Abstract
Umbilical Vein Recanalization (UVR) may occur in patients with long-standing portal hypertension and liver cirrhosis. This study aimed to investigate the clinical significance of UVR.
Medical records of a cohort of patients with cirrhosis (n = 247) who were hospitalized at the Digestive Medicine Center of the Second Affiliated Hospital of Nanchang University from January 2012 to October 2015 were accessed. The UVR diagnosis was made by ultrasound examination and was confirmed by computerized tomography scan.
The UVR incidence was 20.2% (50/247) in the cohort. The size of UVR was 9.9 ± 4.7 mm (range: 5–26.5 mm) in diameter. The UVR and non-UVR groups showed no difference in grades of hepatic encephalopathy (P = .496), Child-Pugh classification (P = .401), the incidence of moderately severe ascites (26% vs 26%, P = 1), the esophageal variceal bleeding rate (32% vs 39%, P = .402), or portal vein thrombosis (8% vs 12%, P = .580). However, the incidence of cavernous transformation of the portal vein was statistically different, that there was 0 case in the UVR group and 8 cases in the non-UVR group (P < .05).
Our results suggested that UVR had little impact on the clinical manifestations of patients with liver cirrhosis, the significance of UVR as an intervention method requires further studies.
Keywords: hepatic cirrhosis, hepatic encephalopathy, portal vein hypertension, portal vein thrombosis, umbilical vein recanalization
1. Introduction
The umbilical vein usually closes within a week after birth and remains intact but without blood flow throughout life.[1] The remnant of the umbilical vein forms the round ligament of the liver, which may reopen under extreme pressure to allow blood to bypass the liver passage of blood. Spontaneous umbilical vein recanalization (UVR) may occur in patients with long-standing portal hypertension or liver cirrhosis, serving as a decompressive portosystemic shunt.[2] Spontaneous UVR can also occur in other conditions like superior vena cava obstruction and rarely, in pancreatitis.[3] The umbilical vein is potential and occluded, directly connects to the left branch of the portal vein through a layer of valve, undoing which by catheter will access to the portal vein system and allow clear radiographic display.[4,5] Computerized tomography (CT) scan can clearly show the splenic veins and mesenteric veins, the pressure gradient of portal and hepatic veins, and liver morphology, providing a basis for the diagnosis of liver diseases.[6] The umbilical vein is used in diagnostic and treatment techniques, such as trans-umbilical vein hepatic venous pressure measurement, trans-umbilical vein hepatoportography, trans-umbilical vein perfusion chemotherapy and chemoembolization, trans-umbilical portosystemic shunt, trans-umbilical vein insertion into the subcutaneous buried chemotherapy pumps, etc.[7,8] Compared with transfemoral vein techniques, transunbilical vein techniques are simpler and easier to perform, cause only mild discomfort, less complications, and less portal thrombosis.[9]
In cirrhosis, neovascularization causes blood circulation disorders, increases resistance to blood flow, and leads to higher pressure in the portal venous system, resulting in portal hypertension.[2] Portal hypertension is responsible for the most severe complications of cirrhosis.[10,11] Collateral circulation recanalization may directly reduce the portal vein pressure, which reduces the incidence of esophageal variceal bleeding and ascites to a certain extent.[12–14] The extrahepatic shunt is mainly related to extrahepatic portal angiogenesis, leading to the reopening of the traffic branch between the usually closed portal-vena cava system, and the collateral circulation being formed between the extracorporeal and the vena cava system.[15,16] One of the reopening channels is the umbilical vein. Finally, part of the portal vein blood flows into the vena cava and back into the heart. However, opposite views posit that collateral circulation recanalization, such as an umbilical vein, splenorenal shunt, portosystemic shunt, will aggravate the development of esophageal varices, and increase the incidence of bleeding events and hepatic encephalopathy (HE).[17,18] Thus far, the clinical significance of UVR remains controversial. This study investigated the relationship between umbilical vein recanalization and portal hypertension with cirrhosis and its complications, portal vein thrombosis, cirrhosis stage classification (Child-pugh classification, model for end-stage liver disease (MELD) scores).
2. Methods
2.1. Patients
Medical records of 247 patients with cirrhosis hospitalized at the Department of Gastroenterology of the Second Affiliated Hospital of Nanchang University from January 2012 to October 2015 were retrospectively screened. The inclusion criteria were according to the CT diagnosis and the clinical diagnostic criteria of cirrhosis;[19,20] Exclusion criteria included: hepatocellular carcinoma; complicated with other systemical diseases (such as heart, brain, lung, kidney, etc.); hematological system diseases. The study protocol was approved by the ethics board of the Second Affiliated Hospital of Nanchang University. In considering the retrospective nature of the study and that informed consents were no longer available from many of the patients, the ethics board waived the requirement for informed consent (Certification No. [2015] 005, Second Affiliated Hospital of Nanchang University).
2.2. UVR diagnosis
The CT consisted of a Somatom Definition Flash dual-source CT scanner (Siemens AG, Germany) and a light speed 64 VCT helical scanner with a scanning parameter of 120KV, 250 mA, a thickness of 5 mm, a layer spacing of 5 mm, a screw pitch of 0.6, and a matrix of 1512 × 512. We performed four hepatic CT dynamic enhanced scans (i.e., equilibrium, artery venous, portal venous, and delay phase). The Beckman Coulter AU5400 automatic biochemical analyzer (USA), Sysmex CA-700 automatic coagulation analyzer (Japan), ARKRAY MJ-11 ammonia detector (Japan), Q450, and the Color Doppler Aplio300 ultrasound system (Toshiba) were applied to diagnostic examinations. An electronic gastroscope (Fujitsu, 900x) was used to diagnose and classify esophageal and gastric varices.[21]
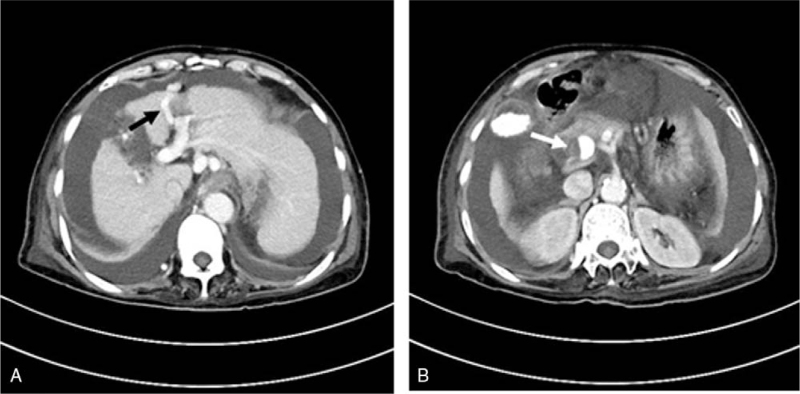
UVR on CT often manifests as a network of collateral vessels connected to the left branch of the portal vein and then extended to the umbilicus. Enhanced CT demonstrates UVR clearly as a tubular structure branching from the left branch of the portal vein during the contrast phase of the portal vein (Fig. 1A),[7] which also allows direct measurement of the diameter of the UVR, the portal vein, spleen vein and superior mesenteric vein. Portal vein thrombosis (PVT) is usually performed as a partial or complete hypodense-filling defect in the portal vein trunk and other branches on CT contrast-enhanced phase (Fig. 1B), extending gradually into the splenic or superior mesenteric veins. The very low-density filling defect can be seen on unenhanced scans.[9] When portal vein lumen is completely obstructed by thrombosis, an “orbit sign” can be observed through the CT-enhanced scan phase.
Figure 1.
(A) Patients with cirrhosis portal vein thrombosis undergoing splenectomy, accompanied by HE, massive ascites, esophageal varicose vein, and umbilical vein recanalization. The black arrow refers to umbilical vein recanalization with an inner diameter of approximately 7.5 mm. (B) Patients with cirrhosis portal vein thrombosis undergoing splenectomy, accompanied by HE, massive ascites, esophageal varicose vein, and umbilical vein recanalization. The white arrow indicates portal vein thrombosis.
2.3. Data collection procedure
During hospitalization, patient data including etiology, vital signs, clinical data, laboratory results, radiological features, treatment and outcomes were recorded into the electronic medical records by the physician. At analysis, we retrieved the medical records by two persons independently. All UVR patients of this cohort were included in the analysis. For the non-UVR group, 50% of the patients were randomly included for the final analysis, to have a comparable sample size of the UVR group.
2.4. Definitions of primary outcomes
Child-Pugh classification and MELD scores were collected for indicators of liver functional reserve. General conditions (age, gender, etiology), clinical symptoms and signs, serum biochemical parameters, such as albumin, bilirubin, creatinine, international normalized ratio, and complications of cirrhosis including ascites, hepatic encephalopathy, gastrointestinal bleeding, esophageal and gastric varices (classified as Mild, Moderate and Severe according to Guidelines for The Diagnosis and Treatment of Esophageal and Gastric Variceal Bleeding in Cirrhotic Portal Hypertension), portal vein thrombosis were compared between groups. The diameters of the portal vein, splenic vein, superior mesenteric vein, and umbilical vein measured by abdominal CT were also compared. All clinical data statistical were analyzed when necessary for assessing the stage, grading and related complications of cirrhosis.
2.5. Statistical analysis
For statistical analysis, a t-test was applied to normally distributed measurement data and the Mann–Whitney U test was applied to non-normally distributed measurement data. Multistage classification statistics were performed using the Mann–Whitney U test. Simple classification statistic materials used a χ2 test or Fisher probability test in 2 × 2 table data. Spearman rank correlation was applied to analyze univariate statistics data. A two-tailed P value < 0.05 was considered statistically significant. Data were analyzed by SPSS version 17.0 software.
3. Results
3.1. General information and etiology of cirrhosis in UVR and non-UVR patients
UVR was found in 20.2% (50/247) of the patients. The demographic characteristics of UVR and non-UVR patients were summarized in Table 1. The size of UVR was 9.9 ± 4.7 mm (range: 5–26.5 mm) in diameter. There was no difference in sex and age between the UVR and non-UVR patient groups. There was no difference in etiology of cirrhosis between the UVR and non-UVR groups, with hepatitis B being the most common etiology of both groups.
Table 1.
General information and etiology of cirrhosis in the cohort.
| UVR (n = 50) | non-UVR (n = 100, randomly selected from the 197 non-UVR patients) | P value | |
| Age (yr) (Mean ± SD) | 50.6 ± 11.6 | 54.0 ± 12.6 | |
| Male, n (%) | 31 (62.0) | 58 (58.0) | |
| Pathogenesis, n (%) | |||
| Hepatitis B | 34 (68.0) | 57 (57.0) | |
| Alcoholic | 7 (14.0) | 20 (20.0) | |
| Autoimmunity | 2 (4.0) | 5 (5.0) | |
| Schistosomia | 3 (6.0) | 5 (5.0) | |
| Other etiology | 4 (8.0) | 13 (13.0) | |
3.2. Esophageal and gastric varices, alimentary tract hemorrhage, and hepatic encephalopathy
As shown in Table 2, patients that were free of esophageal and gastric varices in the UVR group and the non-UVR groups accounted for 10% (5/50) and 11% (11/100), respectively (P > .05). There was no difference in the severe esophageal and gastric varices rate in the UVR group and the non-UVR group (48% vs 54%, P = .398 > .05), indicating that cirrhosis with UVR did not affect the formation and degree of esophageal and gastric varices.
Table 2.
The first major medical complications in the UVR and non-UVR patients.
| UVR Group (n = 50) (%) | non-UVR Group (N = 100, randomly selected from the 197 non-UVR patients) (%) | P value | |
| Hepatic Encephalopathy grading, n (%) | |||
| None | 40 (80) | 86 (86) | |
| I Class | 3 (6) | 0 (0) | |
| II Class | 3 (6) | 2 (2) | .496 |
| III Class | 3 (6) | 6 (6) | |
| IV Class | 1 (2) | 6 (6) | |
| Ascites degree, n (%) | |||
| None | 17 (34) | 23 (23) | |
| Mild | 20 (40) | 42 (42) | .296 |
| Moderate | 3 (6) | 12 (12) | |
| Severe | 10 (20) | 23 (23) | |
| Esophageal and gastric varices∗, n (%) | |||
| None | 5 (10) | 11 (11) | |
| Mild | 9 (18) | 15 (15) | .398 |
| Moderate | 12 (24) | 20 (20) | |
| Severe | 24 (48) | 54 (54) | |
| Alimentary tract hemorrhage, n (%) | 16 (32) | 39 (39) | .402 |
The HE rates for the UVR and non-UVR groups were 20% and 14% (Table 2). HE was divided into 4 grades according to the grading standard, and there was no difference in HE grades between the two groups (P = . 496). The patients free of ascites in the UVR group and the non-UVR group accounted for 34% and 23%, respectively, and there was no statistical difference in the severity of ascites (P = .296), indicating that UVR did not change the clinical manifestations of ascites.
There were 8 cases of esophageal vein ligation (the procedure was performed according to the indications and contraindications, and the patient consent) in the UVR group and 14 cases in the non-UVR group, with no statistical significance between groups (16% vs. 14%, P = .744). In addition, alimentary tract hemorrhage incidence was 36.7% (55/150) in all patients, 32% (16/50) in the UVR group and 39% (39/100) in the non-UVR group, with no statistical significance between groups (P = .402, suggesting that UVR did not affect the occurrence of esophageal varices bleeding.
3.3. PVT incidence and location in the UVR and non-UVR patients
PVT occurred in 16 patients (10.7%), including 4 (8%) and 12 (12%) from the UVR and the non-UVR groups, respectively (P = .580). The main portal vein trunk thrombosis accounted for 43.75%, the portal vein + superior mesenteric vein thrombosis accounted for 12.5%, and other locations accounted for 6.25% (Table 3). The incidence of cavernous transformation of the portal vein was statistically significantly different, that there was 0 case in the UVR group and 8 cases in the non-UVR group (P = .04).
Table 3.
The location of portal vein thrombosis.
| Location | Number | Proportion (%) |
| Portal vein trunk | 7 | 43.75 |
| Right branch | 1 | 6.25 |
| Left branch | 1 | 6.25 |
| Trunk + left + right branch | 1 | 6.25 |
| Trunk + superior mesenteric vein | 2 | 12.5 |
| Superior mesenteric vein | 1 | 6.25 |
| Splenic vein | 1 | 6.25 |
| Right branch +superior mesenteric vein | 1 | 6.25 |
| Left + right + branch + trunk + superior mesenteric Vein + splenic vein | 1 | 6.25 |
| Total | 16 | 100 |
3.4. Liver function of the UVR and non-UVR patients
The liver function was accessed by biochemical criterion, Child-Pugh grading and MELD score. As shown in Table 4, the albumin, bilirubin, International normalized ratio, and creatinine were not statistically different between the UVR and non-UVR patients (Mann–Whitney U test P values: .182, .253, .797, .255, respectively). The Child-Pugh classification was not statistically different in the UVR group and the non-UVR group (P > .401), suggesting that UVR did not affect the liver in Child-Pugh classification. The R factor of the MELD score was divided into four layers: < 10 points, 10–15 points, 15–20 points, and 21–25 points, which was not statistically different between the UVR group and the non-UVR patients. The R factors of the two groups were 7.94 ± 5.87 and 6.67 ± 4.69, respectively (P > .05, Table 4).
Table 4.
Liver function and serum laboratory of the UVR and non-UVR patients.
| UVR Group (N = 50) | non-UVR Group (N = 100) | P value∗ | |
| Child–Pugh grading, n (%) | |||
| A grading | 13 (26) | 37 (37) | |
| B grading | 26 (52) | 40 (40) | .401 |
| C grading | 11 (22) | 23 (23) | |
| MELD score# | .213 | ||
| R value (Mean ± SD) | 7.94 ± 5.87 | 6.67 ± 4.69 | |
| <10 points, n (%) | 33 (66) | 72 (72) | |
| 10∼15 points | 12 (24) | 23 (23) | .755 |
| 15∼20 points | 2 (4) | 3 (3) | |
| 21∼25 points | 3 (6) | 2 (2) | |
| Laboratory | |||
| Albumin (g/L) | 29.93 ± 4.5 | 29.0 ± 4.15 | .182 |
| Bilirubin (mg/dL) | 2.7 ± 3.52 | 1.9 ± 1.65 | .253 |
| International normalized ratio. | 1.33 ± 0.26 | 1.27 ± 0.26 | .797 |
| Creatinine (mg/dL) | 0.85 ± 0.61 | 0.78 ± 0.26 | .255 |
3.5. Portal vein diameter, spleen vein diameter, superior mesenteric vein diameter
The portal vein diameter, spleen vein diameter, and the superior mesenteric vein diameter in the UVR group were 16.98 ± 3.89 mm, 12.22 ± 3.3 mm, and 11.8 ± 2.12 mm and in the non-UVR group were 16.16 ± 3.26 mm, 11.45 ± 3.45 mm, and 11.58 ± 2.71 mm, respectively. The portal vein diameter, spleen vein diameter, and superior mesenteric vein diameter were not statistically different between the UVR and non-UVR groups (P1 = .347, P2 = .338, P3 = .772).
The umbilical vein diameter was 9.89 ± 4.73, with a range of 4.5 mm to 26.5 mm. The Spearman rank correlation analysis showed that umbilical vein diameter was significantly correlated with the portal vein diameter, spleen vein diameter, and superior mesenteric vein diameter (P1 = .025, P2 = .025, P3 = .001 < .05, Table 5). This indicated that the UVR would affect the diameter of the portal vein, splenic vein, and superior mesenteric vein.
Table 5.
Statistics of portal vein, splenic vein, and superior mesenteric vein diameter.
| Portal vein | Splenic vein | Superior mesenteric vein | |
| Mann–Whitney U test | |||
| Z value | –.941 | –.959 | –.290 |
| P value | .347 | .338 | .772 |
| Spearman rank correlation | |||
| P value | .025 | .025 | .001 |
4. Discussion and conclusion
Early-phase cirrhosis is rarely diagnosed, instead, many patients are diagnosed in the decompensated period when their symptoms are complicated with portal hypertension, ascites, bleeding, and collateral circulation recanalization. Studies on the esophageal and gastric variceal and the umbilical or paraumbilical patency are of great significance.[4] In varicose veins caused by portal hypertension, esophageal varices are the most common, followed by UVR[22] UVR is easy to detect and serves as an ideal entry point for studying the collateral circulation of portal hypertension with cirrhosis. A discrepancy has been found in studies on the clinical significance of UVR. In one study, UVR incidence was found to be gradually increased from 0 to 22%, 56%, and 75% in patients with normal and chronic hepatitis patients to patients with compensatory cirrhosis, decompensation cirrhosis, and severe chronic liver disease, suggesting UVR had an impact on disease progress.[16] It was suggested that UVR directs blood entering the systemic circulation without liver detoxification, which may increase HE risks.[23] Based on the phenomenon that collateral circulation increases the incidence of HE, clogging of collateral vessel recanalization may be used to treat HE, and the ascites and esophageal varices do not significantly aggravate the HE incidence.[24] When HE was clinically found, an abdominal CT scan was done to detect whether it was combined with portal vein collateral branch, which was important for developing a reasonable therapeutic strategy. However, our study showed that UVR did not affect the HE incidence.
In our study, UVR did not affect Child-Pugh classification, the incidence of moderately severe ascites, the esophageal variceal bleeding rate, or PVT. Other studies have shown that UVR increases the blood flow of the portal vein outflow tract to reduce the portal vein pressure, reducing risks of esophageal gastric varices, varicose vein rupture, and bleeding.[25] Again, this was not found in our study. The possible reason was that it was a slow and gradual process. As the portal vein pressure gradually increases, the collateral circulation gradually forms and expands, however, the portal vein pressure is not likely to decrease but increases. This process is accompanied by the slow progress of cirrhosis. The umbilical vein does not suddenly expand, and the progressive process would not relieve the portal vein pressure, while the incidence of bleeding and ascites does not decrease. PVT is a common complication of cirrhosis, which may be related to portal hypertension caused by portal blood vein stasis, slow blood flow, and increased blood viscosity.[26–28] PVT was the most common complication after upper gastrointestinal hemorrhage with therapeutic endoscopy, which was also found after splenectomy.[29] PVT was closely related to the severity degree of hypohepatia. Diffuse PVT can develop into the cavernous transformation of the portal vein, limiting its treatment methods, with complications difficult to control, like bleeding and ascites.[30,31] Theoretically, increased portal vein blood flow outflow and velocity in portal vein collateral circulation would reduce the risk of PVT, especially UVR increases the blood flow of portal vein trunk and left branch. However, this study failed to support this theory; because spontaneous UVR was a slow process and unable to relieve portal vein pressure or improve the portal vein blood flow velocity.
If the shunt happens in a short period of time, such as transjugular intrahepatic portosystemic shunt (TIPS) and surgical shunt, the portal pressure is significantly reduced, which helps prevent hemorrhage and to improve ascites.[32,33] TIPS is a man-made portosystemic shunt, which was also an effective treatment for diffuse PVT. The complete recanalization rate of the portal vein was 57%, while TIPS did not reduce the risk of thrombosis,[34] which was similar to the results of the study. It is difficult to control bleeding or ascites during these treatments. Early studies have shown that UVR does not decrease the formation of ascites.[1] In addition, the complexity of the ascites formation mechanism such as hypoalbuminemia and the umbilical vein's gradual expansion do not relieve portal vein pressure. The study is limited in the following aspects: we included a limited number of patients for analysis, and the observation window was a limited period of time. There are other spontaneous portosystemic collaterals in liver cirrhosis with portal hypertension, however, other types of spontaneous portosystemic collaterals such as splenorenal shunt were not discussed.
In summary, the results show that UVR has no significant effect on the complications of cirrhosis or liver function, and it may not relieve portal pressure because it is a gradual process. However, it may act as access for interventional therapy.
Acknowledgments
The authors thank all the patients and their families for participating the study.
Author contributions
Conceptualization: Xiaoling Ye.
Data curation: Qing Shi, Bin Ding.
Formal analysis: Qing Shi, XiaoLing Ye.
Funding acquisition: Xiaoling Ye.
Investigation: Qing Shi, XiaoLing Ye, Bin Ding, Kai Xiong.
Methodology: Kai Xiong.
Project administration: Xiaoling Ye.
Resources: Xiaoling Ye.
Supervision: Xiaoling Ye.
Writing – original draft: Qing Shi, XiaoLing Ye.
Writing – review & editing: Xiaoling Ye.
Correction
When originally published, the corresponding author's information appeared incorrectly as “Department of Interventional Radiology, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, China, 350025” and has been corrected to “The Department of Hepatology, First People Hospital of JiuJiang, JiangXi, China, 332000.” “This work was supported by China National Nature Science Foundation (grant number: 91029720)” has been removed from the funding information. Affiliation “cThe Department of Hepatology, the First People Hospital of JiuJiang, JiangXi, China” has been added and Dr. Xiao Ling's affiliation has been changed from a to c.
Footnotes
Abbreviations: CT = computerized tomography, HE = hepatic encephalopathy, MELD = model for end-stage liver disease, PVT = portal vein thrombosis, TIPS = transjugular intrahepatic portosystemic shunt, UVR = umbilical vein recanalization.
How to cite this article: Shi Q, Xiong K, Ding B, Ye X. Clinical characteristics of cirrhosis patients with umbilical vein recanalization: a retrospective analysis. Medicine. 2021;100:35(e26774).
QS and KX contributed to the work equally.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
UVR = umbilical vein recanalization.
UVR = umbilical vein recanalization.
According to Guidelines for The Diagnosis And Treatment Of Esophageal And Gastric Variceal Bleeding In Cirrhotic Portal Hypertension: Mild Grade 1: Esophageal varices are linear or slightly tortuous without RC. Moderate Grade2: Esophageal varices that are straight or slightly tortuous, with RC or esophageal varices that are serpentine and tortuous but not RC. Severe Grade 3: Esophageal varices that are serpentine and tortuous with RC or esophageal varices that are beaded, nodular, or tuberous (with or without RC).
## UVR Group had 4 patients with splenectomy, non-UVR group had 9 patients with splenectomy.
MELD = model for end-stage liver disease, UVR = umbilical vein recanalization.
The MELD score is divided into four levels.
Each analysis with Bonferroni's correction.
Mann–Whitney U test.
References
- [1].Aagaard J, Jensen LI, Sørensen TI, Christensen U, Burcharth F. Recanalized umbilical vein in portal hypertension. AJR Am J Roentgenol 1982;139:1107–10. [DOI] [PubMed] [Google Scholar]
- [2].Alexander MS, Blake RA, Gelman R. Visualization of a recanalized umbilical vein on hepatobiliary imaging with CT correlation. Clin Nucl Med 1991;16:517–9. [DOI] [PubMed] [Google Scholar]
- [3].Møller S, Bendtsen F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int 2018;38:570–80. [DOI] [PubMed] [Google Scholar]
- [4].Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis 2014;18:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prati D, Colli A. When a liver biopsy is ’norma’. Liver Int 2016;36:21–3. [DOI] [PubMed] [Google Scholar]
- [6].Ma J, Yan Z, Luo J, Liu Q, Wang J, Qiu S. Rational classification of portal vein thrombosis and its clinical significance. PloS One 2014;9:e112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peng Y, Qi X, Dai J, Li H, Guo X. Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med 2015;8:751–7. [PMC free article] [PubMed] [Google Scholar]
- [8].Kondo T, Maruyama H, Sekimoto T, et al. Influence of paraumbilical vein patency on the portal hemodynamics of patients with cirrhosis. J Clin Gastroenterol 2014;48:178–83. [DOI] [PubMed] [Google Scholar]
- [9].Shinkai M, Mochizuki K, Kitagawa N, et al. Usefulness of a recanalized umbilical vein for vascular reconstruction in pediatric hepatic surgery. Pediatr Surg Int 2016;32:553–8. [DOI] [PubMed] [Google Scholar]
- [10].Harding DJ, Perera MT, Chen F, Olliff S, Tripathi D. Portal vein thrombosis in cirrhosis: controversies and latest developments. World J Gastroenterol 2015;21:6769–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang ZH, Zhang JW, He P, Zhou Y, Sun CY. Fondaparinux is effective for acute portal vein thrombosis in decompensated cirrhotic patients. Medicine (Baltimore) 2017;96:e8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dao T, Bouvard N. Prevalence of spontaneous hepatofugal portal flow in liver cirrhosis. Gastroenterology 1991;101:1141–2. [DOI] [PubMed] [Google Scholar]
- [13].Nunez D, Russell E, Yrizarry J, Pereiras R, Viamonte M, Jr. Portosystemic communications studied by transhepatic portography. Radiology 1978;127:75–9. [DOI] [PubMed] [Google Scholar]
- [14].Jungers M, Hillon P, Lebrec D. [Degree of portal hypertension and risk of recurrent gastrointestinal bleeding in patients with cirrhosis (author's transl)]. Gastroenterol Clin Biol 1982;6:318–20. [PubMed] [Google Scholar]
- [15].Amitrano L, Guardascione MA, Brancaccio V, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol 2004;40:736–41. [DOI] [PubMed] [Google Scholar]
- [16].Su ZZ, Shan H, Ke WM, He BJ, Zheng RQ. Portalsystemic hemodynamic changes in chronic severe hepatitis B: an ultrasonographic study. World J Gastroenterol 2008;14:795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lam KC, Juttner HU, Reynolds TB. Spontaneous portosystemic shunt: relationship to spontaneous encephalopathy and gastrointestinal hemorrhage. Dig Dis Sci 1981;26:346–52. [DOI] [PubMed] [Google Scholar]
- [18].Tu R, Xia LP, Yu AL, Wu L. Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT. World J Gastroenterol 2007;13:3956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sundaram V, Kowdley K. Management of chronic hepatitis B infection. BMJ 2015;351:h4263. [DOI] [PubMed] [Google Scholar]
- [20].Lee HK, Park SJ, Yi BH, Yeon EK, Kim JH, Hong HS. Portal vein thrombosis: CT features. Abdom Imaging 2008;33:72–9. [DOI] [PubMed] [Google Scholar]
- [21].Williams MJ, Hayes P. Improving the management of gastrointestinal bleeding in patients with cirrhosis. Expert Rev Gastroenterol Hepatol 2016;10:505–15. [DOI] [PubMed] [Google Scholar]
- [22].Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol 2014;61:912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gupta D, Chawla YK, Dhiman RK, Suri S, Dilawari JB. Clinical significance of patent paraumbilical vein in patients with liver cirrhosis. Dig Dis Sci 2000;45:1861–4. [DOI] [PubMed] [Google Scholar]
- [24].Murakami M, Nishino K, Satou T, et al. Percutaneous transretroperitoneal direct approach to occlude a major shunt in a patient with extrahepatic portal-systemic encephalopathy. Hepatol Res 2009;39:313–7. [DOI] [PubMed] [Google Scholar]
- [25].Caturelli E, Pompili M, Squillante MM, et al. Cruveilhier-Baumgarten syndrome: an efficient spontaneous portosystemic collateral preventing oesophageal varices bleeding. J Gastroenterol Hepatol 1994;9:236–41. [DOI] [PubMed] [Google Scholar]
- [26].Qi X, Han G, Fan D. Management of portal vein thrombosis in liver cirrhosis. Nat Rev Gastroenterol Hepatol 2014;11:435–46. [DOI] [PubMed] [Google Scholar]
- [27].Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010;51:210–8. [DOI] [PubMed] [Google Scholar]
- [28].Ponziani FR, Zocco MA, Campanale C, et al. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol 2010;16:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Winslow ER, Brunt LM, Drebin JA, Soper NJ, Klingensmith ME. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–5. discussion 635-636. [DOI] [PubMed] [Google Scholar]
- [30].Vilgrain V, Condat B, Bureau C, et al. Atrophy-hypertrophy complex in patients with cavernous transformation of the portal vein: CT evaluation. Radiology 2006;241:149–55. [DOI] [PubMed] [Google Scholar]
- [31].Lutz HH, Gassler N, Tischendorf FW, Trautwein C, Tischendorf JJ. Doppler ultrasound of hepatic blood flow for noninvasive evaluation of liver fibrosis compared with liver biopsy and transient elastography. Dig Dis Sci 2012;57:2222–30. [DOI] [PubMed] [Google Scholar]
- [32].Orloff MJ. Fifty-three years’ experience with randomized clinical trials of emergency portacaval shunt for bleeding esophageal varices in Cirrhosis: 1958-2011. JAMA Surg 2014;149:155–69. [DOI] [PubMed] [Google Scholar]
- [33].Perarnau JM, Le Gouge A, Nicolas C, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol 2014;60:962–8. [DOI] [PubMed] [Google Scholar]
- [34].Luca A, Miraglia R, Caruso S, et al. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut 2011;60:846–52. [DOI] [PubMed] [Google Scholar]