Abstract
Pain is most common symptom associated with progressive disorder, chronic kidney disease (CKD), and is usually undertreated during the early stages of CKD. So, present review was conducted to evaluate the challenges for the management of pain in CKD patients and addresses the scope for considering Diclofenac as suitable alternative for pain management in CKD patient. The database PubMed and Google Scholar were searched from 1970 to Dec 2020 for literature published in English and all studies, review articles that examined the use of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in pain management in CKD patients were included. Literatures revealed that there is a considerable challenge in appropriate management of pain in CKD patients include understanding the altered pharmacokinetics and pharmacodynamics of analgesics in CKD patients and the risk of acute interstitial nephritis. The shorter duration of analgesics is acceptable and considered to pose a low risk of acute interstitial nephritis in patients. Considering that Diclofenac has a shorter half-life and high efficacy, it may be well tolerated in patients with CKD. The acceptance of Diclofenac is partly attributed to being a potent COX-2 inhibitor with the lowest IC50 and its rapid onset of action at lowest effective dose. In conclusion, diclofenac may be well tolerated in patients of renal impairment when used at lowest effective dose for shortest dose duration. Diclofenac is worthy of consideration in mild to moderate cases of CKD. For effective pain management, it is vital to evaluate the tolerability and efficacy of the available analgesics critically.
Keywords: Diclofenac, nsaid, chronic kidney disease, pain management, pain
Introduction
Pain is one of the most common and distressing symptoms for patients with chronic kidney disease (CKD) impacting the health-related quality of life and is usually undertreated.[1,2] The disorder is associated with a progressive and gradual loss of kidney function that can result in end-stage renal disease (ESRD).[3] Literature suggests that 70% of patients affected with the disease reported pain.[4,5] Additionally, CKD patients with co-morbid conditions may undergo surgical procedures that require effective pain management. Pain manifested in patients vary in severity and the types. Clinicians need to take cognizance of these aspects for pain management in CKD patients.[6] The literature reported >60-70% of pain prevalence among advanced and end-stage kidney disease patients.[7] Also, in the last two decades, the role of primary healthcare has expanded in minimizing renal morbidity, which was earlier considered under the purview of specialists only.[8] CKD management was integrated in primary healthcare for early diagnosis, and management can be started to intercept disease progression and improve patient outcomes.[9]
The complex nature of the disease, co-morbidities, and associated risk factors make it challenging to select appropriate analgesic treatment for these patients. The present review will discuss the management of pain in CKD patients and potential role of diclofenac in pain control while reducing the drug induced complications. The research and development focus lacks in the area of pain management in renal impairment. Scientific literature demonstrates conflicting results on the impact of non-steroidal anti-inflammatory drugs (NSAIDs) on the pain status of the disease.[10] This could be mainly due their focus on the late stages of CKD and partly due to limitations in their methodology of studies employed. The review aims to evaluate the challenges in managing pain in CKD patients and addresses the scope for considering Diclofenac as a suitable alternative.
Methods
The PubMed database and manual search of google scholar were used to perform the literature search for this review. We included articles published in ‘English’ language from the period 1970 to April 2020. The search was performed using keywords, chronic kidney disease, analgesics, end-stage renal failure, WHO analgesic ladder, WHO pain management, pain in chronic kidney disease, CKD, CKD status, opioids, NSAIDs, cyclooxygenase enzymes, COX enzymes, diclofenac safety, pharmacokinetics of diclofenac, pharmacological properties of diclofenac, dosage and formulations of diclofenac. The present review article is a narrative review; hence entire PRISMA guidelines for reporting systematic reviews were not followed. We screened articles based on the titles and abstracts and include 72 articles to develop this review.
Discussion
The considerable variation in prevalence is attributed to demographic and socioeconomic factors, including age, sex, genetic susceptibility, lifestyle, diet, and environment.[3,11,12,13] Increasing prevalence of diabetes and hypertension, resulting in albuminuria, are the leading causes of CKD contributing to increased prevalence in low and middle-income countries.[14] Various studies using divergent methodologies have reported CKD prevalence in Indian population ranging from 0.8% to 18%.[15,16,17,18]
According to Kafkia et al., symptoms pertaining to pain are more prevalent in stage 5 patients relative to cancer and are associated with higher levels of moderate to severe pain.[19] CKD patients have multiple factors that trigger pain, and management varies balancing between efficacy and tolerability. The type of pain can be ischemic, musculoskeletal, neuropathic, or due to renal osteodystrophy[20] Davison's et al. (2003) suggests that musculoskeletal pain is prevalent in over half of the patients equal in severity to neuropathic and ischemic pain; 75% of the patients report inadequate treatment.[21] Additionally, comorbidities such as diabetes mellitus (DM) and peripheral vascular disease (PVD) further aggravate the pain due to progressive peripheral neuropathy or intermittent claudication.
About 37 to 50% of hemodialysis patients experience chronic pain, of which 82% is moderate to severe in intensity. Pain in elderly dialysis patients is perceived as more challenging to treat. It is prevalent in 42% of patients withdrawn from dialysis. Generally, pain in hemodialysis patients is of different etiologies, and musculoskeletal pain emerges to be the major cause.[21,22] A quantitative analysis of varying etiologies demonstrated that musculoskeletal pain complaints were reported by 54% of patients followed by cramps in 43%, headache in 19%, and chest pain in 10% of patients.[23] Figure 1 highlights the causes of pain in CKD patients.[24]
Figure 1.
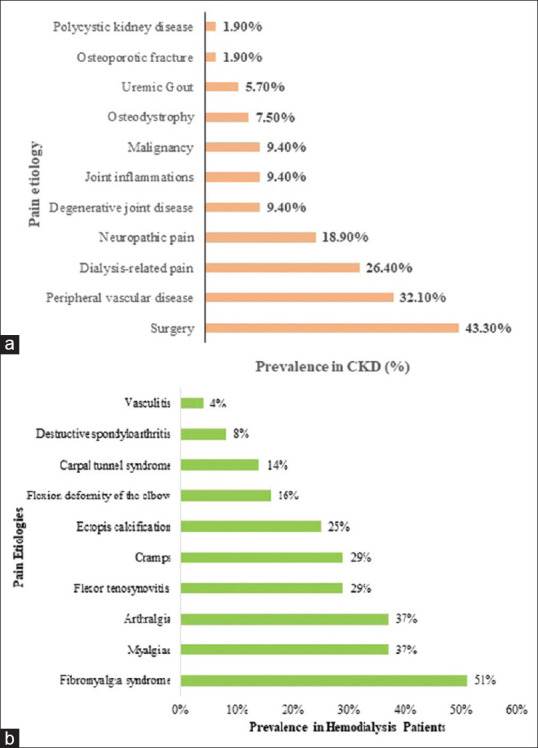
Prevalence of pain etiologies in: 1a: CKD patients.[22] Adapted from Sakata RK et al. Revista Dor. 2014;15:224-9. 1b: Hemodialysis patients.[24] Adapted from Haroon MM et al. Int J Clin Rheumatol. 2018;13(5):7
WHO step-ladder approach for pain management in CKD
According to the WHO step-wise pain management scheme [Figure 2], NSAIDs/non-opioids are the first line of treatment for pain management in CKD patients.[25,26] In severe CKD, in addition to the analgesics, despite serious concerns with addiction and adverse side effects, opioids are considered but with extreme caution.[25,26] Current literature suggests NSAIDs are not the only cause for progression of renal impairment.[27] For any analgesic class considered for long term at cumulative high dosages, there is a potential trade-off with balancing benefits versus harmful side effects.[28]
Figure 2.
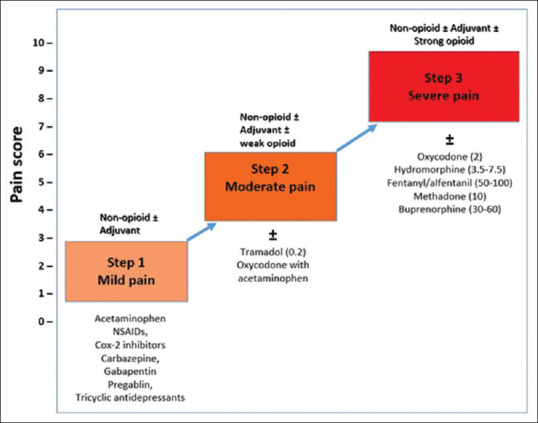
Modified WHO analgesic ladder in patients with CKD.[26] Adapted from Parmar M et al. F1000Research. 2013;2(28)
Acetaminophen is a centrally acting analgesic with no or least impact on associated inflammatory component. Owing to the mode of excretion, it is recommended in CKD patients. Acetaminophen may be used for treating advanced CKD patients with mild or low-grade pain, and its impact on CKD is debatable.[29] Studies have indicated a positive association between CKD and acetaminophen.[30,31,32] Acetaminophen-induced liver necrosis is well studied and overdose of acetaminophen may result in both hepatic and renal failures.[33,34]
Opioids can be considered for pain management only when other alternatives seem inadequate. All guidelines emphasize the potential misuse and abuse of these drugs when used for a longer period for chronic pain management.[7] Most opioids undergo hepatic biotransformation and are primarily eliminated by renal excretion. The toxic effects due to opioid drugs and its metabolites often emerge after 48 h and demand re-evaluation every 24-48 h following treatment.[35] Especially in CKD patients, the accumulation of opioids and their toxic metabolites remains a serious concern as it may result in severe complications such as myoclonus and seizures.[36,37,38] Therefore, careful monitoring of renal function is required.
Accumulation of commonly used opioids like morphine, oxycodone, etc., in advanced CKD patients can lead to profound CNS and respiratory depression.[36,37,38] This could be disastrous for CKD patients with associated metabolic acidosis since they depend on respiratory compensation to maintain acid-base homeostasis. Due to favorable pharmacokinetic profile posing less burden on the kidneys, use of opioids like methadone and fentanyl may be acceptable in CKD patients.[7] However, caution is advised due to lack of evidence on tolerability and efficacy in chronic pain.[39]
A take on NSAIDs in CKD patients
Prolonged use of NSAIDs is considered harmful for CKD patients by most clinicians. Also, the clinical guidelines (KDIGO 2012) recommended to avoid used of NSAIDs in patients with eGFR >30 mL/min/1.73 m2 due to NSAID nephrotoxicity. Literature states that NSAID nephrotoxicity in CKD patients is confounded by factors like severity of underlying health conditions, comorbidities, disease burden, and polypharmacy. Moreover, most epidemiological studies were deficient with respect to the stratification by CKD stage or exclusion of advanced CKD.[40]
Available literature disputes the significant association of NSAIDs with progression of CKD to moderate and severe cases. A review that summarizes the findings from 768 articles suggested that NSAIDs administered at regular dosage are well-tolerated; high-dose and long-term usage might be one of the risk factors for CKD progression.[10] It suggests that unless contraindicated, drugs like diclofenac with a short plasma half-life (2 hours) at regular dosage need not be strictly avoided even in patients with moderate to severe CKD. Long-acting NSAIDs (meloxicam, Piroxicam) with half-life >12 h could inhibit renal vasodilator prostaglandins, therefore, must be avoided to prevent significant reduction in eGFR.[10] Accordingly, diclofenac with a shorter half-life may be an option in CKD patients.[41,42,43,44] Shorter dosing is acceptable as it poses low risk of acute interstitial nephritis in patients with eGFR <30, mL/min/1.73 m2 where dialysis is not needed. Besides, the longer duration can be considered in aforementioned patients with regular monitoring for kidney functions. In hemodialysis patients, the use of NSAIDs may also be acceptable coupled with regular monitoring.[28,39,40,41,42,43,44]
The above pointers suggest that cautious use of NSAIDs and regular monitoring of renal functions may contribute towards effective pain management in CKD patients.[28,39] Responsible pain management strategy for CKD patients would therefore comprise of careful selection of appropriate analgesic and NSAIDs in low doses for shorter duration.
The different classes of NSAIDs comprise of drugs that could selectively inhibit cyclooxygenase (COX) enzymes i.e., COX-1 or COX-2 or both, giving rise to a spectrum of varying effectiveness and adverse effects. Studies demonstrated the role of both COX-1 and 2 in renal homeostasis. Since constitutive COX-2 homeostatic response has been observed in developing brain, kidney, and gut tissues, selective COX-2 inhibitors pose serious adverse renal effects.[45] This has led to the withdrawal of most highly selective COX-2 inhibitors, while others in the market come with a ‘black box’ warning sign since 2015.[45] This has resulted in reverting the preference back to non-selective (ibuprofen, naproxen) and, largely to partially selective diclofenac.
Diclofenac displays analgesic, anti-inflammatory, and anti-pyretic properties. The remarkable acceptance of diclofenac is attributable to being a potent COX-2 inhibitor while retaining similar activity as some of other popular non-selective NSAIDs.[46,47] Indomethacin has seven times greater selectivity to COX-1 than COX-2.[48] This high preponderance to inhibit COX-1 over COX-2 enzymes lends Indomethacin with significant adverse gastric irritation. The in-vitro assays have selectively quantified and compared the COX-2 dependent PGE2 formation by monocytes with that of end product of the COX-1 dependent pathway, thromboxane A2 (TXB2) formation by platelets.[49,50] These assays demonstrated that diclofenac inhibits COX-2 enzyme with higher potency than COX-1 Table 1. This inhibitory action was comparable to that of celecoxib, a potent COX-2 inhibitor. The below table shows that a balanced and optimal COX-2: COX-1 ratio puts Diclofenac in a different context in terms of tolerability balanced with potent efficacy.
Table 1.
Inhibitory action of NSAIDs against COX-1 or COX2 enzymes
Pharmacokinetics profile of diclofenac in CKD patients
Diclofenac has a short biological half-life of two hours and fast elimination rate (mean elimination half-life 1.2–1.8 h).[41,42,43,44] Diclofenac is metabolized into glucuronide and sulfate conjugates and eliminated in urine and bile secretions. A minimal amount of diclofenac (~1%) remains unchanged and excreted through renal system.[54] Since renal excretion is not the primary route for excretion, renal burden imposed by diclofenac is not a significant concern. Therefore, diclofenac at standard dosing may be well tolerated in patients with renal dysfunction without other contraindications.
Diclofenac can diffuse into and out of the synovial fluid. Diclofenac being an acetic acid derivative of NSAIDs, remains highly bound to plasma proteins like albumin. Diclofenac gets concentrated in the systemic circulation and inflammatory response sites. The inflamed tissues pose a weakly acidic environment, thereby reducing diclofenac interactions with plasma proteins. Consequently, concentration of unbound diclofenac increases which can readily diffuse into the intracellular spaces and execute its target response.[55,56] In a concentration gradient dependent manner, diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid. The unique pKa of diclofenac renders higher persistent availability at inflammation site (synovial fluid) than plasma. For instance, Diclofenac sodium was detectable in synovial fluid for up to 11 h following administration of 50-mg enteric-coated tablet and up to 25 h following administration of 100-mg slow-release tablet.[57,58] This persistence at the site of inflammation and its inhibition of COX-2 enzymes in the inflammatory cells contributes to the prolonged therapeutic effect. Administration of high doses could extend pharmacodynamic half-life, thus contributing to the prolonged effect.[59] The therapeutic effect of diclofenac is evidenced as decreased levels of PGE2 in the synovial fluid and of the inflammatory cytokines.[60,61]
Dosage of diclofenac compared to other NSAIDs
The IC50 values of diclofenac Table 1 makes it efficient even at lower doses. According to a study, efficacy of 150 mg of diclofenac was comparable to 500 mg of naproxen and had lower adverse reactions compared to most other NSAIDs.[62] Of the different NSAIDs studied, Indomethacin had the most effect on eGFR.[60]
Diclofenac sodium is demonstrated to be more effective than ibuprofen, and even at doses of 12.5 mg, it is as effective as 500 mg of paracetamol.[62,63,64] Further, the advent of fast-acting formulation, diclofenac potassium displays accelerated absorption, fast relief, and requires much lower dose. The chemical difference between diclofenac being an acetic acid derivative to ibuprofen is a propionic acid derivative accounts for greater ability of diclofenac to bind plasma proteins. Hence diclofenac is considered to be a well-tolerated alternative in managing pain and inflammation.[65]
Tolerability of diclofenac
Diclofenac is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reactions and severe skin reactions), with a history of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Long-term administration of diclofenac may result in renal papillary necrosis. In susceptible patients, the effect of NSAIDs on renal prostaglandins following administration may cause a dose-dependent adverse reaction. The gastrointestinal complications are most common side effects with NSAIDs including Diclofenac[66] Safety warnings associated with diclofenac usage include cardiovascular thrombotic events, gastrointestinal bleeding, ulceration and perforation, hepatotoxicity, renal toxicity, and hyperkalemia. The recommendation is to administer diclofenac at the lowest effective dose for a shorter duration; however, diclofenac is contraindicated in patients with eGFR <15.[66]
Diclofenac formulations
Delayed- and extended-release forms of diclofenac sodium were initially developed to reduce the dose with retained efficacy, and to improve tolerability especially in chronic pain management.[67,68] New drug products consisting of diclofenac potassium salt were associated with faster absorption and rapid onset of pain relief. Various pharmaceutical evolution of formulation of diclofenac offers an additional advantage of dosage regulations. These include immediate-release tablets, liquid-filled soft gel capsules, and powder for oral solution.[69,70] Table 2 presents the key pointers on the role of diclofenac in CKD.
Table 2.
Key pointers on role of Diclofenac in CKD
| Diclofenac is both a COX-1 and COX-2 inhibitor with more potency against COX-2. |
| The drug is metabolized and is excreted by both renal and bile mode with only 2% of unchanged diclofenac. |
| Diclofenac diffuses into and out of the synovial fluid. |
| Pharmacokinetic property including its short plasma half-life, lowest |
| IC50 and its prolonged action of one single dose makes it one among the potent NSAIDs. |
| Different formulations for fast actions [Diclofenac K], varying doses of enteric-coated Diclofenac Sodium, and sustained release make it more patient-friendly. |
| Renal impairment with NSAIDs and Diclofenac is not solely attributed to these drugs; but to patient's underlying medical conditions and renal function status. |
| Diclofenac may be well tolerated in renally impaired patients when used at the lowest effective dose for the shortest dose duration. |
COX-1 - Cyclooxygenase 1; COX-2 - Cyclooxygenase 2; NSAID - Nonsteroidal anti-inflammatory drugs; IC- Inhibitory concentration
Challenges in pain management in CKD
The challenges like comorbid conditions leading to polypharmacy, elderly age, and reduced GFR due to altered pharmacokinetics of drugs, poses a challenge in appropriate pain management. Some challenges are increased risk of toxicity, unwarranted drug interactions, and adverse effects of analgesics. Occasionally, uremic symptoms are confused with the adverse effects of analgesics resulting in inappropriate withdrawal of pain medications. Further, patients fail to seek medical attention until the pain becomes severe and to some extent due to the cost of pain medications.
Another challenge is inadequate pain assessment by healthcare providers or due to lack of staff time and training. The extent and severity of the problem seem to be less recognized by the health care providers mainly because of limited training to consider the compromised course of drug and altered pharmacokinetics in CKD patients.[4,20]
Considering the need for pain management in the CKD patients, the choice of analgesics after careful assessment of risk is warranted. Some of the necessary steps include assessment of the type of pain, underlying cause, intensity and duration of pain, concomitant medications, and co-morbidities. Adjuvant analgesics and non-pharmacological methods of pain control should also be considered.
Some points to ponder
Effective management in CKD patients is challenging due to altered pharmacodynamics and pharmacokinetic properties. However, pain in CKD patients requires early diagnosis and therefore effective management. The current need of the hour is to individualize pain management basis the patients’ underlying condition. The choice of the analgesic needs to be made based on the efficacy, tolerability, duration of treatment, and contraindications. NSAIDs are generally considered as first line of treatment. However, many practitioners have concerns about renal safety with NSAIDs. A recent report by Prof. Nicholas Moore published in April 2020 highlights that renal effects of NSAIDS are dose duration dependent; long-term usage may be a risk factor for renal impairment. This is less certain for the short-term use of lower doses, such as used to relieve common pain.[71] For CKD patients, diclofenac emerges as a well-tolerated alternative with an added advantage of potent efficacy. Less potent analgesics than diclofenac, may be administered for a longer duration for managing pain in CKD patients, pose adverse renal effects. Administering diclofenac with shorter plasma half-life at lowest effective dose for a shorter duration can be considered as a promising option while monitoring for contraindications.
Managing pain in CKD patients can also be achieved through shared decision-making process. A detailed consultation with multiple specialties like nephrologists, orthopedics, surgeons, etc., may help to be pre-emptive of adverse effects associated with the available pharmacological options.
Implications for future research and clinical practice
The narrative review has been opted to cover the issues of pain management and comprehensively discuss the role of diclofenac in pain management. This review does not provide a critical appraisal of the methodologies in existing literature and conclusions pertaining to concerns with using diclofenac in CKD patients. Further work in this regard is needed.
Key Messages
NSAIDS are not the sole cause for progression of renal impairment. Diclofenac with shorter half life is worthy of consideration in CKD along with shared decision and dose adjustments.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Sharad Sheth has attended advisory boards arranged by Novartis and is a speaker for Novartis initiated scientific speaker programs.
Dr. Sneha Thakur, Dr. Anup Thorat and Dr. Pankaj Gupta are full time employees of Novartis India Limited.
Acknowledgement
Authors have received medical writing support from Dr. Pawandeep Kaur Dhawan working with tech observer.
References
- 1.Bailie GR, Mason NA, Bragg-Gresham JL, Gillespie BW, Young EW. Analgesic prescription patterns among hemodialysis patients in the DOPPS: Potential for underprescription. Kidney Int. 2004;65:2419–25. doi: 10.1111/j.1523-1755.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 2.Davison SN. The prevalence and management of chronic pain in end-stage renal disease. J Palliat Med. 2007;10:1277–87. doi: 10.1089/jpm.2007.0142. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–80. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 4.Brown S, Lascarides P, Stickevers S. Treating pain in patients with chronic kidney disease: A review of the literature. Pract Pain Manag. 2014;4 [Google Scholar]
- 5.Pham PC, Toscano E, Pham PM, Pham PA, Pham SV, Pham PT. Pain management in patients with chronic kidney disease. NDT Plus. 2009;2:111–8. doi: 10.1093/ndtplus/sfp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams A, Manias E. A structured literature review of pain assessment and management of patients with chronic kidney disease. J Clin Nurs. 2008;17:69–81. doi: 10.1111/j.1365-2702.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 7.Pham PC, Khaing K, Sievers TM, Pham PM, Miller JM, Pham SV, et al. 2017 update on pain management in patients with chronic kidney disease. Clin Kidney J. 2017;10:688–97. doi: 10.1093/ckj/sfx080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady M, O’Donoghue D. The role of primary care in managing chronic kidney disease. Br J Gen Pract. 2010;60:396–7. doi: 10.3399/bjgp10X502065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wouters OJ, O’Donoghue DJ, Ritchie J, Kanavos PG, Narva AS. Early chronic kidney disease: Diagnosis, management and models of care. Nat Rev Nephrol. 2015;11:491–502. doi: 10.1038/nrneph.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nderitu P, Doos L, Jones PW, Davies SJ, Kadam UT. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: A systematic review. Fam Pract. 2013;30:247–55. doi: 10.1093/fampra/cms086. [DOI] [PubMed] [Google Scholar]
- 11.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: A meta-analysis. Lancet. 2007;369:381–8. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 12.Rettig RA, Norris K, Nissenson AR. Chronic kidney disease in the United States: A public policy imperative. Clin J Am Soc Nephrol. 2008;3:1902–10. doi: 10.2215/CJN.02330508. [DOI] [PubMed] [Google Scholar]
- 13.Duan J, Wang C, Liu D, Qiao Y, Pan S, Jiang D, et al. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in Chinese rural residents: A cross-sectional survey. Sci Rep. 2019;9:10408. doi: 10.1038/s41598-019-46857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 15.Varma PP, Raman DK, Ramakrishnan TS, Singh P, Varma A. Prevalence of early stages of chronic kidney disease in apparently healthy central government employees in India. Nephrol Dial Transplant. 2010;25:3011–7. doi: 10.1093/ndt/gfq131. [DOI] [PubMed] [Google Scholar]
- 16.Singh NP, Ingle GK, Saini VK, Jami A, Beniwal P, Lal M, et al. Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in North India using Cockcroft-Gault and Modification of Diet in Renal Disease equation: An observational, cross-sectional study. BMC Nephrol. 2009;10:4. doi: 10.1186/1471-2369-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal SK, Dash SC, Irshad M, Raju S, Singh R, Pandey RM. Prevalence of chronic renal failure in adults in Delhi, India. Nephrol Dial Transplant. 2005;20:1638–42. doi: 10.1093/ndt/gfh855. [DOI] [PubMed] [Google Scholar]
- 18.Tatapudi RR, Rentala S, Gullipalli P, Komarraju AL, Singh AK, Tatapudi VS, et al. High prevalence of CKD of unknown etiology in Uddanam, India. Kidney Int Rep. 2019;4:380–9. doi: 10.1016/j.ekir.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kafkia T, Chamney M, Drinkwater A, Pegoraro M, Sedgewick J. Pain in chronic kidney disease: Prevalence, cause and management. J Ren Care. 2011;37:114–22. doi: 10.1111/j.1755-6686.2011.00234.x. [DOI] [PubMed] [Google Scholar]
- 20.Salisbury EM, Game DS, Al-Shakarchi I, Chan M, Fishman L, Tookman L, et al. Changing practice to improve pain control for renal patients. Postgrad Med J. 2009;85:30–3. doi: 10.1136/pgmj.2008.071191. [DOI] [PubMed] [Google Scholar]
- 21.Davison SN. Pain in hemodialysis patients: Prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42:1239–47. doi: 10.1053/j.ajkd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Sakata RK, Nunes MH. Analgesics use for kidney failure. Revista Dor. 2014;15:224–9. [Google Scholar]
- 23.Weisbord SD, Fried LF, Arnold RM, Fine MJ, Levenson DJ, Peterson RA, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16:2487–94. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]
- 24.Haroon MM, Sayed S, Al-ghitany A, Ezzat H, Gheita TA. Rheumatic and musculoskeletal manifestations in renal hemodialysis patients. Int J Clin Rheumatol. 2018;13:263–9. [Google Scholar]
- 25.World Health Organization Staff, World Health Organization. Cancer pain relief: With a guide to opioid availability. 2nd ed. World Health Organization. World Health Organization Cancer Pain Relief; 1996. pp. 3–36. Available from: https://apps.who.int/iris/handl/10665/37896 . [Google Scholar]
- 26.Parmar M, Parmar K. Management of acute and post-operative pain in chronic kidney disease. F1000Res. 2013;2:28. doi: 10.12688/f1000research.2-28.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moller B, Scherer A, Forger F, Villiger PM, Finckh A. Anaemia may add information to standardised disease activity assessment to predict radiographic damage in rheumatoid arthritis: A prospective cohort study. Ann Rheum Dis. 2014;73:691–6. doi: 10.1136/annrheumdis-2012-202709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sriperumbuduri S, Hiremath S. The case for cautious consumption: NSAIDs in chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28:163–70. doi: 10.1097/MNH.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 29.Evans M, Fored CM, Bellocco R, Fitzmaurice G, Fryzek JP, McLaughlin JK, et al. Acetaminophen, aspirin and progression of advanced chronic kidney disease. Nephrol Dial Transplant. 2009;24:1908–18. doi: 10.1093/ndt/gfn745. [DOI] [PubMed] [Google Scholar]
- 30.Sandler DP, Smith JC, Weinberg CR, Buckalew VM, Jr, Dennis VW, Blythe WB, et al. Analgesic use and chronic renal disease. N Engl J Med. 1989;320:1238–43. doi: 10.1056/NEJM198905113201903. [DOI] [PubMed] [Google Scholar]
- 31.Perneger TV, Whelton PK, Klag MJ. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N Engl J Med. 1994;331:1675–9. doi: 10.1056/NEJM199412223312502. [DOI] [PubMed] [Google Scholar]
- 32.Pommer W, Bronder E, Greiser E, Helmert U, Jesdinsky HJ, Klimpel A, et al. Regular analgesic intake and the risk of end-stage renal failure. Am J Nephrol. 1989;9:403–12. doi: 10.1159/000168002. [DOI] [PubMed] [Google Scholar]
- 33.Mazer M, Perrone J. Acetaminophen-induced nephrotoxicity: Pathophysiology, clinical manifestations, and management. J Med Toxicol. 2008;4:2–6. doi: 10.1007/BF03160941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem M, Iftikhar H. A rare case of acetaminophen toxicity leading to severe kidney injury. Cureus. 2019;11:e5003. doi: 10.7759/cureus.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallappallil M, Sabu J, Friedman EA, Salifu M. What do we know about opioids and the kidney. Int J Mol Sci. 2017;18:223. doi: 10.3390/ijms18010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: A review of available evidence. Am J Kidney Dis. 2003;42:217–28. doi: 10.1016/s0272-6386(03)00645-0. [DOI] [PubMed] [Google Scholar]
- 37.Murtagh FE, Chai MO, Donohoe P, Edmonds PM, Higginson IJ. The use of opioid analgesia in end-stage renal disease patients managed without dialysis: Recommendations for practice. J Pain Palliat Care Pharmacother. 2007;21:5–16. [PubMed] [Google Scholar]
- 38.Saboory E, Derchansky M, Ismaili M, Jahromi SS, Brull R, Carlen PL, et al. Mechanisms of morphine enhancement of spontaneous seizure activity. Anesth Analg. 2007;105:1729–35. doi: 10.1213/01.ane.0000287675.15225.0b. table of contents. [DOI] [PubMed] [Google Scholar]
- 39.Davison SN. Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin J Am Soc Nephrol. 2019;14:917–31. doi: 10.2215/CJN.05180418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker M, Perazella MA. NSAIDs in CKD: Are they safe. Am J Kidney Dis. 2020;76:546–57. doi: 10.1053/j.ajkd.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33:184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 42.Riess W, Stierlin H, Degen P, Faigle JW, Gerardin A, Moppert J, et al. Pharmacokinetics and metabolism of the anti-inflammatory agent Voltaren. Scand J Rheumatol Suppl. 1978;7:17–29. doi: 10.3109/03009747809097212. [DOI] [PubMed] [Google Scholar]
- 43.Willis JV, Kendall MJ, Flinn RM, Thornhill DP, Welling PG. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur J Clin Pharmacol. 1979;16:405–10. doi: 10.1007/BF00568201. [DOI] [PubMed] [Google Scholar]
- 44.Todd PA, Sorkin EM. Diclofenac sodium: A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1988;35:244–85. doi: 10.2165/00003495-198835030-00004. [DOI] [PubMed] [Google Scholar]
- 45.Kirkby NS, Chan MV, Zaiss AK, Garcia-Vaz E, Jiao J, Berglund LM, et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways. Proc Natl Acad Sci U S A. 2016;113:434–9. doi: 10.1073/pnas.1517642113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowlinson SW, Kiefer JR, Prusakiewicz JJ, Pawlitz JL, Kozak KR, Kalgutkar AS, et al. A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J Biol Chem. 2003;278:45763–9. doi: 10.1074/jbc.M305481200. [DOI] [PubMed] [Google Scholar]
- 47.Van Hecken A, Schwartz JI, Depré M, De Lepeleire I, Dallob A, Tanaka W, et al. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol. 2000;40:1109–20. [PubMed] [Google Scholar]
- 48.Cao H, Yu R, Tao Y, Nikolic D, van Breemen RB. Measurement of cyclooxygenase inhibition using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2011;54:230–5. doi: 10.1016/j.jpba.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patrignani P, Panara MR, Greco A, Fusco O, Natoli C, Iacobelli S, et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J Pharmacol Exp Ther. 1994;271:1705–12. [PubMed] [Google Scholar]
- 50.Patrono C, Ciabattoni G, Pinca E, Pugliese F, Castrucci G, De Salvo A, et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res. 1980;17:317–27. doi: 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 51.Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: Discovery, selectivity and the future. Trends Pharmacol Sci. 1999;20:465–9. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- 52.Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem J. 1999;339(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 53.Dannhardt G, Ulbrich H. In-vitro test system for the evaluation of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) inhibitors based on a single HPLC run with UV detection using bovine aortic coronary endothelial cells (BAECs) Inflamm Res. 2001;50:262–9. doi: 10.1007/s000110050752. [DOI] [PubMed] [Google Scholar]
- 54.Kirchheiner J, Meineke I, Steinbach N, Meisel C, Roots I, Brockmöller J. Pharmacokinetics of diclofenac and inhibition of cyclooxygenases 1 and 2: No relationship to the CYP2C9 genetic polymorphism in humans. Br J Clin Pharmacol. 2003;55:51–61. doi: 10.1046/j.1365-2125.2003.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brune K, Hinz B. The discovery and development of antiinflammatory drugs. Arthritis Rheum. 2004;50:2391–9. doi: 10.1002/art.20424. [DOI] [PubMed] [Google Scholar]
- 56.Brune K, Renner B, Hinz B. Using pharmacokinetic principles to optimize pain therapy. Nat Rev Rheumatol. 2010;6:589–98. doi: 10.1038/nrrheum.2010.141. [DOI] [PubMed] [Google Scholar]
- 57.Fowler PD, Shadforth MF, Crook PR, John VA. Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis. Eur J Clin Pharmacol. 1983;25:389–94. doi: 10.1007/BF01037953. [DOI] [PubMed] [Google Scholar]
- 58.Fowler PD, Dawes PT, John VA, Shotton PA. Plasma and synovial fluid concentrations of diclofenac sodium and its hydroxylated metabolites during once-daily administration of a 100 mg slow-release formulation. Eur J Clin Pharmacol. 1986;31:469–72. doi: 10.1007/BF00613526. [DOI] [PubMed] [Google Scholar]
- 59.Altman R, Bosch B, Brune K, Patrignani P, Young C. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs. 2015;75:859–77. doi: 10.1007/s40265-015-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seppälä E, Nissilä M, Isomäki H, Nuotio P, Nykänen E, Laitinen O, et al. Comparison of the effects of different anti-inflammatory drugs on synovial fluid prostanoid concentrations in patients with rheumatoid arthritis. Clin Rheumatol. 1985;4:315–20. doi: 10.1007/BF02031615. [DOI] [PubMed] [Google Scholar]
- 61.Sacerdote P, Carrabba M, Galante A, Pisati R, Manfredi B, Panerai AE. Plasma and synovial fluid interleukin-1, interleukin-6 and substance P concentrations in rheumatoid arthritis patients: Effect of the nonsteroidal anti inflammatory drugs indomethacin, diclofenac and naproxen. Inflamm Res. 1995;44:486–90. doi: 10.1007/BF01837915. [DOI] [PubMed] [Google Scholar]
- 62.Catalano MA. Worldwide safety experience with diclofenac. Am J Med. 1986;80:81–7. doi: 10.1016/0002-9343(86)90085-9. [DOI] [PubMed] [Google Scholar]
- 63.Silva de Oliveira JC, Grossi de Oliveira GA, Bassi AP. Comparative assessment of the effect of ibuprofen and etodolac on edema, trismus, and pain in lower third molar surgery: A randomized clinical trial. J Oral Maxillofac Surg. 2016;74:1524–30. doi: 10.1016/j.joms.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Kubitzek F, Ziegler G, Gold MS, Liu JM, Ionescu E. Analgesic efficacy of low-dose diclofenac versus paracetamol and placebo in postoperative dental pain. J Orofac Pain. 2003;17:237–44. [PubMed] [Google Scholar]
- 65.Gazal G, Al-Samadani KH. Comparison of paracetamol, ibuprofen, and diclofenac potassium for pain relief following dental extractions and deep cavity preparations. Saudi Med J. 2017;38:284–91. doi: 10.15537/smj.2017.3.16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voltaren® (diclofenac) [Prescribing Information]. U.S. Food and Drug Administration: Novartis Pharmaceutical Corporation. 2009. [Last accessed on 2020 Mar 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019201s038lbl.pdf2009 .
- 67.Gan TJ. Diclofenac: An update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26:1715–31. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- 68.Gan TJ, Daniels SE, Singla N. A novel injectable formulation of diclofenac compared with intravenous ketorolac or placebo for acute moderate-to-severe pain after abdominal or pelvic surgery: A multicenter, double-blind, randomized, multiple-dose study. Anesth Analg. 2012;115:1212–20. doi: 10.1213/ANE.0b013e3182691bf9. [DOI] [PubMed] [Google Scholar]
- 69.Desjardins PJ, Olugemo K, Solorio D, Young CL. Pharmacokinetic properties and tolerability of low-dose SoluMatrix diclofenac. Clin Ther. 2015;37:448–61. doi: 10.1016/j.clinthera.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 70.McKenna F. Diclofenac/misoprostol: The European clinical experience. J Rheumatol Suppl. 1998;51:21–30. [PubMed] [Google Scholar]
- 71.Moore N. Coronary risks associated with diclofenac and other NSAIDs: An update. Drug Saf. 2020;43:301–18. doi: 10.1007/s40264-019-00900-8. [DOI] [PubMed] [Google Scholar]


