Abstract
Road ecology has traditionally focused on the impact of in-situ and functional roads on wildlife. However, road construction also poses a major, yet understudied, threat and the implications for key aspects of animal behaviour are unknown. Badgers (Meles meles) have been implicated in the transmission of tuberculosis to cattle. There are concerns that environmental disturbances, including major road construction, can disrupt badger territoriality, promoting the spread of the disease to cattle. To address these knowledge gaps the ranging behaviour of a medium-density Irish badger population was monitored using GPS-tracking collars before, during, and after a major road realignment project that bisected the study area. We estimated badgers’ home range sizes, nightly distances travelled, and the distance and frequency of extra-territorial excursions during each phase of the study and quantified any changes to these parameters. We show that road construction had a very limited effect on ranging behaviour. A small increase in nightly distance during road construction did not translate into an increase in home range size, nor an increase in the distance or frequency of extra-territorial excursions during road construction. In addition, suitable mitigation measures to prevent badger deaths appeared to ensure that normal patterns of ranging behaviour continued once the new road was in place. We recommend that continuous badger-proof fencing be placed along the entire length of new major roads, in combination with appropriately sited underpasses. Our analysis supports the view that road construction did not cause badgers to change their ranging behaviour in ways likely to increase the spread of tuberculosis.
Introduction
Studies on the impact of roads on animals have tended to focus on their impact on population density; barriers to, or facilitators of, movement; habitat loss and fragmentation; mortality; increase or decrease of food availability; and avoidance behaviour once roads are already constructed and in-situ e.g. [1–3]. However, there have been few studies that have specifically examined the impact of the process of road construction on animals [4]. This lack is surprising because the building of roads could have an extremely high disturbance intensity [5–7], including vegetation clearance, rock-breaking, major excavation, and earth-moving activity. This study provides insights into the impacts of road construction on these key elements of animal behaviour using a large GPS-tracking dataset of European badger (Meles meles). Most of the literature pertaining to the disruptive effects of roads on mustelid ecology e.g. [8], and specifically badger ecology, consider barriers to dispersal, mortality due to road traffic accidents (RTAs) and the mitigation of these effects [9–12]. Little is known about the impact of road construction activities on badger behaviours such as movement and territoriality.
The badger makes an appropriate study species because it is subject to protection under Annex III of the Bern Convention (1979) under which activities capable of causing local disappearance of, or serious disturbance to, populations are prohibited. However, in some European countries, badgers are also subject to purposeful deliberate population control through culling. In both Ireland and the United Kingdom (UK), badgers have been implicated in the spread of Mycobacterium bovis (M. bovis) to cattle, and act as a wildlife reservoir for bovine tuberculosis (TB) [13–16]. TB transmission between badgers and between badgers and cattle is highly complex and multi-factorial, involving many other factors such as cattle-to-cattle transmission, movement of infected livestock between herds, biosecurity, and the poor sensitivity of TB testing in cattle [17, 18]. Nonetheless, in order to understand the dynamics of a disease in a wildlife reservoir, and to control it successfully, as complete a picture as possible of the ranging behaviour of the carrier species is required [19].
Badgers are nocturnal, fossorial animals, generally only emerging to move about above ground under cover of darkness. The organisation of badgers into territorial social groups appears to limit the spread of TB because it lowers disease transmission rates between groups [20–22]. However, badger movements into and out of neighbouring social groups are associated with increased prevalence of TB in those groups [23, 24]. Therefore, the ranging behaviour of badgers is of direct importance to the transmission of TB infection both between individual badgers [25, 26] and between badgers and cattle [27–31].
From studies of disease control, there is evidence for both positive and negative effects of purposeful badger culling on TB breakdown rates in cattle. A breakdown can be defined as a positive reaction to the skin-test during herd testing where two or more skin-test positive cattle are disclosed, or identification of macroscopic TB lesions post-mortem from an animal with histopathogical identification of a TB granuloma, or the culture of M. bovis, of the lesions identified. While Irish studies have found that culling badgers to lower their population density is associated with reduced bovine TB breakdown rates [28, 29, 32, 33], studies in the UK have found that culling may produce a ‘perturbation effect’, i.e. it may disrupt badger ranging behaviour such that TB transmission to cattle increases resulting in an increase in herd breakdowns [34–40]. These disruptions to ranging behaviour include rapid immigration into culled areas, increased social group range size, increased social group range overlap, increased inter-group movements and increased ranging of individuals [39], all of which result in higher TB transmission rates among badgers and between badgers and cattle [16, 38, 41, 42]. In contrast to the perturbation effects found in the UK, Irish studies have reported sustained decreases in the rates of bovine TB breakdowns associated with culling [28, 29, 32, 33], despite some evidence for disruption of ranging behaviour [43, 44].
Other human activities have the potential to disturb badger ranging behaviour and consequently influence the rate of disease transmission. For example, density reduction due to persecution has been found to increase the distance badgers travel, and to reduce territoriality [45, 46] and has been associated with increased risk of TB in cattle and the persistence of TB hotspots [47]. It has been suggested that environmental disturbances, such as clear felling of forests [48, 49] and landscape changes that occur as road density increases [10], may also impact the population density and ranging behaviour of badgers. Disruptive events like these may also have implications for TB transmission among badgers and between badgers and cattle. Recently, the Irish media have reported concerns that the construction of major roads may increase bovine TB breakdowns resulting from an associated increase in movement by badgers e.g. [50, 51].
Ranging behaviour can be described by a variety of movement metrics, including home range size (HR), nightly distance moved (ND), the distance of extra-territorial excursions (ETEs), and the frequency of ETEs (fETE). The distances badgers move each night vary considerably, from an average of 7km per night at low population densities [52] to an average of less than 1km (849m) per night [53] at higher population densities. Within territories, the sizes of home ranges vary seasonally, being smallest in winter [54–56] and largest in summer [53, 54, 57, 58]. HR also varies with population density, from 22km2 in low density populations [59] to 0.26km2 in very high-density populations (Chris Newman, pers. comm.) The majority of Irish populations (76%) are of medium density, averaging 1.4 badgers/km2 with a mean territory size of 1km2 (S1 Table in S1 File) so HR would be expected to vary seasonally around this figure. A large-scale trapping study in Ireland [60] found that movements of >1km, which would take a badger outside its territory, accounted for 57% of all movements detected. Irish badgers have been recorded making such ETEs in all seasons, some of which may be up to 8km from their home territory [61–63].
If road construction activity disrupts badger ranging behaviour, we would expect to see changes in any or all ranging parameters. The distance moved in a night (ND) might increase during the construction phase, when disturbance is greatest, see [39]. Such an increase in ND could result in a corresponding increase in HR and/or an increase in ETE distance and frequency, as badgers seek refuge from the ecological, acoustic, and seismic disturbance of road construction. If extreme, these disturbances might lead to a breakdown in territoriality in the area around the road construction.
The present study monitored the ranging behaviour of a medium-density population of badgers over 6.5 years: before, during, and after a major road upgrade. This allowed us to assess how road-building activity affected the ranging behaviour of badgers, and how rapidly acclimation, if any, occurred. We considered the before/during/after comparisons with respect to their ecological importance, as well as their statistical significance. Such a long-term, detailed study of badger ranging behaviour using GPS tracking technology has never before been conducted. We do not consider here the ranging behaviour of badgers that were in the process of dispersal nor that of super-rangers, with the atypical ranging behaviour of both groups considered elsewhere [64, 65].
Materials and methods
The study was conducted in mixed farmland in Co. Wicklow, Ireland (52.924130 N, -6.117960 W). The study area was a matrix of undulating agricultural land (75%), with patches of mixed and coniferous woodland (14%) and small residential areas and farmyards scattered throughout (7%). A well-developed hedgerow system connected fields. In any given year, on average 68.5% of agricultural land was under pasture, while at least 16.7% was arable crops. This habitat mix provided good foraging opportunities for badgers’ preferred food resources, i.e. Noctuid larvae, Carabid beetles and larvae, Tipulid larvae, and earthworms [66, 67]. Over the course of the study, we trapped 139 badgers, 80 of which wore GPS tracking collars. Details of the trapping and handling of badgers are given elsewhere [64, 68]. The population density in the study area was 1.8 badgers/km2 (adults plus juveniles) and this remained stable over the 6.5-year study period [64]. The average territory size was 1.9km2 and the average distance between main setts (permanently occupied, breeding setts [69]) was 1.3km. The road upgrade involved building a new 16km section of motorway (M11) adjacent to the original national road (N11) [68]. The area within which road construction occurred was long and narrow, on average 100m wide (max. 400m) and 16km in length (S1 Fig in S1 File). Wildlife underpasses were installed under the M11 at appropriate locations following walkover surveys to locate badger paths and crossings, the location of RTAs, and the examination of GPS data from the ‘before’ phase. The entire 16km of new motorway was lined with badger-proof fencing which effectively guided wildlife into underpasses. The mitigation measures were checked by the National Parks and Wildlife Service (NPWS) to ensure functionality and compliance to statutory requirements.
Ethical approval for the project was granted by Trinity College Dublin’s Animal Research Ethics Committee (Project No. 290516) and the Health Products Regulatory Authority (Project No. 7024754). Badgers were captured under licences (NPWS Nos. 101/2009, 04/2010, 13/2010, C123/2010, 03/2011, C040/2011, C03/2013, C005/2013, and C001/2015) as required by the Wildlife Act, 1976. Both cage traps and stopped-restraints conformed to national legislation for humane trapping defined in the Wildlife Act, 1976, Regulations 2003 (S.I. 620 of 2003) [68].
Data collection and management
Badgers were tracked using Tellus Light GPS collars (Followit Wildlife, Lindsberg, Sweden). Data collection began in April 2010 and GPS tracking continued until October 2016, when all collars were removed. We aimed to capture as many badgers as possible within each social group. Captured badgers were anaesthetised in-cage by veterinary practitioners at each trapping event [64]. Badgers were sexed and had their teeth examined and photographed. Age was determined by dentition [70, 71] and general appearance of each badger. Irish badgers give birth from the first week in February to early March with the majority of cubs born in mid-February [72]. For convenience in age classification, the date of birth was assumed to be the 1st of February for all badgers. Age cohorts were defined as follows: cub (a badger in its first year); young adult (a yearling or a 2-year-old); older adult (a 3- or a 4-year-old), and aged adult (badgers ≥5 years old). Records were kept of known badger deaths during the course of the study. These badgers were identified on the basis of their microchip and tattoo numbers, assigned upon first capture.
A social group was defined as the group of badgers which were regularly trapped at the same main sett and whose home ranges overlapped during the time-period in question [53, 73]. Thus, badgers were assigned to a social group based on their most frequent trapping location and, if collared, their GPS tracking data. Badgers were categorised into one of three ranging status categories–traditional rangers, dispersers or super-rangers–on the basis of their behaviour at the time that the GPS location was recorded. Dispersers (confirmed retrospectively) were badgers in the process of moving permanently from one social group to another social group [65]. Super-rangers were those badgers that ranged within an extended territory consisting of their original/natal territory and some, or all, of an adjacent territory or territories [64]. The vast majority of the population were traditional rangers, accounting for ≥80% of movement observations. Both super-rangers and dispersers were excluded from the analyses presented here, as road construction was found to have had no impact on their ranging behaviour, using the same methodology [74], and their numbers pre-, during-, and post-construction did not materially change (S2 Table in S1 File).
Social groups were categorised as adjacent to road construction if they shared a border with the N11/M11 and associated road construction. Those social groups that were further away were categorised as non-adjacent. The timing of road construction was split into three phases: ‘before’ (April 2010-August 2013, 41 months), ‘during’ (September 2013 –June 2015, 22 months) and ‘after’ (July 2015 –August 2016, 14 months) the construction of the road. During the ‘before’ period the area where the new motorway was to be located (S1 Fig in S1 File, Fig 1A) was cleared of trees, hedgerows, and grass/crops and became mostly scrub land. Despite being fenced, it was completely accessible to and regularly used by badgers. In the ‘during’ phase, the scrub within this area was completely cleared, leaving exposed earth. Road construction involved rock-breaking, major excavation and earth-moving activity within the construction zone (Fig 1B). Continuous badger-proof fencing was installed only at the very late stages of the ‘during’ phase. In the ‘after’ phase, badger proof-fencing and underpasses were fully operational (Fig 1C), and badgers did not have access to the area around the M11 and its associated access roads. Underpasses were inspected for accessibility and their use by badgers (and other wildlife) confirmed through the use of camera-traps.
Fig 1. Road construction phases.
a) ‘Before’—satellite image of part of the study area before road construction. The hatched orange area indicates the location of the M11 motorway construction zone adjacent to the N11 road. The N11 is represented by the green line. b) ‘During’—aerial photograph of part of the study area taken during road construction revealing the extent of the change to the habitat. The N11 can be seen to the right-hand side of the photograph. c) ‘After’—aerial photograph of part of the study area taken upon completion of road construction. The motorway and access roads were completely enclosed in continuous badger-proof fencing.
GPS collars were programmed to record four locations a night, at 22:00, 23:00, 01:00, and 02:00, for each collared badger, when badgers were expected to be above ground. GPS locations from the Followit website were converted from the global geodetic system WGS-84 to the Irish Grid projection system using Grid InQuest II (Ordinance Survey Ireland). Data were visualised by mapping the GPS locations onto the World Imagery basemap using the coordinate system TM_75 Irish Grid in ArcMap (ArcGIS version 10.4.1). The study area was digitised by creating a polygon shapefile, which traced the landscape features in the study area such as field boundaries, roads, rivers, forestry, and farmyards. This resulted in a dataset of 81,925 GPS records from April 2010 to August 2016 (77 months) from 80 different individuals.
To calculate ND, for each collared badger, nightly trajectories were created using the package adehabitatLT [75] in R Version 3.4.0 [76], in which a ‘burst’ was defined as a single night’s GPS locations, and the total distance travelled each night was extracted in meters (m). Two or more GPS locations were required to calculate ND, but, because collars could not transmit when the badger was below ground, and badgers did not always emerge from their setts for the full night, badgers did not always send the four scheduled GPS locations in a night. Therefore, our analysis only considered data from those nights on which badgers successfully recorded two or more GPS locations. Our calculations of how far a badger travelled in a night will inevitably be under-estimations for two reasons. Although badgers tend to follow linear features in their landscape [77], their routes can be quite tortuous, particularly when foraging [78]. Our analysis was limited to considering straight line distances between the four GPS locations. Secondly, the dataset is lacking information regarding how far a badger travelled both before and after the first and last GPS locations were recorded. However, as the methods were identical throughout the study, this should not influence our ability to make statistical comparisons between time-periods or groups of badgers. Overlaying nightly trajectories on the study area in ArcMap allowed us to record the number of occasions that badgers crossed roads in the study area.
To generate the HR dataset, monthly home range size was estimated by calculating 95% minimum convex polygons (MCPs) for each badger in each calendar month of each year of the study using the R package adehabitatHR [75]. MCPs could only be calculated when there were >5 records for a badger in a given month. We chose to use 95% MCP as a proxy for home range size as it is used in many earlier badger studies [79], allowing for comparability. However, we acknowledge that more spatially accurate techniques for estimating home range are available, e.g. [53, 80]. Because MCP can include areas not regularly used by an animal, this method will overestimate HR size. However, as the methods were identical throughout the study, this should not influence our ability to make statistical comparisons between time-periods or groups of badgers. Spatial autocorrelation can also be an issue for estimating HR size. However, in the case our dataset where locations are sampled at a minimum of hourly intervals, and given that badgers can cover very large distances in a single night (e.g., >10km), the sampling intervals herein theoretically allows the animal to move to anywhere else in its territory in the intervening period.
ETEs are journeys made by badgers beyond the boundaries of their social group’s territory into neighbouring territories or beyond [63]. In order to calculate the length and frequency of ETEs, the boundaries of social groups needed to be determined. As GPS data became available, it became clear that an earlier bait-marking study of the area did not accurately represent territory boundaries in this population (S2 Fig in S1 File). Badgers reach maximum ranging in summer [52, 53, 57, 59]. Therefore, in areas where social groups are contiguous, as they are in Ireland, the shape of their summer ranges should reflect the physical borders between social groups. For each social group in each year, 95% MCPs were calculated using the summer GPS locations, i.e., June, July, and August, of all the collared members of the social group (summer MCPs). Where we did not have data for these months, we included all available GPS data for social group members in that calendar year.
In order to map more realistic territory boundaries than MCPs provide, summer MCPs were plotted in ArcMap along with all of the GPS locations. A ‘geographical territory boundary’ was digitised based on the location of the summer MCP polygon, the real linear landscape features in the study area (roads, hedgerows, rivers, and observed badger-paths) and the GPS locations of the relevant social group (S1 Text). The boundaries for each year were compared and only changed if it was very clear that members of the social group were ranging differently from the years before/after. Most social groups were relatively stable. However, there were some examples of fission or fusion of social groups [57, 81], and the construction of the M11 altered the position of some territory boundaries adjacent to the road (S3 Fig in S1 File).
To determine ETE distances, the ‘Generate Near Table’ tool in ArcMap was used to calculate the distance (m) between each of a badger’s GPS points and the nearest edge of the polygon representing the boundary of their social group in that year. All points that fell outside the polygon could be considered ETEs. However, as the Followit GPS collar locations can be inaccurate by up to 15m [31], only locations that were >15m from the polygon where identified as ETEs in this study. On a given night, a badger may have recorded more than one GPS location outside its territory boundary. In such cases, only the GPS location that was furthest from the badger’s territory boundary was retained in the dataset. The frequency of ETEs per month (fETE), described as a proportion between 0 and 1, was calculated by dividing the number of active nights in a month by the number of ETEs made in that month. Active nights were defined as nights in which the badger was above ground for long enough to record GPS locations and wore a working collar. The fETE dataset also included collared badgers that never went on ETEs, and these were included as zeros.
For each dataset, in addition to the response variables (ND, HR size, ETE distance and fETE), the following data were included: badger ID, social group ID, sex, age cohort, month, year, road construction phase, and road construction adjacency. The data management process resulted in an ND dataset of 18,954 records, a HR dataset of 890 records, an ETE distance dataset of 3,726 records and a fETE dataset of 892 records.
Statistical analyses
Generalized linear mixed models (GLMMs) were used to analyse the effects of the road on ND, HR, and ETE distance and frequency in R Version 3.4.0 [76]. To investigate the effect of road construction, roadwork phase (three levels: before; during; after) was included as a fixed factor. To investigate whether road construction impacted the behaviour of badgers living in social groups adjacent to road construction to a greater extent than those who were living further away during different construction phases, an interaction between road construction phase and a second fixed factor, adjacency (two levels: yes; no), was also included in the model. Sex (two factor levels: male; female), age cohort (four factor levels: cub; young adult; older adult; aged adult), the interaction between sex and cohort (i.e., whether the behaviour of each age cohort differed between the sexes), and calendar month were included as fixed factors to control for the effect these variables had on ranging behaviour. Badger ID, nested within social group ID, and year (un-nested) were specified as random factors.
GLMMs were fitted using the package lme4 for models with a continuous responses [82] and glmmTMB for proportional data (frequency of ETEs) [83]. Model selection was conducted using the package MuMIn [84]. Model selection was conducted using an information theoretical approach [85, 86]. The most parsimonious model, hereafter the “best model” was selected as the model with the lowest Akaike Information Criteria for small sample sizes (AICc). Models with a difference in AICc of <2 are considered to be plausible alternatives to the best model, and the details of the variables included in these models are given in S3-S6 Tables in S1 File. We include p-values from the best models for reference. However, we caution against a strict interpretation of the 0.05 significance threshold as the framework of p-values is not strictly compatible with multi-model inference [85] (i.e., because multiple models are tested in the process of selecting the best model by AICc, rather than a strictly hypothesis driven approach, in which only a single model was chosen a priori).
Models with continuous responses (ND, HR and ETE distance) were initially fitted with a Gaussian error distribution, and model validation was performed by extracting the normalized Pearson’s residuals and plotting Q-Q and homoscedasticity plots. Where did not meet the assumption of normality of residuals data transformations were conducted (e.g., log, square root, BoxCox [87]), and model residuals retested for normality. BoxCox transformations of the raw data were found to meet normality assumptions for ND and HR. ETE distances did not meet normality assumptions after transformation, and therefore the model for ETE distances was fitted using a Gamma distribution with a log link function. Frequency of ETE’s was initially fitted with a binomial response distribution suitable for proportion data. Overdispersion was assessed following [88], and the residuals were found to be overdispersed. Therefore, the full model was refitted using two alternatives, a beta-binomial distribution which accounts for overdispersion, and a beta-binomial distribution with zero-inflation. The beta-binomial distribution without zero inflation was selected for use as it had the lower AIC value. Following model selection, equal variance was confirmed by plotting the residuals against each explanatory variable. A summary of best model specifications for ND, HR, ETE distance and fETE can be found in S7 Table in S1 File. The glht function in the package multcomp [89] was used to perform Tukey post hoc tests with Holm adjusted p-values.
Due to the skewed and variable nature of our data, results are presented in the text as medians and interquartile ranges (IQR) of individual medians, rather than overall means and standard deviations of the entire datasets, which can be found in S8, S10, S12, S14 Tables in S1 File. The period before road construction commenced was much longer (41 months) than either the ‘during’ or ‘after’ phases (22 and 14 months respectively). In order to assess the biological significance of any changes in ranging behaviour caused by road construction, it is useful to consider how variable the badgers’ ranging was between years before any disturbance. We therefore consider the yearly medians, i.e., the median value for each year, for each of the movement metrics in the period before road construction commenced. This helps to place any changes during and after road construction into the context of natural variation.
Results
Nightly distance travelled
On average, across the study period, badgers travelled a median distance of 657m per night (IQR: 458m-806). The majority of journeys, as determined using the 95th percentile [60], were below 2km (S8 Table in S1 File). The maximum distance a badger travelled in a single night was 11.25km (S8 Table in S1 File). The model that best explained the variation in the nightly distance dataset retained the following explanatory variables; sex and age (in interaction with one another), road construction, adjacency, and month (S3 and S9 Tables in S1 File).
Before road construction commenced, badgers travelled a median distance of 651m (IQR: 382-777m, n = 7713) each night (Fig 2A). During road construction period, the median ND travelled by badgers increased slightly, but significantly, to 673m (IQR: 470-911m, n = 5428) (Table 1). Similarly, a further increase in median ND to 848m (IQR: 675–927, n = 5813) occurred after the new motorway opened (Table 1).
Fig 2. Boxplots for badger movement metrics in each phase of road construction.
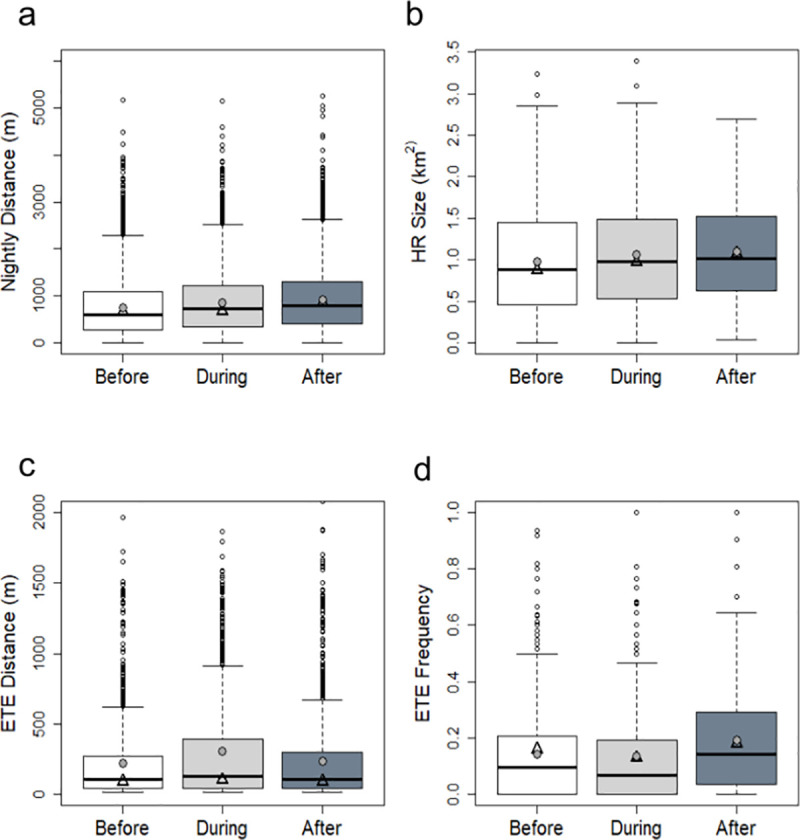
The before phase is represented in white, the during phase in light grey and the after phase in dark grey. Thick black bars represent cohort medians, filled grey circles represent cohort means and open black triangles represent the medians of individual medians. a) Nightly distance (m) by road construction phase, y-axis limited to 6000m for clarity; b) Home range size (km2) by road construction phase; c) ETE distance (m) by road construction phase, y-axis limited to 2000m for clarity and d) ETE frequency (proportion between 0 and 1) by road construction phase.
Table 1. Results of Tukey post hoc tests for multiple comparisons of ND means for the different road construction phases.
| Comparison | Estimate | Std. Error | z value | Pr(>|z|) |
|---|---|---|---|---|
| Before-During | 0.055 | 0.010 | 5.245 | <0.001 |
| Before-After | 0.078 | 0.013 | 6.144 | <0.001 |
| During-After | 0.023 | 0.008 | 2.872 | <0.01 |
Home range size
On average, across the study period, badgers had a monthly median HR size of 0.88km2 (IQR: 0.54–1.32km2). HR size ranged seasonally from 0.04km2 (40m2) to 3.4km2 (S10 Table in S1 File), the former reflecting winter lethargy [90]. We found no evidence for an effect of road construction on HR size, as neither road construction phase nor adjacency to the road, either independently or in interaction with one another, were included in the model that best explained the data (S4 Table in S1 File). The model that best explained the variation in home range size included sex and age (in interaction with one another) and month as explanatory variables (S11 Table in S1 File). Before road construction commenced, badgers had a median HR size of 0.87km2 (IQR: 0.49–1.38km2, n = 403) (Fig 2B). During road construction, median HR size was 0.97km2 (IQR: 0.65–1.35km2, n = 260) while after road construction it was 1.07km2 (IQR: 0.68–1.48km2, n = 227) (Fig 2B).
Distance of extra-territorial excursions
Of the 80 badgers in the study, 91% made ETEs (n = 73). At all times of year, over half of the collared badgers were making ETEs. Across the study period, the median straight-line distance that a badger travelled from its social group boundary was 99m (IQR: 61-167m). Most ETEs, as measured by the 95th percentile, were less than 1km in length (S12 Table in S1 File). The maximum straight-line distance a badger travelled from its territory boundary was 4.2km (S12 Table in S1 File). We found no evidence that road construction influenced ETE distance, as neither road construction phase nor adjacency to the road, either independently or in interaction with one another, were included in the best model (S5 Table in S1 File). The model that best explained the variation in ETE distance included sex and age (in interaction with one another) and month as explanatory variables (S13 Table in S1 File).
Prior to road construction, the median distance of badger excursions was 90m (IQR: 65-127m, n = 1560) beyond their territory boundary (Fig 2C). During road construction, the median distance of badger ETEs was 102m (IQR: 55-272m, n = 951) and after the new motorway was completed, the median ETE distance was 91m (IQR: 56–154, n = 1215) (Fig 2C).
Frequency of extra-territorial excursions
Of the badgers that were recorded leaving their territory, across the study period the median fETE was 0.14 (IQR: 0.10–0.23), which equates to 4.25 ETEs in a month. fETE ranged from 0 to 1, meaning that in a given month, some badgers went on ETEs every night, while others never left their territory (S14 Table in S1 File). Of these data, 83% (n = 742) were from badgers that were wearing functioning collars for the full month and 17% (n = 150) were from badgers whose collars functioned for only part of the month. We allowed for incomplete months by using the proportion of active nights. Only 6% (n = 54) of records were from partial months where the number of active nights was less than 2 weeks. We are therefore confident that our data are representative of typical ETE behaviour in this population. For atypical behaviour of super-rangers and dispersers see [64, 65].
The model that best explained the variation in fETE included sex, age, month, and road construction as explanatory variables (S6 Table in S1 File). Road construction phase had a significant effect on the frequency with which badgers made ETEs (S15 Table in S1 File). Before road construction the median fETE was 0.16 (IQR: 0.10–0.23, n = 294). During road construction the median fETE was 0.13. The difference between the before and during phases was not significant (Table 2). However, badgers made ETEs significantly more frequently after road construction (median fETE 0.18, IQR: 0.10–0.25, n = 200) compared to the periods either before or during roadwork construction (Fig 2D, Table 2). On average, in a given month, badgers made ETEs on 4.9 nights before road construction, on 3.9 nights during road construction, and on 5.5 nights after road construction.
Table 2. Results of Tukey post hoc tests for multiple comparisons of fETE means for the different road construction phases.
| Comparison | Estimate | Std. Error | z value | Pr(>|z|) |
|---|---|---|---|---|
| During–Before | 0.238 | 0.199 | 1.198 | 0.231 |
| After–Before | 0.593 | 0.238 | 2.488 | < 0.05 |
| After–During | 0.355 | 0.156 | 2.277 | < 0.05 |
Table 3 summarises the results of the four movement metrics alongside the yearly medians evidenced in the period before the road construction commenced, placing them in the context of natural variation.
Table 3. Summary of median Nightly Distance (ND), Home Range (HR) size, Extra-Territorial Excursion (ETE) distance and frequency (fETE) during each phase of road construction.
| Median ND (m) | Median HR size (km2) | Median ETE (m) | Median fETE | |
|---|---|---|---|---|
| Before road construction | 651 | 0.87 | 90 | 0.16 |
| Variation in annual median before road construction | 551–640 | 0.72–1.13 | 90–142 | 0.13–0.16 |
| During road construction | 673 *** | 0.97 (ns) | 102 (ns) | 0.13 (ns) |
| After road construction | 848 *** | 1.07 (ns) | 91 (ns) | 0.18 * |
The variation in annual median in the ‘before’ phase is included in the second row for each movement metric. The statistical significance of differences between the ‘during’ and ‘after’ phases when compared with the ‘before’ phase are marked with asterisks (*** < 0.001, * < 0.05), while (ns) signifies no significant difference.
How often did badgers cross the N11/M11 before, during and after road construction?
In addition to the above movement metrics, we also recorded the number of times that badgers were recorded crossing the N11 road before and during road construction, and the M11 after road construction (S16 Table in S1 File). Badgers crossed the N11 road 75 times before road construction (1.8 crossing/month) and 22 times during road construction (1.7 crossings/month). After road construction badgers crossed the M11 motorway, using underpasses, 140 times (10 crossings/month). The badgers that had high levels of road crossing activity in the ‘after’ phase i.e., >3 crossings/month came from just three social groups (outlined in Fig 3A–3D). The majority were badgers from two of these social groups that were using underpasses to maintain access to parts of their territories (The Driving Range and The Briars) that would otherwise have been cut off by the new motorway. Badgers from a third social group (Hawthorn) were using an underpass to make ETEs to a neighbouring territory.
Fig 3. Territory boundaries and road crossings.
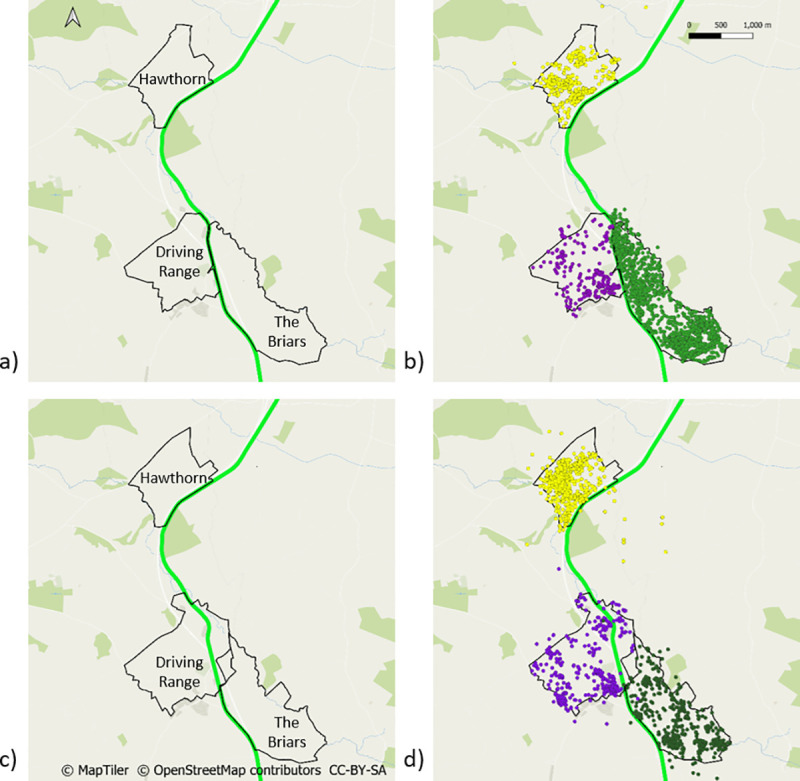
a) Territory boundaries of three of the social groups before road construction outlined in black; b) GPS locations for badgers from each of these social groups before road construction: Hawthorn in yellow, Driving Range in pink and The Briars in green; c) territory boundaries of the same three social groups after road construction outlined in purple and d) GPS locations for badgers from of these each social groups after road construction. The location of the N11 road is represented by a green line. The location of the M11 motorway is represented by a blue line. The locations of underpasses are represented by red dashes intersecting the M11. The N11 has been omitted from map d) for clarity.
Badger mortality on the roads
During the course of the study, we recorded 49 badgers that died in RTAs on the N11 (n = 28) and minor roads (n = 21) within the study area. There were only two badger deaths on the M11 in the 14-month post-construction study phase. Together, these figures give an average mortality rate of 10–15% per year of the population due to RTAs.
Discussion
The disturbance caused by the process of road construction has the potential to impact the ranging behaviour of animals [5–7]. Increased movement of badgers between social groups has been associated with an increase in the prevalence of TB in those social groups [23, 24]. However, the road upgrade considered here had very little effect on the ranging behaviour of badgers in the study area. The period before road construction spanned nearly 3.5 years and therefore acted as a record of normal behaviour. In considering any changes in the four movement metrics, it is helpful to bear in mind the natural variation between years as this gives a context for the values obtained during and after road construction (Table 3).
Nightly distance travelled
The median distance moved by badgers per night increased by 197m in total over the study period, by 22m a night during the construction phase, and by another 175m a night after the construction phase. This increase in ND suggests that the badgers were disturbed to some extent by the construction process and changes to the surrounding landscape. The fact that ND did not return to pre-road construction distances once road construction was completed could indicate that the disturbance caused a permanent change in ranging behaviour, or that a monitoring period of greater than 12 months is required to capture a return to normal behaviour. While the median ND in the ‘before’ phase was 651m, there was a lot of variation in NDs across these years (Table 3). The values obtained during and after road construction fall outside this normal variation, suggesting a biologically significant effect on the badgers’ behaviour. However, it should be noted that the maximum recorded ND was 11.25km (S8 Table in S1 File), so an extra 1.75% (197m) in a night would represent a small proportion of the total distance the badgers were able to travel (11.25km).
It is also likely that the mitigation measures on the motorway (continuous badger-proof fencing and underpasses) had an effect on ND in the ‘after’ phase. Although an interaction between road construction and adjacency was not included in the best model, badgers living in social groups located next to the N11/M11 may have had to travel further within their own territories once road construction was completed to maintain access to all areas of their territory (Fig 3D), since a further increase in ND was seen after road construction was completed. Indeed, ND of badgers living in social groups adjacent to the N11/M11 increased by on average 168m during road construction, and by a further 68m after road construction, a total of 237m. While ND also increased for badgers living in territories that were not adjacent to the N11/M11, the total increase for these badgers was only 78m on average (2m during and 78m after road construction). It is also possible that the increase in ND in badgers living adjacent to the road was due to loss of foraging habitat and an increase in foraging effort. However, the area within which construction occurred was on average 100m wide, and habitat loss was relatively small, and badgers continued to use as much suitable habitat as was available to them (S1 and S4 Figs in S1 File).
Home range size
The small increase in ND did not manifest in a corresponding increase in HR size, suggesting that any extra ranging was confined primarily to within territory boundaries, indicating that territoriality in the area was not disrupted. This interpretation was strengthened by the finding that the median HR size both during and after road construction fell within the yearly range of median HR sizes recorded in the ‘before’ phase (Table 3). While there was no significant change in mean HR size of badgers, there was an inevitable alteration to the boundaries of the social groups immediately adjacent to road construction. Some social groups lost access to land while others gained access to land previously belonging to a different social group, however these changes were relatively small (S3 Fig in S1 File). Potential loss of territory was mitigated for some social groups by the location of underpasses (Fig 3C and 3D) that facilitated continued access to land within their territory.
Extra-territorial excursions
A possible consequence of an increase in ND might be an increase in the distance of ETEs. However, there was no change in ETE distance across the study period (Table 3). In a situation where disturbance leads to a breakdown in territoriality, ETE distance might be expected to increase. However, even though badgers were moving around more within their own territories during and after road construction, they remained as cautious about how far they trespassed into other territories as they had been before road construction commenced. This suggests that territory boundaries continued to be marked and defended as effectively as before the disturbance.
Similarly, any reduction in territoriality might be expected to lead to an increase in the frequency with which badgers undertook ETEs. Before road construction, badgers made, on average, 4.9 ETEs in a given month. However, there was no significant change in the frequency of ETEs during road construction. Once construction had finished and the new motorway was opened, the frequency of ETEs did increase, albeit by less than one additional ETE per month, to on average 5.5 ETEs a month. On average, ETEs were approximately 100m in length, and the majority were less than 1km. The average distance between main setts in the study area was 1.3km. The majority of ETEs were therefore likely to be into adjacent social groups. Our findings suggest that although the frequency of ETEs increased, the potential for badgers to interact with animals with which they had not interacted prior to road construction did not increase, since there was no increase in ETE distance. The availability of underpasses may have facilitated safe and successful ETEs (Fig 3D) across a major road, a journey that was previously very risky.
Badger mortality on the new motorway
A reduction in population density due to culling or persecution has been found to disrupt ranging behaviour in badgers [39, 45, 46]. Roads are a major cause of mortality in badgers [9, 11, 12]. The extensive mitigation measures used in this case have proved successful in preventing badger deaths on the new M11 motorway. There were only two badger deaths on the M11 in the 14-month post-construction study phase. These were due to problems with incomplete fencing. Between 2010 and 2016 i.e., during the course of the study, we know of 49 badgers that died in RTAs on the N11 (n = 28) and minor roads (n = 21) within our study area. This gives us an average mortality rate of 10–15% per year. This is an order of magnitude higher than has previously been reported for rural Ireland [91] and is similar to figures reported for Sweden [92] but is half the mortality rate reported for The Netherlands [12]. We know that the death of a badger in our study area often prompted the movement of other badgers from different social groups into that group. If the new motorway had lacked effective fencing and underpasses, it is likely an increase in RTA-related mortality, along with associated movements between social groups would have occurred. Therefore, we recommend that the high level of mitigation measures used here is employed, and periodically checked, in all major road realignment and road building projects.
Implications of these changes for TB transmission
Taken together, our results suggest that any effect of road construction on ranging behaviour in badgers was very small, and that territoriality was not disrupted in our population. While there was a small but significant increase in ND during road construction, this was not reflected in any corresponding increase of HR size or ETE distance. After the new motorway was completed a further increase in nightly distance, and an increase in the frequency of ETEs was seen. However, this was not accompanied by a corresponding increase in home range size or ETE distance, suggesting that additional interactions within the social network were not formed as a result. It is possible that during the course of road construction, badgers responded to the disturbance by consolidating their own territories by ranging more within them, possibly marking border latrines more frequently. Only after road construction had finished was there evidence that they then began to investigate potential impacts upon adjacent territories through more frequent ETEs, although they did not venture further afield.
From commencement of the study we vaccinated all captured badgers against TB, using Bacille Calmette-Guérin (BCG) vaccine [68]. At the start of the study, based on blood testing and bacteriology, TB prevalence in badgers in the study area was 19%. All captured study area badgers have been vaccinated with BCG vaccine since April 2010 and M. bovis has not been isolated from any badgers since December 2013. Since then, there have been 7 more capture events, and 270 samples submitted for culture. Inside the study area there have only been two herd breakdowns since April 2010 –one high risk breakdown (c.15 reactor animals) on the edge of the study area in 2012, and one low risk breakdown (1 reactor animal) in the middle of the study area in March 2014. Both breakdowns were attributed to badgers. Since 2014, there have been no further breakdowns within the study area. In 2015, there was a complete herd depopulation in a farm directly adjacent to the study area. However, this was conclusively demonstrated to be due to an infected cow being brought into the herd. Vaccination of badgers mitigated against the possible transmission of TB between badgers both through bite wounding, and through activation of latent infections due to stress associated with road construction [93–95].
Due to the fact that we vaccinated the badgers in our study area, we could not investigate whether there were any changes in TB infections of either badgers or local cattle herds directly attributable to a change in badger ranging behaviour, that is, a potential ‘perturbation’ effect [34–40]. Badger movements into and out of neighbouring social groups are associated with increased prevalence of TB in those groups [23, 24]. Given the small degree of disruption to ranging behaviour evidenced, we believe it is unlikely that any increase in TB breakdowns in cattle would have occurred as a result.
It must be noted that the findings herein can only be extrapolated to other projects involving an upgrade of an existing road that is likely to act as a territory boundary [11, 96]. In greenfield sites i.e., the construction of a new road over agricultural land, and potentially through the middle of existing, unvaccinated social groups, we cannot be certain whether ranging behaviour would be disturbed, nor whether there would be an associated perturbation effect on bovine TB in the area. However, the lack of disturbance seen, particularly in those social groups which lost territory sections in this road realignment, lead us to suggest that, where effective mitigation is provided, there is no expectation of a perturbation effect arising from road construction. We recommend that mitigation in the form of continuous badger-proof fencing be placed along the entire length of all major new road-builds and upgrades through areas occupied by badgers, and that underpasses should be located in appropriate places. This should prevent RTAs and minimise disturbance to the ranging behaviour of the badgers. We also recommend BCG vaccination of badgers in advance of major earthworks.
Conclusion
The objective of this project was to ascertain the effects of a major road upgrade and realignment on the ranging behaviour of the badgers in the surrounding area. Road construction, which included vegetation clearance, rock-breaking, major excavation, and earth-moving activity, proved to be of minor consequence to the badgers’ movements or territoriality. Our results demonstrate that badgers can adapt to the considerable environmental disturbance resulting from major road construction. Given the modest disruption in ranging behaviour seen in this badger population, both during and after road construction, we believe it is unlikely that a fully-mitigated major road upgrades would result in a perturbation effect sufficient to increase TB in the local cattle.
Supporting information
This file contains all supporting tables (S1-S16 Tables), text (S1 Text) and figures (S1-S4 Figs).
(DOCX)
Acknowledgments
We would like to thank Mark Foley and Denis Foley for their field work expertise, and all of the landowners for access to their lands.
Data Availability
All data files are available from the Open Science Framework repository at https://doi.org/10.17605/OSF.IO/7UYMG.
Funding Statement
AG was supported by a PhD scholarship provided by the Department of Agriculture, Food and the Marine, Ireland. This project was conceived, carried out and funded by the Department of Agriculture, Food and the Marine, Ireland, and the National Parks and Wildlife Service, Ireland.
References
- 1.Carr LW, Fahrig L, Pope SE. Impacts of landscape transformation by roads. Applying landscape ecology in biological conservation. Springer; 2002. pp. 225–243. [Google Scholar]
- 2.Coffin AW. From roadkill to road ecology: a review of the ecological effects of roads. Journal of Transport Geography. 2007;15: 396–406. [Google Scholar]
- 3.Benítez-López A, Alkemade R, Verweij PA. The impacts of roads and other infrastructure on mammal and bird populations: a meta-analysis. Biological Conservation. 2010;143: 1307–1316. [Google Scholar]
- 4.Torres A, Palacín C, Seoane J, Alonso JC. Assessing the effects of a highway on a threatened species using Before–During–After and Before–During–After-Control–Impact designs. Biological Conservation. 2011;144: 2223–2232. [Google Scholar]
- 5.Kohn B, Frair J, Unger D, Gehring T, Shelley D, Anderson E, et al. Impacts of a highway expansion project on wolves in north-western Wisconsin. Proceedings of the Third International Conference on Wildlife Ecology and Transportation (FL-ER-73-99), Missoula, Montana. 1999. pp. 13–16. [Google Scholar]
- 6.Klar N, Herrmann M, Kramer-Schadt S. Effects and mitigation of road impacts on individual movement behavior of wildcats. The Journal of Wildlife Management. 2009;73: 631–638. [Google Scholar]
- 7.Lesmerises F, Dussault C, St-Laurent M-H. Major roadwork impacts the space use behaviour of gray wolf. Landscape and Urban Planning. 2013;112: 18–25. [Google Scholar]
- 8.Clevenger AP. Mitigating highways for a ghost: Data collection challenges and implications for managing wolverines and transportation corridors. Northwest Science. 2013;87: 257–264. [Google Scholar]
- 9.Davies JM, Roper TJ, Shepherdson DJ. Seasonal distribution of road kills in the European badger (Meles meles). Journal of Zoology. 1987;211: 525–529. [Google Scholar]
- 10.Van der Zee F, Wiertz J, Ter Braak C, Van Apeldoorn R, Vink J. Landscape change as a possible cause of the badger Meles meles decline in The Netherlands. Biological Conservation. 1992;61: 17–22. [Google Scholar]
- 11.Clarke GP, White PCL, Harris S. Effects of roads on badger (Meles meles) populations in south-west England. Biological Conservation. 1998;86: 117–124. [Google Scholar]
- 12.Dekker JJ, Bekker H. Badger (Meles meles) road mortality in The Netherlands: the characteristics of victims and the effects of mitigation measures. Lutra. 2010;53: 81–92. [Google Scholar]
- 13.Gormley E, Costello E. Tuberculosis and badgers: new approaches to diagnosis and control. Journal of Applied Microbiology. 2003;94: 80S–86S. doi: 10.1046/j.1365-2672.94.s1.9.x [DOI] [PubMed] [Google Scholar]
- 14.Murphy D, Gormley E, Costello E, O’Meara D, Corner LAL. The prevalence and distribution of Mycobacterium bovis infection in European badgers (Meles meles) as determined by enhanced post mortem examination and bacteriological culture. Research in Veterinary Science. 2010;88: 1–5. doi: 10.1016/j.rvsc.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 15.Corner LA, Murphy D, Gormley E. Mycobacterium bovis infection in the Eurasian badger (Meles meles): the disease, pathogenesis, epidemiology and control. Journal of Comparative Pathology. 2011;144: 1–24. doi: 10.1016/j.jcpa.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Godfray HC, Donnelly CA, Kao RR, Macdonald DW, McDonald RA, Petrokofsky G, et al. A restatement of the natural science evidence base relevant to the control of bovine tuberculosis in Great Britain. Proceedings of the Royal Society B: Biological Sciences. 2013;280: 20131634. doi: 10.1098/rspb.2013.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor CM, Haydon DT, Kao RR. An ecological and comparative perspective on the control of bovine tuberculosis in Great Britain and the Republic of Ireland. Preventive Veterinary Medicine. 2012;104: 185–197. doi: 10.1016/j.prevetmed.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Allen AR, Skuce RA, Byrne AW. Bovine tuberculosis in Britain and Ireland–A perfect storm? The confluence of potential ecological and epidemiological impediments to controlling a chronic infectious disease. Frontiers in Veterinary Science. 2018;5: 109. doi: 10.3389/fvets.2018.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conner MM, Miller MW. Movement patterns and spatial epidemiology of a prion disease in mule deer population units. Ecological Applications. 2004;14: 1870–1881. [Google Scholar]
- 20.Cheeseman CL, Wilesmith JW, Stuart FA, Mallinson PJ. Dynamics of tuberculosis in a naturally infected badger population. Mammal Review. 1988;18: 61–72. [Google Scholar]
- 21.Delahay RJ, Langton S, Smith GC, Clifton‐Hadley RS, Cheeseman CL. The spatio‐temporal distribution of Mycobacterium bovis (bovine tuberculosis) infection in a high‐density badger population. Journal of Animal Ecology. 2000;69: 428–441. [Google Scholar]
- 22.Davis S, Abbasi B, Shah S, Telfer S, Begon M. Spatial analyses of wildlife contact networks. Journal of The Royal Society Interface. 2015;12: 20141004. doi: 10.1098/rsif.2014.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers LM, Delahay R, Cheeseman CL, Langton S, Smith GC, Clifton-Hadley RS. Movement of badgers (Meles meles) in a high-density population: individual, population and disease effects. Proceedings of the Royal Society B: Biological Sciences. 1998. Available: http://rspb.royalsocietypublishing.org/content/265/1403/1269.short [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riordan P, Delahay RJ, Cheeseman C, Johnson PJ, Macdonald DW. Culling-induced changes in badger (Meles meles) behaviour, social organisation and the epidemiology of bovine tuberculosis. PLoS One. 2011;6. doi: 10.1371/journal.pone.0028904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber N, Carter SP, Dall SR, Delahay RJ, McDonald JL, Bearhop S, et al. Badger social networks correlate with tuberculosis infection. Current Biology. 2013;23: R915–R916. doi: 10.1016/j.cub.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 26.O’Mahony DT. Badger (Meles meles) contact metrics in a medium-density population. Mammalian Biology. 2015;80: 484–490. [Google Scholar]
- 27.Martin SW, Eves JA, Dolan LA, Hammond RF, Griffin JM, Collins JD, et al. The association between the bovine tuberculosis status of herds in the East Offaly Project Area, and the distance to badger setts, 1988–1993. Preventive Veterinary Medicine. 1997;31: 113–125. doi: 10.1016/s0167-5877(96)01111-7 [DOI] [PubMed] [Google Scholar]
- 28.Eves J. Impact of badger removal on bovine tuberculosis in east County Offaly. Irish Veterinary Journal. 1999;52: 199–203. [Google Scholar]
- 29.Griffin JM, Williams DH, Kelly GE, Clegg TA, O’Boyle I, Collins JD, et al. The impact of badger removal on the control of tuberculosis in cattle herds in Ireland. Preventive Veterinary Medicine. 2005;67: 237–266. doi: 10.1016/j.prevetmed.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 30.Woodroffe R, Donnelly CA, Cox D, Gilks P, Jenkins HE, Johnston WT, et al. Bovine tuberculosis in cattle and badgers in localized culling areas. Journal of Wildlife Diseases. 2009;45: 128–143. doi: 10.7589/0090-3558-45.1.128 [DOI] [PubMed] [Google Scholar]
- 31.Mullen EM, MacWhite T, Maher PK, Kelly DJ, Marples NM, Good M. The avoidance of farmyards by European badgers Meles meles in a medium density population. Applied Animal Behaviour Science. 2015;171: 170–176. [Google Scholar]
- 32.Griffin JM, More SJ, Clegg TA, Collins JD, O’Boyle I, Williams DH, et al. Tuberculosis in cattle: the results of the four-area project. Irish Veterinary Journal. 2005;58: 629–636. doi: 10.1186/2046-0481-58-11-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olea-Popelka FJ, Fitzgerald P, White P, McGrath G, Collins JD, O’Keeffe J, et al. Targeted badger removal and the subsequent risk of bovine tuberculosis in cattle herds in County Laois, Ireland. Preventive Veterinary Medicine. 2009;88: 178–184. doi: 10.1016/j.prevetmed.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 34.Cheeseman CL, Mallinson PJ, Ryan J, Wilesmith JW. Recolonisation by badgers in Gloucestershire. In: Hayden T (Ed), The Badger Royal Irish Academy, Dublin. 1993; 78–93. [Google Scholar]
- 35.Tuyttens FAM, Delahay RJ, Macdonald DW, Cheeseman CL, Long B, Donnelly CA. Spatial perturbation caused by a badger (Meles meles) culling operation: implications for the function of territoriality and the control of bovine tuberculosis (Mycobacterium bovis). Journal of Animal Ecology. 2000;69: 815–828. doi: 10.1046/j.1365-2656.2000.00437.x [DOI] [PubMed] [Google Scholar]
- 36.Tuyttens FAM, Macdonald DW, Rogers LM, Cheeseman CL, Roddam AW. Comparative study on the consequences of culling badgers (Meles meles) on biometrics, population dynamics and movement. Journal of Animal Ecology. 2000;69: 567–580. [Google Scholar]
- 37.Donnelly CA, Woodroffe R, Cox DR, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, et al. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature. 2006;439: 843–846. doi: 10.1038/nature04454 [DOI] [PubMed] [Google Scholar]
- 38.Donnelly CA, Wei G, Johnston WT, Cox DR, Woodroffe R, Bourne FJ, et al. Impacts of widespread badger culling on cattle tuberculosis: concluding analyses from a large-scale field trial. International Journal of Infectious Diseases. 2007;11: 300–308. doi: 10.1016/j.ijid.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 39.Carter SP, Delahay RJ, Smith GC, Macdonald DW, Riordan P, Etherington TR, et al. Culling-induced social perturbation in Eurasian badgers Meles meles and the management of TB in cattle: an analysis of a critical problem in applied ecology. Proceedings of the Royal Society B: Biological Sciences. 2007;274. doi: 10.1098/rspb.2007.0998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkins HE, Woodroffe R, Donnelly CA. The effects of annual widespread badger culls on cattle tuberculosis following the cessation of culling. International Journal of Infectious Diseases. 2008;12: 457–465. doi: 10.1016/j.ijid.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 41.Woodroffe R, Donnelly CA, Cox D, Bourne F, Cheeseman C, Delahay R, et al. Effects of culling on badger Meles meles spatial organization: implications for the control of bovine tuberculosis. Journal of Applied Ecology. 2006;43: 1–10. [Google Scholar]
- 42.McDonald RA, Delahay RJ, Carter SP, Smith GC, Cheeseman CL. Perturbing implications of wildlife ecology for disease control. Trends in Ecology & Evolution. 2008;23: 53–56. doi: 10.1016/j.tree.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 43.O’Corry-Crowe G, Hammond R, Eves J, Hayden T. The effect of reduction in badger density on the spatial organisation and activity of badgers Meles meles L. in relation to farms in central Ireland. Biology and Environment: Proceedings of the Royal Irish Academy; 1996. pp. 96B; 3; 147–158. [Google Scholar]
- 44.Costello E, Flynn O, Quigley F, O’Grady D, Griffin J, Clegg T, et al. Genotyping of Mycobacterium bovis isolates from badgers in four areas of the Republic of Ireland by restriction fragment length polymorphism analysis. Veterinary Record. 2006;159: 619–623. doi: 10.1136/vr.159.19.619 [DOI] [PubMed] [Google Scholar]
- 45.Sadlier L, Montgomery I. The impact of sett disturbance on badger Meles meles numbers; when does protective legislation work? Biological conservation. 2004;119: 455–462. [Google Scholar]
- 46.Sleeman DP, Mulcahy MF. Loss of territoriality in a local badger Meles meles population at Kilmurry, Co Cork, Ireland. The Irish Naturalists’ Journal. 2005. Available: http://www.jstor.org/stable/25536611 [Google Scholar]
- 47.Wright DM, Reid N, Montgomery WI, Allen AR, Skuce RA, Kao RR. Herd-level bovine tuberculosis risk factors: assessing the role of low-level badger population disturbance. Scientific reports. 2015;5: 13062. doi: 10.1038/srep13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blumstein DT. Conservation and animal welfare issues arising from forestry practices. Animal Welfare. 2010;19: 151–157. [Google Scholar]
- 49.Mullen E, MacWhite T, Maher P, Gaughran A, Kelly DJ, Good M, et al. How effective are forestry guidelines at protecting badgers and their setts during clearfelling? Lessons from Ireland. In Practice. CIEEM: 39:43. [Google Scholar]
- 50.Hubert T. Farms decimated by TB along Galway motorway site. 18 Dec 2016 [cited 9 May 2018]. Available: https://www.farmersjournal.ie/farms-decimated-by-tb-along-galway-motorway-site-244883/
- 51.Ó Liatháin C. Cork farmers hit with TB outbreak–Department ‘sceptical’ over ‘link’ to motorway building works. 6 Aug 2020 [cited 30 Aug 2020]. Available: https://www.independent.ie/business/farming/news/farming-news/cork-farmers-hit-with-tb-outbreak-department-sceptical-over-link-to-motorway-building-works-39427739.html
- 52.Kowalczyk R, Zalewski A, Jędrzejewska B. Daily movement and territory use by badgers Meles meles in Białowieża Primeval Forest, Poland. Wildlife Biology. 2006;12: 385–391. [Google Scholar]
- 53.Woodroffe R, Donnelly CA, Ham C, Jackson SY, Moyes K, Chapman K, et al. Ranging behaviour of badgers Meles meles vaccinated with Bacillus Calmette Guerin. Journal of Applied Ecology. 2016. doi: 10.1111/1365-2664.12837 [DOI] [Google Scholar]
- 54.Kowalczyk R, Zalewski A, Jedrzejewska B, Jedrzejewski W. Spatial organization and demography of badgers (Meles meles) in Bialowieza Primeval Forest, Poland, and the influence of earthworms on badger densities in Europe. Canadian Journal of Zoology. 2003;81: 74–87. [Google Scholar]
- 55.Do Linh San E, Ferrari N, Weber J-M. Spatio-temporal ecology and density of badgers Meles meles in the Swiss Jura Mountains. European Journal of Wildlife Research. 2007;53. doi: 10.1007/s10344-006-0085-8 [DOI] [Google Scholar]
- 56.Palphramand KL, Newton-Cross G, White PC. Spatial organization and behaviour of badgers (Meles meles) in a moderate-density population. Behavioral Ecology and Sociobiology. 2007;61: 401–413. [Google Scholar]
- 57.Revilla E, Palomares F. Spatial organization, group living and ecological correlates in low-density populations of Eurasian badgers, Meles meles. Journal of Animal Ecology. 2002;71. doi: 10.1046/j.1365-2656.2002.00617.x [DOI] [Google Scholar]
- 58.Kauhala K, Holmala K, Lammers W, Schregel J. Home ranges and densities of medium-sized carnivores in south-east Finland, with special reference to rabies spread. Acta Theriologica. 2006;51: 1–13. [Google Scholar]
- 59.Kowalczyk R, Bunevich AN, Jędrzejewska B. Badger density and distribution of setts in Białowieża Primeval Forest (Poland and Belarus) compared to other Eurasian populations. Acta Theriologica. 2000;45: 395–408. [Google Scholar]
- 60.Byrne AW, Quinn JL, O’Keeffe JJ, Green S, Sleeman DP, Martin SW, et al. Large-scale movements in European badgers: has the tail of the movement kernel been underestimated? The Journal of Animal Ecology. 2014. doi: 10.1111/1365-2656.12197 [DOI] [PubMed] [Google Scholar]
- 61.Sleeman DP. Long-distance movements in an Irish badger population. Wildlife Telemetry. 1992; 670–676. [Google Scholar]
- 62.Sleeman DP, Mulcahy MF. Behaviour of Irish badgers in relation to bovine tuberculosis. In: Hayden T (Ed), The Badger, Royal Irish Academy, Dublin. 1993; 154–65. [Google Scholar]
- 63.Kelly DJ, Gaughran A, Mullen E, MacWhite T, Maher P, Good M, et al. Extra Territorial Excursions by European badgers are not limited by age, sex or season. Scientific Reports. 2020;10: 1–12. doi: 10.1038/s41598-019-56847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaughran A, Kelly DJ, MacWhite T, Mullen E, Maher P, Good M, et al. Super-ranging. A new ranging strategy in European badgers. PLoS One. 2018;13. doi: 10.1371/journal.pone.0191818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaughran A, MacWhite T, Mullen E, Maher P, Kelly DJ, Good M, et al. Dispersal patterns in a medium‐density Irish badger population: Implications for understanding the dynamics of tuberculosis transmission. Ecology and Evolution. 2019;9: 13142–13152. doi: 10.1002/ece3.5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cleary GP, Corner LAL, O’Keeffe J, Marples NM. The diet of the badger Meles meles in the Republic of Ireland. Mammalian Biology—Zeitschrift für Säugetierkunde. 2009;74: 438–447. doi: 10.1016/j.mambio.2009.07.003 [DOI] [Google Scholar]
- 67.Cleary GP, Corner LAL, O’Keeffe J, Marples NM. Diet of the European badger (Meles meles) in the Republic of Ireland: A comparison of results from an analysis of stomach contents and rectal faeces. Mammalian Biology—Zeitschrift für Säugetierkunde. 2011;76: 470–475. doi: 10.1016/j.mambio.2010.10.012 [DOI] [Google Scholar]
- 68.MacWhite T, Maher P, Mullen E, Marples N, Good M. Satellite tracking study of badgers (Meles meles) to establish normal ranging behaviour prior to a road realignment. The Irish Naturalists Journal. 2013;32: 99–105. [Google Scholar]
- 69.Byrne AW, Davenport J, O’Keeffe J, Paddy Sleeman D. The ecology of the European badger (Meles meles) in Ireland: a review. Biology & Environment: Proceedings of the Royal Irish Academy. 2012;112: 105–132. doi: 10.3318/bioe.2012.02 [DOI] [Google Scholar]
- 70.Hancox M. Field age determination in the European badger. Revue D’ecologie (La Terre et La Vie). 1988;43: 399–404. [Google Scholar]
- 71.da Silva J, Macdonald DW. Limitations to the use of tooth wear as a means of ageing Eurasian badgers, Meles meles. Revue D’ecologie (La Terre et La Vie). 1989;44: 275–278. [Google Scholar]
- 72.Corner LA, Stuart LJ, Kelly DJ, Marples NM. Reproductive Biology Including Evidence for Superfetation in the European Badger Meles meles (Carnivora: Mustelidae). PLoS One. 2015;10: e0138093. doi: 10.1371/journal.pone.0138093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macdonald DW, Newman C, Buesching CD, Johnson PJ. Male-biased movement in a high-density population of the Eurasian badger (Meles meles). Journal of Mammalogy. 2008;89: 1077–1086. [Google Scholar]
- 74.Gaughran A. The impact of roadworks on the ranging behaviour of European badgers (Meles meles). PhD, Trinity College Dublin. 2018. Available: http://www.tara.tcd.ie/handle/2262/84998
- 75.Calenge C. Home range estimation in R: the adehabitatHR package. 2011. Available: ftp://mi.mirror.garr.it/pub/1/cran/web/packages/adehabitatHR/vignettes/adehabitatHR.pdf
- 76.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.or/. 2019. [Google Scholar]
- 77.O’Brien J, Elliott S, Hayden TJ. Use of hedgerows as a key element of badger (Meles meles) behaviour in Ireland. Mammalian Biology. 2016;81: 104–110. [Google Scholar]
- 78.Loureiro F, Rosalino LM, Macdonald DW, Santos-Reis M. Path tortuosity of Eurasian badgers (Meles meles) in a heterogeneous Mediterranean landscape. Ecological Research. 2007;22: 837–844. [Google Scholar]
- 79.Elliott S, O’Brien J, Hayden TJ. Impact of human land use patterns and climatic variables on badger (Meles meles) foraging behaviour in Ireland. Mammal Research. 2015;60: 331–342. [Google Scholar]
- 80.O’Hagan MJH, Gordon AW, McCormick CM, Collins SF, Trimble NA, McGeown CF, et al. Effect of selective removal of badgers (Meles meles) on ranging behaviour during a ‘Test and Vaccinate or Remove’ intervention in Northern Ireland. Epidemiology & Infection. 2021;149. doi: 10.1017/S0950268821001096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.MacDonald DW, Newman C, Dean J, Buesching CD. The distribution of Eurasian badger, Meles meles, setts in a high‐density area: field observations contradict the sett dispersion hypothesis. Oikos. 2004;106: 295–307. doi: 10.1111/j.0030-1299.2004.12879.x [DOI] [Google Scholar]
- 82.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67: 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 83.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, et al. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. The R Journal. 2017;9: 378–400. [Google Scholar]
- 84.Barton K. MuMIn: Multi-Model inference. R package verion 1.43.17. 2020. Available: https://CRAN.R-project.org/package=MuMIn
- 85.Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer Science & Business Media; 2003. [Google Scholar]
- 86.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology & Evolution. 2009;24: 127–135. doi: 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 87.Box GE, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society Series B (Methodological). 1964;26: 211–252. [Google Scholar]
- 88.Bolker B. Testing for overdispersion/computing overdispersion factor. [cited 28 Jul 2021]. Available: https://bbolker.github.io/mixedmodels-misc/glmmFAQ.html#overdispersion
- 89.Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A, Scheibe S, et al. Package ‘multcomp.’ Simultaneous inference in general parametric models Project for Statistical Computing, Vienna, Austria. 2016.
- 90.Fowler PA, Racey PA. Overwintering strategies of the badger, Meles meles, at 57 N. Journal of Zoology. 1988. doi: 10.1111/j.1469-7998.1988.tb03763.x [DOI] [Google Scholar]
- 91.Sleeman DP, Collins DM, Davenport J. What proportion of badgers (Meles meles) are killed on roads in rural areas in the Republic of Ireland? Mammal Notes. 2012;6: 1–4. [Google Scholar]
- 92.Seiler A, Helldin J, Seiler C. Road mortality in Swedish mammals: results of a drivers’ questionnaire. Wildlife Biology. 2004;10: 225–233. [Google Scholar]
- 93.Gallagher J, Clifton-Hadley RS. Tuberculosis in badgers; a review of the disease and its significance for other animals. Research in Veterinary Science. 2000;69: 203–217. doi: 10.1053/rvsc.2000.0422 [DOI] [PubMed] [Google Scholar]
- 94.Chambers MA, Rogers F, Delahay RJ, Lesellier S, Ashford R, Dalley D, et al. Bacillus Calmette-Guérin vaccination reduces the severity and progression of tuberculosis in badgers. Proceedings of the Royal Society B: Biological Sciences. 2011;278: 1913–1920. doi: 10.1098/rspb.2010.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.George SC, Smith TE, Mac Cana PS, Coleman R, Montgomery WI. Physiological stress in the Eurasian badger (Meles meles): effects of host, disease and environment. General and Comparative Endocrinology. 2014;200: 54–60. doi: 10.1016/j.ygcen.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 96.Frantz AC, Pope LC, Etherington TR, Wilson GJ, Burke T. Using isolation-by-distance-based approaches to assess the barrier effect of linear landscape elements on badger (Meles meles) dispersal. Molecular Ecology. 2010;19: 1663–74. doi: 10.1111/j.1365-294X.2010.04605.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains all supporting tables (S1-S16 Tables), text (S1 Text) and figures (S1-S4 Figs).
(DOCX)
Data Availability Statement
All data files are available from the Open Science Framework repository at https://doi.org/10.17605/OSF.IO/7UYMG.



