Abstract
Meloxicam is commonly administrated to control postoperative pain in orthopedic surgery, while its efficacy in total knee arthroplasty (TKA) is not clear. Therefore, this study aimed to explore the postoperative analgesic effect and tolerance of meloxicam in knee osteoarthritis (OA) patients undergoing TKA.
Totally, 128 knee OA patients scheduled for TKA were enrolled in this randomized, controlled, double-blind study, then randomized into meloxicam group (N = 65) and control group (N = 63) as 1:1 ratio. Patients took meloxicam or placebo from 4 hours (h) to 72 h after TKA. Patients were followed up at 6 h, 12 h, day (D)1, D2, D3, D7, month (M)1, and M3.
Pain visual analog scale score at rest was decreased in meloxicam group at 12 h, D1 and D3 compared to control group; pain visual analog scale score at flexion was reduced in meloxicam group at 6 h, 12 h, D1, D2, and D3 compared to control group. Additional and total consumption of patient-controlled analgesia were both attenuated in meloxicam group compared to control group. Furthermore, patient satisfaction score was higher on D1, D2, D3 in meloxicam group compared to control group. However, no difference of hospital for special surgery knee score score at M1 or M3 was found between the 2 groups. Moreover, the occurrence of adverse events was similar between the 2 groups.
Meloxicam displays good effect on controlling postoperative pain and improving patient satisfaction, while does not affect long-term knee function recovery or safety profile in knee OA patients undergoing TKA.
Keywords: knee osteoarthritis, meloxicam, pain, safety, total knee arthroplasty
1. Introduction
Knee osteoarthritis (OA), a complicated peripheral joint disorder, is the most popular joint disorder in the world and its prevalence is increasing.[1] In the west, knee OA is a common cause of pain as well as disability among adults.[1,2] Among the treatment strategies of knee OA, total knee arthroplasty (TKA) is a common and beneficial surgery to relieve pain and elevate function of knee for patients who have severe or advanced knee OA.[3,4] However, patients who undergo TKA usually suffer from postoperative pain, which affects rehabilitation, patient satisfaction, and functional outcomes.[4] Currently, opioids are widely and effectively used for pain management after TKA, while it brings some adverse events such as nausea, vomiting, constipation, and even respiratory depression.[4] Therefore, it might be critical to search for a method to relieve the pain of knee OA patients after TKA with high safety.
Nonsteroidal anti-inflammatory drugs (NSAIDs), with good effect on relieving pain and decreasing inflammation, are widely administrated to reduce postoperative pain effectively and safely in orthopedic surgery.[5] Meanwhile, a previous study reveals that NSAIDs have the capability of pain-relieving in arthral surgeries such as arthroscopic rotator cuff surgery.[6] Among the NSAIDs, meloxicam is a selective inhibitor of cyclooxygenase-2 that can ease the pain following total hip arthroplasty.[7,8] Moreover, according to a previous study, meloxicam is able to moderate the pain after arthroscopic knee surgery.[9] Based on the above-mentioned information, we hypothesized that meloxicam might decrease the pain in knee OA patients after TKA. However, the analgesic effect of meloxicam in knee OA patients after TKA is not clear.
Therefore, the aim of this double-blind, randomized, controlled study was to explore the analgesic efficacy and safety of meloxicam in knee OA patients who received TKA.
2. Methods
2.1. Participants
Between March 2018 and September 2020, this double blind, randomized, controlled study consecutively recruited 128 OA patients who scheduled for TKA in our hospital. The eligible patients were required to meet the inclusion criteria: had diagnosis of knee osteoarthritis; age older than 18 years; about to receive TKA; and able to understand the content of the study and volunteer for the study. Patients with any of the following conditions were not eligible for recruitment: allergic or had other contraindications to the study drug; long term use of analgesics; use of analgesics within 1 week prior to TKA; and had other concomitant diseases that may affect the evaluations; history of knee surgeries; and female patients in pregnant or lactating. The Ethics Committee approved this study. All participants signed the informed consents.
2.2. Grouping and treatment
Before TKA, with the use of blocked randomization assignment method in 1:1 ratio, 128 eligible patients were randomly divided into meloxicam group (N = 65) or control group (N = 63). The sample size calculation, randomization design/procedure, and blind realization were carried out by a third party (Shanghai QeeJen Bio-Tech, China). Meloxicam (7.5 mg per tablet) was provided by Shanghai Boehringer Ingelheim Pharmaceutical Co., Ltd. (China), while the placebo was provided by Guangzhou Boji Medical & Biotechnological Co., Ltd. (China). After TKA, all patients were given patient-controlled analgesia (PCA) for 48 hours (h), which was administered as follows: intravenous injection of fentanyl 0.1 mg combined with tolanesetron mesylate 6 mg was used as analgesic load; intravenous indwelling needle was placed subcutaneously at the left deltoid muscle, followed by introducing of PCA pump (100 mL containing fentanyl 1.0 mg, tramadol 50.0 mg, and tolanesetron mesylate 6 mg); the maintenance dose was 1.0 mL/h; the locking time was 15 mins; and the single dose was 1.0 mL.
In the meloxicam group, patients took 15 mg meloxicam (2 tablets) orally at 4 h after TKA, and 7.5 mg (1 tablet) orally at 12 h after TKA, then 7.5 mg (1 tablet) orally every 12 h until 72 h after TKA. In the control group, patients took 2 tablets of placebo orally at 4 h after TKA, and 1 tablet orally at 12 h after TKA, then 1 tablet orally every 12 h until 72 h after TKA.
2.3. Outcome assessment
Basic clinical features including age, gender, and body mass index of patients were recorded at baseline, meanwhile, hospital for special surgery knee score (HSS) at baseline was also assessed. After TKA, pain at rest and flexion were evaluated using a 10 cm visual analog scale (VAS) at 6 h, 12 h, day (D)1, D2, D3, and D7 postoperation, respectively. Additional consumption of PCA (salvage use) and total consumption of PCA after TKA were documented as well. Patient satisfaction was scored from 0 to 10 points, at D1, D2, D3, and D7, respectively. Furthermore, all patients were followed up at month (M)1 and M3, respectively after TKA, during which, HSS score was evaluated again. No patients lost follow-up within 7 days, while there were 24 patients who lost follow-up within 3 months, then these patients were not included in the analysis of HSS score at M1 and M3.
2.4. Statistical analysis
Mean, standard deviation, case number, and percentage were calculated for descriptive analysis. Student t test or Chi-square test was used for difference determination between 2 groups. Graphics were constructed by GraphPad Prism 7.02 (GraphPad Software Inc.), and statistical analyses were completed by SPSS 24.0 (IBM). Statistical significance was concluded if a P value less than .05.
3. Results
3.1. Study flow
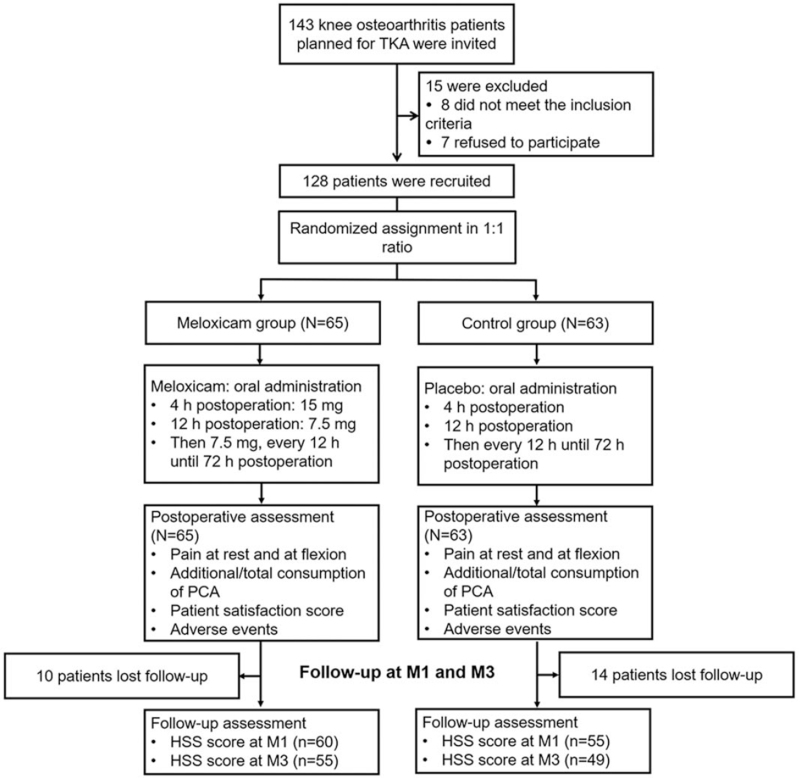
A total of 143 OA patients planned for TKA were invited, and 15 of them were excluded (including 8 patients who did not meet the inclusion criteria, and 7 patients who refused to participate). The remaining 128 eligible patients were recruited and randomized at a ratio of 1:1 into meloxicam group (N = 65) and control group (N = 63). In the meloxicam group, patients were given oral administration of meloxicam (15 mg at 4 h postoperation, 7.5 mg at 12 h postoperation, and then 7.5 mg every 12 h until 72 h postoperation). Meanwhile, in the control group, patients were given oral administration of placebo (at 4 h postoperation, 12 h postoperation, and then every 12 h until 72 h postoperation). Both groups received the same postoperative assessments (including pain at rest and at flexion, additional/total consumption of PCA, patient satisfaction score and adverse events). All patients were followed up at M1 and M3. Over the follow-up period, 10 patients lost follow-up in the meloxicam group and 14 patients lost follow-up in the control group. Finally, a follow-up assessment was made in the meloxicam group [including HSS score at M1 (n = 60) and M3 (n = 55)] and the control group [including HSS score at M1 (n = 55) and M3 (n = 49)] (Fig. 1).
Figure 1.
Flow chart. h = hour, HSS = hospital for special surgery knee score, M = month, PCA = patient-controlled analgesia, TKA = total knee arthroplasty.
3.2. Baseline characteristics
In the control group, the mean age of patients was 67.2 ± 6.2 years, and there were 40 (63.5%) females as well as 23 (36.5%) males. In the meloxicam group, the mean age of patients was 66.3 ± 5.6 years, and there were 47 (72.3%) females as well as 18 (27.7%) males. Further analysis showed that no difference was found in age, gender distribution, body mass index, HSS score before operation, or rate of using chondroitin between the control group and the meloxicam group (all P > .05) (Table 1).
Table 1.
Characteristics of knee osteoarthritis patients.
| Items | Control group (N = 63) | Meloxicam group (N = 65) | P value |
| Age (yr), mean ± SD | 67.2 ± 6.2 | 66.3 ± 5.6 | .638 |
| Gender, no. (%) | .285 | ||
| Female | 40 (63.5) | 47 (72.3) | |
| Male | 23 (36.5) | 18 (27.7) | |
| BMI (kg/m2), mean ± SD | 24.2 ± 2.1 | 24.0 ± 2.0 | .735 |
| HSS score before operation, mean ± SD | 39.9 ± 7.6 | 41.3 ± 8.7 | .215 |
| Previous use of chondroitin, no. (%) | 28 (44.4) | 26 (41.3) | .719 |
BMI = body mass index, HSS = hospital for special surgery knee score, SD = standard deviation.
3.3. Comparison of pain VAS score at rest and at flexion
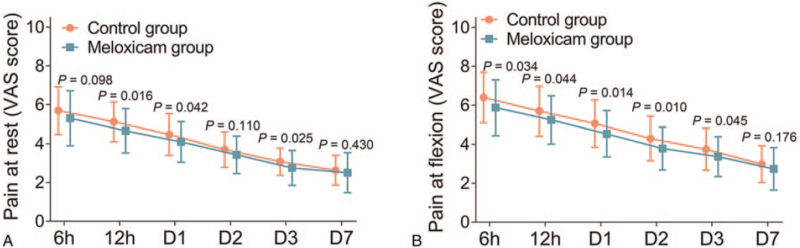
Pain VAS score at rest was elevated in the control group at 12 h (P = .016), D1 (P = .042), and D3 (P = .025) compared to the meloxicam group. However, no difference was found in pain VAS score at rest at 6 h, D2, D7 (all P > .05) between the control group and the meloxicam group (all P > .05) (Fig. 2A). In addition, pain VAS score at flexion was increased in the control group at 6 h (P = .034), 12 h (P = .044), D1 (P = .014), D2 (P = .010), and D3 (P = .045) compared to the meloxicam group. While no difference of pain VAS score at flexion was found at D7 (P = .176) between the control group and the meloxicam group (Fig. 2B).
Figure 2.
Pain VAS score at rest and at flexion. Comparison of the pain VAS score at rest (A) and at flexion (B) between control group and meloxicam group. D = day, VAS = visual analog scale.
3.4. Comparison of PCA consumption
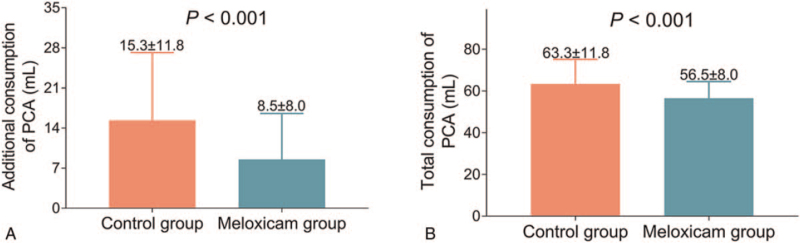
Additional consumption of PCA was enhanced in the control group (15.3 ± 11.8 mL) compared to the meloxicam group (8.5 ± 8.0 mL) (P < .001) (Fig. 3A). In addition, total consumption of PCA was also increased in the control group (63.3 ± 11.8 mL) compared to the meloxicam group (56.5 ± 8.0 mL) (P < .001) (Fig. 3B).
Figure 3.
Additional and total consumption of PCA. Comparison of additional consumption (A) and total consumption (B) of PCA between control group and meloxicam group. PCA = patient-controlled analgesia.
3.5. Comparison of patient satisfaction score
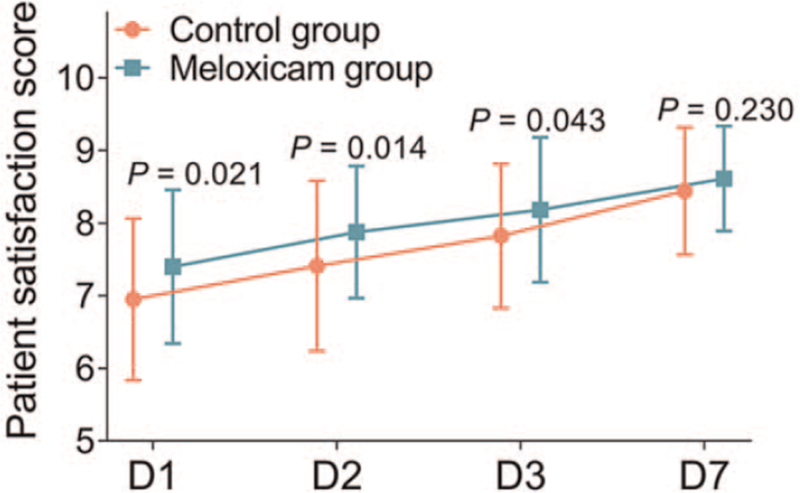
Patient satisfaction score in the meloxicam group was higher on D1 (P = .021), D2 (P = .014), and D3 (P = .043) compared to the control group. However, no difference of patient satisfaction score was found on D7 between the control group and the meloxicam group (P = .230) (Fig. 4).
Figure 4.
Patient satisfaction score. Comparison of patient satisfaction score between control group and meloxicam group. D = day.
3.6. Comparison of HSS score
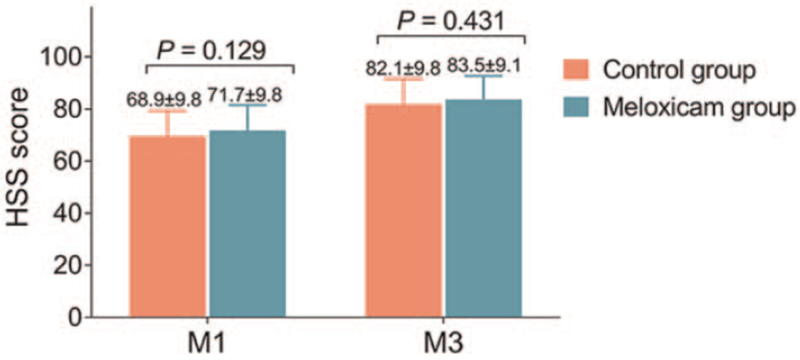
In terms of knee function recovery, which was evaluated by HSS score, it exhibited no difference at M1 (P = .129) or M3 (P = .431) between the control group and the meloxicam group (Fig. 5)
Figure 5.
HSS score. Comparison of HSS score between control group and meloxicam group. HSS = hospital for special surgery knee score, M = month.
3.7. Comparison of adverse events
In the control group, the main adverse events were nausea and constipation, which occurred in 22 (34.9%) patients and 16 (25.4%) patients, respectively. Meanwhile, in the meloxicam group, the main adverse events were also nausea and constipation, which occurred in 17 (26.2%) patients and 12 (18.5%) patients, respectively. Further analyses presented that no difference was found in the occurrences of adverse events between the control group and the meloxicam group (all P > .05) (Table 2).
Table 2.
Adverse events.
| Items | Control group (N = 63) | Meloxicam group (N = 65) | P value |
| Nausea, no. (%) | 22 (34.9) | 17 (26.2) | .281 |
| Vomiting, no. (%) | 8 (12.7) | 6 (9.2) | .530 |
| Constipation, no. (%) | 16 (25.4) | 12 (18.5) | .343 |
| Drowsiness, no. (%) | 3 (4.8) | 5 (7.7) | .494 |
| Dizziness, no. (%) | 3 (4.8) | 4 (6.2) | .729 |
4. Discussion
In this study, we found that: meloxicam attenuated postoperative pain in knee OA patients who received TKA; meloxicam did not affect long-term knee function recovery after TKA in knee OA patients; and meloxicam showed similar safety profile to placebo after TKA in knee OA patients.
Regarding the postoperative analgesic effect of meloxicam, a randomized, double-blind, placebo-controlled trial shows that meloxicam has the analgesic ability following orthopedic surgery including total shoulder replacement and total ankle replacement.[10] It has also been exhibited that meloxicam is able to control postoperative pain in total hip arthroplasty.[7] In terms of TKA, the postoperative analgesic effect of meloxicam is not clear. In this study, we discovered that meloxicam was able to reduce short-term pain in knee OA patients after TKA. Possible explanations could be that the administration of meloxicam was double dosage at first, thus it could achieve analgesic ability rapidly in short term.[11] Meanwhile, meloxicam did not affect long-term postoperative pain in patients who received TKA, which might be because: along with time and the recovery of patient's wound, pain could be reduced[12]; and meloxicam administration was stopped at 72 h after TKA, therefore, no difference of pain was found after D7. Moreover, the additional and total consumption of PCA was reduced by meloxicam administration, which could be explained by that meloxicam had better efficacy on reducing pain than placebo, therefore, patients used less consumption of PCA to control pain after TKA. Furthermore, meloxicam was able to improve short-term patient satisfaction in knee OA patients after TKA. Possible explanations might be that: meloxicam had the efficacy of reducing pain after TKA, which directly increased short-term patient satisfaction[13]; the administration of meloxicam was stopped at 72 h after TKA, thus, its effect on patient satisfaction might be weaken in long term.
Regarding the effect of meloxicam on postoperative recovery, a previous study illustrates that meloxicam does not affect Harris hip score in patients receiving total hip arthroplasty within 6 months.[7] Furthermore, another study also reveals that meloxicam does not affect knee range of motion, International Knee Documentation Committee score and Lysholm score in patients who underwent arthroscopic knee surgery.[9] In our study, we discovered that meloxicam did not affect long-term knee function recovery which assessed by HSS score in knee OA patients who received TKA either, a possible explanation could be that meloxicam was not the main factor that affected the knee function recovery in patients receiving TKA but other factors including the skill of the surgeons and success of TKA were.
As for the safety of postoperative meloxicam, a previous study establishes that meloxicam does not affect the incidence of adverse events in patients receiving orthopedic surgeries such as total hip arthroplasty.[10] In our study, we found that the adverse events were mostly nausea, constipation, vomiting, drowsiness and dizziness. However, no difference of adverse events was found between the administration of meloxicam and placebo in knee OA patients undergoing TKA. This result was consistent with the studies that focus on the application of meloxicam in other joint surgeries.[7,14] In addition, these data also implied that meloxicam might be a safe antalgic in knee OA patients after TKA.
In this study, there were several limitations: the sample size was 128 in our study, which was not big enough and would lead to a less strong statistical power in analyses; patient pain and satisfaction scores were assessed by patients themselves, which might cause objective bias; and for the purpose of reducing confounding factors, we excluded the patients who used long-term analgesics before TKA, thus, the analgesic effect and safety of meloxicam in these patients needed further analyses.
5. Conclusion
To be conclusive, meloxicam attenuates postoperative pain and improves patient satisfaction, while does not affect long-term knee function recovery and tolerance, indicating its potential for postoperative management in knee OA patients undergoing TKA.
Author contributions
Conceptualization: Feng Hu, Guoya Wu.
Data curation: Feng Hu, Guoya Wu.
Formal analysis: Feng Hu, Guoya Wu, Jian Wu.
Investigation: Feng Hu, Guoya Wu, Quan Zhao, Jian Wu.
Methodology: Feng Hu, Guoya Wu, Quan Zhao.
Project administration: Feng Hu, Guoya Wu.
Resources: Feng Hu, Guoya Wu, Quan Zhao, Jian Wu.
Software: Feng Hu, Guoya Wu, Quan Zhao, Jian Wu.
Supervision: Feng Hu, Guoya Wu, Quan Zhao, Jian Wu.
Validation: Feng Hu, Guoya Wu, Quan Zhao, Jian Wu.
Visualization: Feng Hu, Guoya Wu, Quan Zhao, Jian Wu.
Writing – original draft: Feng Hu, Guoya Wu, Quan Zhao, Jian Wu.
Writing – review & editing: Feng Hu, Guoya Wu, Quan Zhao, Jian Wu.
Footnotes
Abbreviations: D = day, h = hours, HSS = hospital for special surgery knee score, M = month, NSAIDs = Nonsteroidal anti-inflammatory drugs, OA = osteoarthritis, PCA = patient-controlled analgesia, TKA = total knee arthroplasty, VAS = visual analog scale.
How to cite this article: Hu F, Wu G, Zhao Q, Wu J. Evaluation of analgesic effect, joint function recovery and safety of meloxicam in knee osteoarthritis patients who receive total knee arthroplasty: a randomized, controlled, double-blind study. Medicine. 2021;100:35(e26873).
FH and GW contributed equality to this work.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article.
References
- [1].Hussain SM, Neilly DW, Baliga S, Patil S, Meek RMD. Knee osteoarthritis: a review of management options. Scott Med J 2016;61:07–16. [DOI] [PubMed] [Google Scholar]
- [2].Giwnewer U, Rubin G, Orbach H, Rozen N. Treatment for osteoarthritis of the knee. Harefuah 2016;155:403–6. [PubMed] [Google Scholar]
- [3].Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012;379:1331–40. [DOI] [PubMed] [Google Scholar]
- [4].Li JW, Ma YS, Xiao LK. Postoperative pain management in total knee arthroplasty. Orthop Surg 2019;11:755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep 2016;20:62. [DOI] [PubMed] [Google Scholar]
- [6].Lu Y, Li Y, Li F, Jiang C. Perspective randomized control study on different NSAIDs drugs after rotator cuff repair. Zhonghua Yi Xue Za Zhi 2015;95:2337–41. [PubMed] [Google Scholar]
- [7].Ren L, Meng L, Yan H, Sun W, Yao D. Preoperative meloxicam versus postoperative meloxicam for pain control, patients’ satisfaction and function recovery in hip osteoarthritis patients who receive total hip arthroplasty: a randomized, controlled study. Inflammopharmacology 2020;28:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khalil NY, Aldosari KF. Meloxicam. Profiles Drug Subst Excip Relat Methodol 2020;45:159–97. [DOI] [PubMed] [Google Scholar]
- [9].Hou J, Li W, Chen Y, Yang L, Li L, Zhao L. Early preoperative versus postoperative administration of meloxicam in pain control, patient global status improvement, knee function recovery of arthroscopic knee surgery. Medicine (Baltimore) 2019;98:e17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sharpe KP, Berkowitz R, Tyndall WA, et al. Safety, tolerability, and effect on opioid use of meloxicam iv following orthopedic surgery. J Pain Res 2020;13:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gates BJ, Nguyen TT, Setter SM, Davies NM. Meloxicam: a reappraisal of pharmacokinetics, efficacy and safety. Expert Opin Pharmacother 2005;6:2117–40. [DOI] [PubMed] [Google Scholar]
- [12].Sobol-Kwapinska M, Plotek W, Mandecki M, et al. Time perspective as a moderator of a relationship between preoperative pain and acute postoperative pain. Psychol Health Med 2019;24:812–8. [DOI] [PubMed] [Google Scholar]
- [13].Shirley ED, Sanders JO. Measuring quality of care with patient satisfaction scores. J Bone Joint Surg Am 2016;98:e83. [DOI] [PubMed] [Google Scholar]
- [14].Krauss E, Cronin M, Dengler N, Segal A. Interaction between low-dose aspirin and nonsteroidal anti-inflammatory drugs can compromise aspirin's efficacy in preventing venous thrombosis following total joint arthroplasty. Clin Appl Thromb Hemost 2020;26:1076029620920373. [DOI] [PMC free article] [PubMed] [Google Scholar]