Supplemental Digital Content is available in the text.
Keywords: blood pressure, clinical trial, hypertension, renin-angiotensin system, surrogate end point, vascular stiffness
Abstract
The SPARTE study (Strategy for Preventing cardiovascular and renal events based on ARTErial stiffness; URL: https://www.clinicaltrials.gov; Unique identifier: NCT02617238) is a multicenter open-label randomized controlled trial with blinded end point evaluation, undertaken at 25 French research centers in university hospitals. Patients with primary hypertension were randomly assigned (1:1) to a therapeutic strategy targeting the normalization of carotid-femoral pulse wave velocity (PWV) measured every 6 months (PWV group, n=264) versus a classical therapeutic strategy only implementing the European Guidelines for Hypertension Treatment (conventional group, n=272). In the PWV group, the therapeutic strategy used preferably a combination of ACE (angiotensin-converting enzyme) inhibitor or angiotensin receptor blocker and calcium channel blockers, as well as maximal recommended doses of ACE inhibitors and angiotensin receptor blockers. The primary outcome was a combined end point including particularly stroke and coronary events. Secondary outcomes included the time-course changes in brachial office blood pressure (BP), ambulatory BP, PWV, and treatments. After a median follow-up of 48.3 months, there was no significant between-group difference in primary outcome (hazard ratio, 0.74 [95% CI, 0.40–1.38], P=0.35). In the PWV group, combinations of renin-angiotensin-system blockers and calcium channel blockers were prescribed at higher dosage (P=0.028), office and ambulatory systolic blood pressure and diastolic blood pressure decreased more (P<0.001 and P<0.01, respectively), and PWV increased less (P=0.0003) than in the conventional group. The SPARTE study lacked sufficient statistical power to demonstrate its primary outcome. However, it demonstrated that a PWV-driven treatment for hypertension enables to further reduce office and ambulatory systolic blood pressure and diastolic blood pressure and prevent vascular aging in patients with hypertension at medium-to-very-high risk, compared with strict application of guidelines.
In patients with hypertension, hypertension mediated organ damage increases cardiovascular risk, independently of blood pressure (BP). However, treatment of hypertension is essentially targeted toward lowering BP, most (but not all) studies demonstrating a reduction in cardiovascular and renal complications.1–3 By comparison, very few studies have tested whether reducing hypertension mediated organ damage translated into a reduction of cardiovascular and renal complications beyond BP reduction. Those studies mainly focused on the regression of left ventricular hypertrophy (LVH) measured by either ECG or echocardiography4,5 and the reduction in urinary albumin excretion.6–8 Thus, LVH and, to a lesser extent, urinary albumin excretion could be qualified as true surrogate end points.9
Whether arterial stiffness is a surrogate, end point for cardiovascular disease has never been directly demonstrated by a controlled clinical trial. Arterial stiffening is the most characteristic clinical feature of the aging process of the arterial system.10,11 Arterial stiffness increases also with hypertension and corresponds to the loss of arterial compliance and changes in large artery wall properties.12–14 Stiff large arteries insufficiently dampen the pulsatility of ventricular ejection, thus high pulsatile pressure and flow are transmitted downstream to the kidney and brain, damaging these organs.12–14 In addition, the backward pulse wave returning to the heart increases the cardiac workload and generates LVH.15 The measurement of aortic stiffness is considered as an integrator of all damages done to the arterial wall during previous years in response to both classical cardiovascular risk factors and poorly identified risk factors, thus allowing to detect early vascular aging.10,11,16,17
In a 2006 consensus document,18 the measurement of carotid-femoral pulse wave velocity (PWV) was considered as a gold standard for the measurement of arterial stiffness. Other authors have shown not only the importance of PWV as an intermediate end point for cardiovascular disease and hypertension mediated organ damage19 but also the association between regression of PWV and regression of LVH.20 The repeated demonstration of the predictive value of carotid-femoral PWV for cardiovascular events21–24 led to its inclusion in the 2013 and 2018 European Society of Hypertension (ESH)/European Society of Cardiology (ESC) Guidelines for the Management of Hypertension.2,3 In a position article from the ESC working group on peripheral circulation,25 carotid-femoral PWV was considered as close to being considered a clinical surrogate end point. A 2019 Consensus Document of the ESC places arterial stiffness at the core vascular pathological changes leading to cardiac disease.26 Finally, a recent call to action of the Lancet Commission on Hypertension27 addressed the global burden of raised BP through a life-course strategy based on the quantification of early vascular ageing, best performed by the measurement of carotid-femoral PWV.10
We set up the SPARTE trial as a (Strategy for Preventing Cardiovascular and Renal Events Based on Arterial Stiffness).28 We hypothesized that a therapeutic strategy targeting the normalization of arterial stiffness in addition to the implementation of the 2007 ESC—ESH Hypertension Guidelines1 would reduce more cardiovascular and renal events compared with the unique implementation of the 2007 ESC-ESH Hypertension Guidelines (current Guidelines at the time of the beginning of the study). Our secondary objectives were to demonstrate that monitoring vascular aging through repeated PWV measurements would result in better intensification of treatment, better prevention of vascular aging, and better control of BP.
Methods
The authors declare that all supporting data are available within the article and in the Data Supplement.
Study Design and Participants
The design and methods of the SPARTE study (URL: https://www.clinicaltrials.gov; Unique identifier: NCT02617238) have been described in details previously.28 Briefly, SPARTE is a multicenter, prospective, open-label randomized controlled trial with blinded end point evaluation (Prospective, Randomized, Open, Blinded End Point design), undertaken at 25 French hypertension centers in university hospitals (Table S1 in the Data Supplement). The coordinating center, which served as a data and biostatistical core center, supervised randomization and inclusion of patients. Patients were adults with primary hypertension, aged 55 to 75 years at inclusion, at medium-to-very high cardiovascular risk, according to the 2007 ESH-ESC Guidelines for the management of hypertension.1 Participants were randomly assigned (1:1) to 2 groups: intervention group aiming at normalization of PWV through a prespecified therapeutic strategy (PWV group) and a control group where ESH-ESC Guidelines were applied, without reference to PWV (conventional group; Figure S1).
Inclusion and exclusion criteria for enrolment (Tables S2 and S3), randomization list, clinical and biological investigation at inclusion, methods for measurement of PWV and central BP, and time schedule of enrolment, interventions, assessments and visits of participants, have been previously detailed.28 Criteria for qualifying at medium to very high risk were, in addition to grade 1 or 2 hypertension, the presence of at least 3 cardiovascular risk factors or any of the following: metabolic syndrome, type 2 diabetes, target organ damage, cardiovascular disease, chronic kidney disease, grade 3 hypertension, isolated systolic hypertension (Tables S4 and S5).
Study Measurements
In both groups, attended seated office BP was measured during each visit, using validated semi-automatic oscillometric medical devices (see Appendix). Ambulatory BP monitoring was performed at baseline and at 6 and 48 months. All ambulatory BP monitoring were performed using brachial cuffs, and recommendations were made to use Omron monitors. Home BP monitoring was encouraged but not mandatory. Carotid-femoral PWV, central BP, and augmentation index (AIx)18,29 were measured by applanation tonometry using the Sphygmocor device (Atcor Medical, Sydney, Australia) as described previously.30,31 Aortic BP was estimated after calibration to mean and diastolic brachial pressures (radial tonometry). The follow-up study duration was 4 years, during which 2 scheduled clinical visits were performed per year for both groups. Additional visits could be performed when deemed necessary as standard of care.
In the intervention PWV group, bimonthly visits were performed during the first 6 months during which treatments were adjusted to target a PWV of <10 m/s. Then PWV (as well as central BP and AIx) was monitored every 6 months. If target PWV of 10 m/s could not be reached after 6 months, treatments were further adjusted at each scheduled visit.
In the conventional group, visits occurred every 6 months. PWV (as well as central BP and AIx) was measured at baseline, 2 years and 4 years. In the PWV group, both patients and investigators were aware of PWV values. In the control group, investigators and patients were strictly blinded for PWV because the results of PWV measurements (performed every 2 years) were masked, thus were not used for adapting therapeutic strategy and only served for comparing groups afterwards. Because SPARTE was an open-label study, blinding applied for the adjudicated end points in both groups (PWV and conventional groups).
Study Interventions
The primary aim in both groups was to control BP and risk factors according to guidelines. The difference between groups was mainly based on intensification and priority of drug-treatment and nonpharmacological interventions, driven by PWV in the intervention group and by BP in the conventional group. Because treatment was intensified based on PWV, we took great care not to over-treat patients. Drugs could be stepped down, even if PWV was uncontrolled, if BP was too low and in case of intolerance, notably orthostatic hypotension.
In the conventional group, we applied mandatory procedures from the 2007 ESH-ESC Guidelines for the Management of Hypertension.1 The objective was to bring office BP below 140/90 mm Hg, targeting 130 to 139 mm Hg for systolic BP (SBP), and 80 to 85 mm Hg for diastolic BP (DBP). We also used targets adapted to daytime ambulatory BP monitoring (135/85 mm Hg). International Guidelines were followed for caring about other risk factors such as diabetes and dyslipidemia, as standard of care.
In the PWV group, the objective was to bring PWV below the target of 10 m/s.29,30 For that purpose, nonpharmacological measures and antihypertensive treatment were adjusted and cardiovascular risk factors corrected until normalization of PWV (Tables S6 and S7). Therapeutic means to be used in the PWV group, and their pharmacological rationale have been previously described in detail.28 In brief, nonpharmacological therapies (physical exercise, dietary measures) were actively implemented at each visit. Combination therapy using a renin angiotensin system (RAS)-blocker (ACE [angiotensin-converting enzyme] inhibitor or angiotensin receptor blocker)32–35 and a calcium channel blocker (CCB)35,36 was recommended as first step.37,38 When a diuretic was indicated, indapamide was preferred.39,40 If BP was not controlled despite a triple combination (ACE inhibitor/angiotensin receptor blocker+CCB+diuretic, second step), or side effects occurred, the third step was to go to the highest recommended doses of ACE inhibitor or angiotensin receptor blocker within the combination.28,33 Betablockers (preferably vasodilating)41 were used as fourth line therapy, unless compelling indication.38,39,42,43 Spironolactone could also be used as fourth line therapy.44 As in the conventional group, other cardiovascular risk factors were taken care of according to international guidelines, using nonpharmacological measures, oral antidiabetic agents, lipid-lowering agents, and antiplatelet agents, as indicated.
Study Outcomes
The primary outcome was a combined end point including stroke and coronary events (myocardial infarction, angioplasty, bypass), fatal or not, peripheral artery disease (angioplasty, bypass, amputation), hospitalization for heart failure, aortic dissection, chronic kidney disease (doubling of creatinine, dialysis), and sudden death. On purpose, were not included transient ischemic attack and new onset of atrial fibrillation. The end point adjudication committee28 adjudicated all components of the primary outcomes of the study in a blinded fashion (allocation group and PWV value).
Secondary outcomes, planned for a prespecified statistical analysis,28 included the following: a restricted combined end point, including fatal cardiovascular events and nonfatal myocardial infarction and stroke; all individual components included in the combined end point; the time-course changes in brachial office and ambulatory BP, PWV, and central BP; and the time-course changes in treatments, in terms of pharmacological class, number of medications and dose.
Statistics
The sample size of the study was calculated from the main criteria (combined end point). A proportion test was used as an approximate estimation for the sample size calculation (2-sided Z test with unpooled variance). Considering a yearly incidence of the combined end point of 10% per year, a 20% risk reduction by the therapeutic strategy targeting PWV, a 4-year follow-up period and an α risk of 5%, a sample size of 750 patients per group gave a 90% power for analyzing the combined end point.
The statistical analysis was performed according to the intention to treat (ITT) principle keeping patients in their randomization group and including protocol violations, and specifically in the modified ITT population, defined as all subjects who had been randomized and had available data for the calculation of the primary end point, that is, patients without any follow-up were excluded from the modified ITT population. A per-protocol sensitivity analysis including only patients who fully complied with the protocol was also performed. The primary analysis focused on the combined primary outcome. In addition, all components of the primary outcome were analyzed separately. Survival analysis was used to calculate the time to the first cardiovascular or renal event. Survival curves were estimated by the Kaplan Meier method, with therapies groups compared with the use of the log-rank test. Therapy effect was estimated using Cox proportional-hazard model, after verification of the hypothesis of proportionality of hazards. Categories of cardiovascular risk were also included in the Cox model as a covariate (stratification factor of randomization). Proportional-hazard assumption was tested using Schoenfeld residuals. All estimates were provided with their 95% CIs.
A repeated-measures mixed model testing group effect, time effect, and group-time interaction was used to analyze the following variables over time: office SBP, DBP, and hazard ratio (HR; at inclusion, 6, 12, 18, 24, 30, 36, 42, and 48 months); ambulatory daytime, night-time, and 24-hour SBP, DBP, and HR (at inclusion, 6 and 48 months); and PWV, central SBP, DBP, and HR, and AIx (at inclusion, 24, and 48 months). Significance was fixed at P<0.05.
We also used a latent variable modeling (and in particular linear growth curve modeling) to analyze time-varying variables, using the R package lavaan. Missing data were handled by full information maximum likelihood: all the available data for each individual were used in obtaining a likelihood function for that person, thus allowing incorporating missing observations: the procedure is embedded in the lavaan package. A linear model was chosen after visual inspection of the data. Office SBP and DBP (mean of three measurements) were analyzed at inclusion visit, 6, 12, 18, 24, 30, 36, 42, and 48 months. Twenty-four hours SBP and 24-hourDBP were analyzed at inclusion visit, 6 and 48 months. Central SBP and DBP and AIx were analyzed at inclusion visit, 24 and 48 months. A model with 2 latent variables (BP at inclusion and slope) was considered. PWV was analyzed at the inclusion visit, at 24 and 48 months. First, a model with 2 latent variables was considered: intercept (PWV at inclusion) and slope (biannual rate of PWV change from inclusion to 48 months visit), with treatment arm as a covariate. Then, mean BP (central pulse pressure/3+DBP) at the 3 visits was added as time-varying covariate too, to investigate BP-independent differences in linear growth between treatment arm.
To detect differences in antihypertensive treatment strategies between conventional and PWV treatment group, a similar procedure (latent variable modeling, linear growth curve modeling) was used to analyze time-varying variables at all available visits (0, 6, 12, 24, 30, 36, 42, and 48 months, real dates). The following treatment-related time-varying variables were used: number of BP-lowering drugs; treatment intensity score, calculated by assigning to each administered drug a coefficient indicating the dosage (1, low; 2, average; and 3, high); and treatment intensity score referring only to RAS-blockers and calcium channel blockers. The percentage of patients treated with a RAS-blocker+CCB combination in each treatment arm and visit was compared by general linear models (factors: visit, treatment arm, visit×treatment arm). Similar analyses were used for detecting differences in lipid lowering and antidiabetic treatments. Analyses were performed using the SAS (SAS Institute, Cary, NC; software version 9.4) and Rstudio (Version 1.2.5042).
All trajectories over time of treatment intensification, BP and PWV, have been obtained with latent variable analysis. Data obtained with the repeated measure mixed model are presented in the Data Supplement.
Role of Funding Sources and Ethical Considerations
The SPARTE study protocol has received approval by the Ethics Committee (CPP) of Ile-de-France XI, on June 14, 2012, that was applicable to all participating centers. This was an investigator generated and driven study and as such was performed in full independence of the study sponsors, that is, Assistance Publique-Hôpitaux de Paris, Direction de la Recherche Clinique et du Développement, and Fondation pour la Recherche en Hypertension Artérielle.
According to the French bioethics law, the patient consent was not required because the SPARTE protocol was aiming at evaluating usual clinical care, by comparing 2 therapeutic approaches using therapeutic means and drugs already recommended by National45 or International Guidelines,1–3 without added risk and with few constraints. However, patients were duly informed, and required to express their nonopposition to participate to the protocol.
Progress of the Study
The first patient was included on July 26, 2013. The last patient-last visit occurred on January 26, 2020. The inclusion rate and consequently the total number of patients were lower than expected because of competing protocols in study centers and insufficient financial support. In January 2016, the steering committee decided to stop the recruitment, in order not to jeopardize the study, and to complete the 4 years follow-up of all patients.
Results
Study Participants and Enrolment
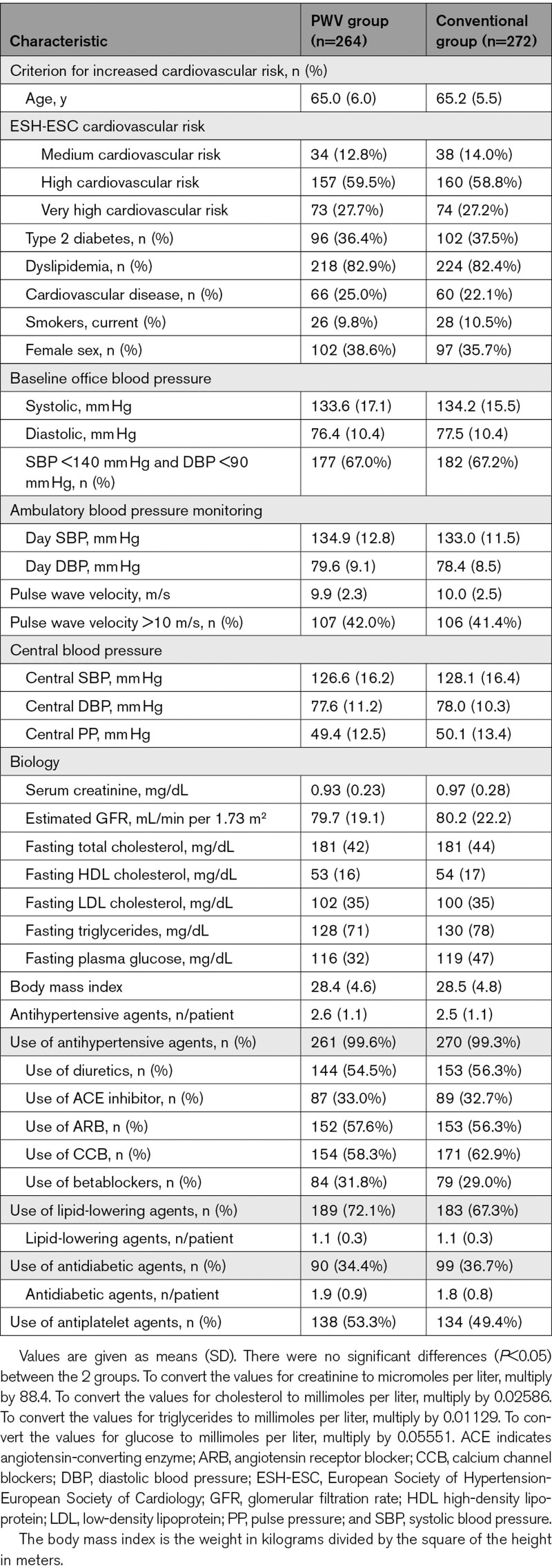
A total of 536 participants were enrolled in the study (264 in the PWV group and 272 in the conventional group) between July 2013 and January 2020 (Figure 1) with a median follow-up of 48.3 months (interquartile range, 46.6–49.8; modified ITT population). The median follow-up (interquartile range) did not differ between groups: 48.3 (45.9–49.7) versus 48.3 (47.1–49.8). Descriptive baseline statistics are presented in Table 1. Patients were young elderly (65 years old), 2/3 were males, most of them were at high to very high risk. Indeed, all were hypertensive with good BP control at entry (134/77 mm Hg at office, similar at ambulatory BP monitoring) with 2.5 antihypertensive drugs. More than 80% had dyslipidemia, 1/3 were diabetics, 1/4 had previously known cardiovascular disease. Baseline characteristics were well balanced between groups.
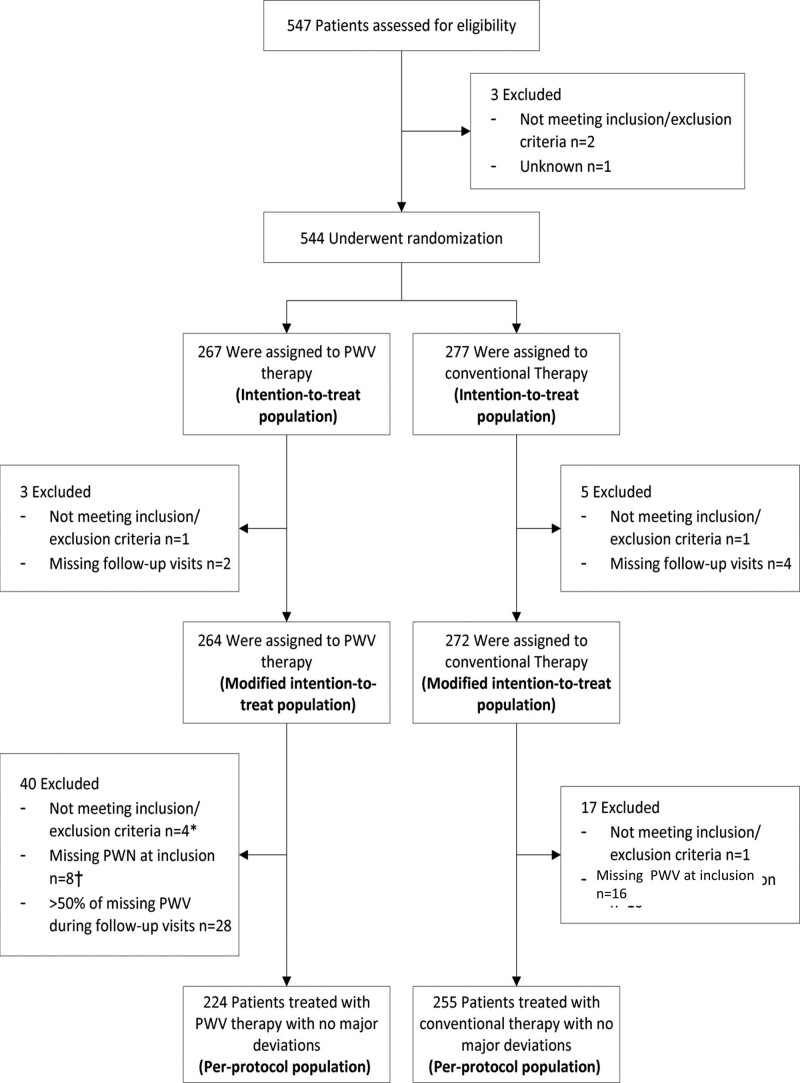
Figure 1.
Eligibility, randomization, and follow-up. The intention-to-treat (ITT) population was defined as all subjects who were randomized. The modified ITT (mITT) population was defined as all subjects who have been randomized and have available data for the calculation of the primary end point, that is, patients without any follow-up were excluded from the mITT population. The per-protocol (PP) population was defined as the set of subjects who did not have any major protocol violation that may interfere with primary criteria evaluation. PWV indicates pulse wave velocity.
Table 1.
Baseline Characteristics of the Study Participants
Clinical Outcomes
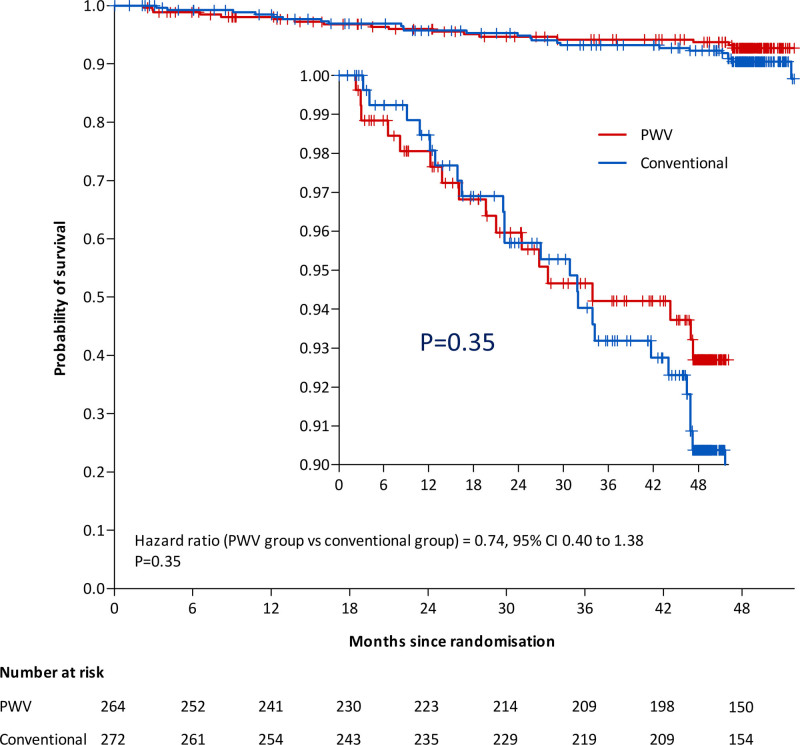
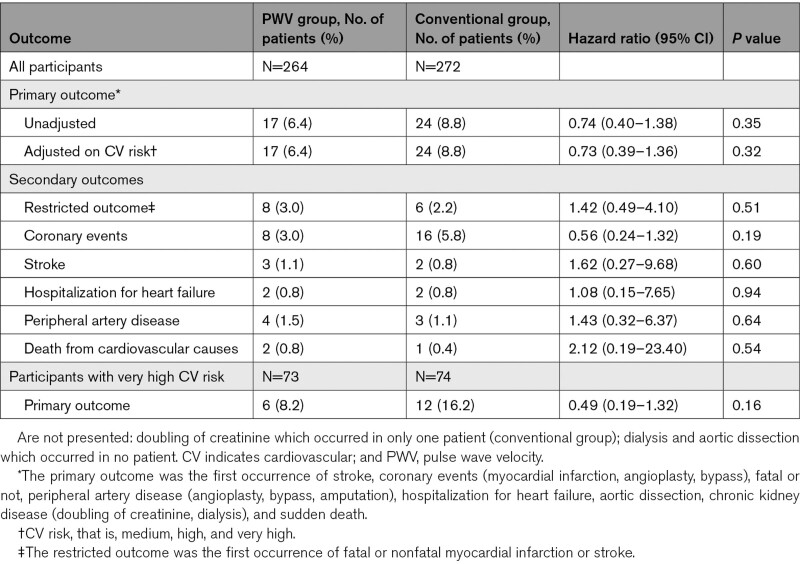
Forty-one participants qualified for a primary outcome event: 17 (1.6% per year) in the PWV group and 24 (2.2% per year) in the conventional group, in the modified ITT analysis. The HR was 0.74, however not significant (95% CI, 0.40–1.38; P=0.35; Figure 2, Table 2). Results were similar when adjusted on cardiovascular risk (HR, 0.73 [95% CI, 0.39–1.36]; P=0.32; Table 2). As prespecified, we stratified the survival analysis according to the level of cardiovascular risk (medium, high, and very-high) on the whole population (independently of treatment group). Because no event was observed for the patients with medium cardiovascular risk, those patients were pooled with high cardiovascular risk. Patients at medium+high cardiovascular risk had lower risk of presenting the primary outcome than very-high cardiovascular risk patients (HR, 0.46 [95% CI, 0.25–0.86], P=0.012; Figure S2). Patients at medium+high cardiovascular risk had similar rates of primary outcome whether they were randomized to PWV monitoring (11 events), or in the conventional group (12 events; HR, 0.99 [95% CI, 0.44–2.24], P=0.97; Figure S3A). A similar analysis performed in patients at very high cardiovascular risk showed that 6 events (2.0% per year) were observed in the PWV group and 12 events (4.0% per year) were observed in the conventional group (HR, 0.49 [95% CI, 0.19–1.32]; P=0.16; Figure S3B). Events contributing to the primary end point in the total study population and the very-high risk sub-population are given in Tables S8 and S9, respectively. In addition, we tested the interaction (not prespecified) between treatment groups (PWV or conventional) and the value of PWV at baseline (PWV> or ≤10 m/s). This interaction was not statistically significant. Finally, no significant between-group difference was observed across prespecified secondary outcomes including restricted outcomes (fatal or nonfatal myocardial infarction or stroke) and the individual components of the primary outcome (Table 2). Results were similar considering the per-protocol population
Figure 2.
Difference in primary outcome events. A primary outcome was confirmed in 41 participants: 17 (1.6% per year) in the pulse wave velocity (PWV) group and 24 (2.2% per year) in the conventional group (hazard ratio, 0.75 [95% CI, 0.40–1.38] P=0.35).
Table 2.
Primary and Secondary Outcomes
Intensification of Treatment
The number of BP-lowering drugs and the treatment intensity score increased over time in the PWV-based group (P=0.004 and P<0.001, respectively), but not in the conventional group (P=0.161 and P=0.271, respectively). Although the proportion of patients treated with a RAS-blocker+CCB combination remained unchanged over time in the 2 treatment groups (Figure S4A), their dosage (similar at inclusion) was progressively increased over time in the PWV-based but not in the conventional treatment group (P=0.007 and P=0.808, respectively). Thus, the trajectories of RAS-blocker+CCB combination treatment intensity score significantly diverged during follow-up (P=0.028; Figure S4B), in favor of the PWV group.
There were no significant differences between groups in lipid lowering and antidiabetic drugs.
Office and Ambulatory Blood Pressure Changes With Treatment
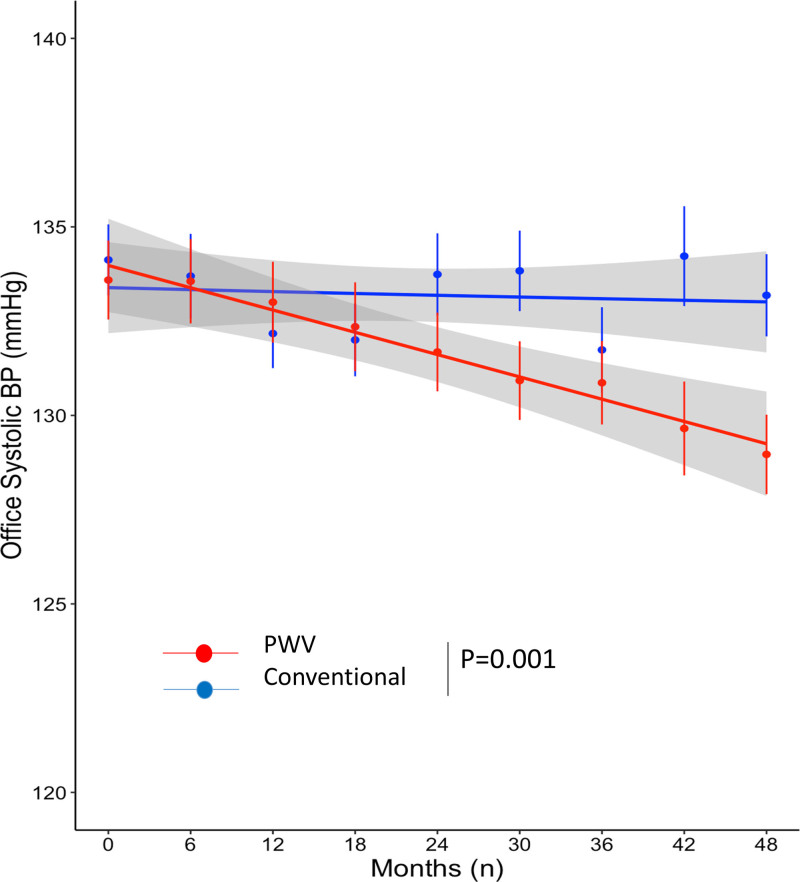
Whereas office SBP and DBP were similar at inclusion in the 2 treatment arms, their trajectories over time were significantly different (P=0.001 for both), with a greater reduction rate in the PWV group than in the conventional group (SBP, −1.08 mm Hg/y versus −0.10 mm Hg/y; DBP, −1.34 mm Hg/y versus −0.61 mm Hg/y; Figures 3 and S5). A repeated-measures mixed model testing group effect, time effect, and group-time interaction gave similar results (Table S10).
Figure 3.
Whereas office systolic blood pressure (SBP) were similar at inclusion in the two treatment arms, their trajectories over time were significantly different (P=0.001), with a greater reduction rate in the pulse wave velocity (PWV) group (in red) than in the conventional group (in blue): −1.08 mm Hg/y vs −0.10 mm Hg/y, respectively. Dots indicate mean values, error bars indicate SDs, lines indicate fitting smoothing spline curves with 95% CIs in gray. Trajectories over time have been obtained with latent variable analysis.
The trajectories of 24-hour SBP and DBP were significantly different, with a greater reduction rate in the PWV group than in the conventional group (24-hour SBP, −1.41 mm Hg/y versus −0.21 mm Hg/y; 24-hour DBP, −1.04 mm Hg/y versus −0.32 mm Hg/y; P=0.004 and P=0.005, respectively). A repeated-measures mixed model testing group effect, time effect, and group-time interaction gave similar results (Table S11).
PWV Changes With Treatment
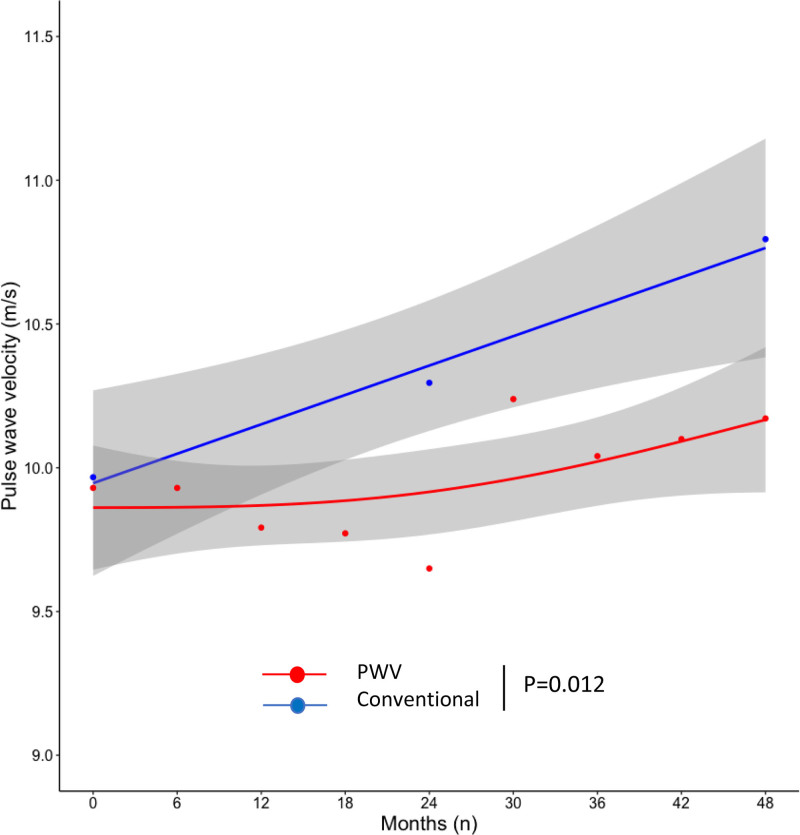
PWV was similar at inclusion in the 2 treatment arms, however, PWV trajectories over time were significantly different (P=0.012; Figure 4). In the conventional arm, PWV increased with a rate of 0.20 m/s/y (P=0.001). This increase was independent from baseline PWV (P=0.916). In the PWV arm, the PWV increase rate was not significant (0.06 m/s/y, P=0.140). The difference between groups remained significant after adjusting for mean BP as time-varying covariate (adjusted PWV increase rate difference 0.14 m/s/y, P=0.041). Central SBP and DBP (but not AIx) significantly decreased during the course of the study. However, we did not observe any significant difference between groups for these parameters (Table S12).
Figure 4.
Trajectories of pulse wave velocity (PWV) in the conventional (in blue) and in the PWV-based (in red) treatment arm. In the conventional arm, PWV increased with a rate of 0.20 m/s/y (P=0.001). In the PWV arm, the PWV increase rate was not significant (0.06 m/s/y, P=0.140). Whereas PWV was similar at inclusion in the 2 treatment arms, PWV trajectories over time were significantly different (P=0.012). Dots indicate mean values. Lines indicate fitting smoothing spline curves with 95% CIs in gray. Trajectories over time have been obtained with latent variable analysis.
Adverse Events
A total of 84 adverse events (42 versus 42, in the PWV and conventional groups, respectively) were observed in 64 patients (33 versus 31, in the PWV and conventional groups, respectively) in the modified ITT population, with no significant difference between groups (Tables S13 and S14). There was no excess of hypotension episodes in the PWV group (Table S14). No syncope occurred. We did not observe renal failure.
Discussion
The SPARTE study was designed to test the hypothesis that, in addition to following guidelines in comparison with following guidelines alone for the management of medium to very-high risk hypertensive patients, targeting PWV is accompanied by a significant reduction of combined cardiovascular events. Even though there was a reduction in the HR of the combined primary outcome (0.74), its 95% CI was large (0.40–1.38), and the difference between the 2 treatment strategies was not significant (P=0.35). Indeed, the number of patients included in the study was much lower than that initially planned and the number of total events was small (total of 41 events), both decreasing the statistical power of the study. Nevertheless, it showed that a therapeutic strategy targeting PWV values lower than 10 m/s, (based on intensification of treatment with high-dose RAS-blockers and CCBs) is associated with a better BP control and is effective in the prevention of vascular aging. This result is obtained without increasing adverse events.
Clinical Outcomes
The SPARTE trial lacked sufficient statistical power to demonstrate a greater reduction in cardiovascular events in the PWV-based compared with the conventional treatment arm. Three main reasons can be identified. First, the SPARTE study included about three times less patients than initially planned (536 instead of 1500), due to competing protocols in study centers and insufficient financial support. Second, the yearly incidence of the primary end point was lower than estimated (10%) in the protocol28 from the Cardio-Sis,46 ACCORD (Action to Control Cardiovascular Risk in Diabetes),47 and STENO (Effect of a Multifactorial Intervention on Mortality in Type 2 Diabetes)48 studies. Indeed, it was only 2.2% in the conventional group as a whole, and 4% in very-high risk patients of this group. Indeed, not all patients of the SPARTE study had LVH or diabetes. Third, the number of cardiovascular deaths which occurred during the SPARTE study was twice lower than predicted from individual Systematic Coronary Risk Evaluation (ie, 4 versus 9).49 Similarly, the number of fatal and nonfatal cardiovascular events which occurred during the SPARTE study was twice lower than predicted from individual Framingham risk scores (ie, 24 versus 51).50 Thus, even if cardiovascular risk was distributed as initially planned (medium risk, 14% of patients; high risk, 59%; very high risk, 27%), a cohort effect may have played a role in reducing cardiovascular risk in the SPARTE population >10 years after the establishment of risk formulas by the SCORE and Framingham equations, as shown in many contemporary studies.51 In addition, patients of the SPARTE study were closely followed-up and treated in hypertension centers of University Hospitals, and this may have contributed to reduce the risk of cardiovascular complications.
The largest individual outcome difference between the PWV and conventional groups was observed in coronary angioplasty (Tables 2 and S8), although this was not significant. In that regard, it is important to note that although indications of coronary angioplasty in France are driven by demonstrated cardiac ischemia, they can still be considered as physician-dependent. However, they were similar in the 2 groups, per the PROBE blinded end point Committee.
The reduction of primary outcome rate observed in the PWV-based treatment arm was in line with our working hypothesis (−25% versus −20%, respectively) and even larger in very-high risk patients (−51%). This suggests that, based on the exploratory findings of the SPARTE study, larger, adequately powered studies can be set up, to finally demonstrate effectiveness of the proposed treatment strategy (PWV-based rather than BP-based) in patients with primary hypertension. In addition, some protocol modifications and simplifications could be suggested. An alternative strategy would be to include patients at very high cardiovascular risk or patients with a PWV that is elevated above that expected for age. Another important issue is whether a trial directed at lowering BP to a lower target in individuals with elevated PWV at baseline might have produced similar results.
Intensification of Treatment, PWV, and BP
The number of antihypertensive drugs and their dosages (ie, treatment intensity score) increased more over time in the PWV-based than in the conventional treatment group, a change mostly driven by titration of RAS-blocker+CCB combination. As a consequence, office and ambulatory SBP and DBP decreased significantly more and PWV increased significantly less in the PWV group than in the conventional group. The PWV difference between groups remained significant after adding mean BP as time-varying covariate. Thus, the intensification of treatment allowed to reduce arterial stiffness independently of BP reduction, as we and other have already demonstrated in small size randomized clinical trials.32–40 Because there were no significant differences in other therapeutic strategies (nonpharmacological, lipid lowering, antidiabetic treatment), it is very likely that the intensification of antihypertensive drugs (increased use of RAS blockers+CCB combinations at optimal dosages) explains the reduction of arterial stiffening, that is, the prevention of vascular aging in the PWV group.
While the importance of the RAS-blocker+CCB combination has been later acknowledged by the 2018 ESC/ESH Guidelines,3 our data point to an extra benefit, in term of BP control and hypertension mediated organ damage, when highest recommended doses are used. If supported by larger studies, this result may change routine hypertension management, since a significant improvement was observed between the trajectories of treatment strategies with quantifiable benefit. Finally, treatment intensification was well tolerated, and we did not observe more adverse events in the PWV intervention group.
SPARTE Study Versus Observational and Longitudinal Studies
This is the first time that PWV was measured every 6 months for such a long period of time (4 years) in such a large group of patients. The prevention of vascular aging was large, since PWV did not significantly increase in the PWV intervention group, whereas it increased by 0.2 m/s/y in the control group, leading to a 1.0 m/s increase at the end of the trial. In the conventional group, but not in the PWV group, the PWV increase rate is consistent with data from cross-sectional30,52 and longitudinal studies53 which included patients at low-to-high risk. Our results are also consistent with a recent analysis of the SPRINT (Systolic Blood Pressure Intervention Trial)-HEART study54 in 337 patients who benefited from a measurement of cfPWV and aortic elastance at baseline and after 18 months follow-up.
In the PWV group of the SPARTE study, the nonsignificant rate of increase in PWV (0.06 m/s/y) suggests that the treatment strategy prevented vascular aging, which was lowered to values observed in low-risk community dwelling volunteers55 for at least the duration of the study. The relationship between arterial stiffness and BP is bidirectional, since any increase in BP can mechanically increase arterial stiffness, and conversely an increase in arterial stiffness is known to increase the probability of incident of hypertension.56 Thus, the sustained increase in arterial stiffness in the conventional group may explain why SBP did not decrease and the reduction in office DBP plateaued after 18 months at a higher level than in the PWV group.
Considerations for Clinical Practice
An important finding of the SPARTE study is that it is possible to further lower BP in patients that were considered, for most of them, as having controlled BP. Indeed, office SBP and DBP at baseline were 134 and 77 mm Hg, and 67% of patients had <140 and 90 mm Hg. The lack of SBP reduction and the limited DBP reduction in the conventional group demonstrate that targeting the normalization of office BP within the 130 to 139/80 to 85 mm Hg range is not effective enough in clinical practice, as lately suggested by the SPRINT trial.57 At variance with, and beyond SPRINT, SPARTE study adds as an original contribution the importance of maximizing doses of de-stiffening drugs (such as RAS-blockers and CCBs), targeting the normalization of arterial stiffness through repeated PWV measurements and using these measurements as a tool for therapeutic education and sensitization of patients and physicians to treatment intensification.
Another important finding is that the intensification of antihypertensive treatment could reduce arterial stiffness and prevent arterial aging not only through BP lowering, but also independently of BP reduction, that is, likely through long-term arterial remodeling.32–41 Thus, the prevention of cardiovascular complications may require not only a good BP control, but also an effective prevention of arterial aging through adequate therapeutic measures including lifestyle changes and intensification of de-stiffening drugs, such as RAS-blockers and CCBs.
Strengths and Limitations
This study has a number of strengths: this is the first attempt to demonstrate that arterial stiffness is a surrogate end point for cardiovascular disease. SPARTE is an intervention trial, performed according to the standards of clinical trials, with long follow-up (4 years) during which repeated measurements of various parameters were performed. The study included a mechanistic approach through the quantification of treatment intensification, PWV changes and BP lowering, as causal mechanisms for the reduction of outcomes. The therapeutic strategy in the PWV group was based on a strong pharmacological rational.
However, SPARTE has limitations. The main limitation is that despite continuing efforts, the recruitment in SPARTE did not reach the expected numbers, resulting in an underpowered study for the primary end point, that is, clinical outcomes. In addition, as a general concern, the question may arise for future studies, when there are 2 colinear cardiovascular risk factors (PWV and SBP), how much the contribution of lower SBP, relative to PWV, made to outcomes.
Perspectives
The SPARTE study has broad implications. First, the present findings can be considered as exploratory ones to plan larger, adequately powered studies aiming at demonstrating the effectiveness of the proposed treatment strategy (PWV-based rather than BP-based) in patients with primary hypertension. Second, if the reduction of outcomes appears significant in replicated studies, it would be possible to demonstrate a therapeutic link between the various steps of our primary hypothesis: intensification of treatment, that is, maximizing doses of de-stiffening drugs such as RAS-blockers and CCBs; reduction of arterial stiffness independently from BP; and reduction of outcomes. Thus, the proof of concept that arterial stiffness is a true surrogate end point would be obtained. Third, from a routine clinical practice point of view, repeated PWV measurements could be used as a tool for therapeutic education and sensitization of patients and physicians to treatment intensification and ultimately better prevention of cardiovascular complications. Finally, clinical trials aiming at demonstrating that arterial stiffness is a surrogate end point may target not only specific hypertensive populations, for instance those with elevated PWV or those at very-high cardiovascular risk, but may also target diabetic patients through intensification of antidiabetic treatment.
Conclusions
A PWV normalization driven strategy, compared with BP driven strategy, did not result in a statistically significant reduction in cardiovascular outcomes despite leading to significant treatment intensification, reduction in office and ambulatory SBP and DBP, and prevention of vascular aging. This study, which has been underpowered for clinical events, should be replicated with a larger number of patients.
Acknowledgments
Chantal Andrieux and Youcef Sekour (CRU of Georges Pompidou Hospital), who acted from 2013 to 2019 as clinical study manager and clinical research associate, respectively; Karim Aïssi, Gilles Barone-Rochette, Jeremy Bellien, Cyrille Bergerot, Jacques Blacher, Gilles Chironi, Gonzagues Claisse, Antoine Cremer, Caroline Dourmap-Colas, Najeh El Esper, Pierre Fessler, Jean-Michel Halimi, Daniel Herpin, Michele Jacob, Robinson Joannides, Fouad Khettab, Pierre Lantelme, Bruno Legallicier, Sylvain Lejeune, Marilucy Lopez-Sublet, Badreddine Merioud, Claire Mounier-Vehier, Patrick Messner-Pelenc, Olivier Ormezzano, Pascal Rossi, Gerald Roul, Camille Roubille, David Rozenbaum, Jean-Michel Tartiere, Anne-Isabelle Tropeano, Alexandra Yanoutsos. S. Laurent, GC, M. Azizi, G. London, J.-J. Mourad, B. Pannier, and P. Boutouyrie contributed to the conception and design of the study. H. Pereira and R.-M. Bruno performed the statistical analysis. H. Khettab performed a large number of arterial stiffness measurements, quality control and technical support to centers. S. Laurent, G. Chatellier, and P. Boutouyrie drafted and wrote the protocol. All authors contributed to the writing and the reviewing of this article, and approved the final draft of the article.
Sources of Funding
French Ministry of Research (PHRC 2011- K110102/N°ID RCB: 2012-A00023-40) and Fondation de Recherche sur l’Hypertension Arterielle (FRHTA). Centers received funding from the Ministry of Research in proportion to the number of patients included in the study, which was paid directly to the hospital and targeted to the salary of a clinical research technician. Centers were also allocated a Sphygmocor device (Atcor Medical, Sydney, Australia) from FRHTA. Patient consent not required. However, patients were duly informed, and should express that they are not opposed to participate to the protocol.
Disclosures
S. Laurent has received honoraria as lecturer for Menarini, Sanofi and Servier, and Axelife, Omron and Withings. M. Azizi has received research grants from the French Ministry of Health, Quantum genomics and European H2020 program; has received grant support and nonfinancial support from ReCor Medical and Idorsia; and has received personal fees from CVRx. G. Choukroun has received honoraria as lecturer, participant to board of experts, and travel grants from Astellas, Sanofi, VIfor Pharma, and Amgen. N. Danchin has received grants, speaking or consulting fees from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Intercept, Merck Shard Dohme, Novartis, NovoNordisk, Pfizer, Sanofi, Servier, Union Chimique Belge, and Vifor. X. Girerd has received honoraria for consultancy or lectures for Bouchara-Recordati, Merck, Sanofi, Boehringer Ingelheim. J.-J. Mourad has received honoraria for consultancy or lectures for Mylan, Pfizer, and Servier. D. Stephan has received honoraria as lecturer for Servier. P. Valensi has received honoraria as lecturer or participant to board of experts, or research grants from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Hikma Pharmaceuticals, Merck Sharp & Dohme, Novo Nordisk, Novartis, Pfizer, Sanofi, and Servier. P. Cunha has received honoraria as lecturer for Merck Shard Dohme, Novartis, Boehringer Ingelheim, and AstraZeneca. K. Narkiewicz has received honoraria from Berlin-Chemie/Menarini, Egis, Idorsia, Gedeon Richter, Krka, Medtronic, Polpharma, Recordati, and Servier. P. Boutouyrie currently serves as President of Association for Research into Arterial Structure and Physiology sponsored by an unconditional grant by SERVIER. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACE
- angiotensin-converting enzyme
- AIx
- augmentation index
- BP
- blood pressure
- CCB
- calcium channel blockers
- DBP
- diastolic blood pressure
- ESC
- European Society of Cardiology
- ESH
- European Society of Hypertension
- HR
- hazard ratio
- ITT
- intention to treat
- LVH
- left ventricular hypertrophy
- PWV
- pulse wave velocity
- RAS
- renin angiotensin system
- SBP
- systolic blood pressure
- SPARTE
- Strategy for Preventing Cardiovascular and Renal Events Based on Arterial Stiffness
A list of all SPARTE Investigators is given in the Data Supplement.
For Sources of Funding and Disclosures, see page 993.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.17579.
Contributor Information
Gilles Chatellier, Email: gilles.chatellier@aphp.fr.
Michel Azizi, Email: michel.azizi@aphp.fr.
David Calvet, Email: d.calvet@ch-sainte-anne.fr.
Gabriel Choukroun, Email: choukroun.gabriel@chu-amiens.fr.
Nicolas Danchin, Email: nicolasdanchin@yahoo.fr.
Pascal Delsart, Email: Pascal.Delsart@chru-Lille.fr.
Xavier Girerd, Email: xavier.girerd@psl.aphp.fr.
Philippe Gosse, Email: philippe.gosse@chu-bordeaux.fr.
Hakim Khettab, Email: hakim.khettab@aphp.fr.
Gerard London, Email: gerardmichellondon@gmail.com.
Jean-Jacques Mourad, Email: jjmourad@hpsj.fr.
Bruno Pannier, Email: brpannier@gmail.com.
Helena Pereira, Email: helena.pereira@egp.aphp.fr.
Dominique Stephan, Email: Dominique.Stephan@chru-strasbourg.fr.
Paul Valensi, Email: paul.valensi@aphp.fr.
Pedro Cunha, Email: pedrogcunha@netcabo.pt.
Krzysztof Narkiewicz, Email: knark@gumed.edu.pl.
Rosa-Maria Bruno, Email: rosa-maria.bruno@inserm.fr.
Pierre Boutouyrie, Email: pierre.boutouyrie@aphp.fr.
Novelty and Significance
What Is New?
First attempt to demonstrate that arterial stiffness is a surrogate end point for cardiovascular disease.
Multicenter open-label randomized controlled trial with blinded end point evaluation.
What Is Relevant?
Aortic stiffness is an integrator of all damages done to the arterial wall during previous years by hypertension and other cardiovascular risk factors.
Summary
A pulse wave velocity normalization driven strategy did not result in a statistically significant reduction in cardiovascular outcomes despite significant treatment intensification, reduction in office and ambulatory blood pressures, and prevention of vascular aging, compared with usual blood pressure driven therapeutic strategy.
References
- 1.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, et al. ; Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. ; Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 4.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlöf B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. doi: 10.1001/jama.292.19.2350 [DOI] [PubMed] [Google Scholar]
- 5.Bang CN, Devereux RB, Okin PM. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction - A LIFE review. J Electrocardiol. 2014;47:630–635. doi: 10.1016/j.jelectrocard.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlöf B, Devereux RB, de Faire U, Fyhrquist F, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a [DOI] [PubMed] [Google Scholar]
- 7.Holtkamp FA, de Zeeuw D, de Graeff PA, Laverman GD, Berl T, Remuzzi G, Packham D, Lewis JB, Parving HH, Lambers Heerspink HJ. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32:1493–1499. doi: 10.1093/eurheartj/ehr017 [DOI] [PubMed] [Google Scholar]
- 8.Inker LA, Levey AS, Pandya K, Stoycheff N, Okparavero A, Greene T; Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient meta-analysis. Am J Kidney Dis. 2014;64:74–85. doi: 10.1053/j.ajkd.2014.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr, Hong Y, Howard BV, et al. ; American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. 2009;54:3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114 [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging. Hypertension. 2019;74:218–228. doi: 10.1161/HYPERTENSIONAHA.119.12655 [DOI] [PubMed] [Google Scholar]
- 12.Safar ME, O’Rourke MF. Handbook of Hypertension, volume 23: Arterial Stiffness in Hypertension. 2006. Elsevier; 598. [Google Scholar]
- 13.Nichols WW, O’Rourke MF. In: McDonald’s Blood Flow in arteries; Theoretical, Experimental and Clinical Principles. 2011Sixth EditionHodder Arnold ed.; 755. [Google Scholar]
- 14.Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, Laurent S, London G, Pannier B, Protogerou A, et al. ; French Study Group on Arterial Stiffness. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72:796–805. doi: 10.1161/HYPERTENSIONAHA.118.11212 [DOI] [PubMed] [Google Scholar]
- 15.Boutouyrie P, Laurent S, Girerd X, Benetos A, Lacolley P, Abergel E, Safar M. Common carotid artery stiffness and patterns of left ventricular hypertrophy in hypertensive patients. Hypertension. 1995;254 Pt 1651–659. doi: 10.1161/01.hyp.25.4.651 [DOI] [PubMed] [Google Scholar]
- 16.Chirinos JA, Segers P, Hughes T, Townsend R. Large-Artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864–886. doi: 10.1161/CIRCRESAHA.121.318061 [DOI] [PubMed] [Google Scholar]
- 18.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- 19.Vasan RS, Short MI, Niiranen TJ, Xanthakis V, DeCarli C, Cheng S, Seshadri S, Mitchell GF. Interrelations between arterial stiffness, target organ damage, and cardiovascular disease outcomes. J Am Heart Assoc. 2019;8:e012141. doi: 10.1161/JAHA.119.012141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Waaij KM, Heusinkveld MHG, Delhaas T, Kroon AA, Reesink KD. Do treatment-induced changes in arterial stiffness affect left ventricular structure? A meta-analysis. J Hypertens. 2019;37:253–263. doi: 10.1097/HJH.0000000000001918 [DOI] [PubMed] [Google Scholar]
- 21.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236 [DOI] [PubMed] [Google Scholar]
- 22.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031 [DOI] [PubMed] [Google Scholar]
- 23.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 24.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–532. doi: 10.1016/j.atherosclerosis.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 26.Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA, De Carlo M, Delgado V, Lancellotti P, Lekakis J, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail. 2019;21:402–424. doi: 10.1002/ejhf.1436 [DOI] [PubMed] [Google Scholar]
- 27.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388:2665–2712. doi: 10.1016/S0140-6736(16)31134-5 [DOI] [PubMed] [Google Scholar]
- 28.Laurent S, Chatellier G, Azizi M, Calvet D, Choukroun G, Danchin N, Delsart P, Gosse P, London G, Mourad JJ, et al. ; on behalf of SPARTE investigators. Protocol of the SPARTE Study: a Strategy for Preventing cardiovascular and renal events based on ARTErial stiffness. Artery Res. 2020;26:250–260. [Google Scholar]
- 29.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, et al. ; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0 [DOI] [PubMed] [Google Scholar]
- 30.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–50. doi: 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbert A, Cruickshank JK, Laurent S, Boutouyrie P; Reference Values for Arterial Measurements Collaboration. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35:3122–3133. doi: 10.1093/eurheartj/ehu293 [DOI] [PubMed] [Google Scholar]
- 32.Tropeano AI, Boutouyrie P, Pannier B, Joannides R, Balkestein E, Katsahian S, Laloux B, Thuillez C, Struijker-Boudier H, Laurent S. Brachial pressure-independent reduction in carotid stiffness after long-term angiotensin-converting enzyme inhibition in diabetic hypertensives. Hypertension. 2006;48:80–86. doi: 10.1161/01.HYP.0000224283.76347.8c [DOI] [PubMed] [Google Scholar]
- 33.Laurent S, Boutouyrie P; Vascular Mechanism Collaboration. Dose-dependent arterial destiffening and inward remodeling after olmesartan in hypertensives with metabolic syndrome. Hypertension. 2014;64:709–716. doi: 10.1161/HYPERTENSIONAHA.114.03282 [DOI] [PubMed] [Google Scholar]
- 34.Mitchell GF, Dunlap ME, Warnica W, Ducharme A, Arnold JM, Tardif JC, Solomon SD, Domanski MJ, Jablonski KA, Rice MM, et al. ; Prevention of Events With Angiotensin-Converting Enzyme Inhibition Investigators. Long-term trandolapril treatment is associated with reduced aortic stiffness: the prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension. 2007;49:1271–1277. doi: 10.1161/HYPERTENSIONAHA.106.085738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutouyrie P, Achouba A, Trunet P, Laurent S; EXPLOR Trialist Group. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension. 2010;55:1314–1322. doi: 10.1161/HYPERTENSIONAHA.109.148999 [DOI] [PubMed] [Google Scholar]
- 36.Stefanadis C, Dernellis J, Vlachopoulos C, Tsioufis C, Tsiamis E, Toutouzas K, Pitsavos C, Toutouzas P. Aortic function in arterial hypertension determined by pressure-diameter relation: effects of diltiazem. Circulation. 1997;96:1853–1858. doi: 10.1161/01.cir.96.6.1853 [DOI] [PubMed] [Google Scholar]
- 37.Ong KT, Delerme S, Pannier B, Safar ME, Benetos A, Laurent S, Boutouyrie P; investigators. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: a meta-analysis of individual data in 294 patients. J Hypertens. 2011;29:1034–1042. doi: 10.1097/HJH.0b013e328346a583 [DOI] [PubMed] [Google Scholar]
- 38.Boutouyrie P, Lacolley P, Briet M, Regnault V, Stanton A, Laurent S, Mahmud A. Pharmacological modulation of arterial stiffness. Drugs. 2011;71:1689–1701. doi: 10.2165/11593790-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 39.Asmar RG, London GM, O’Rourke ME, Safar ME; REASON Project Coordinators and Investigators. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–926. doi: 10.1161/hy1001.095774 [DOI] [PubMed] [Google Scholar]
- 40.London GM, Asmar RG, O’Rourke MF, Safar ME; REASON Project Investigators. Mechanism(s) of selective systolic blood pressure reduction after a low-dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenolol. J Am Coll Cardiol. 2004;43:92–99. doi: 10.1016/j.jacc.2003.07.039 [DOI] [PubMed] [Google Scholar]
- 41.Boutouyrie P, Bussy C, Hayoz D, Hengstler J, Dartois N, Laloux B, Brunner H, Laurent S. Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation. 2000;101:2601–2606. doi: 10.1161/01.cir.101.22.2601 [DOI] [PubMed] [Google Scholar]
- 42.Dhakam Z, Yasmin , McEniery CM, Burton T, Brown MJ, Wilkinson IB. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26:351–356. doi: 10.1097/HJH.0b013e3282f283c9 [DOI] [PubMed] [Google Scholar]
- 43.Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double-blind studies. JAMA. 1996;275:1507–1513. [PubMed] [Google Scholar]
- 44.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. doi: 10.1016/j.jacc.2009.03.066 [DOI] [PubMed] [Google Scholar]
- 45.HAS. Haute Autorité de Santé. Prise en charge de l’hypertension artérielle de l’adulte. Juillet 2005. https://www.has-sante.fr/upload/docs/application/pdf/2011-09/hta_2005_-_recommandations.pdf. Accessed April 2010
- 46.Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, et al. ; Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–533. doi: 10.1016/S0140-6736(09)61340-4 [DOI] [PubMed] [Google Scholar]
- 47.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, et al. ; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245 [DOI] [PubMed] [Google Scholar]
- 49.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, et al. ; SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3 [DOI] [PubMed] [Google Scholar]
- 50.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 51.Pylypchuk R, Wells S, Kerr A, Poppe K, Riddell T, Harwood M, Exeter D, Mehta S, Grey C, Wu BP, et al. Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: a derivation and validation study. Lancet. 2018;391:1897–1907. doi: 10.1016/S0140-6736(18)30664-0 [DOI] [PubMed] [Google Scholar]
- 52.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- 53.Scuteri A, Morrell CH, Orrù M, Strait JB, Tarasov KV, Ferreli LA, Loi F, Pilia MG, Delitala A, Spurgeon H, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension. 2014;64:1219–1227. doi: 10.1161/HYPERTENSIONAHA.114.04127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Upadhya B, Pajewski NM, Rocco MV, Hundley WG, Aurigemma G, Hamilton CA, Bates JT, He J, Chen J, Chonchol M, et al. ; SPRINT Research Group. Effect of intensive blood pressure control on aortic stiffness in the SPRINT-HEART. Hypertension. 2021;77:1571–1580. doi: 10.1161/HYPERTENSIONAHA.120.16676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.