorta or the SpAs. During normal pregnancy, blood volume and circulation increases throughout pregnancy due to systemic vasodilatation.44 In our model, sFLT1-influenced pregnancies seemed to be negatively affected in vascular adaptation regarding not only the SpAs but also the maternal aorta and kidney vessels. This vascular maladaptation upon sFLT1 represents a viable link between the placental phenotype and the formation of hypertension during preeclampsia.
The current study showed a significant influence of sFLT1 overexpression on maternal hypertension. In addition to our study, also other preeclampsia models4,13,15 and clinical studies9–12,45,46 strengthened the link between increased maternal sFLT1 serum levels and severity of maternal hypertension. In accordance with the maternal hypertension, an influence of sFLT1 on maternal aortic physiology was observed, which was also already seen in murine hypertension models without pregnancy47,48 and in human preeclampsia.49 As well, endothelial dysfunction and thus vascular damage in the kidneys are described as glomerular endotheliosis.50 It is known that glomerular VEGF expression is critical for the integrity of an intact filtration barrier and kidney dysfunction during preeclampsia.51 Systemic hsFLT1 overexpression in our model strengthened these findings by inducing glomerular endotheliosis, podocyte damage, and increased albumin/creatine levels.
To sum up, we postulate that circulating maternal hsFLT1 is sufficient to induce a maternal preeclampsia-like syndrome and that maternal vascular maladaptation connects the maternal syndrome to placental characteristics of preeclampsia in mice.
We are aware not to over-interpret the findings since preeclampsia is a human disease, which is here simulated in mice leading to a preeclampsia-like syndrome. As well, although we here demonstrate that systemic sFLT1 overexpression mimics a various number of preeclampsia symptoms in mice, evidence for sFLT1 overexpression as a cause of human preeclampsia is still missing. In addition to viral-transduced sFLT1 models,4,13,15 other preeclampsia models exist targeting among others, the renin-angiotensin system, the nitric oxide synthetase,52 or the uterine perfusion pressure itself.53 However, sFLT1 already serves as a useful clinical biomarker to predict preeclampsia11,12 and is thought to be a key player in preeclampsia progression.8,54 Because all existing sFLT1 models suffer from various shortcomings (1) they must be generated for each individual experiment, which is time and resource-intense and highly variable, (2) they induce other immunologic changes due to viral transduction, etc, (3) or they reveal sFLT1 expression already in blastocysts prior implantation, the usage of this transgenic preeclampsia model could reveal more stable and reproducible results, like in this study the contribution of the maternal and the fetoplacental compartment in preeclampsia pathology.
Perspectives
Preeclampsia is still one of the main reasons for morbidity and mortality among mothers and their children and adequate animal models for research are urgently needed. The current study indicates that sFLT1 overexpression results in impairment of SpA-remodeling, due to sFLT1 presence in maternal decidua and circulation, which is sufficient to induce hypertension during pregnancy. sFLT1 overexpression seems to interfere with vascular adaptation mechanisms, inducing a type of vascular stiffness with reduced potential for vasodilation in maternal aorta and uterine spiral arteries, which could be a vital link to hypertension in dams. Overall, systemic sFLT1 overexpression mimics almost all hallmarks of human preeclampsia, making this model an elegant tool for analyzing: (1) new interventional strategies against preeclampsia/FGR, (2) underlying mechanisms in the progression of preeclampsia, as well as (3) short- and long-term consequences in offspring outcome and maternal future cardiovascular health.
Acknowledgments
A. Gellhaus and R. Vogtmann contributed to experimental design. Following authors conducted investigations: Telemetry: R. Dechend, F. Herse, and K. Kräker; Kidneys: H. Hagmann and M. Matin; Placenta: J. Heupel and R. Vogtmann; Aorta: R. Vogtmann. R. Vogtmann contributed to Data analysis and Figures/Tables. Following authors participated in Discussion: I. Bendix, R. Dechend, A. Gellhaus, F. Herse, H. Hagmann, A. Köninger, K. Kräker, R. Kimmig, M. Matin, R. Vogtmann, and E. Winterhager. A. Gellhaus and R. Vogtmann participated in article drafting. All authors contributed to article revision and approved the submitted version. We thank Gabriele Sehn and Liyan Duan for their excellent technical assistance, Nancy Freitag and Petra Moschansky for technical advice in the Masson Goldner trichrome (MGT) staining, and the team of the Animal Facility for their assistance with the mice.
Sources of Funding
Funding was provided by Mercator Research Center Ruhr (MERCUR) An-2015-0009 to A. Gellhaus and I. Bendix; Programm zur internen Forschungsförderung Essen (IFORES) project grant D/107-81240 to A. Gellhaus (Medical Faculty, University of Duisburg-Essen); German Research Foundation (DFG): GE2223/2-1 to A. Gellhaus; DE 631/15-1 to K. Kräker; HE 6249/5-1 to F. Herse; Else Kröner-Fresenius Foundation (2016-A62) to H. Hagmann.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- Cyto
- cytokeratin
- Dpc
- days postconception
- FGR
- fetal growth restriction
- hsFLT1
- human sFLT1
- MT
- mesometrial triangle
- PAS
- periodic acid-Schiff
- PLGF
- placental growth factor
- rtTA
- reverse tetracycline-controlled trans-activator
- sFLT1
- soluble fms-like tyrosine kinase-1
- SpA
- spiral artery
- TC
- trophoblast cell
- uNK
- uterine natural killer
- VEGF
- vascular endothelial growth factor
- VSMC
- vascular smooth muscle cell
- αSMA
- α-smooth muscle actin
For Sources of Funding and Disclosures, see page 1078.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.17567.
Contributor Information
Rebekka Vogtmann, Email: rebekka.vogtmann@gmail.com.
Jacqueline Heupel, Email: Jacqueline.Heupel@rlk.uk-essen.de.
Florian Herse, Email: florian.herse@charite.de.
Mahsa Matin, Email: mahsa.matin@uk-koeln.de.
Henning Hagmann, Email: henning.hagmann@uk-koeln.de.
Ivo Bendix, Email: Ivo.Bendix@uk-essen.de.
Kristin Kräker, Email: kristin.kraeker@mdc-berlin.de.
Ralf Dechend, Email: ralf.dechend@charite.de.
Elke Winterhager, Email: Elke.Winterhager@uk-essen.de.
Rainer Kimmig, Email: Rainer.Kimmig@uk-essen.de.
Angela Köninger, Email: Angela.Koeninger@barmherzige-regensburg.de.
Novelty and Significance
What Is New?
Using inducible sFLT1 (soluble fms-like tyrosine kinase-1)-transgenic mice to study preeclampsia related to a key factor of anti-angiogenesis, sFLT1.
Systemic sFLT1 overexpression revealed maternal, placental, and fetal preeclampsia-like characteristics.
What Is Relevant?
Systemic maternal sFLT1 overexpression is sufficient to impair spiral artery–remodeling and to induce hypertension during pregnancy.
Summary
Circulating maternal hsFLT1 (human sFLT1) is sufficient to induce preeclampsia-like symptoms in mice and impair spiral artery–remodeling independent from its fetoplacental expression, even if combined maternal/fetoplacental sFLT1 have stronger impact on fetal development. Maternal vascular maladaptation upon sFLT1 connects the maternal syndrome to placental characteristics of preeclampsia in mice.
References
- 1.Gumusoglu SB, Chilukuri ASS, Santillan DA, Santillan MK, Stevens HE. Neurodevelopmental outcomes of prenatal preeclampsia exposure. Trends Neurosci. 2020;43:253–268. doi: 10.1016/j.tins.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am J Physiol Renal Physiol. 2020;318:F1315–F1326. doi: 10.1152/ajprenal.00071.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381 [DOI] [PubMed] [Google Scholar]
- 4.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365. doi: 10.1155/2011/214365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R, Tsigas EZ, Callaghan WM. Health and economic burden of preeclampsia: no time for complacency. Am J Obstet Gynecol. 2017;217:235–236. doi: 10.1016/j.ajog.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol. 2019;134-135:1–10. doi: 10.1016/j.jri.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 8.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–1112. doi: 10.1161/CIRCRESAHA.118.313276 [DOI] [PubMed] [Google Scholar]
- 9.Agrawal S, Cerdeira AS, Redman C, Vatish M. Meta-Analysis and Systematic Review to Assess the Role of Soluble FMS-Like Tyrosine Kinase-1 and Placenta Growth Factor Ratio in Prediction of Preeclampsia: the SaPPPhirE Study. Hypertension. 2018;71:306–316. doi: 10.1161/HYPERTENSIONAHA.117.10182 [DOI] [PubMed] [Google Scholar]
- 10.Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci (Lond). 2012;122:43–52. doi: 10.1042/CS20110097 [DOI] [PubMed] [Google Scholar]
- 11.Schrey-Petersen S, Stepan H. Anti-angiogenesis and Preeclampsia in 2016. Curr Hypertens Rep. 2017;19:6. doi: 10.1007/s11906-017-0706-5 [DOI] [PubMed] [Google Scholar]
- 12.Cerdeira AS, Agrawal S, Staff AC, Redman CW, Vatish M. Angiogenic factors: potential to change clinical practice in pre-eclampsia? BJOG. 2018;125:1389–1395. doi: 10.1111/1471-0528.15042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A. 2011;108:1451–1455. doi: 10.1073/pnas.1011293108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kühnel E, Kleff V, Stojanovska V, Kaiser S, Waldschütz R, Herse F, Plösch T, Winterhager E, Gellhaus A. Placental-Specific Overexpression of sFlt-1 Alters Trophoblast Differentiation and Nutrient Transporter Expression in an IUGR Mouse Model. J Cell Biochem. 2017;118:1316–1329. doi: 10.1002/jcb.25789 [DOI] [PubMed] [Google Scholar]
- 15.Stojanovska V, Dijkstra DJ, Vogtmann R, Gellhaus A, Scherjon SA, Plösch T. A double-hit pre-eclampsia model results in sex-specific growth restriction patterns. Dis Model Mech. 2019;12:dmm035980. doi: 10.1242/dmm.035980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogtmann R, Kühnel E, Dicke N, Verkaik-Schakel RN, Plösch T, Schorle H, Stojanovska V, Herse F, Köninger A, Kimmig R, et al. Human sFLT1 leads to severe changes in placental differentiation and vascularization in a transgenic hsFLT1/rtTA FGR mouse model. Front Endocrinol (Lausanne). 2019;10:165. doi: 10.3389/fendo.2019.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda). 2009;24:58–71. doi: 10.1152/physiol.00033.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 19.Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31:465–474. doi: 10.1016/j.placenta.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda). 2005;20:180–193. doi: 10.1152/physiol.00001.2005 [DOI] [PubMed] [Google Scholar]
- 21.Fraser R, Whitley GS, Thilaganathan B, Cartwright JE. Decidual natural killer cells regulate vessel stability: implications for impaired spiral artery remodelling. J Reprod Immunol. 2015;110:54–60. doi: 10.1016/j.jri.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaynor LM, Colucci F. Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front Immunol. 2017;8:467. doi: 10.3389/fimmu.2017.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brosens A. The role of the spiral arteries in the pathogenesis of preeclampsia [j]. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 24.Khong T, De Wolf F, Robertson W, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x [DOI] [PubMed] [Google Scholar]
- 25.Staff AC, Fjeldstad HE, Fosheim IK, Moe K, Turowski G, Johnsen GM, Alnaes-Katjavivi P, Sugulle M. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am J Obstet Gynecol. 2020:S0002-9378(20)31116-9. doi: 10.1016/j.ajog.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 26.Sato Y. Endovascular trophoblast and spiral artery remodeling. Mol Cell Endocrinol. 2020;503:110699. doi: 10.1016/j.mce.2019.110699 [DOI] [PubMed] [Google Scholar]
- 27.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–769. doi: 10.1016/j.placenta.2004.02.011 [DOI] [PubMed] [Google Scholar]
- 28.Possomato-Vieira JS, Khalil RA. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv Pharmacol. 2016;77:361–431. doi: 10.1016/bs.apha.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrientos G, Tirado-González I, Freitag N, Kobelt P, Moschansky P, Klapp BF, Thijssen VL, Blois SM. CXCR4(+) dendritic cells promote angiogenesis during embryo implantation in mice. Angiogenesis. 2013;16:417–427. doi: 10.1007/s10456-012-9325-6 [DOI] [PubMed] [Google Scholar]
- 31.Gellhaus A, Schmidt M, Dunk C, Lye SJ, Kimmig R, Winterhager E. Decreased expression of the angiogenic regulators cyr61 (ccn1) and nov (ccn3) in human placenta is associatedwith pre-eclampsia. Mol Hum Reprod.. 2006;12:389–399. doi: 10.1093/molehr/gal044 [DOI] [PubMed] [Google Scholar]
- 32.Golic M, Stojanovska V, Bendix I, Wehner A, Herse F, Haase N, Kräker K, Fischer C, Alenina N, Bader M, et al. Diabetes mellitus in pregnancy leads to growth restriction and epigenetic modification of the Srebf2 gene in rat fetuses. Hypertension. 2018;71:911–920. doi: 10.1161/HYPERTENSIONAHA.117.10782 [DOI] [PubMed] [Google Scholar]
- 33.Han L, Yang Z, Li K, Zou J, Li H, Han J, Zhou L, Liu X, Zhang X, Zheng Y, et al. Antepartum or immediate postpartum renal biopsies in preeclampsia/eclampsia of pregnancy: new morphologic and clinical findings. Int J Clin Exp Pathol. 2014;7:5129–5143. [PMC free article] [PubMed] [Google Scholar]
- 34.Qian T, Hernday SE, Bao X, Olson WR, Panzer SE, Shusta EV, Palecek SP. Directed differentiation of human pluripotent stem cells to podocytes under defined conditions. Sci Rep. 2019;9:2765. doi: 10.1038/s41598-019-39504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pijnenborg R. Establishment of uteroplacental circulation. Reprod Nutr Dev. 1988;286B1581–1586. doi: 10.1051/rnd:19881004 [DOI] [PubMed] [Google Scholar]
- 36.Pawlak JB, Bálint L, Lim L, Ma W, Davis RB, Benyó Z, Soares MJ, Oliver G, Kahn ML, Jakus Z, et al. Lymphatic mimicry in maternal endothelial cells promotes placental spiral artery remodeling. J Clin Invest. 2019;129:4912–4921. doi: 10.1172/JCI120446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tayade C, Black GP, Fang Y, Croy BA. Differential gene expression in endometrium, endometrial lymphocytes, and trophoblasts during successful and abortive embryo implantation. J Immunol. 2006;176:148–156. doi: 10.4049/jimmunol.176.1.148 [DOI] [PubMed] [Google Scholar]
- 38.Tong W, Giussani DA. Preeclampsia link to gestational hypoxia. J Dev Orig Health Dis. 2019;10:322–333. doi: 10.1017/S204017441900014X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, et al. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension. 2010;56:304–310. doi: 10.1161/HYPERTENSIONAHA.110.153163 [DOI] [PubMed] [Google Scholar]
- 40.Cerdeira AS, Kandzija N, Pargmae P, Cooke W, James T, Redman C, Vatish M. Circulating soluble fms-like tyrosine kinase-1 is placentally derived in normal pregnancy: first in vivo evidence. Pregnancy Hypertens. 2019;16:145–147. doi: 10.1016/j.preghy.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 41.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15. doi: 10.1159/000341264 [DOI] [PubMed] [Google Scholar]
- 42.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884 [DOI] [PubMed] [Google Scholar]
- 43.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J bstet Gynecol. 2018;218:287–293. e1. doi: 10.1016/j.ajog.2017.11.561 [DOI] [PubMed] [Google Scholar]
- 44.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029 [DOI] [PubMed] [Google Scholar]
- 45.Dröge LA, Perschel FH, Stütz N, Gafron A, Frank L, Busjahn A, Henrich W, Verlohren S. Prediction of preeclampsia-related adverse outcomes with the sflt-1 (soluble fms-like tyrosine kinase 1)/plgf (placental growth factor)-ratio in the clinical routine: a real-world study. Hypertension. 2021;77:461–471. doi: 10.1161/HYPERTENSIONAHA.120.15146 [DOI] [PubMed] [Google Scholar]
- 46.Leaños-Miranda A, Graciela Nolasco-Leaños A, Ismael Carrillo-Juárez R, José Molina-Pérez C, Janet Sillas-Pardo L, Manuel Jiménez-Trejo L, Isordia-Salas I, Leticia Ramírez-Valenzuela K. Usefulness of the sFlt-1/PlGF (Soluble fms-Like Tyrosine Kinase-1/Placental Growth Factor) Ratio in Diagnosis or Misdiagnosis in Women With Clinical Diagnosis of Preeclampsia. Hypertension. 2020;76:892–900. doi: 10.1161/HYPERTENSIONAHA.120.15552 [DOI] [PubMed] [Google Scholar]
- 47.Bersi MR, Bellini C, Wu J, Montaniel KRC, Harrison DG, Humphrey JD. Excessive adventitial remodeling leads to early aortic maladaptation in angiotensin-induced hypertension. Hypertension. 2016;67:890–896. doi: 10.1161/HYPERTENSIONAHA.115.06262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arribas SM, Hinek A, González MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther. 2006;111:771–791. doi: 10.1016/j.pharmthera.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 49.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703–714. doi: 10.1161/CIRCULATIONAHA.113.003664 [DOI] [PubMed] [Google Scholar]
- 50.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005 [DOI] [PubMed] [Google Scholar]
- 51.Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE. Glomerular structure and function require paracrine, not autocrine, vegf–vegfr-2 signaling. J Am Soc Nephrol. 2010;21:1691–1701. doi: 10.1681/ASN.2010030295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsukimori K, Komatsu H, Fukushima K, Kaku T, Nakano H, Wake N. Inhibition of nitric oxide synthetase at mid-gestation in rats is associated with increases in arterial pressure, serum tumor necrosis factor-alpha, and placental apoptosis. Am J Hypertens. 2008;21:477–481. doi: 10.1038/ajh.2007.80 [DOI] [PubMed] [Google Scholar]
- 53.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;351 Pt 2367–372. doi: 10.1161/01.hyp.35.1.367 [DOI] [PubMed] [Google Scholar]
- 54.Zeisler H, Llurba E, Chantraine FJ, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet Gynecol. 2019;53:367–375. doi: 10.1002/uog.19178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.