Abstract
To evaluate the diagnostic values of shear wave elastography (SWE) alone and in combination with the Toronto clinical scoring system (TCSS) on diabetic peripheral neuropathy (DPN) in patients with type 2 diabetes mellitus (T2DM).
The study included 41 DPN patients, 42 non-DPN patients, and 21 healthy volunteers. Conventional ultrasonography and SWE were performed on the 2 sides of the tibial nerves, and cross-sectional area (CSA) and nerve stiffness were measured. TCSS was applied to all patients. A receiver operating characteristic curve analysis was performed.
The stiffness of the tibial nerve, as measured as mean, minimum or maximum elasticity, was significantly higher in patients in the DPN group than the other groups (P < .05). The tibial nerve of subjects in the non-DPN group was significantly stiffer compared to the control group (P < .05). There was no significant difference of the tibial nerve CSA among the 3 groups (P > .05). Mean elasticity of the tibial nerve with a cutoff of 71.3 kPa was the most sensitive (68.3%) and had a higher area under the curve (0.712; 0.602–0.806) among the 3 shear elasticity indices for diagnosing DPN when used alone. When combining SWE with TCSS in diagnosing DPN, the most effective parameter was the EMax, which yielded a sensitivity of 100.00% and a specificity of 95.24%.
SWE is a better diagnostic tool for DPN than the conventional ultrasonic parameter CSA, and a higher diagnostic value is attained when combining SWE with TCSS.
Keywords: cross-sectional area, diabetic peripheral neuropathy, shear wave elastography, Toronto clinical scoring system
1. Introduction
Diabetic peripheral neuropathy (DPN) is one of the most common chronic medical complications of diabetes; the prevalence of DPN in patients with type 2 diabetes mellitus (T2DM) is approximately 45%.[1] Severe DPN can lead to foot ulcers, gangrene and even amputation, which can be difficult to treat and impair quality of life. It is worth noting that the onset of DPN in T2DM patients can occur approximately 4 to 7 years prior to clinical diagnosis.[2] Therefore, early detection of DPN is of great importance for diabetic patients to prevent or postpone the negative clinical outcomes. The diagnosis of DPN is based mainly on its symptoms and signs as well as electrophysiological examinations. Because it yields objective and quantifiable results, electrophysiological examination is considered to be the gold standard in the diagnosis of DPN; however, the invasiveness, high cost and time intensiveness of this technique have limited its consistent use. In addition, patients with DPN commonly yield normal results upon electrophysiological examination. This situation is known as “subelectrophysiological DPN”.[3]
Recently, ultrasonography has been used increasingly as a method that is complementary to electrophysiological examination in the diagnosis of DPN. Ultrasonography holds many distinct advantages over other imaging methods in evaluation of peripheral neuromuscular disorders. For example, it is relatively convenient and cost efficient, it requires zero exposure to radiation and it provides dynamic assessment with relative ease.[4,5]
In previous studies, diagnosis of peripheral neuropathy using ultrasonography was mostly based on measurements of cross-sectional area (CSA).[6] Some reports demonstrated that enlargement of affected nerves could be attributed to the process of attempted remyelination and resulting edema.[7] However, previous studies demonstrated a limited applicability to diagnosis of DPN due to the wide range of cutoff values (from 0.09–0.24 cm2) and relative low sensitivity and specificity (the sensitivity was 73.8% and specificity 68.7% at a cutoff value of 0.13 cm2.).[8–10]
Shear wave elastography (SWE), on the other hand, is a new noninvasive ultrasound method for evaluating the elastic properties of tissues quantitatively. It has been widely used in examination of breast, liver and prostate diseases, and there is increasing interest in the use of SWE for the evaluation of neuromuscular pathologies, including DPN.[11,12] Notably, nerve stiffness tends to increase in peripheral neuropathy in a process that is consistent with the replacement of relatively compliant myelin with connective tissue.[12] We propose that the changes of nerve stiffness can be detected by SWE. In addition, we propose that SWE-mediated diagnosis can be augmented with the Toronto clinical scoring system (TCSS), which was proposed by Perkins et al[13] in 2001 and which has been used extensively in screening and assessment of the severity of DPN. The purpose of this study, then, was to evaluate the diagnostic usefulness of SWE alone and in combination with TCSS in patients with T2DM.
2. Methods
The study was performed with the approval of the Ethics Committee of the Second Affiliated Hospital of Soochow University. Before conducting the examinations, voluntary informed consent was obtained from each participant included in this study.
2.1. Participants
A total of 83 T2DM patients (55 male and 28 female) were recruited from the Second Affiliated Hospital of Soochow University from December 2017 to December 2019, and 21 healthy volunteers (8 male and 13 female) were enrolled as controls. The patients were divided into 2 groups based on results of electrophysiological examination. Diabetic patients with positive electrophysiological examination results were assigned to the DPN group, which consisted of 41 patients. The nondiabetic peripheral neuropathy (NDPN) group comprised 42 diabetic patients with negative electrophysiological examination results. General information of all subjects was obtained, such as sex, age, height, weight, body mass index (BMI), and history of smoking. In addition, the duration of history of diabetes mellitus (DM), the albumin-to-creatinine ratio and levels of hemoglobin A1c (HbA1c), fasting C-peptide, fasting blood glucose (FBG), and low density lipoprotein (LDL) were recorded for all patients.
The diagnostic criteria of DM was in accordance with the criteria of the American Diabetes Association.[14]
The inclusion criteria for the DPN group were confirmation of T2DM as described and confirmation of DPN by electrophysiological examination. The definition of DPN was in accordance with electrophysiologic criteria set by the American Association of Neurology, the American Academy of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation: at least 1 abnormal nerve conduction study parameter involving both the sural and peroneal nerves.[15]
The control group participants came from the population without DM. Electrophysiological examinations were not performed in healthy volunteers.
Exclusion criteria for all participants included the presence of type 1 DM. In addition, patients with multiple neuropathy caused by other reasons, such as heredity, alcohol, metabolism, inflammation or poisoning, were excluded. Patients with a known history of leg or ankle fracture or operation were also excluded.
2.2. Electrophysiological examinations
All examinations were performed in the electromyography laboratory at the Second Affiliated Hospital of Soochow University. Routine electrophysiological examinations were conducted with a standard electromyography system (ZET-100, Digital Electromyography; Zhongren, Shanghai, China) and conventional procedures. Recordings were performed at an ambient temperature of 25°C and skin surface temperatures of 32 to 34°C.
2.3. Sonographic examinations
2.3.1. Patient positioning
All sonographic examinations were performed within 1 week after the electrophysiological examinations by a sonographer with 20 years of experience in ultrasound and 5 years of experience in musculoskeletal elastography. The examiner was blind to the clinical histories and electrophysiological examination results of all subjects.
Examinations were performed in a quiet room with a constant temperature. The positions of all subjects were standardized for comparison. The subjects were placed in the supine position with ankles positioned in slight plantar flexion and external rotation. The participants were asked relax and to not move their feet or ankles during the examination, as these motions may increase ankle soft tissue pressure.
2.3.2. Conventional ultrasound examinations and SWE measurements
All sonographic examinations were performed with a 4 to 15 MHz linear array transducer (Aixplorer; Supersonic Imagine, Aix en Provence, France) under the musculoskeletal condition. A stabilizer was used to keep the transducer stationary during acquisitions (Fig. 1). Transverse images of the tibial nerve were obtained at 4 cm above the medial malleolus to avoid the bifurcation of tibial nerve, while the flexor digitorum longus, flexor pollicis longus and the posterior tibial vessels played a role in confirming the position of the tibial nerve.
Figure 1.
A stabilizer was used to keep the transducer stationary during the whole ultrasonic examinations process.
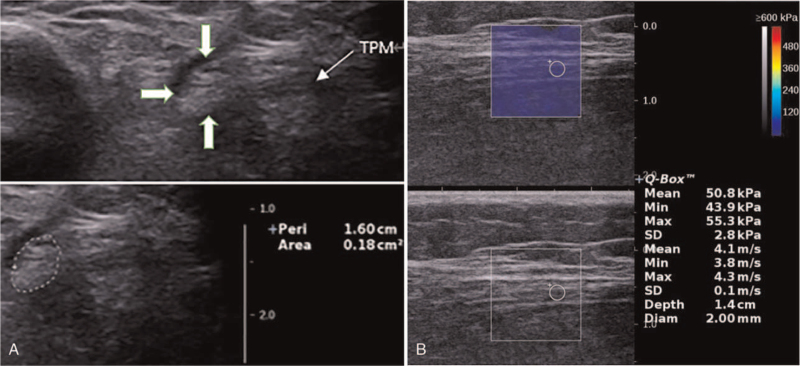
There are 2 sonographic methods for measuring CSA of the nerve: the direct method (tracing) and the indirect method (ellipsoid formula). The areas calculated by the direct and indirect methods agreed to a high correlation (r = 0.99); consequently, we used the easier, direct, method by tracing the inner border of the thin hyperechoic epineurial rim.[3] The CSA of the bilateral tibial nerve was measured 3 times, and the average value used in subsequent analyses. Fig. 2A illustrates the measuring of the tibial nerve CSA of a 68-year-old female in the control group.
Figure 2.
Sonographic findings of the tibial nerve of a 68-year-old female in control group. A Tracing the inner border of the thin hyperechoic epineurial rim to measure tibial nerve CSA (CSA = 0.18 cm2). B The longitudinal level. SWE measurement showed the tibial nerve stiffness (EMean = 50.8kPa, EMin = 43.9kPa, EMax = 55.3kPa). CSA = cross-sectional area, EMax = maximum elasticity, EMean = mean elasticity, EMin = minimum elasticity, SWE = shear wave elastography, TPM = tendon of tibialis posterior muscle.
Following CSA measurements, the transducer was rotated 90° to obtain a sagittal image that was parallel to the tibial nerve fibers. Quantitative SWE measurements were performed by placing a 2 mm diameter region of interest (ROI) in the most rigid part of the tibial nerve. The image was frozen when it became steady, and the elastic modulus of the ROI was measured. The mean (EMean), minimum (EMin), and maximum (EMax) elasticity indices (in kilopascal [kPa]) within the ROI were then obtained automatically with the integrated SWE software. Three iterative SWE measurements were performed, and then EMean, EMin, and EMax values of the tibial nerve within each ROI were obtained by calculating the mean of 3 SWE measurements in each ankle. The size of the SWE-measurement window was 2 cm2. The elastography scale was set between 0 and 600 kPa for the elastic modulus. The use of ample ultrasonic coupling gel was essential in order to minimize the effects of compression. We still performed reliability testing before enrollment of participants. In fact, nearly none of the elastogram boxes had color filling defects, at least in the tibial nerve level, which might technically influence the precision of elasticity.
2.4. Statistical analyses
Statistical analyses were performed with SPSS software (version 22.0; IBM). The x2 test was applied for the comparisons of categorical variables. The Kolmogorov–Smirnov test was used for analyses of normal distribution. Normal variables were expressed as means ± standard deviations, and the t test and one-way analysis of variance test were used to compare variables among 2 and 3 groups, respectively. Correspondingly, nonparametric variables were expressed as medians (quartile), and the differences were evaluated with the Mann–Whitney U test (between 2 groups) and Kruskal–Wallis test (among 3 groups). The Wilcoxon signed-rank test was used to compare CSA and the stiffness of the tibial nerve between the left and right sides. Correlations were expressed by Spearman correlation coefficients.
Receiver operating characteristic (ROC) curves for all parameters were obtained, and sensitivity, specificity, Yoden index, positive predictive value, negative predictive value, area under the curve (AUC) and the optimal cutoff value were calculated. The AUC was compared by a Z test. P < .05 indicated a statistically significant difference.
3. Results
3.1. Clinical characteristics
The clinical characteristics of the subjects are summarized in Table 1. No significant differences in the sex, age, BMI or history of smoking were found among the 3 groups. The HbA1c levels in the DPN group were higher than in the NDPN group (P < .05). The differences between the courses of DM, the albumin-to-creatinine ratio and the levels of fasting C-peptide, FBG, and LDL were nonsignificant between the DPN and NDPN groups.
Table 1.
Baseline characteristics of study population.
| Parameter | CG (n = 21) | NDPN (n = 42) | DPN (n = 41) | P |
| Sex, female/male | 13/8 | 15/27 | 13/28 | .058 |
| Age, yr | 56.05 ± 8.59 | 58.50 ± 9.32 | 59.05 ± 8.99 | .453 |
| BMI, kg/m2 | 23.46 ± 2.68 | 24.75 (22.5, 27.05) | 24.72 ± 3.27 | .065 |
| Presence of smoking, % | 23.8 | 33.3 | 51.2 | .075 |
| DM duration, years | NA | 8.44 ± 5.96 | 9.90 ± 6.57 | .295 |
| HbA1c, % | NA | 8.26 ± 2.29 | 9.34 ± 2.53 | .047 |
| ACR, mg/g | NA | 21.55 (12.28, 45.78) | 33.25 (11.33, 15.08) | .428 |
| C-peptide, ng/mL | NA | 1.76 (1.12, 2.29) | 1.59 (1.03, 2.40) | .549 |
| FPG, mmol/L | NA | 7.71 (5.78, 1.41) | 7.8 (6.33, 11.47) | .524 |
| LDL, mmol/L | NA | 2.91 ± 1.19 | 2.82 ± 1.11 | .730 |
Values are presented as mean ± standard deviation or median (interquartile range). Statistically significant at P < .05. ACR = albumin to creatinine ration, BMI = body mass index, CG = control group, DM = diabetes mellitus, DPN = diabetic peripheral neuropathy, FBG = fasting blood glucose, HbA1c = hemoglobin A1c, LDL = low density lipoprotein, NA = not applicable, NDPN = nondiabetic peripheral neuropathy.
3.2. Conventional ultrasound examinations
Bilateral comparisons showed that there was no significant difference in the CSA of the left and right tibial nerves (P > .05). There was also no significant difference of the tibial nerve among the 3 groups (P > .05). These data are summarized in Table 2.
Table 2.
Comparison of the CSA in tibial nerve in different groups.
| CSA (cm2) | |||
| LT | RT | P | |
| DPN | 0.22 (0.20, 0.26) | 0.21 (0.17, 0.26) | .46 |
| NDPN | 0.21 (0.18, 0.26) | 0.20 ± 0.05 | .43 |
| CG | 0.21 (0.18, 0.23) | 0.20 ± 0.05 | .76 |
| P (DPN vs NDPN vs control) | .441 | .434 | - |
Values are presented as mean ± standard deviation or median (interquartile range). Statistically significant at P < .05. CG = control group, CSA, cross-sectional area, DPN = diabetic peripheral neuropathy, LT = left tibial nerve, NDPN = nondiabetic peripheral neuropathy, RT = right tibial nerve.
3.3. SWE measurements
In all of the 3 groups, there was no significant difference in the elasticity of the tibial nerve between the left and right side (P > .05). However, the DPN group had significantly higher stiffness values than did the other 2 groups (P < .001), and the tibial nerve of the NDPN group was also significantly stiffer than that of the control group (P < .05). These data are shown in Table 3. Figs. 2B, 3 and 4 demonstrated the tibial nerve SWE image of people came from control, NDPN, and DPN group respectively.
Table 3.
Comparison of the SWE in tibial nerve in different groups.
| EMean | EMin | EMax | |||||||
| LT | RT | P | LT | RT | P | LT | RT | P | |
| DPN | 80.50 (62.40, 99.50) | 79.20 (59.15, 93.00) | .66 | 59.10 (46.68, 77.78) | 58.90 (48.40, 74.40) | .81 | 91.45 (79.53, 115.10) | 91.20 (73.75, 113.25) | .48 |
| NDPN | 60.04 ± 16.22 | 61.71 ± 15.17 | .52 | 44.59 ± 15.00 | 48.96 ± 13.29 | .64 | 70.53 ± 19.28 | 72.25 ± 17.64 | .90 |
| CG | 49.35 ± 16.34 | 43.39 ± 17.63 | .26 | 36.47 ± 14.29 | 31.45 ± 16.44 | .44 | 59.46 ± 18.57 | 52.94 ± 22.56 | .52 |
| P (DPN vs NDPN) | <.001 | .011 | - | <.001 | .042 | - | <.001 | .014 | - |
| P (DPN vs CG) | <.001 | <.001 | - | <.001 | <.001 | - | <.001 | <.001 | - |
| P (NDPN vs CG) | .007 | .008 | - | .026 | .002 | - | .018 | .016 | - |
Values are presented as mean ± standard deviation or median (interquartile range). Statistically significant at P < .05. CG = control group, DPN = diabetic peripheral neuropathy, EMax = maximum elasticity, EMean = mean elasticity, EMin = minimum elasticity, LT = left tibial nerve, NDPN = nondiabetic perineuropathy, RT = right tibial nerve, SWE = shear wave elastography.
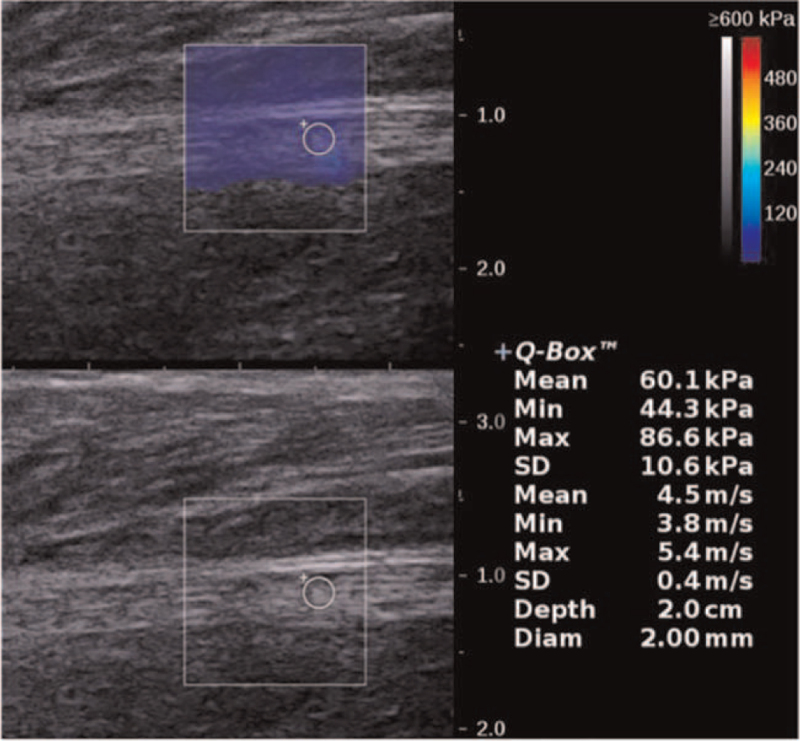
Figure 3.
SWE image of the tibial nerve in a 59-year-old female diabetic patient without DPN (EMean = 60.1kPa, EMin = 44.3kPa, EMax = 86.6kPa). DPN = diabetic peripheral neuropathy, EMax = maximum elasticity, EMean = mean elasticity, EMin = minimum elasticity, SWE = shear wave elastography.
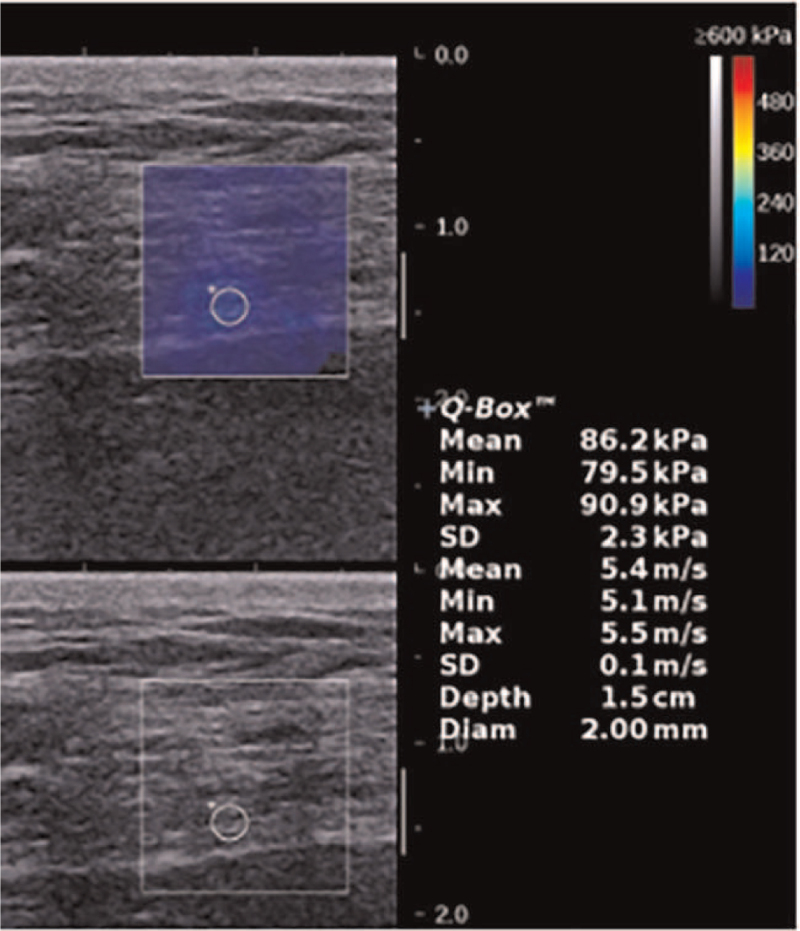
Figure 4.
SWE image of the tibial nerve in a 54-year-old female diabetic patient with DPN (EMean = 86.2kPa, EMin = 79.5kPa, EMax = 90.9kPa). DPN = diabetic peripheral neuropathy, EMax = maximum elasticity, EMean = mean elasticity, EMin = minimum elasticity, SWE = shear wave elastography.
3.4. Application of TCSS
There was no significant difference in TCSS scores between the DPN (5.27) and NDPN (5.24) groups (P > .05).
3.5. Correlation of SWE, clinical characteristics, and TCSS
The results of analyses of correlations between the stiffness and other characteristics in the tibial nerve are shown in Table 4. EMean was significantly correlated with TCSS (r = 0.235; P < .05) and BMI (r = 0.240; P < .05). EMax was also significantly correlated with TCSS (r = 0.225; P < .05) and BMI (r = 0.240; P < .05). EMin was significantly correlated with TCSS (r = 0.282; P < .01) but not with BMI (r = −0.009; P > .05). There existed no significant correlation between the stiffness and other characteristics analyzed in this study (including the duration of DM, the albumin-to-creatinine ratio and levels of HbA1c, fasting C-peptide, FBG, and LDL). Finally, we tried to verify if there existed correlation between the stiffness and the DM duration only in DPN patients. However, no significant correlation has been detected between the stiffness and the DM duration (EMean: P = .452, r = 0.122; EMin: P = .309, r = 0.165; EMax: P = .564, r = 0.094) (See Table S1, Supplemental Digital Content).
Table 4.
Correlation between the stiffness and characteristics in the tibial nerve.
| Correlation coefficient | |||
| Parameter | EMean | EMin | EMax |
| BMI | 0.240∗ | −0.009 | 0.240∗ |
| DM duration | 0.056 | 0.060 | 0.091 |
| HbA1c | 0.041 | 0.037 | −0.007 |
| ACR | 0.081 | 0.008 | 0.012 |
| C-peptide | 0.076 | 0.031 | 0.084 |
| FBG | 0.011 | −0.039 | 0.029 |
| LDL | −0.010 | −0.032 | 0.004 |
| TCSS | 0.235∗ | 0.282∗∗ | 0.225∗ |
ACR = albumin to creatinine ration, BMI = body mass index, DM = diabetes mellitus, EMax = maximum elasticity, EMean = mean elasticity, EMin = minimum elasticity, FBG = fasting blood glucose, HbA1c = hemoglobin A1c, LDL = low density lipoprotein, TCSS = Toronto clinical scoring system. The elasticity of the tibial nerve was compared with the participant's BMI, DM duration, HbA1c level, ACR, C-peptide, FBG, LDL, and TCSS by Spearman correlation coefficients.
P < .05.
P < .01.
3.6. The accuracy of SWE alone and in combination with TCSS for diagnosing DPN
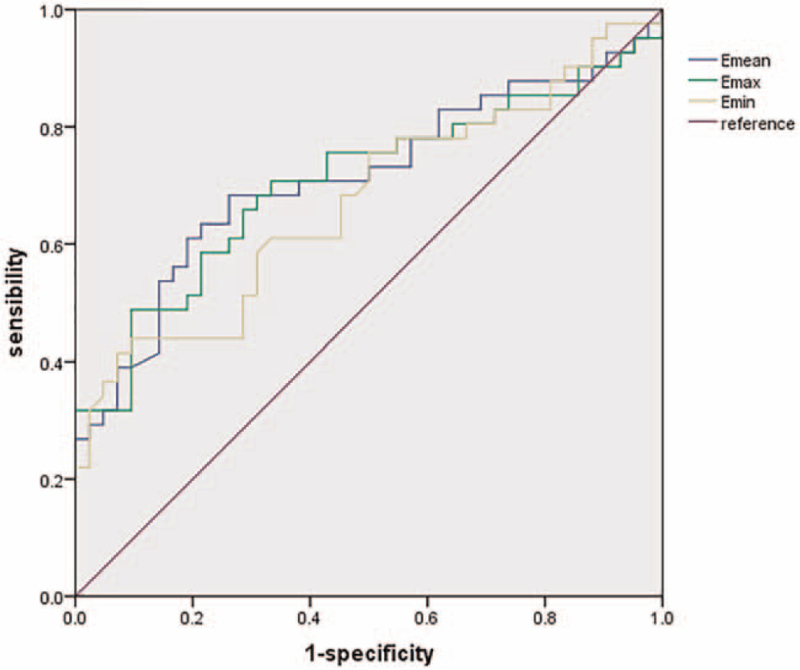
The ROC curve analysis for the diagnosis of DPN based on SWE are shown in Fig. 5. The following cutoff values corresponded to the highest diagnostic accuracy: 71.3 kPa, 63.0 kPa, and 93.0 kPa for the mean, minimum, and maximum elasticity of the tibial nerve, respectively. Table 5 summarizes sensitivity, specificity, Yoden index, positive and negative likelihood ratio, and the AUC when using the optimal cutoff values. Clearly, EMean of the tibial nerve, with the cutoff of 71.3 kPa, was the most valuable predictor in terms of sensitivity (68.3%) and AUC 0.712 (0.602–0.806). EMin and EMax of the tibial nerve demonstrated similar sensitivities.
Figure 5.
ROC curve for the diagnosis of DPN based on SWE. DPN = diabetic peripheral neuropathy, ROC = receiver operating characteristic, SWE = shear wave elastography.
Table 5.
Sensitivity, specificity, Yoden index, positive, and negative likelihood ratio according to cutoff values.
| Cutoff value (kPa) TCSS (score) | Sensitivity (%) | Specificity (%) | Yoden index | LR (+) (%) | LR (−) (%) | AUC | |
| EMean | 71.3 | 68.3 | 73.8 | 0.4210 | 2.61 | 0.43 | 0.712 |
| EMin | 63.0 | 43.9 | 90.5 | 0.3438 | 4.61 | 0.62 | 0.673 |
| EMax | 93.0 | 48.8 | 90.5 | 0.3926 | 5.12 | 0.57 | 0.706 |
| TCSS | 4.0 | 56.1 | 38.1 | 0.0580 | 0.91 | 1.15 | 0.518 |
| EMean + TCSS | - | 100.0 | 81.0 | 0.8950 | 5.25 | 0.00 | - |
| EMin + TCSS | - | 100.0 | 90.5 | 0.9050 | 10.50 | 0.00 | - |
| EMax + TCSS | - | 100.0 | 95.2 | 0.9520 | 21.00 | 0.00 | - |
AUC = area under the receiver operating curve, EMax = maximum elasticity, EMean = mean elasticity, EMin = minimum elasticity, LR (−) = negative likelihood ratio, LR (+) = positive likelihood ratio, TCSS = Toronto clinical scoring system.
EMean VS EMin: AUC 0.712 VS 0.673; Z = 1.184; P =.2363.
EMeanVS EMax: AUC 0.712 VS 0.706; Z = 0.442; P =.6586.
EMinVS EMax: AUC 0.673 VS 0.706; Z = 0.838; P =.4020.
Table 5 also summarizes the sensitivity, specificity, Yoden index, and positive and negative likelihood ratios when the optimal cutoff elasticity values were used in combination with TCSS to diagnose DPN. When the criteria of TCSS or the EMean from SWE for a DPN diagnosis were met, the sensitivity, specificity, Yoden index, positive likelihood ratio, and negative likelihood ratio were 100.00%, 80.95%, 0.895%, 5.250%, and 0.000%, respectively. When TCSS was combined with EMin from SWE, these values were 100.00%, 90.48%, 0.905%, 10.500%, and 0.000%. When TCSS was combined with the EMax from SWE, these values were 100.00%, 95.24%, 0.952%, 21.000%, and 0.000%.
4. Discussion
DPN is a common complication of diabetes; it occurs in approximately 30% to 50% of diabetic patients. And it is generally accepted that the duration of diabetes plays a major role in the pathogenesis of DPN. As a result, we explored the correlation between the stiffness and the DM duration (whether or not he is a DPN patient) using Spearman correlation test. However, there existed no significant correlation between the stiffness and the DM duration in this study. We also tried to verify if there existed correlation between the stiffness and the DM duration only in DPN patients. However, no significant correlation has been detected between the stiffness and the DM duration either.
Consequently, we concluded that there existed no significant correlation between the stiffness and the DM duration in this study regardless of DPN history. This finding was compatible with a previous similar study,[16] which reported that the duration of diabetes mellitus was not associated with the elasticity of the tibial nerve. Nonetheless, there still have been some mixed results. Jiang et al[17] demonstrated that the duration of diabetes (r = 0.23–0.27) were slightly significant associated with elasticity indices by Spearman correlation coefficient. Patients in Jiang et al[17] had a longer duration of diabetes than those in Ishibashi et al[16] and our study (mean, 15.0 years vs 9.7 years and 9.9 years). It is unclear now that if longer duration of diabetes corresponds to stiffer tibial nerve more than 1 particular duration of diabetes. And also, more than 1 factor have potential influence on the elasticity of the tibial nerve, eg, age [16] and height [17] of patients. The points mentioned above could be the potential cause of the difference between the various study results. As a consequence, more studies are indispensable to make more consistent conclusions in the near future.
Electrophysiological examinations remain the cornerstone of the diagnosis of neuropathy, providing detailed information about the dysfunction of affected nerves. However, they are time-consuming and are not tolerated well as repeated evaluations.[18–20]
Ultrasonography has been extensively utilized in diagnosing many forms of peripheral nerve disorders, such as the carpal tunnel syndrome, for it can provide reliable morphological information and can allow clinicians to clearly visualize the location and range of the lesion.[10,21] However, it has been limited in the assessment of DPN. In previous studies, ultrasound diagnosis of peripheral neuropathy was based mainly on CSA measurement.[3,22] Generally, CSA of the nerves in DPN patients are commonly thought to be larger than those in healthy individuals; however, in an earlier study, Hobson-Webb et al[7] found that there was no significant difference in CSA between DPN patients and patients in a normal control group, which was consistent with us. For the above paradoxical findings, we were considering another ultrasound method-SWE-to evaluate DPN.
As a noninvasive ultrasound evaluation technique, SWE can evaluate the stiffness of tissues quantitatively.[23] Elastography has been applied to evaluate several musculoskeletal tissues and related injuries, including the rotator cuff tendon[24] and pertinent disorders and chronic myofascial pain.[25] SWE is predominantly used in evaluating liver fibrosis and differentiating malignant and benign tumors, but its use in peripheral neuropathies has lagged.[12] The shear waves that characterize SWE are generated by an acoustic radiation force impulse or controlled external vibration after tissue excitation, and the velocity of the shear wave is related to tissue stiffness, with stiffer tissues associated with faster shear wave propagation.[12] As a result, SWE can assess tissue elasticity more objectively than strain elastography, which quantifies structure displacement under the manual stress exerted by the operator.[26]
Recently, SWE has been applied to the field of neuromuscular research, including studies in carpal tunnel syndrome, neuropathy of the median and ulnar nerves, peripheral polyneuropathy and other disorders.[12,27,28] Previous studies have measured the median nerve stiffness by this means in patients with carpal tunnel syndrome;[29] however, assessment of DPN with elastography has been limited. Thus, we explored the diagnostic value of SWE in patients with T2DM and found that the DPN group demonstrated a significantly higher tibial nerve stiffness as compared with healthy patients and as compared with T2DM patients without DPN. This finding was in agreement with the findings of Ishibashi et al[16] which indicated that measurement of nerve stiffness by way of SWE can reflect the nerve changes in DPN patients indirectly.
Several pathophysiological mechanisms may account for the increased tibial nerve stiffness in DPN patients. It is generally believed that DPN arises from a process in which edema within the nerve fascicle increases intraneural pressure, increasing the stiffness of the nerve. The increased stiffness leads to further compression of the microvasculature and the reduction of blood flow. These changes have been shown to play an important etiologic role in DPN, and they lead to focal demyelination and axonal degeneration with the fibrotic response,[30,31] and then result in the proliferation of scar tissue and an increase in the speed of shear wave propagation in the tibial nerve.[17,26] These mechanisms have been investigated by studies both in diabetic patients and in rats.[32,33]
The stiffer tibial nerve in the NDPN group relative to that of control patients illustrates that increases of the stiffness of the tibial nerve may appear earlier than can be seen in abnormal electrophysiological examinations. DPN is prone to be confined to small nerve fibers in the early part of the course of the disorder, while electrophysiological examination mainly detects the changes of the large nerve fibers.[34,35]. Despite normal electrophysiological examination results, these diabetic patients may have already suffered some nerve damage.[32] Therefore, SWE has potential value in the early detection of subelectrophysiological DPN, in which some nerve changes have occurred but electrophysiological examination remains negative. Early diagnosis of DPN is essential in that it allows for initiating treatment at the earliest stages of DPN with subsequent minimization of future complication, which decrease both short-term and long-term morbidity.[34,36,37]
According to the ROC curve analysis, we found that the AUC of the EMean of the tibial nerve with the optimal cutoff value of 71.3 kPa was 0.712 (95% CI 0.602–0.806), with a sensitivity of 68.3% and a specificity of 73.8%. Beyond the EMean of the tibial nerve, we also examined the diagnostic value of the minimum and maximum elasticity of the tibial nerve using the ROC curve analysis. Clearly, the EMean of the tibial nerve was the most sensitive and most effective according to the AUC. With regard to specificity, EMin and EMax of the tibial nerve were almost equal. In an earlier study,[17] among the 3 different SWE elasticity indices (EMean, EMin, and EMax) used in the diagnosis of DPN, a cutoff of 45.7 kPa on EMin had the highest sensitivity (74.0%) and AUC 0.867 (0.808–0.913), while EMean had a better specificity (95%) with the threshold of 60.1 kPa. Different sample sizes, operators, and machines may contribute to the differences in results between our study and others. In addition, in our study, the electrophysiological examination results were considered as the only criteria in the diagnosis of DPN; DPN symptoms and signs were not included. As a result, some subelectrophysiological patients were grouped into NDPN group rather than DPN group, resulting in a higher overall cutoff value. In addition, our controls were recruited randomly from the outpatients of the Second Affiliated Hospital of Soochow University, and we only excluded patients with a history of DM, multiple neuropathy unrelated to DM or a history of leg or ankle fracture or operation. They were not evaluated for the presence of thyroid disorders and hyperlipidemia which may also had some influence on the tibial nerves.[17,38] And also, we did not previously consider electrophysiological examinations of these patients. All of these reasons may play important roles in the variable outcomes of different studies.
TCSS is widely used in evaluating the function of peripheral nerves, especially in the alienation or elimination of small nerve fibers. In the early stages of DPN, small nerve fibers tend to be damaged, so TCSS is suitable for early screening of DPN. In our study, a correlation analysis was carried out between tibial nerve stiffness and TCSS. It was found that there was a positive correlation between the tibial nerve stiffness and TCSS: the coefficients of correlation with TCSS were 0.235, 0.282, and 0.225 for the EMean, EMin, and EMax, respectively. We suggest that SWE may also detect the damage of small nerve fibers in the early stages of DPN-subelectrophysiological DPN, which is in line with our results.
When combining SWE with TCSS in the diagnosis of DPN, the most effective parameter was the EMax of the tibial nerve on the basis of the cutoff values noted above. This combination yielded a sensitivity of 100.00% and a specificity of 95.24%, compared with 100.00% and 80.95% with the EMean and 100.00% and 90.48% with the EMin. We found that the sensitivity, specificity, Yoden index, and positive likelihood ratio were significantly improved in the diagnosis of DPN compared with SWE alone. These results suggest that the combination can significantly improve the diagnostic values and can be used as an effective auxiliary means for improved diagnosis of DPN.
We acknowledge several limitations to this study. First, patients with type 1 DM were not enrolled in this study due to its relatively low incidence, and the number of DPN patients was also limited. We plan to explore differences in the sonographic findings between the 2 different types of DM in the near future. Second, as a multiple peripheral nerve disease, DPN may affect many more peripheral nerves in addition to the tibial nerve, such as the median and sural nerve; investigation of other nerves and parts of the same nerve will be performed shortly. Furthermore, we have not yet conducted a follow-up study of the NDPN patients to determine how long it took for them to develop neuropathy. Finally, because nerve biopsies were not performed, we could not identify changes of DPN stiffness from a pathological point of view.
5. Conclusions
Tibial nerve stiffness is markedly higher in T2DM patients with and without DPN. SWE is a better diagnostic tool for DPN than the conventional ultrasonic parameter CSA, and SWE has a potential role in detecting subelectrophysiological DPN. Among the 3 SWE elasticity indices, EMean has the highest accuracy for identifying DPN alone, but a higher diagnostic value is acquired when combining EMax with TCSS.
Acknowledgments
We thank the study participants and their relatives and the clinical staff for their support and contribution to this study. We did not have any writing assistance.
Author contributions
Conceptualization: Ji Hu, Chen Fang, Qi Ma.
Funding acquisition: Qi Ma.
Investigation: Miao Zheng, Qi Ma.
Methodology: Fei Wang, Miao Zheng, Tong Chen, Yunyan Zhu, Qi Ma, Honghong Zhang, Xin Song.
Resources: Miao Zheng, Ji Hu, Qi Ma.
Software: Fei Wang.
Supervision: Ji Hu, Qi Ma.
Visualization: Fei Wang, Miao Zheng.
Writing – original draft: Fei Wang, Miao Zheng.
Writing – review & editing: Chen Fang, Meng Wang, Qi Ma.
Supplementary Material
Footnotes
Abbreviations: AUC = area under the curve, BMI = body mass index, CSA = cross-sectional area, DM = diabetes mellitus, DPN = diabetic peripheral neuropathy, EMax = maximum elasticity, EMean = mean elasticity, EMin = minimum elasticity, FBG = fasting blood glucose, HbA1c = hemoglobin A1c, kPa = kilopascal, LDL = low density lipoprotein, NDPN = nondiabetic peripheral neuropathy, ROC = receiver operating characteristic, ROI = region of interest, SWE = shear wave elastography, T2DM = type 2 diabetes mellitus, TCSS = Toronto clinical scoring system.
How to cite this article: Wang F, Zheng M, Hu J, Fang C, Chen T, Wang M, Zhang H, Zhu Y, Song X, Ma Q. Value of shear wave elastography combined with the Toronto clinical scoring system in diagnosis of diabetic peripheral neuropathy. Medicine. 2021;100:35(e27104).
FW and MZ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Zilliox L, Russell JW. Treatment of diabetic sensory polyneuropathy. Curr Treat Options Neurol 2011;13:143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Said G. Diabetic neuropathy–a review. Nat Clin Pract Neurol 2007;3:331–40. [DOI] [PubMed] [Google Scholar]
- [3].Watanabe T, Ito H, Sekine A, et al. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med 2010;29:697–708. [DOI] [PubMed] [Google Scholar]
- [4].Wu WT, Chen LR, Chang HC, Chang KV, Özçakar L. Quantitative ultrasonographic analysis of changes of the suprascapular nerve in the aging population with shoulder pain. Front Bioeng Biotechnol 2021;9:640747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chang PH, Chen YJ, Chang KV, Wu WT, Özçakar L. Ultrasound measurements of superficial and deep masticatory muscles in various postures: reliability and influencers. Sci Rep 2020;10:14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen J, Wang CL, Wu S, He S, Ren J. The feasibility of using high-resolution ultrasonography to assess ulnar nerve in patients with diabetes mellitus. J Ultrason 2017;17:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hobson-Webb LD, Massey JM, Juel VC. Nerve ultrasound in diabetic polyneuropathy: correlation with clinical characteristics and electrodiagnostic testing. Muscle Nerve 2013;47:379–84. [DOI] [PubMed] [Google Scholar]
- [8].Riazi S, Bril V, Perkins BA, et al. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? A cross-sectional study. Diabet Care 2012;35:2575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cartwright MS, Passmore LV, Yoon JS, Brown ME, Caress JB, Walker FO. Cross-sectional area reference values for nerve ultrasonography. Muscle Nerve 2008;37:566–71. [DOI] [PubMed] [Google Scholar]
- [10].He Y, Xiang X, Zhu BH, Qiu L. Shear wave elastography evaluation of the median and tibial nerve in diabetic peripheral neuropathy. Quant Imaging Med Surg 2019;9:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].İnal M, Tan S, Yumusak EM, Şahan MH, Alpua M, Örnek K. Evaluation of the optic nerve using strain and shear wave elastography in patients with multiple sclerosis and healthy subjects. Med Ultrason 2017;19:39–44. [DOI] [PubMed] [Google Scholar]
- [12].Wee TC, Simon NG. Ultrasound elastography for the evaluation of peripheral nerves: a systematic review. Muscle Nerve 2019;60:501–12. [DOI] [PubMed] [Google Scholar]
- [13].Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabet Care 2001;24:250–6. [DOI] [PubMed] [Google Scholar]
- [14].Standards of medical care in diabetes–2010. Diabetes care 2010;33: Suppl 1: S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199–207. [DOI] [PubMed] [Google Scholar]
- [16].Ishibashi F, Taniguchi M, Kojima R, Kawasaki A, Kosaka A, Uetake H. Elasticity of the tibial nerve assessed by sonoelastography was reduced before the development of neuropathy and further deterioration associated with the severity of neuropathy in patients with type 2 diabetes. J Diabetes Investig 2016;7:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiang W, Huang S, Teng H, et al. Diagnostic performance of two-dimensional shear wave elastography for evaluating tibial nerve stiffness in patients with diabetic peripheral neuropathy. Eur Radiol 2019;29:2167–74. [DOI] [PubMed] [Google Scholar]
- [18].Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 2012;28: Suppl 1: 08–14. [DOI] [PubMed] [Google Scholar]
- [19].Bae JS, Kim BJ. Subclinical diabetic neuropathy with normal conventional electrophysiological study. J Neurol 2007;254:53–9. [DOI] [PubMed] [Google Scholar]
- [20].Radziwill AJ, Steck AJ, Renaud S, Fuhr P. Distal motor latency and residual latency as sensitive markers of anti-MAG polyneuropathy. J Neurol 2003;250:962–6. [DOI] [PubMed] [Google Scholar]
- [21].Goedee HS, Brekelmans GJ, van Asseldonk JT, Beekman R, Mess WH, Visser LH. High resolution sonography in the evaluation of the peripheral nervous system in polyneuropathy–a review of the literature. Eur J Neurol 2013;20:1342–51. [DOI] [PubMed] [Google Scholar]
- [22].Kelle B, Evran M, Balli T, Yavuz F. Diabetic peripheral neuropathy: correlation between nerve cross-sectional area on ultrasound and clinical features. J Back Musculoskelet Rehabil 2016;29:717–22. [DOI] [PubMed] [Google Scholar]
- [23].Wei M, Ye X. Feasibility of point shear wave elastography for evaluating diabetic peripheral neuropathy. J Ultrasound Med 2020;39:1135–41. [DOI] [PubMed] [Google Scholar]
- [24].Chiu YH, Chang KV, Chen IJ, Wu WT, Özçakar L. Utility of sonoelastography for the evaluation of rotator cuff tendon and pertinent disorders: a systematic review and meta-analysis. Eur Radiol 2020;30:6663–72. [DOI] [PubMed] [Google Scholar]
- [25].Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 2015;41:1126–47. [DOI] [PubMed] [Google Scholar]
- [26].Chu CA, Chen YJ, Chang KV, Wu WT, Özçakar L. Reliability of sonoelastography measurement of tongue muscles and its application on obstructive sleep apnea. Front Physiol 2021;12:654667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miyamoto H, Halpern EJ, Kastlunger M, et al. Carpal tunnel syndrome: diagnosis by means of median nerve elasticity–mproved diagnostic accuracy of US with sonoelastography. Radiology 2014;270:481–6. [DOI] [PubMed] [Google Scholar]
- [28].Paluch Ł, Noszczyk B, Nitek Ż, Walecki J, Osiak K, Pietruski P. Shear-wave elastography: a new potential method to diagnose ulnar neuropathy at the elbow. Eur Radiol 2018;28:4932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kantarci F, Ustabasioglu FE, Delil S, et al. Median nerve stiffness measurement by shear wave elastography: a potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur Radiol 2014;24:434–40. [DOI] [PubMed] [Google Scholar]
- [30].Dellon AL. Neurosurgical prevention of ulceration and amputation by decompression of lower extremity peripheral nerves in diabetic neuropathy: update 2006. Acta Neurochir Suppl 2007;100:149–51. [DOI] [PubMed] [Google Scholar]
- [31].Ibrahim I, Khan WS, Goddard N, Smitham P. Carpal tunnel syndrome: a review of the recent literature. Open Orthop J 2012;6:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen R, Wang XL, Xue WL, et al. Application value of conventional ultrasound and real-time shear wave elastography in patients with type 2 diabetic polyneuropathy. Eur J Radiol 2020;126:108965. [DOI] [PubMed] [Google Scholar]
- [33].Chen RJ, Lin CC, Ju MS. In situ biomechanical properties of normal and diabetic nerves: an efficient quasi-linear viscoelastic approach. J Biomechan 2010;43:1118–24. [DOI] [PubMed] [Google Scholar]
- [34].Asadov R, Erdal A, Buğdayci O, Gündüz OH, Ekinci G. The effectiveness of ultrasonography and ultrasonographic elastography in the diagnosis of carpal tunnel syndrome and evaluation of treatment response after steroid injection. Eur J Radiol 2018;108:172–6. [DOI] [PubMed] [Google Scholar]
- [35].Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108–11. [DOI] [PubMed] [Google Scholar]
- [36].Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabet Res Clin Pract 2001;54:115–28. [DOI] [PubMed] [Google Scholar]
- [37].Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at high risk for lower-extremity amputation in a primary health care setting. A prospective evaluation of simple screening criteria. Diabet Care 1992;15:1386–9. [DOI] [PubMed] [Google Scholar]
- [38].Aslan M, Aslan A, Emeksiz HC, et al. Assessment of peripheral nerves with shear wave elastography in type 1 diabetic adolescents without diabetic peripheral neuropathy. J Ultrasound Med 2019;38:1583–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.