Abstract
Background
Progression along the HIV care continuum has been a key focus for improving outcomes for people with HIV (PWH). Transgender women with HIV (TGWWH) have not made the same progress as their cisgender counterparts.
Methods
All PWH identifying as transgender women receiving care at our clinic from 1/1/2015 to 12/31/2019 were identified from the electronic health records (EHRs) using International Classification of Diseases (ICD) codes. Demographics, laboratory data, prescription of gender-affirming hormone therapy (GAHT), and visit history were abstracted from the EHR. Retention in care and viral suppression were defined using Centers for Disease Control and Prevention definitions. The proportions of TGWWH who were consistently retained in care or virally suppressed over time were calculated using a binary response generalized mixed model including random effects and correlated errors.
Results
Of the 76 PWH identified by ICD codes, 2 were excluded for identifying as cisgender and 15 for insufficient records, leaving 59 TGWWH included for analysis. Patients were on average 35 years old and Black (86%), with a median CD4 count of 464 cells/µL. There were 13 patients on GAHT at study entry and 31 receiving GAHT at any point during the study period. Fifty-five percent were virally suppressed at study entry and 86% at GAHT initiation. The proportion of TGWWH who were consistently virally suppressed over time was greater among those receiving GAHT compared with those who were not (P = .04).
Conclusions
Rates of viral suppression were significantly greater among TGWWH receiving GAHT when compared with those who were not. More research to evaluate the reasons behind this effect is needed.
Keywords: HIV, transgender women, gender-affirming hormone therapy, HIV care continuum
The HIV care continuum describes the process in which an individual moves through HIV infection, diagnosis, linkage to care, retention in care, prescription of antiretroviral therapy (ART), and ultimately HIV viral suppression [1, 2]. Numerous studies have shown improved health care outcomes associated with progression along the care continuum. Early initiation of ART, improved retention in care, and the achievement of viral suppression have been associated with improved immunologic function, fewer opportunistic infections, and longer life expectancy/reduced mortality [3–5]. Not only does viral suppression improve individual outcomes, it also reduces the risk of transmission to seronegative partners [6, 7]. As such, global organizations have identified achieving viral suppression as a key goal in reducing the incidence of HIV and improving clinical outcomes for people with HIV (PWH) [8, 9].
Although rates of viral suppression within the United States have significantly improved from 28% in 2011 to 56% in 2018, there is still significant work to be done [1, 2]. Notably, there are several patient groups that have made much more modest improvements over this same time frame. Transgender women with HIV (TGWWH) in particular progress along the HIV care continuum at significantly lower rates than cisgender PWH [10–13]. The reason for this is complex and multifactorial but is believed to be associated in part with higher rates of housing instability, mental health disorders, and medical mistrust or perceived stigma [10–13].
Studies with short-term follow-up suggest that prescription of gender-affirming hormone therapy (GAHT) may improve progression along the HIV care continuum [14, 15], but long-term follow-up is lacking. While factors affecting HIV care for TGWWH have been briefly described previously, there are limited data for this population in the South, where perceived stigma for marginalized populations can be greater [16, 17]. We therefore performed a retrospective cohort study of all TGWWH in our clinic in Memphis, Tennessee, from January 1, 2015, through December 31, 2019, to assess the effects of GAHT on retention in care and viral suppression.
METHODS
Study Population
This study included all transgender individuals between January 1, 2015, and December 31, 2019, who were listed on the Adult Special Care Center’s Ryan White grant, which collects data from the electronic health records (EHRs) of PWH who receive care at the Adult Special Care Center within the Regional One Health System. The Regional One Health System cares for a primarily underserved population in the metropolitan Memphis area in Tennessee and includes the Regional One Medical Center and the Adult Special Care Center. The Adult Special Care Center is a Ryan White–funded outpatient HIV specialty clinic; it served >2000 PWH during the study period. All patients attending the clinic have HIV; in addition to providing HIV care, the clinic also provides primary care services to the majority of patients. The medical providers in this clinic responsible for managing HIV and primary care concerns are also responsible for gender-affirming care, including prescription of GAHT. The data were accessed in August 2020, and all individuals with an International Classification of Diseases, Ninth Edition (ICD-9) or Tenth Edtion (ICD-10), code for transgender were included for manual chart review to confirm eligibility for inclusion in the study. Patient demographics, laboratory data, treatment, and outcomes (retention in care and viral suppression) were abstracted from the EHR. This work was reviewed and approved by the University of Tennessee Health Science Center institutional review board and the Regional One Health Office of Medical Research before any research activities were performed.
Case Definitions
All PWH receiving care from the clinic were considered for possible inclusion in this study. Cases were initially identified from the EHR by searching for ICD-9 and ICD-10 codes for transgender individuals, F64.0. The EHRs for these individuals were then manually reviewed to confirm the individual identified as a transgender woman or transgender man during the study period as documented in the clinic notes. The study entry date was defined as the first attended clinic visit for that individual within the study period.
The definitions for baseline demographics are as follows. Poverty was defined by the ZIP code of residence, with the 5 poorest ZIP codes in the metropolitan area classified as impoverished [18]. This method was chosen because individual income data were not available and because high rates of poverty within the community in which an individual resides have been shown to negatively affect HIV-specific outcomes [19, 20]. Moderate alcohol use was defined as the consumption of 1 alcoholic beverage on average daily by patient report, with heavy use defined as 2 or more daily drinks [21]. Tobacco and illicit drug use were defined by self-report and as documented within the EHR.
Gender-affirming hormone therapy was defined as documentation of the prescription of GAHT, whether present at study entry or at a later point within the study period. The hormones prescribed, as well as the use of an anti-androgen agent, were recorded. GAHT was recorded as either present at study entry or started at a later date. The date of the clinic visit where GAHT was prescribed was recorded if started after the individual’s study entry visit. If this date occurred after June 1 of that calendar year, the participant was coded as not receiving GAHT for that calendar year and as receiving GAHT the following year.
The primary outcomes of retention in care and viral suppression were defined as follows. Retention in care was defined as attending 2 clinic appointments 90 days apart within that calendar year, in accordance with the Centers for Disease Control and Prevention (CDC) definitions [1]. Viral suppression was defined as a viral load of <200 copies/mL, in accordance with CDC definitions [1], for all values within that calendar year.
Data Analysis
All statistical tests were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA). A descriptive analysis of baseline characteristics was performed on all TGWWH and was repeated for those who never received GAHT and those who received GAHT at any time during the study. Additional descriptive analyses on the type of GAHT used for individuals receiving GAHT were also performed. A binary response generalized mixed model including random effects and correlated errors was performed to compare the proportion retained in care and the proportion virally suppressed over time between TGWWH who were receiving GAHT and those who were not. These models allowed for group crossover if an individual started GAHT midstudy after the study entry date and these models did not view each year’s result as independent outcomes. A P value of <.05 was considered statistically significant.
RESULTS
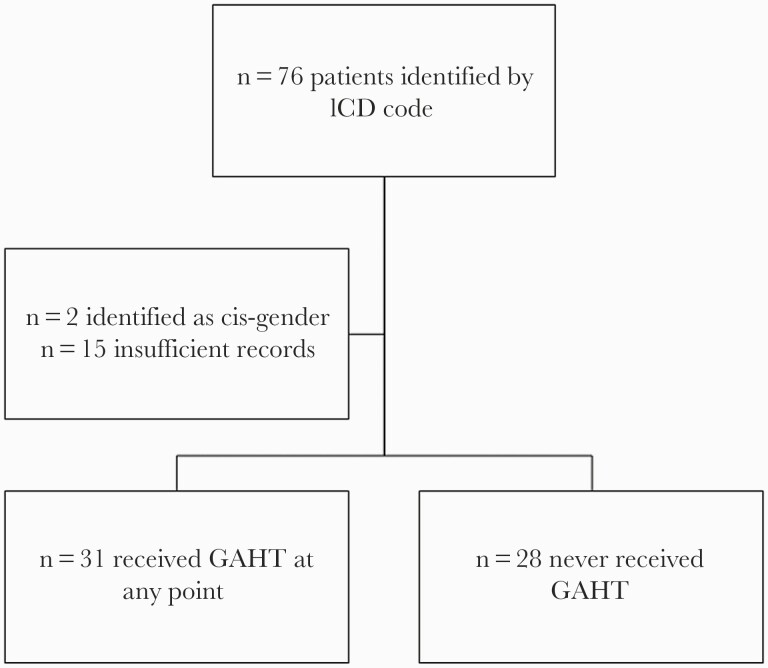
There were 76 patients with an ICD-9 or ICD-10 code for transgender initially identified between January 1, 2015, and December 31, 2019, at our clinic. Two patients were excluded as records indicated they identified as cisgender, and 15 patients were excluded for insufficient records for manual chart review. All participants identified as transfeminine. This yielded 59 total cases, of whom 31 received GAHT at any point within the study period and 28 never received GAHT (Figure 1).
Figure 1.
Study schema. Abbreviations: GAHT, gender-affirming hormone therapy; ICD, International Classification of Diseases.
Patient demographics at study entry are shown in Table 1. The majority (86%) of patients were Black, with a mean age of 35 years old, and provided a mean follow-up time of 3 years. Nearly 80% were on ART at study entry, with a median CD4 cell count of 464 cells/µL. Approximately 55% were virally suppressed at study entry. Among those with a detectable viral load at study entry, the median viral load was 23 540 copies/mL. Approximately 17% lived in areas with poverty, 54% currently smoked cigarettes at study entry, 12% had heavy alcohol use, and 29% smoked marijuana. The demographics between those who never received GAHT and those who received GAHT at any point during the study were largely similar, with the exception that those who received GAHT at any point during the study were observed to be more likely to be White (16.13% vs 7.14%), less likely to smoke cigarettes (54.84% vs 28.57%), and more likely to be virally suppressed (61.29% vs 46.43%) at study entry.
Table 1.
Patient Demographics at Time of Study Entry (n = 59)
| Category | Overall (n = 59) | No GAHT (n = 28) | GAHT (n = 31)a |
|---|---|---|---|
| Age, mean (SD), y | 34.70 (11.28) | 33.31 (11.96) | 35.95 (10.66) |
| Ethnicity, No. (%) | |||
| Hispanic | 3 (5.08) | 0 | 3 (9.68) |
| Race, No. (%) | |||
| Black | 51 (86.44) | 26 (92.86) | 25 (80.65) |
| White | 7 (11.86) | 2 (7.14) | 5 (16.13) |
| Other | 1 (1.69) | 0 | 1 (3.23) |
| Length of follow-up, mean (SD), y | 3.07 (1.82) | 2.82 (1.83) | 3.30 (1.80) |
| Poverty,b No. (%) | 10 (16.95) | 3 (10.71) | 7 (22.58) |
| Tobacco use, No. (%) | |||
| Current cigarette use | 32 (54.24) | 19 (67.86) | 13 (41.94) |
| Former | 2 (3.39) | 1 (3.57) | 1 (3.23) |
| Never | 25 (42.37) | 8 (28.57) | 17 (54.84) |
| Alcohol use,c No. (%) | |||
| None | 27 (45.76) | 10 (35.71) | 17 (54.84) |
| Moderate | 25 (42.37) | 13 (46.43) | 12 (38.71) |
| Heavy | 7 (11.86) | 5 (17.86) | 2 (6.45) |
| Illicit drug use, No. (%) | |||
| None | 31 (52.54) | 14 (50) | 17 (54.84) |
| Marijuana | 17 (28.81) | 8 (28.57) | 9 (29.03) |
| Cocaine | 4 (6.78) | 2 (7.14) | 2 (6.45) |
| Multiple | 7 (11.86) | 4 (14.29) | 3 (9.68) |
| CD4, median (Q1, Q3), cells/µL | 464 (314, 722) | 419.5 (294.5, 689) | 485 (338, 764) |
| Virally suppressedd (n = 58), No. (%) | 32 (55.17) | 13 (46.43) | 19 (61.29) |
| Viral load (n = 58), median (Q1, Q3), copies/mL | 130 (0, 19 380) | 395 (0, 32 660) | 120 (0, 650) |
| Excluding suppressed (n = 26), median (Q1, Q3) | 23 540 (1050, 76 340) | 31 100 (4630, 121 530) | 3110 (460, 76 340) |
| On ART, No. (%) | 47 (79.66) | 22 (78.57) | 25 (80.65) |
| Initial ART,e No. (%) | |||
| NNRTI | 6 (10.17) | 3 (10.71) | 3 (9.68) |
| PI | 16 (27.12) | 9 (32.14) | 7 (22.58) |
| INSTI | 31 (52.54) | 13 (46.43) | 18 (58.06) |
| Other | 6 (10.17) | 3 (10.71) | 3 (9.68) |
Abbreviations: ART, antiretroviral therapy; GAHT, gender-affirming hormone therapy; HRT, hormone replacement therapy; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
aDefined as receiving HRT at any time during the study; n = 13 participants were on HRT at study entry.
bDefined by ZIP code of residence; residing in the poorest 5 ZIP codes in the metropolitan area was classified as poverty.
cModerate alcohol use was defined as 0–1 beverages/day, heavy as 2 or more beverages/day.
dDefined as viral load <200 copies/mL at study entry.
eInitial regimen prescribed within the study period. All “other” regimens were either combination PI + INSTI (6) or NNRTI + PI (1).
Details outlining the use of GAHT are shown in Table 2. There were 13 (22%) patients receiving GAHT at study entry and a total of 31 (53%) patients receiving GAHT at any point during the study period. The majority of these patients (27/31, 87%) received estrogen-containing GAHT, with a smaller proportion receiving progesterone (2/31, 6%) or combined estrogen/progesterone (2/31, 6%) GAHT. Anti-androgen therapy with spironolactone was given to 27/31 (70%) patients. Among patients who had viral load testing performed at the time of GAHT initiation, 86% (19/22) were virally suppressed.
Table 2.
Details of Gender-Affirming Hormone Therapy Used (n = 31)
| Category | Value |
|---|---|
| Prescribed GAHT at study entry, No. (%) | 13 (22.03) |
| Prescribed GAHT at any point, No. (%) | 31 (52.54) |
| GAHT type (n = 31), No. (%) | |
| Estrogen | 27 (87.10) |
| Progesterone | 2 (6.45) |
| Combination | 2 (6.45) |
| Anti-androgen (n = 27), No. (%) | 21 (70) |
| Virally suppressed at GAHT initiation (n = 22),a No. (%) | 19 (86.36) |
Abbreviations: GAHT, gender-affirming hormone therapy; HRT, hormone replacement therapy.
aDefined as viral load <200 copies/mL at the time of HRT initiation when viral load data were available.
Outcomes for retention in care and viral suppression are shown in Table 3. Annual trends for the proportions of TGWWH retained in care for the overall group, those not receiving GAHT, and those receiving GAHT for each calendar year during the study period are shown. Although the proportions of patients retained in care were observed to be higher among those receiving GAHT in the latter 3 years (93% vs 84%, 79% vs 69%, 80% vs 55%), these differences were not statistically significant (P = .54). Annual trends for the proportions of TGWWH with consistent viral suppression for the overall group, those not receiving GAHT, and those receiving GAHT for each calendar year during the study period are also shown. The proportions of patients virally suppressed were greater among those receiving GAHT when compared with those not receiving GAHT (P = .04).
Table 3.
Progression Along the HIV Care Continuum Over Time
| Category | Overall | No GAHT | GAHT | P Value |
|---|---|---|---|---|
| Retention in carea | .5435b | |||
| 2015, No. (%) | 29/35 (82.86) | 23/27 (85.19) | 6/8 (75) | |
| 2016, No. (%) | 35/44 (79.55) | 27/33 (81.82) | 8/11 (72.73) | |
| 2017, No. (%) | 41/47 (87.23) | 27/32 (84.38) | 14/15 (93.33) | |
| 2018, No. (%) | 37/51 (72.55) | 22/32 (68.75) | 15/19 (78.95) | |
| 2019, No. (%) | 40/59 (67.80) | 16/29 (55.17) | 24/30 (80) | |
| Viral suppressionc | .0398 b | |||
| 2015, No. (%) | 19/35 (54.29) | 15/27 (55.56) | 4/8 (50) | |
| 2016, No. (%) | 27/43 (62.79) | 19/32 (59.38) | 8/11 (72.73) | |
| 2017, No. (%) | 26/44 (59.09) | 17/30 (56.67) | 9/14 (64.29) | |
| 2018, No. (%) | 23/45 (51.11) | 12/27 (44.44) | 11/18 (61.11) | |
| 2019, No. (%) | 28/50 (56) | 8/22 (36.36) | 20/28 (71.43) |
Abbreviation: GAHT, gender-affirming hormone therapy.
aDefined as attending 2 clinic appointments 90 days apart within that calendar year.
bCalculated using a generalized linear mixed model, including random effects and correlated errors.
cDefined as viral load <200 copies/mL for all values in that calendar year.
DISCUSSION
In this retrospective observational cohort study of TGWWH in the Southern United States, receiving GAHT was associated with significantly higher rates of sustained HIV viral suppression over time, with an average of 3 years of follow-up. There were also observed higher rates of retention in care among TGWWH receiving GAHT in the final 3 years of this study, though this difference was not significant. Identifying a readily modifiable factor such as the prescription of GAHT that may improve sustained progression along the HIV care continuum in an often-marginalized patient group is noteworthy.
The importance of achieving and sustaining HIV viral suppression cannot be overstated. Achieving viral suppression leads to improved individual health outcomes [3–5] and reduces transmission to seronegative partners [6, 7], and as such has become a primary focus of international HIV efforts. An often-overlooked factor in these benefits from viral suppression, however, is that viral suppression must be consistent and sustained to provide these individual and societal benefits. Many patients fall in and out of care over time, and studies with long term-follow up suggest that this churn may be quite common [22–24]. In our work, we attempted to address this by defining viral suppression strictly with a viral load <200 copies/mL for every value within that calendar year rather than at 1 specific point in time. We additionally had >3 years of follow-up time on average per participant, increasing our likelihood of observing this churn. Despite these strict definitions and long follow-up, we observed higher rates of viral suppression over time among TGWWH receiving GAHT.
The mechanism by which prescribing GAHT affected HIV care outcomes is not clear but may be related to an improved patient–provider relationship. Previous works have identified perceived HIV stigma and the patient–provider relationship as integral factors affecting progression along the HIV care cascade in the Southern United States [25, 26]. Transgender women with HIV in particular may face additional stigma based on their gender identity and as such have noted GAHT and the patient–provider relationship to be instrumental in HIV care outcomes [10, 12, 13]. Qualitative studies have identified gender-affirming care as an important factor for both HIV preventative services [27] and HIV care [28]. It therefore may be that the prescription of GAHT improved the patient–provider relationship in our study, with resultant improvement in HIV viral suppression. The results from our work contribute to this body of work by being one of the few cohorts of TGWWH in a Southern US city evaluating the use of GAHT over long-term follow-up.
There are several limitations to this work. One notable point is that 19 of 22 (86%) TGWWH were virally suppressed at the time GAHT was initiated. As a retrospective cohort, it is difficult to determine whether this occurred by chance, whether this represents a selection bias, or whether the prescribing provider withheld GAHT until viral suppression was achieved. The authors would like to explicitly advocate against this last option, as withholding medical care is unethical and may be associated with the use of GAHT obtained from alternative sources [28, 29], without appropriate safety monitoring. In addition to ethical and safety concerns, prescription of GAHT has been associated with improvements in mental health and potentially even reduced risk of death by suicide [30, 31]. If GAHT was withheld until viral suppression was achieved, however, one might expect the rates of viral suppression between the 2 groups to reconverge over time once GAHT was started. The opposite finding was seen, however, with greater observed differences in retention in care and viral suppression in the final 3 years of the study.
Further, it may be that both viral suppression and initiation of GAHT are the results of a common precursor. It may be that TGWWH who have better access to care, trust in the health care system, or closer patient–provider relationships are more likely to both seek out GAHT and progress along the HIV care continuum. Rather than GAHT leading to improved rates of viral suppression through an unidentified mechanism, it could be that the unidentified mechanism leads to both initiation of GAHT and viral suppression. As a retrospective observational cohort study, causation cannot be determined, and as such we are only able to identify a correlation between prescription of GAHT and viral suppression. Future prospective studies evaluating factors affecting prescription of GAHT and viral suppression, including medical mistrust, perceived stigma, and the patient–provider relationship, may provide further insight into these connections.
Additionally, although our overall study size is relatively large for this specific patient population, the small number of patients limits the statistical power and may bias toward the null. A small sample size also limits the ability to perform quality multivariable regressions and control for confounders due to small overall numbers in each subgroup. Also, by relying on ICD-9 and ICD-10 codes for participant identification, it is possible that we may have missed TGWWH who would have qualified for inclusion but did not have the codes we searched [32]. Lastly, as a retrospective cohort study, it is impossible to control for unmeasured confounders or to determine causality. That said, a randomized controlled trial evaluating GAHT would have significant practical and ethical considerations. As such, we believe our results should be viewed as practice-informing and hypothesis-generating.
Despite significant advances improving progression along the HIV care continuum, rates of retention in care and viral suppression for TGWWH have lagged. We found that prescribing GAHT was significantly associated with improved rates of viral suppression within a population historically excluded from clinical care and research. The mechanism by which prescribing GAHT affected HIV care outcomes is not clear but may be related to an improved patient–provider relationship. Future qualitative studies evaluating the interaction between GAHT and patient–provider relationships and subsequent HIV care outcomes are needed.
Acknowledgments
The authors would like to acknowledge Maretta Cox for her help in obtaining the data set, as well as Rongshun Zhu and Elizabeth Tolley for their guidance on the analysis.
Financial support. There was no funding for this study.
Potential conflicts of interest. No competing financial incentives exist. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. No consent was needed due to the retrospective nature of this work. This work was reviewed and approved by the University of Tennessee Health Science Center institutional review board and Regional One Health Office of Medical Research before any research activities were performed.
References
- 1.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States 2014–2018. HIV Surveillance Supplemental Report. 2020. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-1.pdf. Accessed 1 July 2020. [Google Scholar]
- 2.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis 2007; 44:441–6. [DOI] [PubMed] [Google Scholar]
- 4.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis 2009; 48:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samji H, Cescon A, Hogg RS, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodger AJ, Cambiano V, Bruun T, et al. ; PARTNER Study Group. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019; 393:2428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: an ambitious target to help end the AIDS epidemic.2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. Accessed May 2020.
- 9.Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 10.Kalichman SC, Hernandez D, Finneran S, et al. Transgender women and HIV-related health disparities: falling off the HIV treatment cascade. Sex Health 2017; 14:469–76. [DOI] [PubMed] [Google Scholar]
- 11.Santos GM, Wilson EC, Rapues J, et al. HIV treatment cascade among transgender women in a San Francisco respondent driven sampling study. Sex Transm Infect 2014; 90:430–3. [DOI] [PubMed] [Google Scholar]
- 12.Sevelius JM, Carrico A, Johnson MO. Antiretroviral therapy adherence among transgender women living with HIV. J Assoc Nurses AIDS Care 2010; 21:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevelius JM, Saberi P, Johnson MO. Correlates of antiretroviral adherence and viral load among transgender women living with HIV. AIDS Care 2014; 26:976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baguso GN, Turner CM, Santos GM, et al. Successes and final challenges along the HIV care continuum with transwomen in San Francisco. J Int AIDS Soc 2019; 22:e25270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen JG, Malik M, Cooney EE, et al. Antiretroviral treatment interruptions among Black and Latina transgender women living with HIV: characterizing co-occurring, multilevel factors using the gender affirmation framework. AIDS Behav 2019; 23:2588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton LA, Kalichman SC, Price D, et al. Stigma and conspiracy beliefs related to pre-exposure prophylaxis (PrEP) and interest in using PrEP among Black and White men and transgender women who have sex with men. AIDS Behav 2017; 21:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reif S, Wilson E, McAllaster C, Pence B. The relationship of HIV-related stigma and health care outcomes in the US deep south. AIDS Behav 2019; 23:242–50. [DOI] [PubMed] [Google Scholar]
- 18.Delavega E, Blumenthal GM. The 2019 update of the Memphis Poverty Fact Sheet. 2019. Available at: https://www.memphis.edu/socialwork/research/2019povertyfactsheet.pdf. Accessed 1 July 2020.
- 19.Chandran A, Edmonds A, Benning L, et al. Longitudinal associations between neighborhood factors and HIV care outcomes in the WIHS. AIDS Behav 2020; 24:2811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shacham E, Lian M, Önen NF, et al. Are neighborhood conditions associated with HIV management? HIV Med 2013; 14:624–32. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Agriculture, US Department of Health and Human Services. Dietary guidelines for Americans, 2020–2025. 9th ed. 2020. Available at: dietaryguidelines.gov. Accessed 1 July 2020. [Google Scholar]
- 22.Colasanti J, Kelly J, Pennisi E, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis 2016; 62:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner EM, Daniloff E, Thrun MW, et al. Initial linkage and subsequent retention in HIV care for a newly diagnosed HIV-infected cohort in Denver, Colorado. J Int Assoc Provid AIDS Care 2013; 12:384–90. [DOI] [PubMed] [Google Scholar]
- 24.Byrd KK, Furtado M, Bush T, Gardner L. Reengagement in care after a gap in HIV care among a population of privately insured persons with HIV in the United States. AIDS Patient Care STDS 2016; 30:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colasanti JA, Armstrong WS. Challenges of reaching 90-90-90 in the Southern United States. Curr Opin HIV AIDS 2019; 14:471–80. [DOI] [PubMed] [Google Scholar]
- 26.Kempf MC, McLeod J, Boehme AK, et al. A qualitative study of the barriers and facilitators to retention-in-care among HIV-positive women in the rural Southeastern United States: implications for targeted interventions. AIDS Patient Care STDS 2010; 24:515–20. [DOI] [PubMed] [Google Scholar]
- 27.Nieto O, Fehrenbacher AE, Cabral A, et al. Barriers and motivators to pre-exposure prophylaxis uptake among Black and Latina transgender women in Los Angeles: perspectives of current PrEP users. AIDS Care 2021; 33:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowshen N, Lee S, Franklin J, et al. Access to medical and mental health services across the HIV care continuum among young transgender women: a qualitative study. Transgend Health 2017; 2:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metastasio A, Negri A, Martinotti G, Corazza O. Transitioning bodies. The case of self-prescribing sexual hormones in gender affirmation in individuals attending psychiatric services. Brain Sci 2018; 8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achille C, Taggart T, Eaton NR, et al. Longitudinal impact of gender-affirming endocrine intervention on the mental health and well-being of transgender youths: preliminary results. Int J Pediatr Endocrinol 2020; 2020:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc 2021; 5:bvab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foer D, Rubins DM, Almazan A, et al. Challenges with accuracy of gender fields in identifying transgender patients in electronic health records. J Gen Intern Med 2020; 35:3724–5. [DOI] [PMC free article] [PubMed] [Google Scholar]