Abstract
Background
To improve early detection of emerging infectious diseases in sub-Saharan Africa (SSA), many of them zoonotic, numerous electronic animal disease-reporting systems have been piloted but not implemented because of cost, lack of user friendliness, and data insecurity. In Kenya, we developed and rolled out an open-source mobile phone-based domestic and wild animal disease reporting system and collected data over two years to investigate its robustness and ability to track disease trends.
Methods
The Kenya Animal Biosurveillance System (KABS) application was built on the Java® platform, freely downloadable for android compatible mobile phones, and supported by web-based account management, form editing and data monitoring. The application was integrated into the surveillance systems of Kenya’s domestic and wild animal sectors by adopting their existing data collection tools, and targeting disease syndromes prioritized by national, regional and international animal and human health agencies. Smartphone-owning government and private domestic and wild animal health officers were recruited and trained on the application, and reports received and analyzed by Kenya Directorate of Veterinary Services. The KABS application performed automatic basic analyses (frequencies, spatial distribution), which were immediately relayed to reporting officers as feedback.
Results
Of 697 trained domestic animal officers, 662 (95%) downloaded the application, and >72% of them started reporting using the application within three months. Introduction of the application resulted in 2- to 14-fold increase in number of disease reports when compared to the previous year (relative risk = 14, CI 13.8–14.2, p<0.001), and reports were more widely distributed. Among domestic animals, food animals (cattle, sheep, goats, camels, and chicken) accounted for >90% of the reports, with respiratory, gastrointestinal and skin diseases constituting >85% of the reports. Herbivore wildlife (zebra, buffalo, elephant, giraffe, antelopes) accounted for >60% of the wildlife disease reports, followed by carnivores (lions, cheetah, hyenas, jackals, and wild dogs). Deaths, traumatic injuries, and skin diseases were most reported in wildlife.
Conclusions
This open-source system was user friendly and secure, ideal for rolling out in other countries in SSA to improve disease reporting and enhance preparedness for epidemics of zoonotic diseases.
Introduction
An effective animal disease surveillance system is important for detecting and monitoring of endemic and emerging diseases, including zoonotic diseases that can rapidly spread to humans [1–5]. Among livestock farmers of sub-Saharan Africa (SSA), most of them inhabiting rural regions with high human and livestock interactions, early detection of livestock diseases can also reduce the socio-economic impact associated with loss of productivity or deaths of the animals [6]. Some of these rural areas are also inhabited by diverse species of wildlife, creating a conducive environment for emergence and transmission of pathogens across the wildlife-livestock-human interface [2,7,8].
According to the Food and Agriculture Organization, effective surveillance for animal diseases in SSA is limited by absence of surveillance systems in the wildlife sector, inadequate trained human capacity in the domestic animal sector, and lack of user-friendly electronic data capture tools [3]. Whereas numerous electronic animal disease-reporting tools have been piloted in SSA, none has been rolled out because of cost, lack of user friendliness and analytical capabilities, and data insecurity [3,9]. Most of the platforms were also proprietary, increasing concerns around data security and access by countries [3]. Mobile phones offer a unique opportunity to develop data collection tools that can be easily integrated into existing surveillance systems with minimal resources [10–13]. Approximately 75% of the world’s population has access to mobile phones, including communities living in rural areas of developing countries [14,15]. In Kenya, 83.9% of the population has access to smartphone and the country leads the African continent in smartphone usage [16].The flexibility, portability, connectivity and geo-location features of smart phones makes them an effective tool for electronic collection and submission of animal health data in real-time. Such capabilities may enhance the early detection of an emerging disease and strengthen preparedness, response, and mitigation activities [13].
During the 2015–2016 El Niño rains when Kenya faced the threat of a Rift Valley fever (RVF) outbreak, the Directorate of Veterinary Service created an enhanced phone-based syndromic surveillance system targeting RVF hotspots in the country to provide early warning for the disease [17]. The system collected weekly data on RVF-like syndromes in cattle, sheep, goats, and camels from farmers located in high-risk counties during the four-month flooding season [17]. We built on this initiative by developing a national mobile phone-based data collection system for Kenya, referred to as Kenya Animal Biosurveillance System (KABS), utilizing smart phone mobile technologies for animal health data collection initially developed in the United States [18]. Here, we describe how the KABS reporting application was customized and rolled out to report disease syndromes among domestic and wild animal populations in Kenya. We also present data collected during the 2017–2019 period.
Methods
KABS platform design
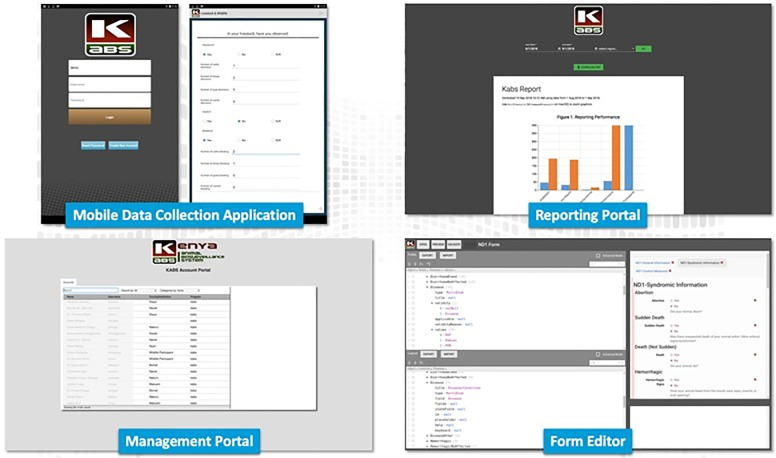
The Kenya Animal Biosurveillance system (KABS) application encompasses a mobile application for data collection, a web-based application for account management and data collection form editing, and a web-based dashboard for data viewing, monitoring, and download. We built the KABS mobile application on the Java® platform for use on Android compatible mobile phones and made it available in the Google Play Store (Sun Microsystems, Santa Clara, CA). The application has four components; data collection that includes tools currently used by the Kenya livestock sector, account management, form editor, and reporting portals (Fig 1).
Fig 1. Illustration of the four components of the KABS mobile phone application platform used to report domestic and wild animal disease in Kenya.
Customization of the application for Kenya
To integrate KABS within the disease surveillance system at the Kenya Directorate of Veterinary Services (KDVS), we added the disease surveillance forms currently used by KDVS (the ND-1 and zero report forms) in the application. Since the Kenya Wildlife Service (KWS) did not have a disease surveillance system, we jointly developed a wildlife disease-reporting tool. Working with veterinarians from the KDVS and KWS, two separate lists of domestic animals (Table 1) and wildlife (Table 2) disease syndromes for reporting were developed. Selection of reporting syndromes was guided by the World Organization for Animal Health’s (OIE) list of notifiable diseases, important trans-boundary animal diseases in sub-Saharan Africa, and endemic and emerging zoonotic diseases in the region [19].
Table 1. Syndromes in domestic animals automatically reported through KABS mobile phone application in Kenya, 2017–2019.
| Syndrome | Definition | Associated diseases |
|---|---|---|
| Abortion | Termination of pregnancy after organogenesis is complete but before the expelled fetus can survive | RVF, brucellosis, BVD, BT, campylobacteriosis, Q fever, leptospirosis, toxoplasmosis |
| Sudden death | Unexplained death in healthy animals within 12–24 hours without prior illness or symptoms | Anthrax, heart water, ECF, black quarter, RVF |
| Hemorrhagic | Non-traumatic bleeding that can occur internally and blood leaks through natural orifices | RVF, ASF, hemorrhagic septicemia, leptospirosis |
| Neurologic | Behavioral changes, seizures, tremors, ataxia, aggression, excessive salivation, vocalization, and paralysis of one or more limbs | Rabies, BSE, heartwater, anaplasmosis, babesiosis, tetanus, Scrapie, NCD |
| Respiratory | Difficult breathing, coughing, wheezing, sneezing and eye and nasal discharges. | CCPP, CBPP, ASF, trypanosomiasis, IB, PPR, ECF, CRD |
| Gastrointestinal | Diarrhoea, vomiting or abdominal pain | ECF, PPR, NCD, ASF, blackquarter, Johne’s disease, rinderpest, IBD |
| Cutaneous | Scaly skin, hair loss, changes in pigmentation or visible growths | LSD, sheep pox, goat pox, and camel pox, bovine papillomatosis, fowl pox |
| Animal bites | Bite that results in bruising or deep wound | Rabies, snake bites |
| Oral and foot lesions | Vesicles, ulcerations and erosion of oral and nasal mucosa, tongue, coronary band or teats | FMD,VS, PPR, bluetongue, orf |
ASF = African swine fever, BSE = bovine spongiform encephalopathy, BT = Bluetongue, BVD = bovine viral diarrhea, CBPP–contagious bovine pleuropneumonia, CCPP = contagious caprine pleuropneumonia, CRD = chronic respiratory disease, ECF = East coast fever, FMD = foot and mouth disease, IB = infectious bronchitis, IBD = infectious bursal disease, LSD = Lumpy skin diseases, PPR = Peste des petits ruminants, NCD = Newcastle disease, RVF = Rift valley fever, VS = vesicular stomatitis.
Table 2. Syndromes in wildlife automatically reported using KABS mobile phone application in Kenya, 2017–2019.
| Syndrome | Description | Associated diseases |
|---|---|---|
| Death | Animals found dead with or without any prior sign of disease | Anthrax, AI, NCD |
| Cutaneous/skin lesions | Animal with multiple lumps, hair loss, pox lesions on the body | Mange, pox lesions |
| Growths | Any abnormal protrusions (swellings) on the skin surface | Warts, tumors, abscesses, pox |
| Respiratory | Animal having difficulty breathing, has a cough, sneezing, wheezing or eye and nasal discharge | Pneumonia |
| Severe emaciation | Abnormally thin and weak | |
| Diarrhoea | Passing of loose stool, more frequently than normal resulting in matting in perianal area | |
| Lameness, hyper salivation | Visible limping, excessive drooling of saliva | FMD, VS, rabies |
| Aggression | Unusual signs of aggression, attacking humans and biting objects | Rabies |
| Blindness | Vision loss exhibited by loss of orientation and direction | Rinderpest |
AI = avian influenza, FMD = foot and mouth disease, NCD = Newcastle disease, VS = vesicular stomatitis.
The ND-1 report form was used to report the presence of nine domestic animal disease syndromes from domestic animals (Table 1), whereas the zero report form reported the absence of important transboundary diseases including RVF, Rinderpest, Peste des petit ruminants (PPR), contagious caprine pleuropneumonia (CCPP), contagious bovine pleuropneumonia (CBPP), and avian influenza. The wildlife disease reporting form reported nine wildlife disease syndromes (Table 2). Given the diversity of wildlife species in East Africa (>2000 different animal species), we categorized them into five broad groups for reporting purposes; herbivores, carnivores, avian, aquatic mammals, and non-human primates. Under each group, important animal species were identified and included in a drop-down menu.
Data security
The KDVS received, stored, and managed all data, with exclusive rights to grant access. Accessing the reporting portal required approval, thus limiting end-user access to data from one or more counties (i.e., for local/regional monitoring) while allowing national leaders access to national data. The Veterinary Epidemiology and Economic Unit at KDVS approved all requests for new accounts, which came with personal identifiers of the end-user for verification of reports. Only the senior surveillance officers of the KDVS and the Kenya Wildlife Services, and county directors of veterinary services could view all data, while sub-county officers and end-users viewed data from their localities.
Disease data collection, transmission, and feedback to end-user
Using the KABS data collection interface, a surveillance officer could select and complete the appropriate disease report tool during any farm (livestock) or wildlife conservation (wildlife) visit anywhere in the country. A definition of each disease syndrome was available in the surveillance tool to ensure homogeneity of data collected (Tables 1 & 2). Among data collected were geographical coordinates, animal species, number at risk and affected, and provisional diagnosis. A surveillance officer was able to enter data in KABS when in areas with no internet connectivity, allowing these data to be transmitted to the server once internet connectivity was regained.
Submitted data were received and stored at the KDVS central server at Kabete, Nairobi, and backed up on the Kenya Ministry of Agriculture, Livestock, and Fisheries central database in Nairobi. The KAB dashboard had an automated analytical tool designed to aggregate data and create spatial maps of the syndromic data, displayed as frequency histograms, tables, and maps, and automatically distributed to end-users for action, and to senior regional and national animal health officers (Fig 1). Due to initial bandwidth limitation, roll out of the feedback module started in January 2020. The reporting portal also provided capabilities for user-driven filtration of data to a certain date or region of the country, including downloading of the raw data for further analysis.
User training
To be recruited and trained, animal surveillances officers from both government and private sectors needed to have a personal android-based smart phone. The five-day training included hands-on sessions to download the application, create user accounts, and input data using simulated scenarios. The KABS application was initially piloted in three counties, and feedback used to make improvements before rollout to the rest of the country. Immediately after each training session, a WhatsApp group of the users was maintained for 3 months to promote timely troubleshooting and experience sharing by system users (WhatsApp Ince, Mountain View California, USA). Between April 2017 the end of December 2020, we had trained domestic animal surveillance officers from 35 of the 47 counties in Kenya, and wildlife officers from all wildlife conservations in the country.
Data analysis
We analyzed the uptake of the KABS application among the trained surveillance officers to determine its acceptance. To determine improvement in reporting domestic animal diseases, we compared reporting one year before (data collected in fiscal year 2016/ 2017) and one year after (data collected in fiscal year 2018/ 2019) the introduction of KABS in six counties. These data were exported into the R statistical package and compared using interrupted time series ITS) approach [20]. For ITS, a time series of reporting of livestock and wildlife syndromes was used to establish an underlying trend, which was ‘interrupted’ by the roll-out of KABS against a counterfactual scenario in the absence of the intervention. In addition, descriptive analysis of domestic and wild animal disease data collected through the application was performed. No systematic wildlife disease data were collected prior to introduction of KABS to enable comparison of reporting rates.
Ethical approval
The development and rolling out of KABS application were led by the KDVS and approved by the Kenya Ministry of Agriculture, Livestock and Fisheries as non-research.
Results
Uptake of KABS application
By December 2020, 697 domestic animal health officers from 35 out of 47 counties had been trained on KABS, including 502 (72.0%) government and 195 (28.0%) private animal health professionals. Of those trained, 95% (N = 662) downloaded and installed the application into their smartphones. Among those who installed, 72.1% (477/662) submitted a report through the application within 3 months, including 75.8% (357/471) government and 62.8% (120/191) private animal health professionals. We trained 47 wildlife officers including veterinarians and research scientists; 42 (89.4%) of them from the government and five (10.6%) from private sector. All trained wildlife officers installed the application into their smartphones; and 57.4% (27/47) submitted a report through the application within 3 months.
Spatial and temporal trends on disease reporting
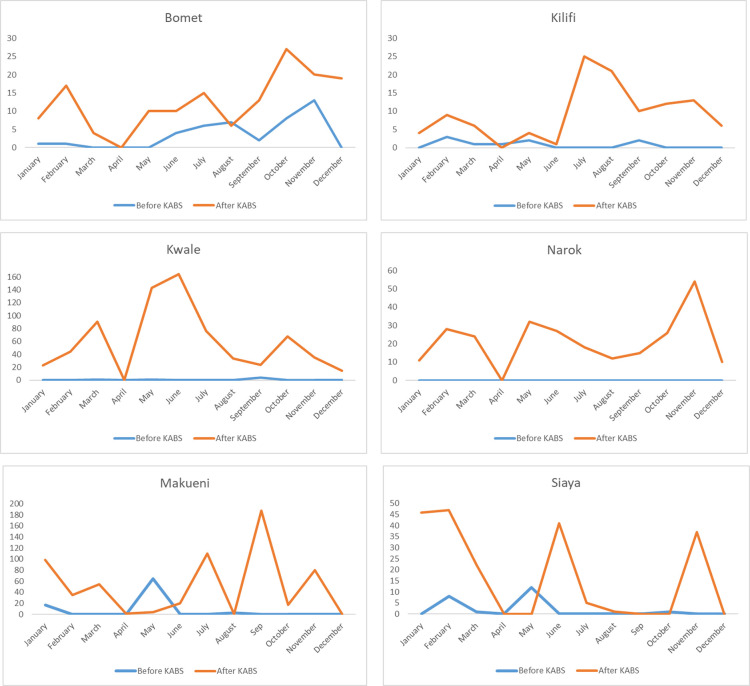
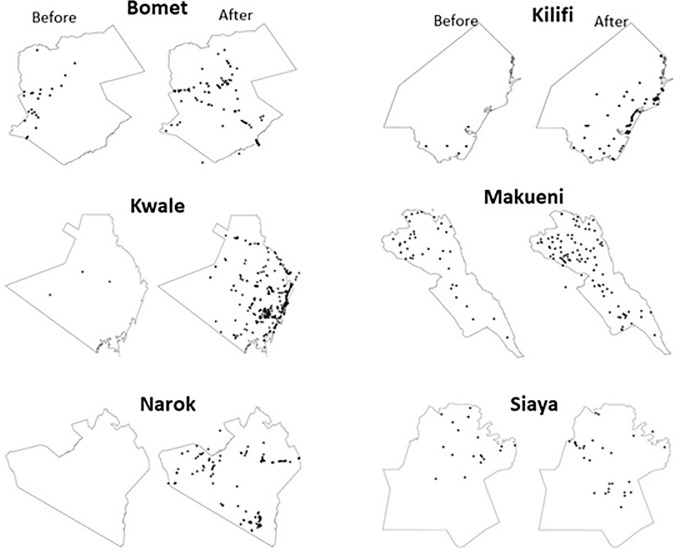
To assess the impact of KABS rollout on disease reporting in domestic animals, we compared number and spatial distribution of reports received from six counties where KABS reporting had occurred for at least 12 months, including Bomet, Kilifi, Kwale, Nandi, Makueni, and Siaya. As shown in Fig 2 there was 2- to 14-fold increase in number of reports upon introduction of KABS across the six counties when compared to the previous year (relative risk = 14, confidence interval 13.8–14.2; p<0.001)). Similarly, reports were more spatially distributed indicating that areas under effective surveillance had been broadened (Fig 3).
Fig 2. Frequency of disease reporting before (blue line) and after (red line) KABS mobile phone application introduction in Bomet, Kilifi, Kwale, Makueni, Narok, and Siaya counties of Kenya.
Fig 3. Spatial distribution of disease reporting before and after KABS mobile phone application introduction in Bomet, Kilifi, Kwale, Makueni, Narok, and Siaya Counties of Kenya.
Summary of reported domestic and wild animal disease syndromes
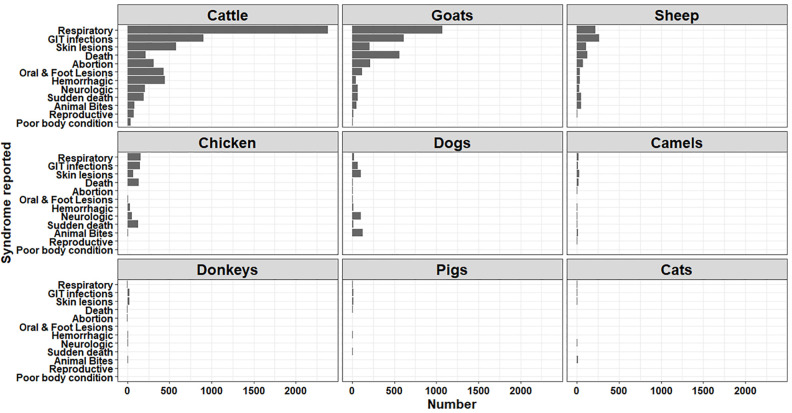
As of December 2019, 11,399 domestic and 205 wild animal disease reports were received through KABS. All reports were submitted and received within 24 hours of surveillance officer collection. Kenya has 146.6 million food animals, including 57.9 million poultry, 35.1 million goats, 27.4 million sheep, 20,9 million cattle, 4.7 million camels, and 0.6 million pigs. These food animals accounted for >90% of the reports, including cattle (51.4%), goats (25.6%), sheep (8.7%), and chicken (6.5%) (Table 3). Camel and pig reports were low and comparable to those observed from non-food animals (dogs, cats, and donkeys). In cattle, sheep, goats, and chicken, respiratory syndromes reports accounted for >50% of the reports, followed by gastrointestinal and skin conditions, and death (Fig 4).
Table 3. Number of disease reports from domestic and wild animals using KABS mobile phone application in Kenya, 2017–2019.
| Domestic animals | Wildlife | ||||
|---|---|---|---|---|---|
| Species | No. of reports | % | Species/Group | No. of reports | % |
| Cattle | 5861 | 51.4 | Zebras | 30 | 14.6 |
| Goats | 3044 | 26.7 | Buffaloes | 27 | 13.2 |
| Sheep | 995 | 8.7 | Elephants | 26 | 12.7 |
| Chicken | 740 | 6.5 | Giraffes | 24 | 11.7 |
| Dogs | 466 | 4.1 | Lions | 22 | 10.7 |
| Camels | 114 | 1.0 | Antelopes (gazelles, eland, dik-dik) | 21 | 10.2 |
| Donkeys | 83 | 0.7 | Monkeys | 19 | 9.3 |
| Pigs | 68 | 0.5 | Birds | 12 | 5.9 |
| Cats | 28 | 0.2 | Cheetahs | 9 | 4.4 |
| Total | 11399 | Hyenas, jackals, wild dogs | 9 | 4.4 | |
| Crocodiles & fish | 3 | 1.5 | |||
| Warthogs | 2 | 1.0 | |||
| Rhinos | 1 | 0.5 | |||
| Total | 205 | ||||
Fig 4. Trends in disease syndromes reported in domestic animals in Kenya using the KABS mobile phone application, (2017–2019).
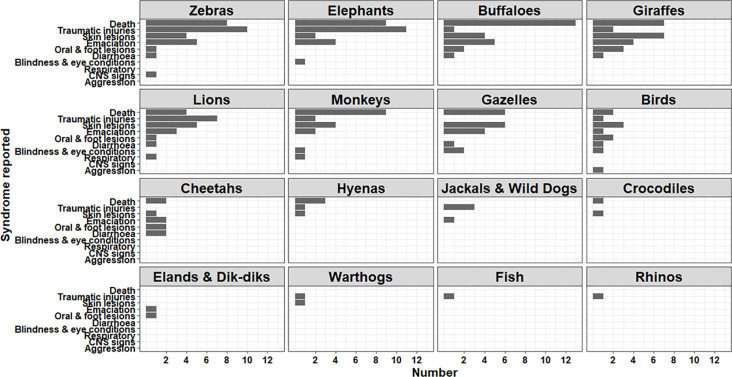
Among wildlife, herbivores including zebra, buffalos, elephants, giraffes, and antelopes accounted for >60% of the reports, followed by carnivores (lions, cheetahs, hyenas, jackals, and wild dogs) with approximately 20% of the reports (Table 3). Deaths, traumatic injuries, and skin conditions were the most reported syndromes in wildlife (Fig 5).
Fig 5. Trends in disease syndromes reported in wildlife in Kenya using the KABS mobile phone application, (2017–2019).
Discussion
KABS is among the first electronic disease surveillance reporting applications to be rolled-out nationwide in SSA, providing near-real-time (within 24 hours) animal disease data collection and transmission that enhanced early detection of disease events. Within three years of introduction (2017–2020), the application had been rolled out in >70% of the country, including the entire wildlife sector. The effectiveness of application was demonstrated in 2018 when KABS detected a RVF outbreak that affected livestock and humans across three counties [21]. Disease surveillance in the domestic animal sectors in Kenya and SSA has been primarily paper-based, whereas the wildlife sectors rarely conducted routine disease surveillance and reporting. Therefore, the roll out of KABS in the wildlife sector represented a major milestone and an opportunity for detecting and responding to diseases before spillover to livestock and humans.
The high installation rate of KABS by domestic animal health officers (>95%) reflected both the acceptance of the application and dominance of android smartphones in the market. Over 70% of the officers submitted a report within three months of training, rates comparable to those observed when a similar system was rolled out in the United States [18]. Similarly in the wildlife sector, there was 57% reporting by veterinarians and research officers. By using their own phone for surveillance, and data capture tools routinely used, the application became easier to accept by all cadres of animal health professionals interested in reporting disease. The rapid submission of data and automated analytic capability allowed generation of reports for immediate feedback to the end users for action. This feedback should be a motivation to keep reporting [18].
For the government of Kenya, KABS addressed several long-standing challenges. First, the application was open-source and free, removing the cost constraints presented by previous applications. The only cost was associated with customizing the application to the Kenya animal disease surveillance system and training. Second, the data were stored and managed by the government of Kenya, providing important assurance to the country on ownership and security. Third, the application was user friendly, in part because the data collection tools were familiar, and syndromes selected for reporting covered important and notifiable transboundary animal diseases that the government was required to report. Reports were submitted immediately, eliminating the lag time experienced with other systems that require an additional step of data compilation, and thus enhancing Kenya’s ability to submit data to the OIE in a timely manner [3,22–24]. Further, the application worked well in remote areas with no internet connectivity, allowing login and data collection while offline and automatic transmission of results to the KDVS once connectivity was restored. The automated collection of geographic coordinates of reported disease events enhanced follow-up by the county and national animal health officers.
The rollout of KABS resulted in up to 10-fold increase in reporting and an exponential increase in spatial distribution of reports. In contrast with the previous animal disease reporting system that relied on government officers, we recruited both government and private animal health professionals as surveillance officers, resulting in an increased rate of reporting and a broader spatial distribution of reports. In fact, we think that the increase was limited by the number of surveillance officers trained in each county. Our team is developing a training-of-trainers program that provides training materials to the current surveillance officers in each county to encourage them to train others for broader usage. The cumulative monthly reporting across the country varied, increasing during the April to June and September to November rainy seasons, and decreasing during the December to March and July to August dry seasons (Fig 2). This trend is similar to those reported over the years by the government of Kenya; however, the overall magnitude of reports has increased [23]. The low reporting in certain areas (e.g. western Kilifi) was low because the region is not inhabited by humans and livestock but instead occupied by a national park.
As shown in Fig 4 the number of reports were higher for food animals, particularly cattle, goats, sheep, and chicken than for non-food animals (dogs, cats, donkeys). Similar trends have been reported in other studies, associated with farmers attaching greater social-economic value to these animals [24]. Among wildlife, the variable number of reports across species is likely due to several factors including the size of animal populations, higher number of surveillance officers in some conservation areas, and the ease of sighting and observing some species. Thus, it was easier to sight, observe and report disease conditions in larger terrestrial wild mammals (elephants, buffalos, zebras, giraffes, lions) than in birds and aquatic animals. Respiratory, gastrointestinal, and cutaneous syndromes were the most reported among all domestic animals, whereas death, cutaneous lesions, traumatic injuries and emaciations were the most common conditions among wildlife.
The project had some limitations. The required ownership of android smartphone for reporting officers eliminated those lacking this application from training and reporting. Adding a testing and diagnosis module to the application will improve its utility in both domestic and wild animal sectors. Broader use of the application in SSA can be enhanced by linking KABS reports to the human health system through the emergency operation centers, thus providing early warning of potential outbreaks of zoonotic infectious diseases from both domestic and wild animals. To realize this step, standardization of syndromic and disease classification across countries and between the human and animal sectors would need to be achieved [25]. The low human capital in the wildlife sector was a setback for increasing the number of disease reports from that sector.
Acknowledgments
We thank the Kenya government domestic and wild animal health officers for supporting the development and rolling out of application. The willingness of the senior leadership at the Kenya Directorate of Veterinary Services to adopt the KABS application as the primary disease-reporting tool was critical.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States’ Centers for Disease Control and Prevention, United States’ Department of Agriculture or the Government of Kenya.
Data Availability
The data underlying the disease syndromes presented are available from the Kenya Directorate of Veterinary Services. Contact Kenya Director of Veterinary Services, email: veeu.dvs@gmail.com. The authors were granted access to the data by the Kenya Directorate of Veterinary Services. Other would need to be granted access by the same agency. The authors had no special access privileges to the data others would not have.
Funding Statement
The project was funded by US Center for Disease Control and Prevention (CDC; Grant # GH001717) under the Global Health Security Agenda, and Food and Agriculture Organization (FAO)-Kenya Office to MKN. The CDC and FAO scientists listed as co-authors were involved in the design and rolling out of KABS application, including data interpretation, and manuscript writing. However, the funder had no role in the data collection and analysis for this manuscript.
References
- 1.Greger M. The human/animal interface: emergence and resurgence of zoonotic infectious diseases. Crit Rev Microbiol. 2007;33: 243–299. doi: 10.1080/10408410701647594 [DOI] [PubMed] [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451: 990–993. doi: 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Agriculture Organization. Challenges of animal health information systems and surveillance for animal diseases and zoonoses. 2011. Available: http://www.fao.org/3/i2415e/i2415e00.pdf.
- 4.Gates MC, Holmstrom LK, Biggers KE, Beckham TR. Integrating novel data streams to support biosurveillance in commercial livestock production systems in developed countries: challenges and opportunities. Front Public Hlth. 2015;3: 74. doi: 10.3389/fpubh.2015.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christaki E. New technologies in predicting, preventing and controlling emerging infectious diseases. Virulence. 2015/06/11. 2015;6: 558–565. doi: 10.1080/21505594.2015.1040975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McElwain TF&, Thumbi SM. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev Sci Tech Off Int Epiz. 2017;36: 423–433. doi: 10.20506/rst.36.2.2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen T, Murray KA, Zambrana-Torrelio C, Morse SS, Rondinini C, Di Marco M, et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun. 2017;8: 1124. doi: 10.1038/s41467-017-00923-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995. doi: 10.3201/eid0101.950102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenya Directorate of Veterinary Services. Annual Livestock Disease Reports. Kabete, Nairobi; 2019.
- 10.Asiimwe C, Gelvin D, Lee E, Ben Amor Y, Quinto E, Katureebe C, et al. Use of an innovative, affordable, and open-source short message service-based tool to monitor malaria in remote areas of Uganda. Am J Trop Med Hyg. 2011;85: 26–33. doi: 10.4269/ajtmh.2011.10-0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson C, Sawford K, Daniel SLA, Nelson TA, Stephen C. Mobile phone-based infectious disease surveillance system, Sri Lanka. Emerg Infect Dis. 2010;16: 1524–1531. doi: 10.3201/eid1610.100249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madder M, Walker JG, Van Rooyen J, Knobel D, Vandamme E, Berkvens D, et al. e-Surveillance in Animal Health: use and evaluation of mobile tools. Parasitology. 2012;139: 1831–1842. doi: 10.1017/S0031182012000571 [DOI] [PubMed] [Google Scholar]
- 13.Mtema Z, Changalucha J, Cleaveland S, Elias M, Ferguson HM, Halliday JEB, et al. Mobile Phones As Surveillance Tools: Implementing and Evaluating a Large-Scale Intersectoral Surveillance System for Rabies in Tanzania. PLoS Med. 2016;13: e1002002. doi: 10.1371/journal.pmed.1002002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Bank. Information and Communications for Development 2012: Maximizing Mobile, Washington DC: World Bank. 2012. [Google Scholar]
- 15.Tomlinson M, Rotheram-Borus MJ, Swartz L, Tsai AC. Scaling Up mHealth: Where Is the Evidence? PLoS Med. 2013;10: e1001382. doi: 10.1371/journal.pmed.1001382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenya Leads Africa in Smartphone usage. 11 Mar 2019.
- 17.Oyas H, Holmstrom L, Kemunto NP, Muturi M, Mwatondo A, Osoro E, et al. Enhanced surveillance for Rift Valley Fever in livestock during El Niño rains and threat of RVF outbreak, Kenya, 2015–2016. PLoS Negl Trop Dis. 2018;12: e0006353. doi: 10.1371/journal.pntd.0006353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson CW, Holmstrom L, Biggers K, Wall J, Beckham T, Coats M, et al. Improving Animal Disease Detection Through an Enhanced Passive Surveillance Platform. Heal Secur. 2016;14: 264–71. doi: 10.1089/hs.2016.0016 [DOI] [PubMed] [Google Scholar]
- 19.World Organization for Animal Health (OIE). OIE Listed diseases, infections and infestations in force in 2020. [cited 28 Sep 2020]. Available: https://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2020/.
- 20.Hudson J, Fielding S. Ramsay CR. Methodology and reporting characteristics of studies using interrupted time series design in healthcare. BMC Med Res Methodol 2019; 19, 137. doi: 10.1186/s12874-019-0777-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan A, Muturi M, Mwatondo A, Omolo J, Bett B, Gikundi S, et al. Epidemiological investigation of a Rift Valley fever outbreak in humans and livestock in Kenya, 2018. Am J Trop Med Hyg. 2020;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenya Directorate of Veterinary Services. Annual Livestock Disease Reports. Kabete, Nairobi; 2017.
- 23.Kenya Directorate of Veterinary Services. Annual Livestock Disease Report. Kabete, Nairobi; 2013.
- 24.Thumbi SM, Njenga MK, Otiang E, Otieno L, Munyua P, Eichler S, et al. Mobile phone-based surveillance for animal disease in rural communities: Implications for detection of zoonoses spillover. Philos Trans R Soc B Biol Sci. 2019;374. doi: 10.1098/rstb.2019.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dórea FC, Vial F. Animal health syndromic surveillance: a systematic literature review of the progress in the last 5 years (2011–2016). Vet Med (Auckland, NZ). 2016;7: 157–170. doi: 10.2147/VMRR.S90182 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying the disease syndromes presented are available from the Kenya Directorate of Veterinary Services. Contact Kenya Director of Veterinary Services, email: veeu.dvs@gmail.com. The authors were granted access to the data by the Kenya Directorate of Veterinary Services. Other would need to be granted access by the same agency. The authors had no special access privileges to the data others would not have.