Introduction
The ongoing Coronavirus Disease 2019 (COVID-19) pandemic has taken a toll on millions of lives worldwide. Currently, India has the second highest number of active COVID-19 cases and ranks third for the total number of deaths worldwide. While few vaccines are currently approved for use in India and elsewhere, there is still an urgent need for complementary approaches to tackle the current disease burden. Human monoclonal antibodies (mAbs) that neutralize Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and its variants provide an attractive treatment strategy. To this end, very recently, a formulation of human mAbs (casirivimab and imdevimab) against SARS-CoV-2 was approved for passive immunotherapy in mild and moderately severe COVID-19 cases in India and elsewhere. Thus, it is valuable and timely to summarize the specificity and reactivity of human mAbs against SARS-CoV-2 and its rapidly emerging variants.
Primary targets of SARS-CoV-2 therapeutic neutralizing antibodies
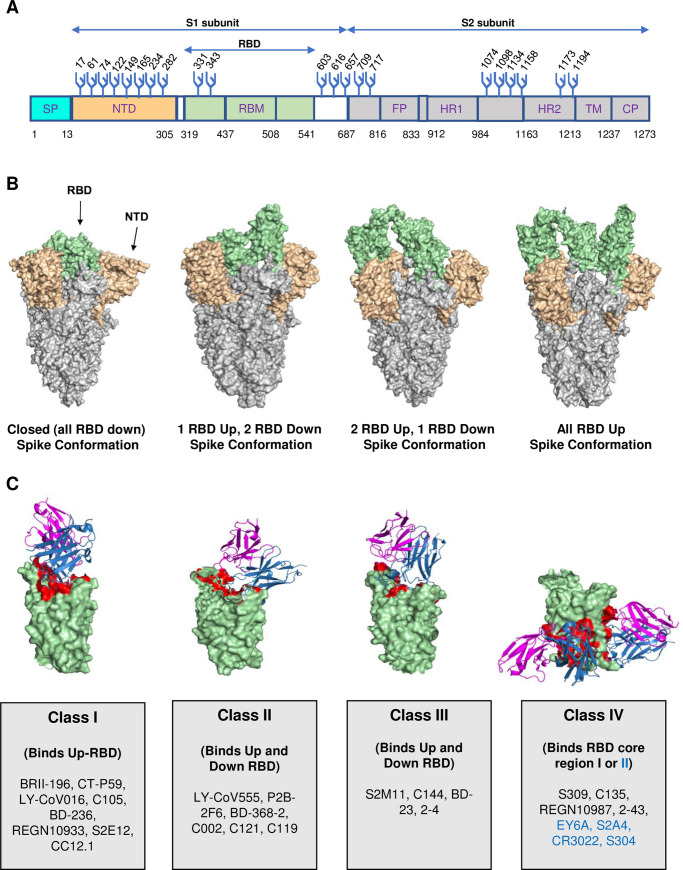
The spike (S) protein of SARS-CoV-2 is the primary target of neutralizing antibodies (NAbs) (Fig 1A). Therefore, NAbs against SARS-CoV-2 that have either been deployed for therapy or are in advanced stage trials, for the most part, either target the receptor-binding domain (RBD) or the N-terminal domain (NTD) of the spike glycoprotein (Fig 1A and 1B) [1,2]. The S protein exists in different conformations within the host, and their nomenclatures are based on the position of RBD protein—an “up” or “down” position (Fig 1B). To this end, based on the epitope recognition and binding mode, RBD-specific NAbs are categorized into 4 major classes (I, II, III, and IV) [3–5]. Class I and II NAbs bind the angiotensin converting enzyme 2 (ACE2) binding region or “receptor-binding motif” (RBM) region of the RBD on the spike glycoprotein [3]. The RBM region is responsible for the primary contact with host ACE2 to initiate the entry of the virus [6]. MAbs that block this RBM–ACE2 interaction are “ACE2 blockers.” The class I NAbs bind RBD in “up” conformation only and block ACE2 binding, whereas class II NAbs block ACE2 binding, and recognize both “up” and “down” RBDs (Fig 1B and 1C). On the other hand, the class III NAbs block ACE2 binding site, recognize spike protein with both “up” and “down” RBD conformations, and can interact with adjacent RBD protomers. The class IV NAbs do not overlap with ACE2 binding site and bind conserved region in RBD (core I region) or RBD in “up” conformation only (core II region) (Fig 1B and 1C). A complete description of these 4 classes of RBD-dependent mAbs is shown in Fig 1C. Class IV core I region-dependent NAbs have broad neutralizing activity against SARS-CoV-2, its variants, and other related coronaviruses [1–3,7]. Very recently, NAbs targeting new epitopes on the S2 domain (stem helix region) of spike have also been identified that are broadly neutralizing, i.e., neutralize SARS-related and other human coronaviruses (hCoVs) [8–11].
Fig 1. SARS-CoV-2 spike protein structure, conformation, and targets of RBD-dependent mAbs.
(A) SARS-CoV-2 spike protein regions with amino acid position numbering are shown, which includes S1 domain regions: the SP, NTD, RBD, and RBM and S2 domain regions: FP, HR1 and HR2, TM, and CP. The glycosylation sites are numbered and marked with Y-like structures in blue. (B) Different conformations of spike protein (PDB: 7DF3, 6XKL, 7EB5, and 7KML, left to right). (C) Representation of 4 classes of SARS-CoV-2 RBD dependent mAbs. Antibody variable heavy chain region (sky blue) and light chain variable region (magenta) are marked. Antibody constant regions were removed from the bound Fab for clarity. The RBD is shown in (pale green) color and antibody contacts on RBD are marked in red (PDB ID: left to right, 7CM4 (CT-P59), 7CHF (BD-368-2), 7K90 (C144), and 6R6X (S304 (left) and S309 (right)). RBD class IV core II region targeting mAbs are shown in blue. CP, cytoplasmic tail; FP, fusion peptide; HR1, heptad repeat 1; HR2, heptad repeat 2; mAb, monoclonal antibody; NTD, N-terminal domain; PDB, Protein Data Bank; RBD, receptor-binding domain; RBM, receptor-binding motif; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SP, signal peptide; TM, transmembrane domain.
Therapeutic COVID-19 mAbs in the clinic and in clinical trials
Therapeutic mAbs for COVID-19 treatment have been developed in accelerated time and the pace has been unprecedented for any disease. The approvals were obtained in a record time of only 10 months, including 3 to 4 months of clinical grade mAbs production since the discovery of mAbs [12]. Currently, 8 SARS-CoV-2 RBD-specific potent NAbs have been approved by the Food and Drug Administration (FDA) under an emergency use authorization (EUA) to treat COVID-19 nonhospitalized patients at high risk of severe illness. The following COVID-19 mAbs are in clinical use: bamlanivimab (LY-CoV555) [13]; bamlanivimab (LY-CoV555) and etesevimab (LY-CoV016 or JS016) [14] from Eli Lilly; casirivimab (REGN10933) and Imdevimab (REGN10987) [15] from Regeneron; cilgavimab (COV2-2130 or AZD1061) and tixagevimab (COV2-2196 or AZD8955) [16] from AstraZeneca; monotherapy-based NAbs sotrovimab (VIR-7831) [17] from GSK and Vir Biotechnology; and regdanvimab (CT-P59) [18] from Celltrion. Another set of monotherapy and combination Nabs-based therapies are under Phase III trials: 2B04 [19] and 47D11 [20] from AbbVie; BRII-196 and BRII-198 from Brii Biosciences [2]; and TY027 from Tychan are also in Phase III trials [2]. A comprehensive list of NAbs that are currently in Phase I, II, and III trials and in clinic is summarized in Fig 2A. These therapeutic mAbs are used/administered in a range of 0.5 g to 1.2 g per dose, within 10 days of symptoms onset, as monotherapy or 2.4 g as a cocktail [21–23]. No dose-dependent effect was observed when these mAbs were tested at different doses (1.2 g to 8 g dose). The COVID-19 mAbs have demonstrated high efficacy in trials with a reduction of 70% to 85% in hospitalization or death [21,23,24]. Presently, these mAbs are used for intravenous administration; however, their intramuscular or subcutaneous administration testing is underway to facilitate larger access by overcoming the requirement of hospital settings. Presently, these mAbs are being produced in large-scale bioreactors of 15,000 L capacity, sufficient to provide 100 to 200,000 doses [12,25]. However, a single-dose regimen of these therapeutic mAbs continues to be expensive, particularly for low- and middle-income countries.
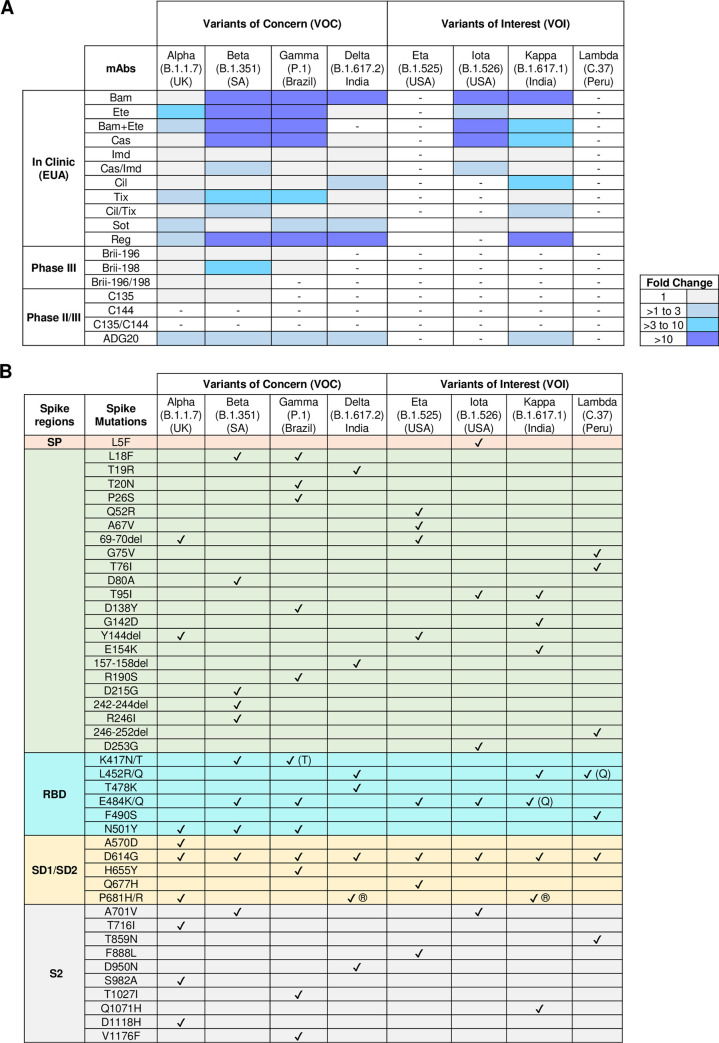
Fig 2. Neutralization potential of therapeutic mAbs against SARS-CoV-2 VOCs and VOIs.
(A) Neutralization potential of SARS-CoV-2 mAbs at various stages of development/clinic against VOCs and VOIs. Here, fold change represents the reduction in IC50 values of SARS-CoV-2 variant neutralization in comparison to wild-types virus. The abbreviations for mAbs in the clinic (EUA) are the following: Bam, Bamlanivimab (LY-CoV555); Ete, Etesevimab (LY-CoV016 or JS016 or CB6); Bam/Ete, Bamlanivimab+Etesevimab; Cas, Casirivimab (REGN10933); Imd, Imdevimab (REGN10987); Cas/Imd, Casirivimab+imdevimab (REGN-COV2); Cil, Cilgavimab (COV2-2130 or AZD1061); Tix, Tixagevimab (COV2-2196 or AZD8895); Tix/Cil, Tixagevimab+Cilgavimab; Sot, Sotrovimab (Vir-7831 or S309); Reg, Regdanvimab (CT-P59). (B) List of mutations present in the current SARS-CoV-2 VOCs and VOIs. EUA, emergency use authorization; mAb, monoclonal antibody; RBD, receptor-binding domain; S2, S2 subunit; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SD1, subdomain 1; SD2, subdomain 2; SP, signal peptide; VOC, variant of concern; VOI, variant of interest.
What is the efficacy of SARS-CoV-2 mAbs against emerging variants?
Several SARS-CoV-2 variants are being reported from different parts of the world. According to the World Health Organization (WHO), a recognized mutation is elevated to a “variant of concern” (VOC) when the acquisition of a new mutation allows for increased viral transmission, increased fatality, and a significant decrease in the effectiveness of therapy and vaccines. A “variant of interest” (VOI) is a variant with a new mutation capable of affecting disease severity, transmissibility, immune and diagnostic escape. The current VOCs are Alpha (B.1.1.7, identified in the United Kingdom) [26], Beta (B.1.351, identified in South Africa) [27], Gamma (P.1, identified in Brazil) [28], and Delta (B.1.617.2, identified in India) [29]. The VOIs are Eta (B.1.525, identified in UK/Nigeria), Iota (B.1.526, identified in the United States of America) [30], Kappa (B.1.617.1, identified in India) [29], and Lambda (C.37, identified in Peru) [31] (Fig 2B). Recently, Epsilon (B.1.427/429, identified in the USA) [32], Zeta (P.2, identified in Brazil), and Theta (P.3, identified in the Philippines) [33] variants have been excluded from the category of VOIs by WHO due to their declining prevalence. Ideally, an effective antiviral therapeutic strategy should have the ability to prevent infection/disease by new variants while simultaneously maintaining breadth against existing multiple viral strains/variants. Recent studies have reported that many NTD-specific NAbs are relatively less effective to all emerging variants, whereas RBD-specific NAbs are variably effective against emerging variants and VOCs [2,34,35]. The majority of the potent therapeutic NAbs as monotherapy showed complete abrogation or reduced neutralizing activity against SARS-CoV-2 emerging variants that contain the E484K/Q or L452R mutations [34–37]. Bamlanivimab (LY-CoV555) was ineffective against all VOCs and thus was no longer considered for EUA. Currently, combination therapies comprising a cocktail of NAbs targeting distinct nonoverlapping epitopes on RBD have demonstrated exceptional potency and promising correlates of protection against SARS-CoV-2 and its variants (Fig 2B) [36,38]. Additionally, newly identified RBD core-binding NAbs SARS2-38 [39] and LY-CoV1404 [40] as monotherapy potently neutralize all SARS-CoV-2 VOCs. Therefore, several options of NAbs targeting conserved RBD epitopes are emerging as promising and attractive therapeutic candidates to tackle the disease burden caused by SARS-CoV-2 or its variants.
What is the role of antibody Fc portion in therapeutic antibodies against SARS-CoV-2?
Although anti-viral functions of NAbs against rapidly emerging variants are being studied extensively, there is also a need to focus on understanding the role of the Fc portion of NAbs in providing protection against SARS-CoV-2 and emerging variants. The role of the Fc region is secondary when NAbs are administered as prophylaxis, but is critical for optimal therapeutic protection. A recent study demonstrated that therapeutic NAbs (REGN, Abbvie, AstraZeneca, and Vir Biotechnology) with intact Fc region reduced viral load and lung disease in animal models in comparison to NAbs without Fc effector functions (LALA-PG mutation) [41]. It is well appreciated that for optimal in vivo protection, NAbs with intact Fc region can mediate downstream effector functions via interaction with Fc receptors resulting in antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis. Moreover, Fc-mediated complement activation can exert a broad range of immunomodulatory functions, with activation of C1q resulting in antibody-mediated complement activation, and complement-dependent cytotoxicity. However, a recent study showed the diminished role of the Fc region in protecting against lethal SARS-CoV-2 infections in K18-hACE2 transgenic mice [42]. This study showed that potent NAbs do not rely on Fc effector functions to provide optimal protection when administered as therapy. Moreover, the role of Fc-mediated antibody-dependent enhancement (ADE) observed in vitro is yet to be fully elucidated in vivo [43,44].
Benefits of multispecific antibodies
Recently, a few bispecific NAbs have been developed by combining the antibody chains of 2 independent nonoverlapping antibodies [45,46]. These bispecific NAbs neutralize wild-type SARS-CoV-2, its VOCs, and escape mutants and have shown to be protective in mice models. This suggests that bispecific NAbs are promising next-generation cost-effective therapeutics against SARS-CoV-2 and its VOCs. Such variant-resistant next-generation or combination of broadly reactive ultrapotent NAbs-based safe therapeutics are desperately needed globally. These mAb-based therapeutics should be globally accessible and affordable in low-middle income countries where more than of 85% human populations reside. Therefore, the development of a panel of well-characterized, clinically developable ultrapotent NAbs could be established rapidly to combat current and rapidly emerging SARS-CoV-2 variants.
Conclusions
Here we have summarized the current status of mAb-based therapy for COVID-19 and have shed light on the ongoing development of mAbs-based therapeutics against emerging SARS-CoV-2 variants. Due to the potential of newly emerging SARS-CoV-2 variants in the future, vaccines will need to be constantly reassessed for their efficacy. The mAb biotherapeutics are a promising strategy for immediate treatment/prophylaxis or in situations where vaccines are less effective—such as in immunocompromised individuals, young, elderly, and vaccine-hesitant individuals. MAbs can also be rapidly tailored, selected, or mined towards new variants. For this, we need more intensive studies to track viral evolution, analyze the human antibody repertoire, identify and develop pan-coronavirus NAbs that target evolutionarily conserved epitopes. These efforts will enable rapid and dynamic reconfiguration of existing NAb cocktails to cull new surges that are driven by SARS-CoV-2 variants.
Funding Statement
We are thankful to the Indian Council of Medical Research (ICMR), India (https://www.icmr.gov.in/) for funding provided to VIR/COVID-19/02/2020/ECD-1 to AC and AS. SK is supported by the DBT/Wellcome Trust India Alliance Early Career Fellowship grant IA/E/18/1/504307 (https://www.indiaalliance.org). The funders had no role in the decision to publish or preparation of the manuscript.
References
- 1.Raybould MIJ, Kovaltsuk A, Marks C, Deane CM. CoV-AbDab: the coronavirus antibody database. Bioinformatics. 2021May5;37(5):734–5. doi: 10.1093/bioinformatics/btaa739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzou PL, Tao K, Nouhin J, Rhee S-Y, Hu BD, Pai S, et al. Coronavirus Antiviral Research Database (CoV-RDB): An Online Database Designed to Facilitate Comparisons between Candidate Anti-Coronavirus Compounds. Viruses. 2020Sep;12(9):1006. doi: 10.3390/v12091006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes CO, Jette CA, Abernathy ME, Dam K-MA, Esswein SR, Gristick HB, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020Dec;588(7839):682–7. doi: 10.1038/s41586-020-2852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelstein MT, Mermelstein AG, Parker Miller E, Seth PC, Stancofski E-SD, Fera D. Structural Analysis of Neutralizing Epitopes of the SARS-CoV-2 Spike to Guide Therapy and Vaccine Design Strategies. Viruses. 2021Jan19;13(1):134. doi: 10.3390/v13010134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021Jun10;184(12):3086–108. doi: 10.1016/j.cell.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020Apr16;181(2):281–92.e6. doi: 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto D, Park Y-J, Beltramello M, Walls AC, Tortorici MA, Bianchi S, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020Jul;583(7815):290–5. doi: 10.1038/s41586-020-2349-y [DOI] [PubMed] [Google Scholar]
- 8.Song G, He W, Callaghan S, Anzanello F, Huang D, Ricketts J, et al. Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nat Commun. 2021May19;12(1):2938. doi: 10.1038/s41467-021-23074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, van Haperen R, Gutiérrez-Álvarez J, Li W, Okba NMA, Albulescu I, et al. A conserved immunogenic and vulnerable site on the coronavirus spike protein delineated by cross-reactive monoclonal antibodies. Nat Commun. 2021Mar17;12(1):1715. doi: 10.1038/s41467-021-21968-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauer MM, Tortorici MA, Park Y-J, Walls AC, Homad L, Acton OJ, et al. Structural basis for broad coronavirus neutralization. Nat Struct Mol Biol. 2021May;12:1–9. [DOI] [PubMed] [Google Scholar]
- 11.Pinto D, Sauer MM, Czudnochowski N, Low JS, Tortorici MA, Housley MP, et al. A human antibody that broadly neutralizes betacoronaviruses protects against SARS-CoV-2 by blocking the fusion machinery. bioRxiv. 2021May10. doi: 10.1101/2021.05.09.442808 [DOI] [Google Scholar]
- 12.Kelley B. Developing therapeutic monoclonal antibodies at pandemic pace. Nat Biotechnol. 2020May;38(5):540–5. doi: 10.1038/s41587-020-0512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An EUA for Bamlanivimab-A Monoclonal Antibody for COVID-19. JAMA. 2021Mar2;325(9):880–1. doi: 10.1001/jama.2020.24415 [DOI] [PubMed] [Google Scholar]
- 14.An EUA for bamlanivimab and etesevimab for COVID-19. Med Lett Drugs Ther. 2021Apr5;63(1621):49–50. [PubMed] [Google Scholar]
- 15.An EUA for casirivimab and imdevimab for COVID-19. Med Lett Drugs Ther. 2020Dec28;62(1614):201–2. [PubMed] [Google Scholar]
- 16.Dong J, Zost SJ, Greaney AJ, Starr TN, Dingens AS, Chen EC, et al. Genetic and structural basis for recognition of SARS-CoV-2 spike protein by a two-antibody cocktail. bioRxiv. 2021Mar1. doi: 10.1101/2021.01.27.428529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid MA, Agostini ML, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2021May13. doi: 10.1101/2021.03.09.434607 [DOI] [Google Scholar]
- 18.Kim C, Ryu D-K, Lee J, Kim Y-I, Seo J-M, Kim Y-G, et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021Jan12;12(1):288. doi: 10.1038/s41467-020-20602-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alsoussi WB, Turner JS, Case JB, Zhao H, Schmitz AJ, Zhou JQ, et al. A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection. J Immunol. 2020Aug15;205(4):915–22. doi: 10.4049/jimmunol.2000583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus ADME, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020May4;11(1):2251. doi: 10.1038/s41467-020-16256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med [Internet]. 2020. Oct 28 [cited 2021 Jun 7]. Available from: https://www.nejm.org/doi/10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2021Feb16;325(7):632–44. doi: 10.1001/jama.2021.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med [Internet]. 2020. Dec 17 [cited 2021 Jun 7]. Available from: https://www.nejm.org/doi/10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A, Gonzalez-Rojas Y, Juarez E, Casal MC, Moya J, Falci DR, et al. Early Covid-19 Treatment With SARS-CoV-2 Neutralizing Antibody Sotrovimab. medRxiv. 2021May28. doi: 10.1101/2021.05.27.21257096 [DOI] [PubMed] [Google Scholar]
- 25.Kelley B, Renshaw T, Kamarck M. Process and operations strategies to enable global access to antibody therapies. Biotechnol Prog. 2021;37(3):e3139. doi: 10.1002/btpr.3139 [DOI] [PubMed] [Google Scholar]
- 26.Frampton D, Rampling T, Cross A, Bailey H, Heaney J, Byott M, et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis [Internet]. 2021. Apr 12 [cited 2021 Aug 3]. Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00170-5/abstract doi: 10.1016/S1473-3099(21)00170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020Dec22. doi: 10.1101/2020.12.21.20248640 [DOI] [Google Scholar]
- 28.Voloch CM, da Silva FR, de Almeida LGP, Cardoso CC, Brustolini OJ, Gerber AL, et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J Virol. 2021Mar1. doi: 10.1128/JVI.00119-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh J, Rahman SA, Ehtesham NZ, Hira S, Hasnain SE. SARS-CoV-2 variants of concern are emerging in India. Nat Med. 2021Jul;27(7):1131–3. doi: 10.1038/s41591-021-01397-4 [DOI] [PubMed] [Google Scholar]
- 30.West AP, Wertheim JO, Wang JC, Vasylyeva TI, Havens JL, Chowdhury MA, et al. Detection and characterization of the SARS-CoV-2 lineage B.1.526 in New York. bioRxiv. 2021Apr22. doi: 10.1101/2021.02.14.431043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wink PL, Volpato FCZ, Monteiro FL, Willig JB, Zavascki AP, Barth AL, et al. First identification of SARS-CoV-2 Lambda (C.37) variant in Southern Brazil. medRxiv. 2021Jun23. doi: 10.1101/2021.06.21.21259241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCallum M, Bassi J, Marco AD, Chen A, Walls AC, Iulio JD, et al. SARS-CoV-2 immune evasion by variant B.1.427/B.1.429. bioRxiv. 2021Apr1.doi: 10.1101/2021.03.31.437925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutat,ions, and immune escape. Nat Rev Microbiol. 2021Jul;19(7):409–24. doi: 10.1038/s41579-021-00573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021Apr;27(4):717–26. doi: 10.1038/s41591-021-01294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021May;593(7857):130–5. doi: 10.1038/s41586-021-03398-2 [DOI] [PubMed] [Google Scholar]
- 36.Diamond M, Chen R, Winkler E, Case J, Aziati I, Bricker T, et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Res Sq. 2021Apr23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of infectious SARS-CoV-2 variant B.1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals. bioRxiv. 2021May27. doi: 10.1101/2021.05.26.445838 [DOI] [Google Scholar]
- 38.Liu H, Yuan M, Huang D, Bangaru S, Zhao F, Lee C-CD, et al. A combination of cross-neutralizing antibodies synergizes to prevent SARS-CoV-2 and SARS-CoV pseudovirus infection. Cell Host Microbe. 2021May12;29(5):806–18. e6. doi: 10.1016/j.chom.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanBlargan LA, Adams LJ, Liu Z, Chen RE, Gilchuk P, Raju S, et al. A potently neutralizing anti-SARS-CoV-2 antibody inhibits variants of concern by binding a highly conserved epitope. bioRxiv. 2021Apr26. doi: 10.1101/2021.04.26.441501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westendorf K, Žentelis S, Foster D, Vaillancourt P, Wiggin M, Lovett E, et al. LY-CoV1404 potently neutralizes SARS-CoV-2 variants. bioRxiv. 2021May4. doi: 10.1101/2021.04.30.442182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler ES, Gilchuk P, Yu J, Bailey AL, Chen RE, Chong Z, et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell. 2021Apr1;184(7):1804–20. e16. doi: 10.1016/j.cell.2021.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noy-Porat T, Edri A, Alcalay R, Makdasi E, Gur D, Aftalion M, et al. Fc-independent neutralization of SARS-CoV-2 by recombinant human monoclonal antibodies. bioRxiv. 2021May15. doi: 10.1101/2021.05.15.443978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Edwards RJ, Manne K, Martinez DR, Schäfer A, Alam SM, et al. The functions of SARS-CoV-2 neutralizing and infection-enhancing antibodies in vitro and in mice and nonhuman primates. bioRxiv. 2021Feb18. doi: 10.1101/2020.12.31.424729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Liu Z, Li S, Xu W, Zhang Q, Silva IT, et al. Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD. Cell Rep. 2021Feb2;34(5):108699. doi: 10.1016/j.celrep.2021.108699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Gasparo R, Pedotti M, Simonelli L, Nickl P, Muecksch F, Cassaniti I, et al. Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice. Nature. 2021May;593(7859):424–8. doi: 10.1038/s41586-021-03461-y [DOI] [PubMed] [Google Scholar]
- 46.Cho H, Gonzales-Wartz KK, Huang D, Yuan M, Peterson M, Liang J, et al. Ultrapotent bispecific antibodies neutralize emerging SARS-CoV-2 variants. bioRxiv. 2021Apr1. doi: 10.1101/2021.04.01.437942 [DOI] [PMC free article] [PubMed] [Google Scholar]