Abstract
Background:
Chronic suppurative otitis media (CSOM) is a major cause of hearing disability and morbidity in poor socioeconomic developing countries with prevalence of 4%. Indiscriminate use of antibiotics and poor follow up of patients has resulted in treatment failure, emergence of resistant strains, super infection, intra-cranial and extra-cranial complications in CSOM patients. Staphylococcus aureus, P. aeruginosa, Proteus spp. and Klebsiella spp. are most common organisms causing infection. Knowledge of microbial profile and susceptibility pattern is essential for efficacious treatment of this disorder.
Objective:
To determine the clinico-bacteriological profile of CSOM, to analyze the susceptibility pattern of various antibiotics and to evaluate the in vitro efficacy of aminoglycosides over fluoroquinolones against the aerobic bacterial isolates from CSOM.
Methods:
We studied 153 clinically suspected CSOM cases from March 2018 to October 2018 in Microbiology and Otorhinolaryngology department. The ear swabs were aerobically cultured and identification of the isolate was done by standard bacteriological methods.
Results:
Safe type CSOM was a major cause of disease. Moderate (35.3%) and mild degree (32.7%) of hearing loss was seen in most of the CSOM cases. The culture positivity rate was 82.4% and the most common isolate was P. aeruginosa (55.8%) followed by S. aureus (27.5%). P. aeruginosa, A. baumannii and Enterobacteriaceae spp. showed high sensitivity to colistin, piperacillin-tazobactam, ceftazidime-tazobactam and good sensitivity for cefepime and amikacin; 33.3% S. aureus isolates were Methicillin-resistant which was sensitive to gentamicin, vancomycin and linezolid.
Conclusion:
Knowledge of the spectrum of microorganisms causing ear discharge is important for effective treatment.
Keywords: Antibiotic drug resistance, CSOM, hearing loss, P. aeruginosa, poor hygiene, S. aureus
Introduction
Chronic suppurative otitis media (CSOM) is a common cause of hearing impairment, disability and poor scholastic performance in children in poor and developing countries. It is the major cause of morbidity all over the world.[1] The worldwide prevalence of CSOM is 65-330 million people, and 39-200 million (60%) have clinically significant hearing impairment.[2] The overall incidence is estimated to be around 9 per 100,000 people.[1] CSOM is the persistent inflammation of the middle ear or mastoid cavity and characterized by recurrent or persistent ear discharge (otorrhea) over 2-6 weeks through a perforation of the tympanic membrane.[1,2] Frequent upper respiratory tract infections and poor socioeconomic conditions (overcrowded housing and poor hygiene and nutrition) are often associated with the development of CSOM.[1] The deafness caused by CSOM of safe type was usually considered to be purely of conductive type.[2] In unsafe type of CSOM, the sensorineural deafness is known usually due to labyrinthitis and cholesteatoma. Occasionally, in fatal condition, CSOM can lead to fatal intracranial infections and acute mastoiditis.[2,3]
Due to misuse and overuse of antibiotics, antibiotic drug resistance (ADR) is increasing among the pathogens causing CSOM which makes this mandatory for periodic surveillance of microbiological and sensitivity profile of CSOM.[1] So, this study was planned to determine the clinico-bacteriological profile of CSOM, to analyze the susceptibility pattern of various antibiotics and to evaluate in vitro efficacy of aminoglycoside antibiotic over fluoroquinolones against the aerobic bacterial isolate from CSOM at a tertiary care hospital in western Rajasthan.
Material and Methods
This is a prospective cross-sectional study conducted from March 2018 to October 2018 at a tertiary care hospital. This study was approved by the Institutional Ethical Committee, AIIMS, Jodhpur with letter no. AIIMS/IEC/2017/946. Total 153 patients, who were clinically diagnosed with CSOM, were enrolled for the study after their consent. Patients presenting with tympanic perforation and ear discharge of more than 3 months and those patients who were not on any antibiotics (oral and systemic) in the previous 7 days were included in the study. Patient having ear discharge with intact tympanic membrane and on antibiotic therapy was excluded from the study. Informed consent was obtained at the enrolment of the patient and before collecting the aural discharge without touching external auditory canal. The middle ear discharge was then aseptically collected by the Otorhinolaryngologist from the tympanic cavity with a thin sterile cotton swab (HiMedia, Mumbai, India) after cleaning with normal saline. The specimens so collected were transported immediately to the microbiology laboratory for further processing. The swabs were inoculated onto blood agar, chocolate agar and MacConkey agar for aerobic culture and the inoculated plates were incubated at 37°C for 24–48 hours with 5% carbon dioxide given to blood agar and chocolate agar plates. Antimicrobial susceptibility testing for aerobic bacterial isolates was done by Kirby-Bauer disc diffusion method[4] on Mueller Hinton agar (HiMedia, India) as per Clinical Laboratory Standards Institute (CLSI) guidelines 2018.[5] The following antibiotics with specific concentrations were used: Trimethoprim-sulfamethoxazole/cotrimoxazole (25 μg), gentamicin (10 μg), amikacin (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), cefoxitin (30 μg), ceftazidime (30 μg), piperacillin (10 Units), tazobactam (100/10 μg), colistin sulphate (10 μg), erythromycin (15 μg), clindamycin (2 μg), imipenem (10 μg), vancomycin (30 μg), linezolid (30 μg), clindamycin (2 ug), erythromycin (15 ug), high-level gentamicin (120 μg) from HiMedia Laboratories, India. AIIMS/IEC/2017/946 dated 15/12/2017.
Automated Siemens 2 Microscan-Beckman Coulter automated identification and antimicrobial susceptibility testing (AST) analyzer was used to evaluate minimum inhibitory concentration (MIC) of antibiotic which needs for confirmation. Methicillin resistance among Staphylococcus aureus strains was detected by cefoxitin disc diffusion test as cefoxitin is considered as a surrogate marker of mecA resistance in Staphylococcus aureus.[5]
Data analysis and interpretation
Data were entered and analyzed using SPSS version 20 software. Results were presented through graphs and tables. The statistical significance of association was measured by using the Chi-square test. A P value < 0.05 was considered as statistically significant.
Results
In the present study, a total of 153 patients with clinical diagnosis of CSOM were enrolled in the study. Out of 153 cases, Safe- and Unsafe-type CSOM was found in 92 (60.1%) and 61 (39.9%) cases, respectively. Males, 80 (52.3%), were predominantly affected as compared to females, 73 (47.7%). The maximum incidence of CSOM was observed in patients of 21-30 years age group (25.5%) as seen in Table 1.
Table 1.
Age-wise distribution of CSOM patient
| Age (Years) | Number | Percentage |
|---|---|---|
| 1-10 | 15 | 9.8% |
| 11-20 | 33 | 21.6% |
| 21-30 | 39 | 25.5% |
| 31-40 | 24 | 15.7% |
| 41-50 | 16 | 10.4% |
| 51-60 | 13 | 8.5% |
| 61-70 | 10 | 6.5% |
| 71-80 | 3 | 2% |
| Total | 153 | 100% |
Figures 1 and 2 show status of hygiene and degree of hearing loss in clinically diagnosed patients of CSOM. A majority of patients had either poor or borderline hygiene status (38.6%). A 31.4% patient suffered from moderately severe hearing loss and the rest of them had moderate (35.3%) and mild (32.7%) loss of hearing. However, the statistical correlation to see the association between severity of hearing loss and hygiene status could not be done.
Figure 1.
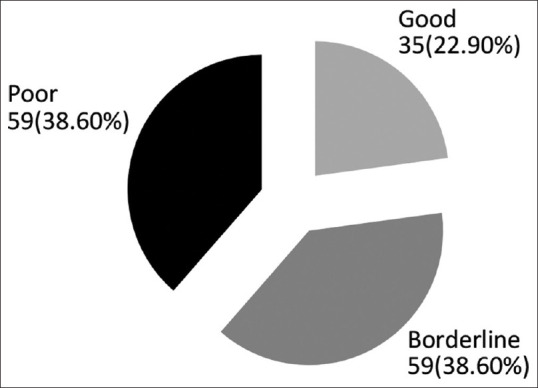
Hygiene status among all clinically diagnosed cases of CSOM (n = 153)
Figure 2.
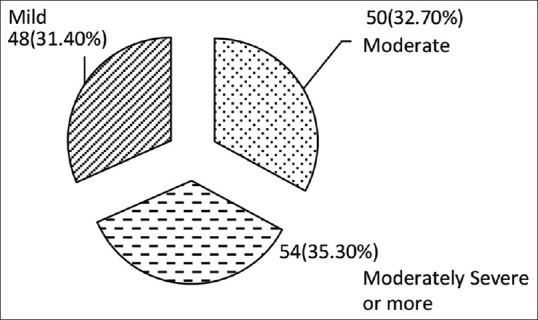
Hearing loss among clinically diagnosed cases of CSOM (n = 152)
Out of 153 samples cultured, bacterial growth was obtained in 126 (82.4%) and 27 (17.6%) showed no growth. In positive cultures, 109 (86.5%) isolates were pathogenic and 14 (11.1%) were identified as commensals and the remaining 3 (2.4%) had growth of more than three types of organisms. Amongst these 14 cases, 8 (57.1%) were Coagulase negative Staphylococci (CoNS), 4 (28.6%) Coryneform species and 2 (14.3%) were Micrococcus species, thus, excluded from the study.
Out of the total 109 pathogenic isolates, mono-microbial growth was seen in 99 (90.8%) samples and 10 (9.2%) with polymicrobial growth as shown in Figure 3. The total bacterial isolates obtained were 120 that included all isolates obtained from mono-microbial and polymicrobial growth. Gram negative bacteria 83 (69.2%) far exceeded Gram positive bacteria 37 (30.8%).
Figure 3.
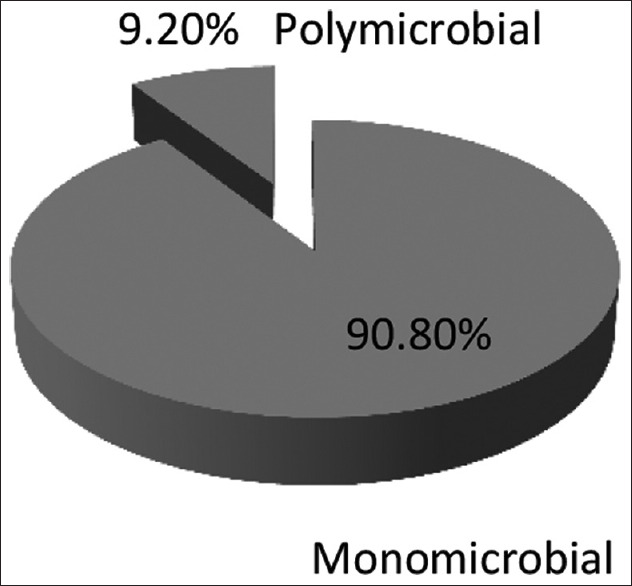
Microbial growth in CSOM cases (n = 109)
Figure 4 shows the distribution of polymicrobial isolates in CSOM cases. Pseudomonas aeruginosa and Klebsiella pneumoniae combination were more commonly isolated followed by Proteus mirabilis and Pseudomonas aeruginosa.
Figure 4.
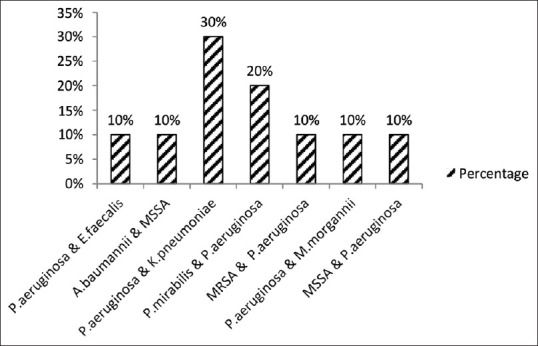
Distribution of polymicrobial isolates in CSOM cases (n = 10)
Among the total bacterial isolates (mono-microbial and polymicrobial), the most common isolate was Pseudomonas aeruginosa 67 (55.8%) followed by Staphylococcus aureus 33 (27.5%). Other isolates found were Klebsiella pneumoniae and Proteus mirabilis 5 (4.2%) each, Acinetobacter baumannii 3 (2.5%), Enterococcus faecalis 2 (1.7%) and Escherichia coli, Citrobacter koseri, Morganella morganii, Streptococcus pyogenes, Streptococcus pneumoniae 1 (0.8%) each displayed in in Figure 5. The organisms were also distributed between safe and unsafe CSOM disease type; however, the difference was not statistically significant (P-value = 0.542), the difference was also not found to be statistically significant among hygiene status.
Figure 5.
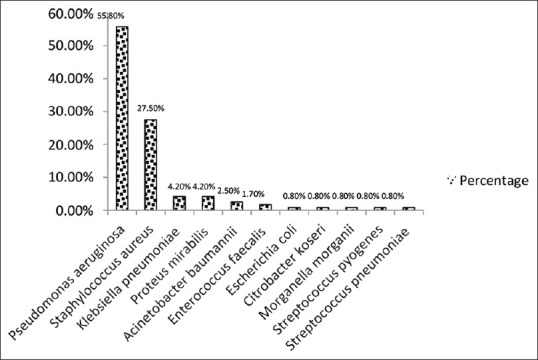
Frequency of bacterial isolates in CSOM (n = 120)
Figure 6 shows the distribution of organisms among various degree of hearing loss, however, the association between them was not found to be statistically significant (P-value = 0.233).
Figure 6.
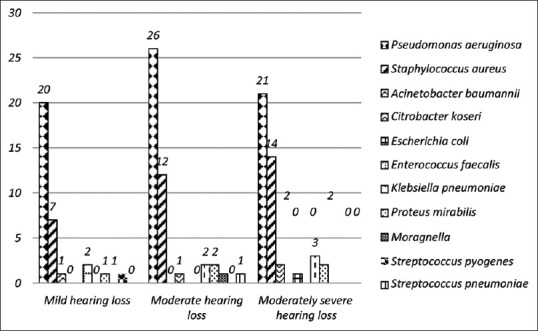
Microbial profile in various degree of hearing loss in CSOM infection (n = 120)
Figures 7 and 8 show antibiotic susceptibility pattern among P. aeruginosa and A. baumannii. P. aeruginosa showed 100% susceptibility to ceftazidime and colistin followed by piperacillin tazobactam (95.5%), ceftazidime-tazobactam (92.9%) and cefepime (81.8%). It showed moderate sensitivity to aztreonam, aminoglycosides, fluoroquinolones and imipenem. Acinetobacter species was found highly sensitive (100%) to ceftazidime-tazobactam and colistin, however, it was 66.7% sensitive to gentamicin and had low sensitivity for amikacin. It was fully resistant to cotrimoxazole and levofloxacin.
Figure 7.
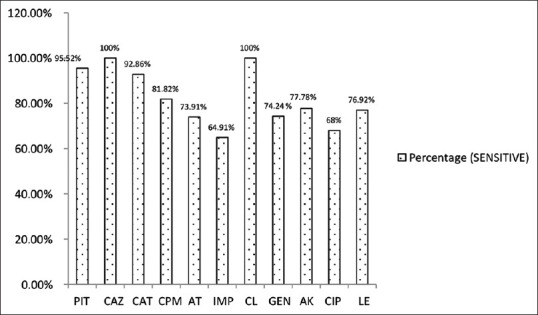
Antibiotic susceptibility pattern in Pseudomonas aeruginosa (n = 67). PIT – Piperacillin tazobactam, CAZ – Ceftazidime, CAT – Ceftazidime-tazobactam, AT – Aztreonam, IMP – Imipenem, CL – Colistin, GEN – Gentamicin, AK – Amikacin, CIP – Ciprofloxacin, LE - Levofloxacin
Figure 8.
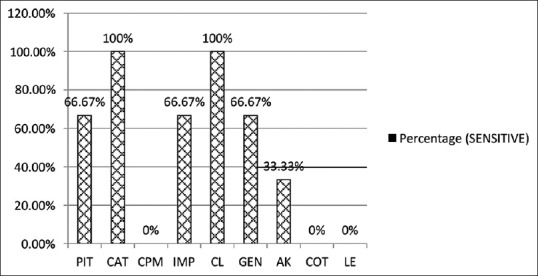
Antibiotic susceptibility pattern in Acinetobacter baumannii (n = 3). COT – Cotrimoxazole
Table 2 shows antibiotic susceptibility pattern among Enterobacteriaceae. Most of the isolates showed 100% sensitivity to Imipenem (IMP), Piperacillin tazobactam (PIT), Ceftazidime-tazobactam (CAT), amikacin (AK), Gentamicin (GEN), Levofloxacin (LE). Moderate sensitivity was seen for ceftriaxone (CTR), Ciprofloxacin (CIP). K. pneumoniae was fully resistant to CAT, E. coli resistance was for cefepime (CPM), CTR and LE. C. koseri was not sensitive to IMP (0%). M. morganii was found to be fully sensitive to netilmicin (NET) (100%).
Table 2.
Antibiotic susceptibility pattern among Enterobacteriaceae
| Organism | PIT | CPM | IMP | CL | AK | GEN | CIP | LE | CTR | CFS | CAT | NET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Klebsiella pneumoniae (n=5) | 100% | 80% | 100% | 100% | 100% | 100% | 75% | 100% | 75% | - | 0% | - |
| Escherichia coli (n=1) | 100% | 0% | 100% | - | - | 100% | - | 0% | 0% | - | 100% | - |
| Citrobacter koseri (n=1) | 100% | 100% | 0% | - | - | 100% | - | 100% | 100% | 100% | - | - |
| Proteus mirabilis (n=5) | 100% | 100% | 100% | IR* | - | 100% | 50% | 100% | 75% | 100% | 100% | - |
| Morganella morganii (n=1) | 100% | - | 100% | IR* | - | 100% | - | 100% | - | - | 100% | 100% |
IR* – Intrinsic resistance, CTR – ceftriaxone, NET – netilmicin, CFS- cefoperazone sulbactam
Among Gram positive isolates, 11 (33.3%) S. aureus isolates were Methicillin resistant (MRSA) and 22 (66.7%) were Methicillin sensitive (MSSA). No resistance was seen to vancomycin and linezolid in S. aureus. Good sensitivity (93.9%) was seen for gentamicin. Cotrimoxazole was susceptible in 59.1% cases. A very low sensitivity was observed for penicillin, erythromycin and fluoroquinolones as shown in Figure 9 Enterococcus species was fully susceptible to P, CIP, GEN (High level), VAN and Linezolid (LZ). These isolates were resistant to E. No P resistance was observed in S. pyogenes. It was also found to be susceptible to CD. S. pneumoniae was 100% sensitive to P, LE, VAN and LZ.
Figure 9.
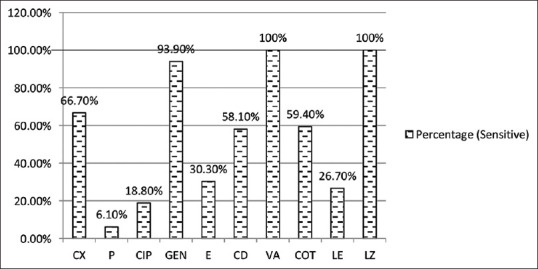
Antibiotic susceptibility in Staphylococcus aureus (n = 33). CX – Cefoxitin, P – Penicillin, E- Erythromycin, CD – Clindamycin, VA – Vancomycin, LZ - Linezolid
When we compared the resistance of aminoglycoside over fluoroquinolones in Gram positive and Gram negative isolates, we found that the aminoglycosides were more susceptible over fluoroquinolones as shown in Figures 10 and 11.
Figure 10.
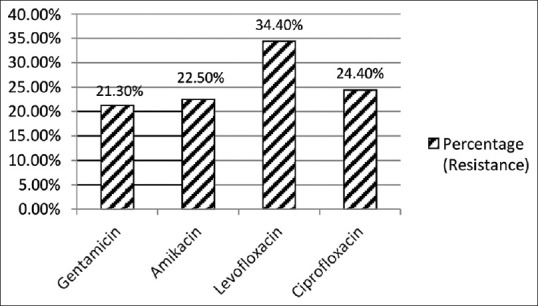
Aminoglycosides resistance over Fluoroquinolones in Gram negative isolates (n = 83)
Figure 11.
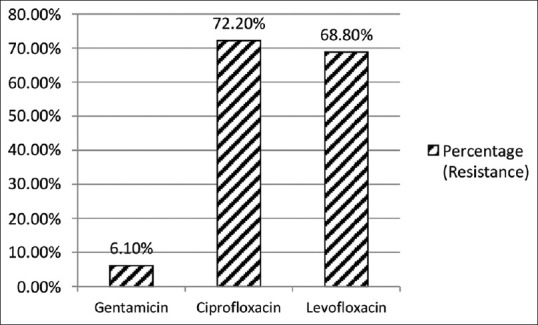
Aminoglycosides resistance over Fluroquinolones in Gram positive isolates (n = 37)
Discussion
CSOM is a major public health problem in poor and developing countries like India. According to a report by WHO, India belongs to the highest (>4%) CSOM-prevalent countries.[1] Hence, early diagnosis, knowledge of regional etiological agents and an effective antibiotic policy can curtail the development of CSOM in fatal cases.
In the present study, safe type CSOM contributed to majority 92 (60.1%) of cases followed by unsafe type 61 (39.9%). The findings are consistent with the study done by Bhan C, et al.[6] Males were predominantly (52.3%) affected as compared to females (47.7%), this was in agreement with various studies.[7,8] However, Shrestha BL, et al.[9] reported female predominance. As young adult males are more engaged in outdoor activities and travelling which expose them to recurrent upper respiratory tract infections and later to CSOM. Maximum number of patients were in the age group 21-30 years followed by 11-20 years (21.6%). Young children may develop CSOM due to unhygienic condition and over gathering in school premises. Similar findings were reported by Agarwal R, et al.[10] and Rathi S, et al.[7] In contrast, maximum number of patients in the age group of 0-8 years (72%) were observed by Chavan P et al.[11]
Majority of patients in the study had either poor or borderline hygiene status (38.6%). Overcrowding, poor hygiene, low socioeconomic status, inadequate housing, altered immunity, recurrent upper respiratory tract infections have been well documented as important risk factors for CSOM.[12]
Hearing loss in CSOM is classified into mild (26-40decibel,(db), moderate (41-55 db) and moderately severe (56-70 db).[13] In the present study, moderately severe hearing loss was observed in 31.4% cases followed by moderate (35.3%) and Mild degree (32.7%) of hearing loss. In a study conducted by Bhan C, et al.[6] hearing loss was observed in 87.7% patients. It is an established fact that hearing loss is not associated with any specific species of bacteria but with duration of disease, low socioeconomic status and its complications.[6] In the present study, statistical association between severity of hearing loss and poor hygiene practices could not be done.
Out of 153 samples cultured, bacterial growth was obtained in 126 (82.4%) and 27 (17.6%) showed no growth. Culture positivity rate varying from 84% to 91.18% have been reported in different Indian studies.[14,15] the reason of culture negativity (17.6%) can be due prior antibiotic therapy or infections by anaerobes, Mycoplasma and Chlamydia. Culture negativity of 12.6% and 16.9% has been reported in other studies from India.[16]
In positive cultures, 109 (86.5%) isolates were pathogenic and 14 (11.1%) were identified as commensals and the remaining 3 (2.4%) had growth of more than three types of organisms. Amongst these 14 cases, 8 (57.1%) were Coagulase negative Staphylococci (CoNS), 4 (28.6%) coryneform species and 2 (14.3%) were Micrococcus species. Similar findings were documented by Khatoon A, et al.[17] while Harshika et al.[18] reported a 3.8% growth of skin contaminants (Micrococcus).
Out of 109 total isolates, mono-microbial growth was seen in 90.8% samples and 9.2% with polymicrobial growth. Gram negative bacteria (69.2%) exceeded Gram positive bacteria (30.8%). The findings were in well agreement with a study done by Rathi et al.[7] However, Samanth TU, et al.[19] observed predominance of Gram positive bacteria in their study.
The combination of Pseudomonas aeruginosa and Klebsiella pneumoniae was more commonly isolated followed by Proteus mirabilis and Pseudomonas aeruginosa from samples having polymicrobial growth. Rangaiah et al.[20] observed the predominance of Escherichia coli and Pseudomonas aeruginosa combination among polymicrobial growth.
The most common isolate found in the present study was Pseudomonas aeruginosa (55.8%) followed by Staphylococcus aureus (27.5%), Klebsiella pneumoniae and Proteus mirabilis (4.2%) each, Acinetobacter baumannii (2.5%), Enterococcus faecalis (1.7%) and Escherichia coli, Citrobacter koseri, Morganella morganii, Streptococcus pyogenes, Streptococcus pneumoniae 1 (0.8%) each. These observations are in accordance with the studies conducted by some authors[10,21,22] but in contrast with the other studies who have reported Staphylococcus aureus, Proteus mirabilis and Streptococcus pyogenes as the commonest etiological agents of CSOM.[11,19,23] The probable reason for high prevalence of Pseudomonas aeruginosa could be due to the hot and sandy dry environment in this area which accelerate more sweating condition. Pseudomonas aeruginosa also requires minimal nutrition for survival and have the ability to produce self-defense products like pyocyanin, bacteriocin and pyoverdine. Staphylococcus aureus, Pseudomonas aeruginosa and Proteus species are common organisms causing CSOM in India whereas in the western countries Streptococcus pneumoniae, Hemophilus influenzae, Streptococcus pyogenes, Pseudomonas species, Enterobacter ales and Branhamella catarrhalis have been implicated as the commonest organisms.[24]
The variation in incidence of various causative organisms can be explained by geographical distribution and patient population.
This study also provides insights into the susceptibility profile of bacteria isolated from ear infections. Pseudomonas aeruginosa was found to be highly sensitive to ceftazidime, colistin (100%), piperacillin tazobactam (95.5%) and ceftazidime-tazobactam (92.9%); and good sensitivity for cefepime (81.8%), amikacin (77.8%), gentamicin (74.2%) and levofloxacin (76.9%). Aztreonam, ciprofloxacin and imipenem were sensitive in 73.9%, 68%, 64.9% cases, respectively. A study done by Khatoon et al.[17] reported maximum sensitivity for colistin, piperacillin-tazobactam and ceftazidime, while Soumya et al.[25] observed that the most effective antibiotics for Pseudomonas aeruginosa were piperacillin and piperacillin tazobactam.
In the present study, Acinetobacter baumannii was highly sensitive (100%) to ceftazidime-tazobactam and colistin. It was 66.7% sensitive to gentamicin and had a low sensitivity for amikacin. It was fully resistant to cefepime, cotrimoxazole and levofloxacin. A study done by Sahu et al.[26] reported the organism to be highly susceptible to aminoglycosides and fluoroquinolones.
Among Enterobacteriaceae, the most effective antibiotics were imipenem, piperacillin tazobactam, ceftazidime-tazobactam, amikacin, gentamicin and levofloxacin (100%). However, Citrobacter koseri was resistant to imipenem. The observations are comparable with the study of Harshika et al.[18]
Among Gram positive isolates, MRSA was found in 33.3% cases and 66.7% were MSSA. The most effective antibiotics were gentamicin, vancomycin and linezolid. Cotrimoxazole was susceptible in only 59.1% cases. However, a very low sensitivity was observed for penicillin, erythromycin and fluoroquinolones. These results were comparable with the studies done by Samanth TU, et al.[19] and Kaur P, et al.[24]
Enterococcus faecalis was fully susceptible to ampicillin, ciprofloxacin, gentamicin (high level), vancomycin and linezolid. Kashyap S, et al.[16,27] reported ciprofloxacin as the most effective antibiotic and the isolates were 50% sensitive to gentamicin and fully resistant to ampicillin Streptococcus pyogenes was penicillin sensitive and Streptococcus pneumoniae was found to be sensitive to penicillin, levofloxacin, vancomycin and linezolid.
The most widely used topical antibiotics for CSOM are fluoroquinolones and aminoglycosides.[28]
A systemic review compared quinolones versus aminoglycosides in topical treatment of CSOM and found very low certainty of the evidence which indicates it is debatable if or not one intervention is better or worse than the other.[29]
When we compared resistance of aminoglycoside over fluoroquinolones in Gram negative bacteria, we found sensitivity pattern as gentamicin (78.7%), amikacin (77.5%), ciprofloxacin (75.6%) and levofloxacin (65.6%). In Gram positive bacteria, fluoroquinolones had low sensitivity as compared to aminoglycosides. Based on best-practice recommendations, quinolones should be used in the treatment of otitis media.[30] When gentamicin ear drops are indicated, otoscopic examination is essential, because aminoglycoside ear drops are contraindicated in patients with perforated tympanic membrane. The treatment duration should be as short as possible, often less than 7 days, and the drug should be stopped immediately if ototoxic symptoms develop. Patients should be assessed for adverse effects after the first 5–7 days of use, and regularly thereafter, if the treatment is prolonged.[28] Although ototoxicity is rare and not well established, it poses a dilemma for the prescribing physician. Therefore, other safer options should be looked for based on local antibiogram.
The increased susceptibility in comparison to fluoroquinolones is an interesting finding noted in the study. Usually, empirical treatment of CSOM is done with fluoroquinolone drops, however, we anticipate inappropriate and wide use of empirical ear drops has led to this change in the microbial dynamics. Aminoglycosides being a reserve drug for ear and skull base infections, this finding is alarming and change of practice from empirical therapy to culture guided therapy of CSOM is warranted to address this difficult situation of developing antimicrobial resistance in this reason. This study will guide general family practitioners regarding appropriate management of CSOM in the western region of India which can help in avoiding the indiscriminate use of antibiotics. However, in the present study, in vivo utility of aminoglycosides was not studied.
The other limitation of this study is that the fungal culture and anaerobic culture were not done.
Conclusion
Pseudomonas aeruginosa followed by Staphylococcus aureus were observed as the principle causes of CSOM in this study. Knowledge of the spectrum of microorganisms causing ear discharge is important for the treatment of patients which decides whether to start antibacterial agents which helps to reduce treatment cost. Aminoglycoside had high susceptibility compared to fluoroquinolones in both Gram positive and Gram negative isolates due to over-the-counter and high use of fluoroquinolones. This study can help to formulate local antibiotic policy and will guide the clinician on appropriate management of CSOM infection in this area.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rajput MS, Rajput MS, Arain AA, Zaidi SS, Hatem A, Akram S. Mucosal type of chronic suppurative otitis media and the long-term impact on hearing loss. Cureus. 2020;12:1–8. doi: 10.7759/cureus.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muftah S, Mackenzie I, Faragher B, Brabin B. Prevalence of chronic suppurative otitis media (CSOM) and associated hearing impairment among school-aged children in Yemen. Oman Med J. 2015;30:358–65. doi: 10.5001/omj.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellad SA, Kavi A, Mudhol RS. Prevalence of chronic suppurative otitis media among school children residing in Rural Area of Belagavi, South India. Indian J Otolaryngol Head Neck Surg. 2019;71:1549–52. doi: 10.1007/s12070-019-01627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koneman EW, Allen SD, Janda MW, Schreckenberger PC, Winn WC. Colour Atlas in Textbook of Diagnostic Microbiology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 1997. Mycology; pp. 1153–232. [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. Wayne: Clinical and Laboratory Standards Institute; 2021. CLSI document M100-Ed31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhan C, Purohit K, Purohit JP, Kumar V, Yadav HS. Clinical vs bacteriological and mycological evaluation in chronic suppurative otitis media. Int J Contemporary Med Res. 2016;3:1443–7. [Google Scholar]
- 7.Rathi S, Jaiswal AA, Sharma N, Banerjee PK, Garg AK. Bacteriological profile and drug sensitivity patterns inchronic suppurative otitis media patientsat J.L.N. Hospital and Drug research centre, Bhilai, Chattisgarh State, India. IP Indian Journal of Anatomy and Surgery of Head, Neck and Brain. 2018;4:27–37. [Google Scholar]
- 8.Agrawal A, Kumar D, Goyal A, Goyal S, Singh N, Khandelwal G. Microbiological profile and their antimicrobial sensitivity pattern in patients of otitis media with ear discharge. Indian J Otol. 2013;19:5–8. [Google Scholar]
- 9.Shrestha BL, Amatya RC, Shrestha I, Ghosh I. Microbiological profile of chronic supurative otitis media. Nepalese Journal of ENT Head and Neck Surgery. 2011;2:6–7. [Google Scholar]
- 10.Agrawal R, Khatri P, Parihar R, Shah H. Microbial assessment of chronic suppurative otitis media in a tertiary care center of Rajasthan. Int J Health Sci Res. 2017;7:120–6. [Google Scholar]
- 11.Malkappa SK, Kondapaneni S, Surpam RB, Chakraverti TK. Study of aerobic bacterial isolates and their antibiotic susceptibility pattern in chronic suppurative otitis media. Indian Journal of Otology. 2012;18:136. [Google Scholar]
- 12.Kumar H, Seth S. Bacterial and fungal study of 100 cases of chronic suppurative otitis media. J Clin Diagn Res. 2011;5:1224–7. [Google Scholar]
- 13.Shariff ME. Analysis of hearing loss by pure tone audiometry in patients with chronic suppurative otitis media. Natl J Physiol Pharm Pharmacol. 2019;9:515–8. [Google Scholar]
- 14.Srivastava A, Singh RK, Varshney S, Gupta P, Bist SS, Bhagat S, et al. Microbiological evaluation of an active tubotympanic type of chronic suppurative otitis media. Nepalese Journal of ENT Head and Neck Surgery. 2010;1:14–6. [Google Scholar]
- 15.Shaheen MM, Raquib A, Ahmad SM. Chronic suppurative otitis media and its association with socio-econonic factors among rural primary school children of Bangladesh. Indian J Otolaryngol Head Neck Surg. 2012;64:36–41. doi: 10.1007/s12070-011-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashyap S, Pandey A, Thakuria B, Saxena AK, Asthana AK, Madan M. Resistant microorganisms isolated from cases of chronic suppurative otitis media: A therapeutic concern. Natl. lab. med. 2017;6:M001–6. [Google Scholar]
- 17.Khatoon A, Rizvi M, Sultan A, Khan F, Sharma M, Shukla I, et al. Chronic suppurative otitis media: A clinico-microbiological menace. Int J Res Med Sci. 2015;3:1932–6. [Google Scholar]
- 18.Harshika YK, Sangeetha S, Prakash R. Microbiological profile of CSOM and their antibiotic sensitivity pattern in a tertiary care hospital. Int J Curr Microbiol App Sci. 2015;4:735–43. [Google Scholar]
- 19.Samanth TU, Jha SG, Sinha V, Dadhich S. Bacteriology and drug susceptibility in chronic suppurative otitis media in Ear, Nose, and Throat outpatient and inpatient department of tertiary care Hospital, Bhavnagar. Indian J Otol. 2017;23:252–5. [Google Scholar]
- 20.Rangaiah ST, Dudda R, Prasad MH, Balaji NK, Sumangala B, Gudikote MM. Bacteriological profile of chronic suppurative otitis media in a tertiary care hospital. Int J Otorhinolaryngol Head Neck Surg. 2017;3:601–5. [Google Scholar]
- 21.Malkappa SK, Kondapaneni S, Surpam RB, Chakraverti TK. Study of aerobic bacterial isolates and their antibiotic susceptibility pattern in chronic suppurative otitis media. Indian J Otol. 2012;18:136–9. [Google Scholar]
- 22.Smitha NR, Jnaneshwara KB, Patil AB, Harshika YK, Medegar S. A study of aerobic bacteriological profile of chronic suppurative otitis media in a tertiary care hospital, South India. Indian Journal of Microbiology Research. 2020;5:470–5. [Google Scholar]
- 23.Suresh Chander VC, Kavinkumar A. Microbiological profile of chronic suppurative otitis media presenting to a tertiary care teaching hospital-A cross-sectional study. International Archives of Integrated Medicine. 2019;6:5–11. [Google Scholar]
- 24.Kaur P, Sood AS, Sharma S, Awal G. Microbiological profile and antimicrobial susceptibility pattern of chronic suppurative otitis media in a Tertiary care centre. Tropical Journal of Pathology and Microbiology. 2018;4:1–13. [Google Scholar]
- 25.Soumya S, Vagrali M, Nagmoti JM, Hogade S. Prevalence and antibiogram of pseudomonas aeruginosa in Chronic suppurative otitis media (CSOM) J Med Sci Clin Res. 2018;6:999–1005. [Google Scholar]
- 26.Sahu MC, Swain SK. Surveillance of antibiotic sensitivity pattern in chronic suppurative otitis media of an Indian teaching hospital. World J Otorhinolaryngol Head Neck Surg. 2019;5:88–94. doi: 10.1016/j.wjorl.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta P, Varshney S, Kumar SK, Mohanty A, Jha MK. Chronic suppurative otitis media: A microbiological review of 20 years. Indian J Otol. 2020;26:59. [Google Scholar]
- 28.Harris AS, Elhassan HA, Flook EP. Why ototopical aminoglycosides are still first-line therapy for chronic suppurative otitis media. A systematic review and discussion of aminoglycosides versus quinolones. J Laryngol Otol. 2016;130:2–7. doi: 10.1017/S0022215115002509. [DOI] [PubMed] [Google Scholar]
- 29.Brennan-Jones CG, Head K, Chong LY, Burton MJ, Schilder AG, Bhutta MF. Topical antibiotics for chronic suppurative otitis media. Cochrane Database Syst Rev. 2020;1:CD013051. doi: 10.1002/14651858.CD013051.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wooltorton E. Ototoxic effects from gentamicin ear drops. CMAJ. 2002;167:56. [PMC free article] [PubMed] [Google Scholar]


