Abstract
With an estimated worldwide volume of 266 million surgeries in 2015, the call for general inhalation anesthesia is considerable. However, widely used volatile anesthetics such as N2O and the highly fluorinated gases sevoflurane, desflurane, and isoflurane are greenhouse gases, ozone-depleting agents, or both. Because these agents undergo minimal metabolism in the body during clinical use and are primarily (≥95%) eliminated unchanged via exhalation, waste anesthetic gases (WAGs) in operating rooms and postanesthesia care units can pose a challenge for overall elimination and occupational exposure. The chemical properties and global warming impacts of these gases vary, with atmospheric lifetimes of 1−5 years for sevoflurane, 3−6 years for isoflurane, 9−21 years for desflurane, and 114 years for N2O. Additionally, the use of N2O as a carrier gas for the inhalation anesthetics and as a supplement to intravenous (IV) anesthetics further contributes to these impacts. At the same time, unscavenged WAGs can result in chronic occupational exposure of health care workers to potential associated adverse health effects. Few adverse effects associated with WAGs have been documented, however, when workplace exposure limits are implemented. Specific measures that can help reduce occupational exposure and the environmental impact of inhaled anesthetics include efficient ventilation and scavenging systems, regular monitoring of airborne concentrations of waste gases to remain below recommended limits, ensuring that anesthesia equipment is well maintained, avoiding desflurane and N2O if possible, and minimizing fresh gas flow rates (eg, use of low-flow anesthesia). One alternative to volatile anesthetics may be total intravenous anesthesia (TIVA). While TIVA is not associated with the risks of occupational exposure or atmospheric pollution that are inherent to volatile anesthetic gases, clinical considerations should be weighed in the choice of agent. Appropriate procedures for the disposal of IV anesthetics must be followed to minimize any potential for negative environmental effects. Overall, although their contributions are relatively low compared with those of other human-produced substances, inhaled anesthetics are intrinsically potent greenhouse gases and pose a risk to operating-room personnel if not properly managed and scavenged. Factors to reduce waste and minimize the future impact of these substances should be considered.
See Article, p 825
Since the 1950s, the climate system has warmed, causing changes that are projected to have an increasing effect on environmental and social determinants of health such as the need for clean air, safe drinking water, sufficient food, and secure shelter.1,2 According to a 2014 report by the World Health Organization (WHO), such effects are expected to cause an additional 250,000 deaths/y in the coming decades.2 With the human influence on global climate becoming clearer over the last several decades, integrated evidence-based responses from individuals, institutions, and governments are needed more than ever to mitigate the ecological and health effects of climate change. A key contributor to climate change is the emission of greenhouse gases (GHGs),1,3 which includes release of waste anesthetic gases (WAGs) from surgical procedures into the environment (Figure 1). Although anesthesia gases contribute a relatively small portion of GHGs, a strong body of evidence supports the importance of minimizing WAG release into the environment to limit contributions to climate change and associated health risks on the global level and, on an individual level, to minimize occupational exposure and risk of adverse effects.
Figure 1.
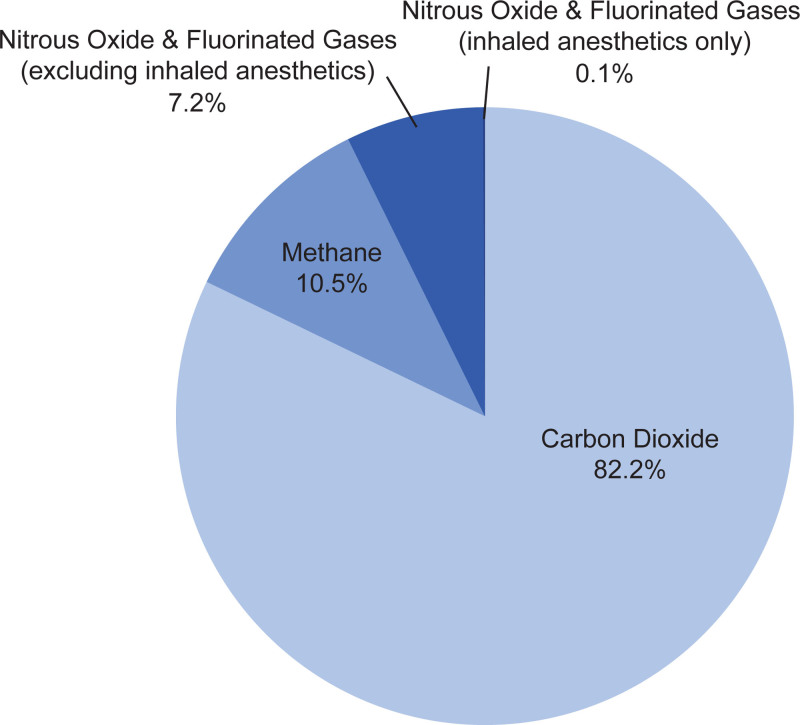
The total annual US GHG emission in 2012 was 6.2 gigatons of CO2 equivalent, of which 6.8% comprised N2O (4.3%) and fluorinated gases (3%; hydrofluorocarbons, perfluorocarbons, sulfur hexafluoride, and nitrogen trifluoride).4 The contributions of inhaled anesthetics (N2O, desflurane, isoflurane, and sevoflurane) to US GHG emissions for 2011−2013 were estimated to be 5.6 million tons of CO2 equivalent (excluding dental, laboratory, and veterinary medicine),5,6 comprising approximately 1% of GHG emissions from the US health care sector6 and approximately 0.1% of total US GHG emissions.4,6 GHG indicates greenhouse gas.
The importance of this issue is further supported by the considerable worldwide volume of surgical procedures, many of which call for general inhalation anesthesia. A 2019 study of global surgery metrics estimated that 266 million surgeries were performed worldwide in 2015, with a global median of 4171 procedures per 100,000 individuals.7 Just in the United States, an estimated 36 million surgeries were performed in 2015, corresponding to 11,113 surgeries per 100,000 individuals. This large surgical volume exposes a broad range of health care workers, including anesthesiologists, dentists/dental personnel, nurse anesthetists, operating-room nurses, operating-room technicians/personnel, recovery-room nurses/personnel, and surgeons, to volatile anesthetics.8 In the United States alone, during 2015, more than a quarter of a million health care workers were potentially exposed to anesthetic gases that leak during procedures (ie, WAGs) and are consequently at risk for associated adverse health effects.9
Given these considerations, this narrative review aimed to summarize the current understanding of the environmental (climate change health effects, greenhouse effect/gases that impact the atmosphere, and effect of anesthetic gases released into the atmosphere) and occupational (agents used, early research on exposure and health impact, exposure limits, and modern exposure and health effects) exposure aspects of volatile anesthetic gases. In this context, specific strategies and recent innovations for hospital anesthesia waste-minimization efforts are also discussed (volatile/inhaled and intravenous [IV] anesthetic alternatives and current strategies to minimize environmental and occupational exposure, including “greening the operating room/operating theater”).
SEARCH STRATEGY
A PubMed search for English-language articles published from January 1, 2000, to June 30, 2020, using the search string (anesthesia and “greenhouse gas”) was conducted to ensure inclusion of the most current literature. Of the 23 articles identified by the PubMed search, 7 did not discuss environmental or occupational exposure to inhaled anesthetic gases or the mitigation thereof and were therefore excluded. The remaining relevant articles were retrieved and reviewed, and relevant references cited in retrieved articles were also reviewed.
VOLATILE ANESTHETICS: ENVIRONMENTAL RELEASE AND POTENTIAL IMPACT
Of the volatile anesthetics, the most widely used include N2O and the highly fluorinated gases sevoflurane (eg, Ultane/Sevorane, AbbVie Inc, North Chicago, IL), desflurane (eg, Suprane, Baxter Healthcare Corporation, Deerfield, IL), and isoflurane (eg, Forane, Baxter Healthcare Corporation) (Figure 2A), all of which have been recognized during the last decade to contribute to climate change by altering the photophysical properties of the atmosphere (Table 1).14,15 N2O and halogenated gases containing chlorine or bromine, such as isoflurane and the older drug halothane, can deplete ozone and diminish the ultraviolet radiation–shielding effect of the ozone layer.15–17 While halogenated gases that lack chlorine or bromine, such as sevoflurane and desflurane, do not catalytically destroy ozone, they remain important examples of climate-harming GHGs because trace amounts in the earth’s atmosphere absorb and reduce outgoing infrared thermal energy, thereby warming the environment. Although the contribution of volatile anesthetics to total GHG emissions is small (0.1%) compared with CO2 (82.2%; Figure 1), it is still important to consider the long-term, cumulative impact of inhaled anesthetics on climate change and pursue strategies to minimize the introduction of these agents into the environment.15,18
Figure 2.
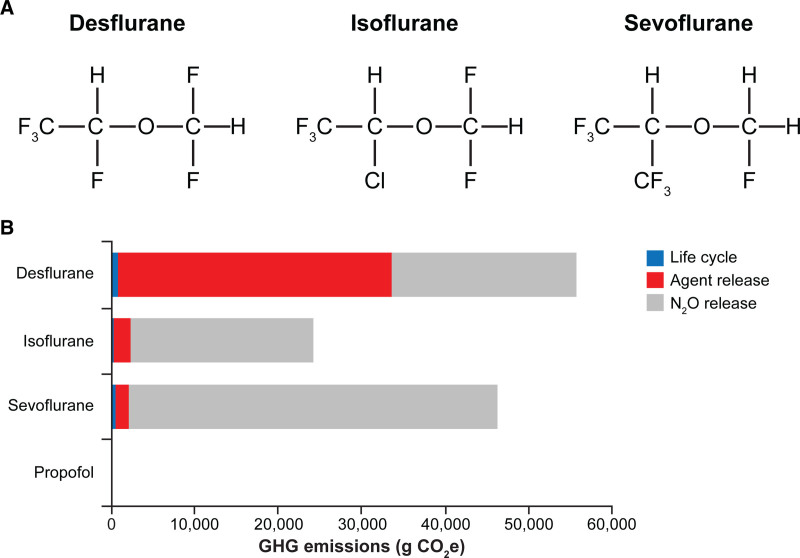
Structure and life-cycle GHG emissions of flurorinated volatile anesthetics. A, Structural formulas of the most frequently used fluorinated volatile anesthetics.10–12 B, Life-cycle GHG emissions of anesthetics, including waste anesthetic gas emissions of halogenated drugs and N2O.13 Life-cycle GHG emissions shown in (B) are based on a functional unit of 1 MAC or MAC-equivalent for propofol, for maintenance anesthesia for an average 70-kg adult patient for 1 h (1 MAC-h). Panel B and 1 MAC-h definition have been reprinted from Sherman J, et al.13 by permission of Wolters Kluwer Health on behalf of the International Anesthesia Research Society. GHG indicates greenhouse gas; MAC, minimum alveolar concentration.
Table 1.
Volatile Anesthetics That Are Ozone Depleters, Greenhouse Gases, or Both
| Anesthetic | Chemical formula | Ozone depleter | Greenhouse gas |
|---|---|---|---|
| Nitrous oxide | N2O | ✓ | ✓ |
| Halothane | CF3CHBrCl | ✓ | ✓ |
| Isoflurane | CHF2OCHClCF3 | ✓ | ✓ |
| Sevoflurane | CH2FOCH(CF3)2 | ✓ | |
| Desflurane | CHF2OCHFCF3 | ✓ |
During clinical use, volatile anesthetics undergo little metabolism in the body and are, for the most part, eliminated via exhalation.14,19–22 For example, ≥95% of N2O, desflurane, isoflurane, and sevoflurane are exhaled unchanged.10–12,23 These agents are typically scavenged in operating rooms from patient exhalation to minimize occupational exposure. However, these medical waste gases are then released into the atmosphere with little to no further processing, where they function as GHGs.22,24
The contributions of GHGs and other agents to climate change are quantified using the global warming potential (GWP), which takes into account the radiative and atmospheric properties of a particular agent.1,15,22,25 Because global warming is assessed in terms of CO2, GWP compares the contribution of a GHG with the same mass of CO2 over a given period of time.
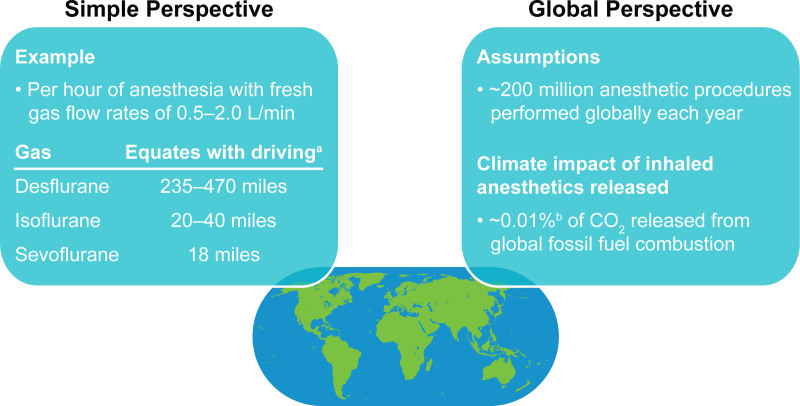
Atmospheric lifetimes vary among volatile anesthetics; of the 3 most commonly used highly fluorinated drugs, sevoflurane has the shortest lifetime (1–5 years) and a lower estimated GWP compared with isoflurane (3–6 years) or desflurane (9–21 years) (Table 2).14,15,26 Overall, life-cycle GHG emissions with desflurane are 15 times larger than those with sevoflurane and 20 times higher than those with isoflurane (Figure 2B).13,18 The use of N2O as a carrier gas for volatiles and as a supplemental with IV anesthetics further contributes to these impacts.13,18 An Australian study confirmed the disproportionate contribution of desflurane to GHG emissions compared with sevoflurane and isoflurane—while desflurane represented a small proportion (21%) of inhaled anesthetic bottles purchased for use in public hospitals in 2011, it accounted for the majority (81%) of the total annual GHG emissions attributed to these inhaled anesthetics.27 In contrast, sevoflurane and isoflurane contributed much smaller proportions (17% and 2%, respectively) of the total annual GHG emissions. The 100-year GWP was calculated to be 893 CO2 equivalents/kg for desflurane, compared with 48 for sevoflurane and 191 for isoflurane.27 Furthermore, a US-based calculation has contextualized GWPs of these anesthetics by estimating that 1 hour of anesthesia with desflurane is equivalent to automobile emissions from driving a distance of 235–470 miles, whereas 1 hour of isoflurane or sevoflurane equates to driving 20−40 or 18 miles, respectively (Figure 3).18
Table 2.
Atmospheric Lifetime of Trace Gases, Including Common Volatile Anesthetics
| Compound | Lifetime (y) |
|---|---|
| N2O | 114 |
| CFCs | 50−100 |
| CO2 | 5−200 |
| Desflurane | 8.9−21.0 |
| Halothane | 1.0−7.0 |
| Isoflurane | 2.6−5.9 |
| Sevoflurane | 1.1−5.2 |
Figure 3.
Global warming impact of inhaled anesthetics in perspective.15,18 aAssumes a US automobile average for CO2 emissions of 398 g/mile. bDetermining the precise climate impact of worldwide anesthetic procedures is complicated because of limited available data on usage or production of anesthetic agents.
To provide additional context around the contributions of volatile anesthetics to climate change, the discussions by Sulbaek Andersen et al15,28 on the climate impact of isoflurane, desflurane, and sevoflurane assumed that in the region of 200 million procedures involving these gases are performed each year. In their report, they tabulated best estimates of atmospheric lifetimes, ozone-depletion potential, radiative efficiencies, and GWP for N2O, halothane, enflurane, isoflurane, desflurane, and sevoflurane.15 Based on these data, they concluded that, although inhaled anesthetics are estimated to represent a small contribution relative to CO2 and total GHG emissions (around 0.1% of CO2 released from global fossil fuel combustion [Figure 1]), it remains important to consider the long-term, cumulative impact of inhaled anesthetics on climate change (Figure 3).15
VOLATILE ANESTHETICS: OCCUPATIONAL EXPOSURE AND POTENTIAL IMPACT
Minimizing the impact of anesthetic gases not only contributes to the protection of the environment but also takes into account the potential health hazard to individuals who experience chronic risk of occupational exposure from waste gases.
Potential Occupational Hazards Associated With Volatile Anesthetics
Early survey-based studies from the 1970s suggested some risk of health hazards (eg, liver disease, renal disease, neurologic disease, cancer, spontaneous miscarriage, or congenital abnormalities) among health care personnel exposed to inhaled anesthetics (primarily N2O, diethyl ether, halothane, and enflurane29) in working environments with poor or inadequate scavenging of inhaled anesthetics.30–34 Compared with the anesthetics and scavenging systems presently used, exposure levels of anesthetic gases in the operating room were, in general, higher in the era in which these studies were conducted (eg, halothane and N2O at levels >2 and >25 ppm, respectively, with reports as high as 85 and 7000 ppm [time-weighted averages]).32,35–37 More recently, the potential for genetic damage and oxidative stress caused by exposure to WAGs has been recognized,37 and guidance on exposure limits has been put in place to decrease health risks associated with occupational exposure.38–40
Governmental Implementation of Exposure Limits
To ensure occupational safety around inhaled anesthetics, several countries have established recommended exposure limits (Table 3).39 In 1977, the US National Institute for Occupational Safety and Health (NIOSH) recommended that occupational exposure to halogenated anesthetics agents should not exceed 2 ppm or N2O >25 ppm within a 1-hour period (time-weighted average for exposure duration) and that anesthetic gas machines, nonrebreathing systems, and T-tube devices have effective scavenging devices to collect all WAGs.35 The current guidance from the US Department of Labor Occupational Safety and Health Administration (OSHA) for workplace exposures, created in 1999 and last revised in 2000, recommends minimizing exposure to all waste and trace gases for worker health and safety.41 Following these safety measure regulations in the United States, many other countries have followed suit with their own guidelines,37 although occupational exposure to inhaled anesthetics has been shown to exceed exposure limits in some circumstances (eg, 8-hour time-weighted averages of halothane and N2O of 10 and 100 ppm, respectively).42,43
Table 3.
Recommended Exposure Limits for Volatile Anesthetics (Daily Exposure Limits in ppm)
| Country | N2O | Halothane | Desflurane | Isoflurane | Sevoflurane |
|---|---|---|---|---|---|
| Finland | 100 | 1 | 10 | 10 | 10 |
| Sweden | 100 | 5 | 10 | 10 | 10 |
| Denmark | 50 | 5 | 5 | 5 | 5 |
| Norway | 50 | 0.02 | 20 | 2 | 20 |
| Austria | 100 | 5 | - | 10 | 10 |
| Germany | 100 | 5 | - | - | - |
| United Kingdom | 100 | 10 | - | 50 | - |
| Switzerland | 100 | 5 | - | 10 | - |
| Belgium | 50 | 50 | - | - | - |
| Spain | 50 | 50 | - | 50 | - |
| United States (NIOSH) | 25a | 2a | 2a | 2a | 2a |
Adapted and reprinted from Molina Aragonés et al39 with permission from the Oxford University Press on behalf of the Society of Occupational Medicine.
Abbreviation: NIOSH, National Institute for Occupational Safety and Health.
Exposure level that cannot be exceeded during a 1-h period.
Impact of Waste Anesthesia Gas Regulations in the Workplace
With the implementation of guidelines limiting workplace exposure, studies have confirmed little to no increase in adverse effects associated with WAGs when gases are scavenged effectively.37,39,44 A 2016 systematic review of occupational exposure showed that evidence for adverse effects due to volatile anesthetics for personnel at risk of exposure is scarce and inconsistent, with evidence from many studies weakened by flaws in methodology and data collection.39 Furthermore, no compelling evidence of significant adverse effects (eg, genotoxicity, congenital anomalies, and biomarkers of dysfunction) was found when environmental levels were kept within recommended exposure limits by using adequately designed and appropriately maintained facilities and exposure-minimization approaches.39 However, some studies in facilities with poor air control or scavenging efficiency or in developing countries have demonstrated oxidative stress, genotoxicity, and adverse health effects resulting from occupational exposure to anesthetics.37,45–47 Therefore, a potential health risk may remain for individuals chronically exposed to inhaled anesthetics in nonscavenged working environments or in workplaces with poor or inadequate air control where WAG exposure may exceed recommended limits.41
STRATEGIES FOR ENVIRONMENTAL AND OCCUPATIONAL IMPROVEMENT: HOSPITAL ANESTHESIA AND MINIMIZATION OF WASTE AND EXPOSURE
Around the world, the identification of environmental and occupational hazards for WAGs spurred the implementation of regulations by many governmental authorities. Consequently, hospitals and other settings that deliver inhaled anesthesia have increasingly sought to mitigate negative effects of WAGs through a variety of strategies and recent innovations.
Approaches to Minimize the Environmental Impact of Volatile Anesthetic Gases
Updated strategies recommended by the American Society of Anesthesiologists and other experts to decrease WAGs include avoiding N2O as a carrier gas and minimizing fresh gas flow (FGF) rates.5,18,48–51 Ryan and Nielsen18 have estimated that the best approximations of ideal FGF rates would be achieved by reducing FGF to 2 L/min with sevoflurane and to 0.5−1 L/min with desflurane and isoflurane. Use of closed-circle breathing systems and low-flow anesthesia further increases the efficiency of administration and reduces the amount of inhaled agents used and associated environmental and occupational exposure.52
Because N2O is both an ozone depleter and a GHG, with an atmospheric lifetime of 114 years, use of N2O as a carrier gas versus air/oxygen substantially increases the global warming effects of sevoflurane and isoflurane.18 Compared with sevoflurane and isoflurane, desflurane has much higher life-cycle GHG emissions (15 and 20 times higher, respectively), owing to a combination of higher required concentration and higher radiative forcing effect.5,13 Therefore, avoidance of both N2O and desflurane is recommended, unless use of either could reduce morbidity and mortality compared with other anesthetics.5,50
New technologies are also being investigated to reduce WAG release into the atmosphere. In a study comparing manual versus automated control of end-tidal anesthetic gases, automated control significantly reduced GHG emissions by 44%.53 A second study demonstrated that changing from a traditional CO2 absorbent to one that is nonreactive allowed for further reduction of FGF rates, which reduced both the amount of volatile anesthetic needed as well as the amount vented into the environment.54 A recent proof-of-concept study of a photochemical exhaust gas destruction system demonstrated efficient removal of desflurane and sevoflurane, although removal of N2O requires further optimization.55 These and similar strategies provide valuable reductions in the environmental impact of volatile anesthetic gases that can often be implemented in a cost-neutral or even cost-saving fashion.53–55
Approaches to Minimize Occupational Exposure and Potential Health Impact of Volatile Anesthetic Gases
To manage and minimize occupational exposure to WAGs, NIOSH and others highlight the pivotal importance of using an efficient air ventilation and scavenging system,8,23,56,57 and, although a full list of countries is not available, reports suggests that this approach is being adopted around the world.56,58,59 Survey data have shown that approximately 97% of anesthetic administrators report consistent use of a waste gas scavenging system.9
Scavenging systems need to be in place not only in operating rooms but also in postanesthesia care units (PACUs) where residual gases exhaled by patients also need to be removed by effective ventilation methods.56 Scavenging devices/anesthetic conserving devices have been shown to limit occupational exposure in recovery units following use of fluorinated inhaled anesthetics (ie, maintaining sevoflurane and desflurane time-weighted averages <2 ppm).60,61 Although trace amounts of sevoflurane have been detected in PACUs equipped with controlled air exchange systems, occupational limits were not exceeded.62
Overall, regular monitoring of airborne waste gas concentrations should be performed in all personnel breathing zones.8 This may include not only operating facilities or recovery rooms/PACUs with no (or suboptimal) ventilation/scavenging systems but also similar settings with good scavenging/venting systems in place. Even in the latter case, health care workers may be exposed as a result of anesthetic breathing circuit leaks (eg, connectors, tubing, and valves), gas hookup and disconnection issues, gas seepage from patient mask or endotracheal apparatus (eg, during pediatric anesthesia if the mask is poorly fitted), induction leaks, or other dental surgery issues.8,42 Daily checks for leaks of anesthetic gases and the correct functioning of the scavenging and ventilation systems are required, and regular maintenance of all equipment, including preventive maintenance, should be performed and documented.8,63
In conjunction with facility-based leakage monitoring, a medical surveillance program of all staff exposed to waste gases is also recommended.8 For example, in the United States, NIOSH recommends obtaining baseline values and periodic monitoring of hepatic and renal function for exposed personnel as well as documentation of pertinent medical history information such as pregnancy outcomes for both female workers and female partners of male workers.8
To minimize WAGs in settings that administer volatile anesthetics, preventive measures are discussed in the current US guidance on WAGs from NIOSH and include the following:
A complete anesthesia apparatus check should be performed each day/before each use8,41
Face masks must fit properly and provide an effective seal8,23,56
Cuffs on tracheal tubes and laryngeal masks must be inflated adequately8,23
Vaporizers should be carefully filled in well-ventilated areas8
Vaporizers with a closed filling system should be used (risk for accidental spillage and leakage is negligible)59
Before disconnecting a patient from a breathing system, residual gases should be eliminated through the scavenging system as much as possible8,41
Added protection may also be gained from use of filters in anesthesia machines. Charcoal filters are often used as the final capture device to prevent halogenated anesthetic agents from dispersing into the atmosphere.5,64–66 Use of a closed filling system for vaporizers has also been shown to decrease ambient air contamination by inhaled anesthetics. One vaporizer system with an integral valve filling adaptor that connects directly into the vaporizer reduced sevoflurane contamination of ambient air by approximately 60% compared with other systems that used screw cap closures and vaporizer-specific adaptors for filling.67 This closed filling system also maintained sevoflurane exposure (0.10 ppm) in the operator breathing zone that was well below the recommended maximum levels (2 ppm for 1 hour or 20 ppm for 15 minutes) and may help minimize occupational exposure.68
Total Intravenous Anesthesia
Based on the issues associated with volatile anesthetics discussed above, total intravenous anesthesia (TIVA) may be considered as an alternative to volatile/inhaled anesthetics.13,42,51,57 By its nature, TIVA is not associated with the risks of occupational exposure inherent to volatile anesthetic gases; however, TIVA is not entirely devoid of potential negative environmental effects and the total environmental impact of TIVA must be taken into account.50
Prediction of environmental hazards and the potential environmental impact of pharmaceuticals can be categorized by their impact on an aquatic environment according to a precautionary principle composed of 3 characteristics69: persistence (P; ability to resist degradation in the aquatic environment), bioaccumulation (B; accumulation in adipose tissue of aquatic organisms), and toxicity (T; potential to poison aquatic organisms). Based on these characteristics, the “hazard score” indicates the environmental hazard associated with a particular substance such as a certain volatile or total IV anesthetic, calculated by assigning a numerical value of 0–3: P: 0 (not persistent) or 3 (persistent); B: 0 (does not bioaccumulate) or 3 (does bioaccumulate); T: 0–3, (nontoxic, very toxic, highly toxic). When summed, these characteristics provide a total PBT index that ranges from 0 to 9, with a higher value indicating a greater environmental hazard.69 For example, if a compound is “P,” “B,” and “T” (ie, fulfills the specific criteria for all 3 properties), then it is automatically categorized as hazardous to the environment. Likewise, if a compound fulfills the criteria for “very persistent” and “very bioaccumulative” (vB), then it is also considered hazardous to the environment, regardless of predicted levels of exposure and toxicity. Because insufficient evidence exists to assess risk, desflurane, isoflurane, sevoflurane, and N2O do not have hazard scores, indicating that environmental risk cannot be excluded.69–71 In comparison, although the widely used IV anesthetic propofol has demonstrated toxicity in aquatic organisms and disposal via incineration is recommended,72 propofol has a hazard score of 4, indicating low environmental risk.73,74 However, studies have shown that 32%–49% of dispensed propofol is unused and disposed of as waste,75,76 and not all institutions incinerate unused propofol.77 While improper disposal methods and subsequent release into the environment may add to the negative impact of TIVA with agents such as propofol, accumulation of propofol in the environment has not been reliably estimated.13,76,77 Regional or multimodal anesthesia, either alone or in combination with TIVA or inhaled anesthetics, may also be viable alternatives for some procedures.51,78,79
CONCLUSIONS
In providing a narrative overview of the impact of anesthetic waste and the significance of overall hospital waste, we have noted that inhaled anesthetics contribute to GHG emissions, although their contributions are lower than those of other human-produced substances. This notwithstanding, these volatile agents may also pose a potential health risk to operating-room personnel if not properly managed and scavenged.
Overall, factors to reduce waste and minimize the future impact of these substances should be considered. Specific measures that can be implemented to help reduce occupational exposure and the environmental impact of inhaled anesthetics include utilizing an efficient ventilation and scavenging system, regularly monitoring airborne concentrations of waste gases to maintain environmental levels below recommended limits, ensuring that anesthesia equipment is well maintained without leaks, avoiding desflurane and N2O if possible, and using appropriate FGF rates. TIVA may also be an alternative to inhaled anesthetics, because it is not associated with risks from occupational exposure, but agents such as propofol must be disposed of appropriately. In addition, use of these mitigation measures has demonstrated not only reduced environmental and occupational impact but also reduced financial impact.80 Further research may be needed to understand fully the long-term impacts and occupational exposure risk and outcomes associated with such exposure, and an increased focus on education and awareness among individuals, institutions, and governments may help to mitigate the environmental and occupational health footprint associated with global surgical use of volatile anesthetics.
DISCLOSURES
Name: Shane Varughese, MD.
Contribution: This author participated in the drafting, review, and approval of the publication and had access to all relevant data.
Conflicts of Interest: S. Varughese is a full-time employee of AbbVie and owns AbbVie stock.
Name: Raza Ahmed, MD.
Contribution: This author participated in the drafting, review, and approval of the publication and had access to all relevant data.
Conflicts of Interest: R. Ahmed is a full-time employee of AbbVie and owns AbbVie stock.
This manuscript was handled by: Ken B. Johnson, MD.
GLOSSARY
- B
- bioaccumulation
- CFC
- chlorofluorocarbon
- FGF
- fresh gas flow
- GHG
- greenhouse gas
- GWP
- global warming potential
- IV
- intravenous
- MAC
- minimum alveolar concentration
- NIOSH
- National Institute for Occupational Safety and Health
- OSHA
- Occupational Safety and Health Administration
- P
- persistence
- PACU
- postanesthesia care unit
- T
- toxicity
- TIVA
- total intravenous anesthesia
- vB
- very bioaccumulative
- WAG
- waste anesthetic gas
- WHO
- World Health Organization
Published ahead of print April 15 2021.
Funding: AbbVie is the manufacturer of Ultane/Sevorane (sevoflurane) and funded this work; contributed to its design; and was involved in the collection, analysis, and interpretation of the data and in the writing, review, and approval of the publication. Medical writing support was provided by C. L. Mitchell and J. Matsuura of ICON (North Wales, PA) and was funded by AbbVie.
Conflicts of Interest: See Disclosures at the end of the article.
Listen to this Article of the Month podcast and more from OpenAnesthesia.org® by visiting http://journals.lww.com/anesthesia-analgesia/pages/default.aspx.
Reprints will not be available from the authors.
REFERENCES
- 1.Pachauri RK, Meyer LAIntergovernmental Panel on Climate Change; Core Writing Team; Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 2015. Accessed July 23, 2020. https://www.ipcc.ch/report/ar5/syr/. [Google Scholar]
- 2.World Health Organization. Climate Change and Health. Fact Sheet 266. Last updated February 1, 2018. Accessed July 23, 2020. www.who.int/mediacentre/factsheets/fs266/en/.
- 3.World Resources Institute. World Greenhouse Gas Emissions. 2016. Accessed July 23, 2020. https://www.wri.org/resources/data-visualizations/world-greenhouse-gas-emissions-2016.
- 4.World Resources Institute. CAIT Climate Data Explorer. 2015. Accessed July 23, 2020. http://cait.wri.org.
- 5.Sherman J, McGain F. Environmental sustainability in anesthesia: pollution prevention and patient safety. Adv Anesth. 2016;34:47–61. [Google Scholar]
- 6.Sherman JD, Schonberger RB, Eckelman M. Estimate of carbon dioxide equivalents of inhaled anesthetics in the United States [abstract A3196]. Presented at the Anesthesiology Annual Meeting. October 11–15, 2014; New Orleans, LA. [Google Scholar]
- 7.Holmer H, Bekele A, Hagander L, et al. Evaluating the collection, comparability and findings of six global surgery indicators. Br J Surg. 2019;106:e138–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Occupational Safety and Health. Waste Anesthetic Gases: Occupational Hazards in Hospitals. 2007.Department of Health and Human Services, Centers for Disease Control and Prevention; Publication No. 2007-151 [Google Scholar]
- 9.Boiano JM, Steege AL. Precautionary practices for administering anesthetic gases: a survey of physician anesthesiologists, nurse anesthetists and anesthesiologist assistants. J Occup Environ Hyg. 2016;13:782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forane™ (isoflurane). Full Prescribing Information. 2017.Baxter Healthcare Corporation; [Google Scholar]
- 11.Ultane® (sevoflurane). Full Prescribing Information. 2017.AbbVie Inc, [Google Scholar]
- 12.Suprane® (desflurane). Full Prescribing Information. 2019.Baxter Healthcare Corporation, [Google Scholar]
- 13.Sherman J, Le C, Lamers V, Eckelman M. Life cycle greenhouse gas emissions of anesthetic drugs. Anesth Analg. 2012;114:1086–1090. [DOI] [PubMed] [Google Scholar]
- 14.Ishizawa Y. General anesthetic gases and the global environment. Anesth Analg. 2011;112:213–217. [DOI] [PubMed] [Google Scholar]
- 15.Sulbaek Andersen MP, Nielsen OJ, Wallington TJ, Karpichev B, Sander SP. Medical intelligence article: assessing the impact on global climate from general anesthetic gases. Anesth Analg. 2012;114:1081–1085. [DOI] [PubMed] [Google Scholar]
- 16.Langbein T, Sonntag H, Trapp D, et al. Volatile anaesthetics and the atmosphere: atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane. Br J Anaesth. 1999;82:66–73. [DOI] [PubMed] [Google Scholar]
- 17.Fahey DW, Hegglin MI. Twenty Questions and Answers About the Ozone Layer: 2010 Update. World Meteorological Organization; Accessed July 23, 2020. https://www.esrl.noaa.gov/csl/assessments/ozone/2010/twentyquestions/. [Google Scholar]
- 18.Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92–98. [DOI] [PubMed] [Google Scholar]
- 19.Shiraishi Y, Ikeda K. Uptake and biotransformation of sevoflurane in humans: a comparative study of sevoflurane with halothane, enflurane, and isoflurane. J Clin Anesth. 1990;2:381–386. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda N, Lockhart SH, Eger EI, II, et al. Kinetics of desflurane, isoflurane, and halothane in humans. Anesthesiology. 1991;74:489–498. [DOI] [PubMed] [Google Scholar]
- 21.Kharasch ED. Biotransformation of sevoflurane. Anesth Analg. 1995;81:S27–S38. [DOI] [PubMed] [Google Scholar]
- 22.Sherman JD, Ryan S. Ecological responsibility in anesthesia practice. Int Anesthesiol Clin. 2010;48:139–151. [DOI] [PubMed] [Google Scholar]
- 23.Yasny JS, White J. Environmental implications of anesthetic gases. Anesth Prog. 2012;59:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mchaourab A, Arain SR, Ebert TJ. Lack of degradation of sevoflurane by a new carbon dioxide absorbent in humans. Anesthesiology. 2001;94:1007–1009. [DOI] [PubMed] [Google Scholar]
- 25.Van Norman GA, Jackson S. The anesthesiologist and global climate change: an ethical obligation to act. Curr Opin Anaesthesiol. 2020;33:577–583. [DOI] [PubMed] [Google Scholar]
- 26.Bosenberg M. Anaesthetic gases: environmental impact and alternatives. South Afr J Anaesth Analg. 2011;17:345–348. [Google Scholar]
- 27.Weinberg L, Tay S, Aykanat V, et al. Changing patterns in volatile anaesthetic agent consumption over seven years in Victorian public hospitals. Anaesth Intensive Care. 2014;42:579–583. [DOI] [PubMed] [Google Scholar]
- 28.Sulbaek Andersen MP, Sander SP, Nielsen OJ, Wagner DS, Sanford TJ, Jr, Wallington TJ. Inhalation anaesthetics and climate change. Br J Anaesth. 2010;105:760–766. [DOI] [PubMed] [Google Scholar]
- 29.Whalen FX, Bacon DR, Smith HM. Inhaled anesthetics: an historical overview. Best Pract Res Clin Anaesthesiol. 2005;19:323–330. [DOI] [PubMed] [Google Scholar]
- 30.Cohen EN, Bellville JW, Brown BW., Jr.Anesthesia, pregnancy, and miscarriage: a study of operating room nurses and anesthetists. Anesthesiology. 1971;35:343–347. [DOI] [PubMed] [Google Scholar]
- 31.Knill-Jones RP, Rodrigues LV, Moir DD, Spence AA. Anaesthetic practice and pregnancy. Controlled survey of women anaesthetists in the United Kingdom. Lancet. 1972;1:1326–1328. [DOI] [PubMed] [Google Scholar]
- 32.American Society of Anesthesiologists. Occupational disease among operating room personnel: a national study. Report of an Ad Hoc Committee on the Effect of Trace Anesthetics on the Health of Operating Room Personnel. Anesthesiology. 1974;41:321–340. [PubMed] [Google Scholar]
- 33.Knill-Jones RP, Newman BJ, Spence AA. Anesthetic practice and pregnancy. Controlled survey of male anaesthetists in the United Kingdom. Lancet. 1975;2:807–809. [PubMed] [Google Scholar]
- 34.Cohen EN, Gift HC, Brown BW, et al. Occupational disease in dentistry and chronic exposure to trace anesthetic gases. J Am Dent Assoc. 1980;101:21–31. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Occupational Safety and Health. Criteria Document 77-140: Criteria for a Recommended Standard: Occupational Exposure to Waste Anesthetic Gases and Vapors. 1977. Accessed March 25, 2021. https://www.cdc.gov/niosh/docs/77-140/default.html.
- 36.Hemminki K, Kyyrönen P, Lindbohm ML. Spontaneous abortions and malformations in the offspring of nurses exposed to anaesthetic gases, cytostatic drugs, and other potential hazards in hospitals, based on registered information of outcome. J Epidemiol Community Health. 1985;39:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucio LMC, Braz MG, do Nascimento Junior P, Braz JRC, Braz LG. Occupational hazards, DNA damage, and oxidative stress on exposure to waste anesthetic gases. Rev Bras Anestesiol. 2018;68:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weimann J. Toxicity of nitrous oxide. Best Pract Res Clin Anaesthesiol. 2003;17:47–61. [DOI] [PubMed] [Google Scholar]
- 39.Molina Aragonés JM, Ayora Ayora A, Barbara Ribalta A, et al. Occupational exposure to volatile anaesthetics: a systematic review. Occup Med (Lond). 2016;66:202–207. [DOI] [PubMed] [Google Scholar]
- 40.Lew V, McKay E, Maze M. Past, present, and future of nitrous oxide. Br Med Bull. 2018;125:103–119. [DOI] [PubMed] [Google Scholar]
- 41.Occupational Safety and Health Administration. Anesthetic Gases: Guidelines for Workplace Exposures. 2020. Accessed July 23, 2020. https://www.osha.gov/dts/osta/anestheticgases/index.html.
- 42.Irwin MG, Trinh T, Yao CL. Occupational exposure to anaesthetic gases: a role for TIVA. Expert Opin Drug Saf. 2009;8:473–483. [DOI] [PubMed] [Google Scholar]
- 43.Henderson KA, Matthews IP. An environmental survey of compliance with occupational exposure standards (OES) for anaesthetic gases. Anaesthesia. 1999;54:941–947. [DOI] [PubMed] [Google Scholar]
- 44.Tankó B, Molnár L, Fülesdi B, Molnár C. Occupational hazards of halogenated volatile anesthetics and their prevention: review of the literature. J Anesth Clin Res. 2014;5:1–7. [Google Scholar]
- 45.Guirguis SS, Pelmear PL, Roy ML, Wong L. Health effects associated with exposure to anaesthetic gases in Ontario hospital personnel. Br J Ind Med. 1990;47:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saurel-Cubizolles MJ, Estryn-Behar M, Maillard MF, Mugnier N, Masson A, Monod G. Neuropsychological symptoms and occupational exposure to anaesthetics. Br J Ind Med. 1992;49:276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teschke K, Abanto Z, Arbour L, et al. Exposure to anesthetic gases and congenital anomalies in offspring of female registered nurses. Am J Ind Med. 2011;54:118–127. [DOI] [PubMed] [Google Scholar]
- 48.Feldman JM. Managing fresh gas flow to reduce environmental contamination. Anesth Analg. 2012;114:1093–1101. [DOI] [PubMed] [Google Scholar]
- 49.Hönemann C, Hagemann O, Doll D. Inhalational anaesthesia with low fresh gas flow. Indian J Anaesth. 2013;57:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Axelrod D, Bell C, Feldman J, et al. Greening the Operating Room and Perioperative Arena: Environmental Sustainability for Anesthesia Practice. 2014. American Society of Anesthesiologists. Accessed June 2, 2020. https://www.asahq.org/about-asa/governance-and-committees/asa-committees/committee-on-equipment-and-facilities/environmental-sustainability/greening-the-operating-room. [Google Scholar]
- 51.Petre MA, Malherbe S. Environmentally sustainable perioperative medicine: simple strategies for anesthetic practice. Can J Anaesth. 2020;67:1044–1063. [DOI] [PubMed] [Google Scholar]
- 52.Brattwall M, Warrén-Stomberg M, Hesselvik F, Jakobsson J. Brief review: theory and practice of minimal fresh gas flow anesthesia. Can J Anaesth. 2012;59:785–797. [DOI] [PubMed] [Google Scholar]
- 53.Tay S, Weinberg L, Peyton P, Story D, Briedis J. Financial and environmental costs of manual versus automated control of end-tidal gas concentrations. Anaesth Intensive Care. 2013;41:95–101. [DOI] [PubMed] [Google Scholar]
- 54.Epstein RH, Dexter F, Maguire DP, Agarwalla NK, Gratch DM. Economic and environmental considerations during low fresh gas flow volatile agent administration after change to a nonreactive carbon dioxide absorbent. Anesth Analg. 2016;122:996–1006. [DOI] [PubMed] [Google Scholar]
- 55.Rauchenwald V, Rollins MD, Ryan SM, et al. New method of destroying waste anesthetic gases using gas-phase photochemistry. Anesth Analg. 2020;131:288–297. [DOI] [PubMed] [Google Scholar]
- 56.Byhahn C, Wilke HJ, Westpphal K. Occupational exposure to volatile anaesthetics: epidemiology and approaches to reducing the problem. CNS Drugs. 2001;15:197–215. [DOI] [PubMed] [Google Scholar]
- 57.Wyssusek KH, Keys MT, van Zundert AAJ. Operating room greening initiatives - the old, the new, and the way forward: a narrative review. Waste Manag Res. 2019;37:3–19. [DOI] [PubMed] [Google Scholar]
- 58.Asefzadeh S, Raeisi A, Mousavi A. Risk management status of waste anesthetic gases using ECRI institute standards. Iran J Public Health. 2012;41:85–91. [PMC free article] [PubMed] [Google Scholar]
- 59.Oliveira CR. Occupational exposure to anesthetic gases residue. Rev Bras Anestesiol. 2009;59:110–124. [DOI] [PubMed] [Google Scholar]
- 60.González-Rodríguez R, Muñoz Martínez A, Galan Serrano J, Moral García MV. Health worker exposure risk during inhalation sedation with sevoflurane using the (AnaConDa®) anaesthetic conserving device. Rev Esp Anestesiol Reanim. 2014;61:133–139. [DOI] [PubMed] [Google Scholar]
- 61.Tallent R, Corcoran J, Sebastian J. Evaluation of a novel waste anaesthetic gas scavenger device for use during recovery from anaesthesia. Anaesthesia. 2018;73:59–64. [DOI] [PubMed] [Google Scholar]
- 62.Heiderich S, Thoben C, Dennhardt N, et al. Low anaesthetic waste gas concentrations in postanaesthesia care unit: a prospective observational study. Eur J Anaesthesiol. 2018;35:534–538. [DOI] [PubMed] [Google Scholar]
- 63.Sub-Committee of ASA Committee on Equipment and Facilities. ASA Recommendations for Pre-Anesthesia Checkout. 2020. Accessed October 2019. https://www.asahq.org/standards-and-guidelines/2008-asa-recommendations-for-pre-anesthesia-checkout.
- 64.Enderby DH, Bushman JA, Askill S. Investigations of some aspects of atmospheric pollution by anaesthetic gases. II: aspects of adsorption and emission of halothane by different charcoals. Br J Anaesth. 1977;49:567–573. [DOI] [PubMed] [Google Scholar]
- 65.Højkjaer Larsen V, Severinsen I, Waaben J. Removal of halogenated anaesthetics from a closed circle system with a charcoal filter. Acta Anaesthesiol Scand. 1989;33:374–378. [DOI] [PubMed] [Google Scholar]
- 66.Division of Occupational Health and Safety (DOHS) WAG Program Manager. Office of Research Services: Waste Anesthetic Gas. 2019. National Institutes of Health, Division of Occupational Health and Safety, Technical Assistance Branch. Accessed July 23, 2020. https://www.ors.od.nih.gov/sr/dohs/Documents/Waste%20Anesthetic%20Gas%20(WAG)%20Surveillance%20Program.pdf. [Google Scholar]
- 67.Heijbel H, Bjurstöm R, Jakobsson JG. Personnel breathing zone sevoflurane concentration adherence to occupational exposure limits in conjunction with filling of vaporisers. Acta Anaesthesiol Scand. 2010;54:1117–1120. [DOI] [PubMed] [Google Scholar]
- 68.Varughese S, Bacher HP. Validation of waste anaesthetic gas exposure limits when using a closed vaporizer filling system: a laboratory-based study. Adv Ther. 2020;37:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stockholm City Council. Pharmaceuticals and Environment. 2019. Accessed July 23, 2020. https://www.janusinfo.se/beslutsstod/lakemedelochmiljo/pharmaceuticalsandenvironment.4.7b57ecc216251fae47487d9a.html.
- 70.Stockholm City Council. Pharmaceuticals and Environment: Desflurane. 2016. Accessed July 23, 2020. https://www.janusinfo.se/beslutsstod/lakemedelochmiljo/pharmaceuticalsandenvironment/databaseenven/desflurane.5.30a7505616a041a09b063b10.html.
- 71.Stockholm City Council. Pharmaceuticals and Environment: Isoflurane. 2016. Accessed July 23, 2020. https://www.janusinfo.se/beslutsstod/lakemedelochmiljo/pharmaceuticalsandenvironment/databaseenven/isoflurane.5.30a7505616a041a09b063c4f.html.
- 72.AstraZeneca Pty Ltd. Material Safety Data Sheet: Diprivan® (Propofol) 1% & 2%. 2012. Accessed September 21, 2020. https://www.astrazeneca.com.au/content/dam/az-au/Material%20Safety%20Data/Diprivanltsupgt174ltsupgt.pdf.
- 73.Stockholm City Council. Pharmaceuticals and Environment: Propofol. 2020. Accessed July 23, 2020. https://www.janusinfo.se/beslutsstod/lakemedelochmiljo/pharmaceuticalsandenvironment/databaseenven/propofol.5.30a7505616a041a09b063eac.html.
- 74.AstraZeneca. Environmental Risk Assessment Data: Propofol. 2020. Accessed September 21, 2020. https://www.astrazeneca.com/content/dam/az/our-company/Sustainability/2017/Propofol.pdf.
- 75.Gillerman RG, Browning RA. Drug use inefficiency: a hidden source of wasted health care dollars. Anesth Analg. 2000;91:921–924. [DOI] [PubMed] [Google Scholar]
- 76.Mankes RF. Propofol wastage in anesthesia. Anesth Analg. 2012;114:1091–1092. [DOI] [PubMed] [Google Scholar]
- 77.Schneider D, Ponto J, Martin E. Propofol disposal in the anesthesia setting: overcoming barriers. AANA J. 2017;85:417–423. [PubMed] [Google Scholar]
- 78.Savitha KS, Dhanpal R, Shilpa J. The effect of multimodal analgesia on minimum alveolar concentration of isoflurane for skin incision at constant bispectral index. Anesth Essays Res. 2016;10:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuvadia M, Cummis CE, Liguori G, Wu CL. ‘Green-gional’ anesthesia: the non-polluting benefits of regional anesthesia to decrease greenhouse gases and attenuate climate change. Reg Anesth Pain Med. 2020;45:744–745. [DOI] [PubMed] [Google Scholar]
- 80.Zuegge KL, Bunsen SK, Volz LM, et al. Provider education and vaporizer labeling lead to reduced anesthetic agent purchasing with cost savings and reduced greenhouse gas emissions. Anesth Analg. 2019;128:e97–e99. [DOI] [PubMed] [Google Scholar]