Abstract
We report in this work a highly sensitive and nonradioactive PCR method for the detection of the four species of parasite causing human malaria. Plasmodium-specific primers corresponding to the small-subunit rRNA genes of the malaria parasite were used, and a 291-bp fragment was amplified. Our results showed a high specificity for the four human Plasmodium species, and we were able to detect one parasite in 50 μl of whole blood. The responses of 12 patients infected with Plasmodium falciparum to antimalarial therapy were monitored by PCR diagnosis and examination of thick blood film for at least 20 min by an experienced microscopist. For one patient this study allowed early diagnosis of therapeutic failure, confirmed 7 days later by examination of the thick blood film. A total of 134 samples were examined; 94 were positive by PCR, and among these 68 were positive by thick blood film examination. The sensitivity of the thick blood film was 72.3% compared to PCR and 60.7% compared to dot blot hybridization.
In recent years, new techniques for visualizing malaria parasites have been proposed. The quantitative buffy coat malaria test and acridine orange-stained thick or thin blood film (7, 12) use acridine orange as a fluorochrome to stain parasite nucleic acids, but this fluorochrome is not specific and stains nucleic acids from all cell types. The dipstick test (Parasight F test) is an immunological method specific for the detection of Plasmodium falciparum, based on the presence in the blood of parasite histidine-rich protein 2 antigen (4); consequently, Parasight F gives negative results for samples containing Plasmodium vivax, Plasmodium ovale, or Plasmodium malariae. Therefore, the specificity limitations presented by these procedures leave the microscopic examination of Giemsa-stained blood smears as the main method for diagnosis of malaria infection in the field (6). However, this technique is not a very satisfactory standard, as it is time-consuming and requires an experienced microscopist and because its sensitivity in detecting very low-level parasitemia is limited.
To overcome this problem, enzymatic amplification of DNA by PCR and amplification by the reverse transcriptase PCR (1, 13) have been successfully developed and used as highly sensitive diagnostic methods. The first studies of nucleic acid-based malaria detection used the repetitive genomic DNA as a target (3, 22). To date, the main sequences of DNA that are amplified are those in the genes encoding the surface antigens (19, 24, 28), the 18S rRNA gene (10, 15), and the dihydrofolate reductase-thymidylate synthetase gene (2, 26).
In this study we have developed a simple and highly sensitive DNA diagnostic method without organic extraction and using a digoxigenin-11–dUTP-labeled probe. We chose the rRNA as a diagnostic target, particularly the small-subunit (SSU) rRNA gene. The sequence of the SSU rRNA gene is composed of a mosaic of conserved and variable regions; this type of arrangement allows the amplification of a sequence from a sample with primers that are conserved within every member of the genus Plasmodium (13, 27). The chosen primers allowed the amplification of both asexual and sexual sequences of the 18S rRNA genes of the four human malaria parasites; the length of amplified DNA was predicted at 291 bp. This method was used to monitor antimalarial therapy in 12 patients. Results of thick blood films and PCR for each sample were compared.
MATERIALS AND METHODS
Genus specificity.
To amplify the DNA of the four human Plasmodium species, blood samples containing P. vivax (5 samples), P. ovale (12 samples), P. malariae (3 samples), and P. falciparum (56 samples) from patients with acute malaria were collected. Each sample was collected from a single patient and no mixed infection was found. Moreover, for the genus specificity, we lysed in vitro cultures of other protozoa such as Toxoplasma gondii, Leishmania infantum, and Trypanosoma cruzi for DNA amplification.
Level of detection. (i) Absolute sensitivity.
To determine the sensitivity of the PCR, asynchronous cultures of P. falciparum NF54 were cultured by the method of Trager and Jensen (23) and harvested when the level of parasitemia was between 5 and 10%. DNA was extracted by the procedure described by Goman et al. (8). Briefly, infected erythrocytes 1× SSC solution (0.15 M NaCl, 0.015 M sodium citrate [pH 7]) containing 0.01% saponin were lysed at 37°C for 20 min. The lysate was digested with 1 mg of proteinase K (Boehringer Mannheim, Meylan, France) per ml in the presence of 4% sodium N lauroylsarcosine (Sigma) in 1× SSC by incubation for 1 h at 37°C. Parasite DNA was extracted with phenol-chloroform and precipitated with ethanol according to the method described by Maniatis et al. (14). The DNA concentration was determined spectrophotometrically, and 10-fold serial dilutions of P. falciparum DNA (10 to 0.001 pg) were used as positive controls.
To establish the minimum number of parasites that could be detected, blood samples from three patients infected with P. falciparum were collected. The parasitemia was adjusted to 1% and hematocrit was standardized to 50%. The infected blood was diluted with uninfected erythrocytes from healthy individuals. Ten-fold serial dilutions were made to obtain a final parasitemia level of 10−8% (one parasite/1010 erythrocytes). Samples were treated in duplicate.
(ii) Relative sensitivity. (a) Blood sample collection and microscopy.
Blood samples were collected by venipuncture from 12 nonimmune P. falciparum-infected patients admitted to the Pitié-Salpêtrière Hospital with acute uncomplicated P. falciparum malaria. These patients were included in a randomized, double-blind, parallel-group clinical trial to compare the efficacy of a new combination of antimalarial drugs with that of halofantrine. The patients were hospitalized for 3 days and blood samples were collected for each patient. Thick and thin peripheral blood smears were taken at 0, 4, 8, 12, 24, 32, 48, 52, 56, and 60 h after the start of treatment and subsequently on days 8, 15, 21, and 29. Thin and thick blood films (one slide each) were Giemsa stained and examined by an experienced microscopist for at least 20 min before the samples were declared negative. The microscopist was unaware of the patient treatment status. Parasitemia was expressed as number of parasites per microliter by using the leukocyte count per microliter and number of infected erythrocytes (thin blood film) or number of parasites (thick blood film) per 200 leukocytes.
(b) Sample treatment.
Fifty microliters of whole blood was transferred into a 0.5-ml centrifuge tube containing 500 μl of lysis buffer (0.2% NaCl, 1% Triton X-100, 1 mM EDTA) and mixed at room temperature by inverting the tube to ensure complete lysis of the erythrocytes. The mixture was centrifuged at 11,300 × g (Mikroliter 2041; Hettich) at 4°C for 10 min and the supernatant was removed. The pellet was washed with 300 μl of PCR buffer (10 mM Tris-HCl [pH 9], 50 mM KCl, 2.5 mM MgCl2, 0.01% gelatin, 0.1% Triton X-100) and centrifuged at 10,000 rpm for 5 min. The pellet of each sample was stored at −20°C before PCR amplification.
Oligonucleotide primers and labeled probe.
Oligonucleotides were synthesized by Eurogentec (Seraing, Belgium). Two primers were designed according to the primary sequence of the SSU rRNA gene of P. falciparum (9). The forward primer corresponded position 919 to 939 of the sequence (5′-AGTTACGATTAATAGGAGTAG-3′) (10), and the reverse primer corresponded to position 1180 to 1201 on the complementary strand (5′-CCAAAGACTTTGATTTCTCAT-3′). An internal oligonucleotide probe (5′-GAACGAAAGTTAAGGGAG TGAAGACG-3′) was labeled with digoxigenin-11–dUTP by using the digoxigenin oligonucleotide tailing kit (Boehringer Mannheim) according to the manufacturer’s instructions.
PCR amplification.
Amplification was carried out in a DNA thermal cycler (Hybaid) according to the method of Saiki et al., with modifications (18). The washed pellets were resuspended in 80 μl of the PCR buffer described above containing a 0.2 μM concentration of each primer. The samples were overlaid with 70 μl of mineral oil and boiled at 100°C for 10 min before addition of 1 U of Taq polymerase (ATGC, Noisy le Grand, France) and a 200 μM concentration of each deoxynucleotide triphosphate in 20 μl of PCR buffer. Samples were amplified in duplicate. The PCR program ran for 40 cycles under the following conditions: 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C, with exception of the first cycle, in which the denaturation was carried out for 5 min, and for the last cycle, in which the elongation time was extended to 10 min. Each experiment included control tubes corresponding to a serial dilution of (i) positive controls consisting of P. falciparum genomic DNA and (ii) a negative control containing no target DNA.
Detection of PCR products.
Twenty microliters of the PCR product was electrophoresed in a 2% agarose gel containing 0.1 μg of ethidium bromide per ml, and bands were visualized by UV transillumination. For Southern blot hybridization, amplified DNA was transferred from the agarose gel to a positively charged nylon membrane (Hybond+; Amersham, Les Ulis, France) by the method of Southern (21). The PCR sample was prehybridized for 1 h at 50°C in hybridization buffer containing 5× SSC, 0.1% (wt/vol) N-lauroylsarcosine, 0.02% (wt/vol) sodium dodecyl sulfate (SDS), and a 1% concentration of the blocking reagent recommended by Boehringer Mannheim. Hybridization was carried out overnight at 50°C in the same buffer with 0.5 or 1 pmol of the labeled probe per ml. The blot was then washed twice for 5 min each time in 2× SSC–0.1% SDS at room temperature and twice for 15 min each time in 0.5× SSC–0.1% SDS at 50°C. Bound digoxigenin-11–dUTP-labeled probe was detected by using a digoxigenin luminescent detection kit (Boehringer Mannheim). The membrane was autoradiographed on Hyperfilm ECL (Amersham) for 15 to 30 min.
RESULTS
Genus specificity.
The chosen primers were able to amplify both asexual and sexual sequences of the 18S rRNA genes of the four human malaria parasites. The 291-bp PCR fragments of the four human Plasmodium species were visualized by agarose gel electrophoresis, and the amplified DNA gave a strong signal with the oligonucleotide digoxigenin-labeled probe in Southern blot hybridization. No signal was detected by PCR or Southern blot analysis for the other parasites tested (Toxoplasma, Leishmania, and Trypanosoma) or with the human DNA (Fig. 1).
FIG. 1.
Determination of the genus specificity of PCR. (A) Analysis by agarose gel electrophoresis and ethidium bromide staining. (B) Southern blot and hybridization with a digoxigenin-11–dUTP-labeled probe. Lane 1, φX174 DNA cleaved with HaeIII as a molecular size marker, lane 2, L. infantum (106 organisms); lane 3, T. gondii (106 organisms); lane 4, T. gondii DNA (1 μg); lane 5, human DNA (1 μg); lane 6, T. cruzi (106 organisms); lane 7, P. vivax (0.3%); lane 8, P. ovale (0.1%); lane 9, P. malariae (1%); lane 10, pBR322 DNA cleaved with HaeIII as a molecular size marker; lane 11, P. falciparum (0.5%). Arrows at the right indicate the 291-bp PCR product.
Level of detection. (i) Absolute sensitivity.
Over 20 tests, the sensitivity of the PCR was determined with P. falciparum DNA obtained by phenol-chloroform extraction with a 10-fold dilution standard down to 1 fg (10−15 g). A visible signal was detected in 30% of cases at 0.001 pg of DNA and in 55% of cases at 0.01 pg of DNA (a genome equivalent of the parasite is 0.02 pg according to Goman et al. [8]) (Table 1).
TABLE 1.
Absolute sensitivity of PCR
| Quantity of DNA (pg) | Genome equivalent | Frequency (%) of positive results |
|---|---|---|
| 10 | 500 | 100 |
| 1 | 50 | 100 |
| 0.1 | 5 | 85 |
| 0.01 | 0.5 | 55 |
| 0.001 | 0.05 | 30 |
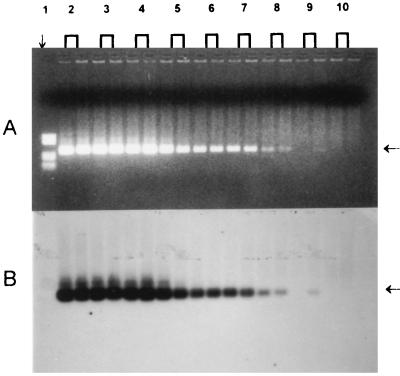
Sensitivity was also measured by using a limited dilution of a single P. falciparum isolate, from 1 to 10−8%. The PCR was able to detect 5 parasites (patient 1), 12.5 parasites (patient 2), and 0.25 parasite (patient 3) (corresponding to 0.1, 0.25, and 0.005 parasite/μl) in 50 μl of whole blood at 50% hematocrit (5 × 106 erythrocytes/μl). Results for patient 3 are showed in Fig. 2.
FIG. 2.
Determination of the absolute sensitivity of PCR. (A) Analysis by agarose gel electrophoresis. (B) Southern blot hybridization with a digoxigenin-11–dUTP-labeled probe. Lane 1, pBR322 DNA cleaved with HaeIII as a molecular size marker; lanes 2 to 10, 10-fold dilutions of a single isolate with uninfected erythrocytes (1 to 10−8%). Arrows at the right indicate the 291-bp PCR product.
(ii) Relative sensitivity.
Our technique was applied to compare the results of the thick blood film and PCR in a series of 135 samples from 12 patients treated with a new antimalarial combination. The results showed that the thick blood film had a lower sensitivity than the PCR diagnosis. We therefore calculated the sensitivity of Giemsa staining with respect to the PCR results (Table 2). The thick blood film was negative for 27.7% of samples shown to be PCR positive by ethidium bromide staining. For all PCR-negative samples the thick blood films were also negative. Samples (n = 134) from these patients were tested by dot blot hybridization (Table 3), and the 291-bp segment specific for the Plasmodium genus was amplified in samples from all 12 patients at day 0. We observed that PCR yields positive results longer than microscopic examinations. The median times to clearance of parasite DNA were 54 h with ethidium bromide staining and 192 h by dot blot hybridization. According to thick blood film the median total clearance time was 32 h. For one patient PCR after ethidium bromide staining or dot blot hybridization yielded positive results until day 19, although the microscopic examination was negative at day 5. One week later, this patient, who had received the combination of antimalarial drugs, consulted the department again with headache and asthma without fever. P. falciparum was diagnosed by microscopic examination (600 trophozoites/μl). To cure the relapse he was given one dose of halofantrine. The thick blood films were negative on days 18, 21, and 28, but the PCR yielded a positive result on day 18, giving negative results only on days 21 and 28. For another patient dot blot hybridization yielded positive results until day 15 and a thick blood film was negative at day 2, but no failure of treatment was observed more than 4 weeks after the start of therapy.
TABLE 2.
Sensitivities of the thick blood filma and PCR diagnosis with ethidium bromide staining
| Thick blood film result | No. of samples with PCR result
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 68 | 0 | 68 |
| Negative | 26 | 41 | 67 |
| Total | 94 | 41 | 135 |
Thick blood film sensitivity is 72.3%.
TABLE 3.
Sensitivities of the thick blood filma and PCR diagnosis with dot blot hybridization with a digoxigenin-11–dUTP-labeled probe
| Thick blood film result | No. of samples with PCR hybridization result
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 68 | 0 | 68 |
| Negative | 44 | 22 | 66 |
| Total | 112 | 22 | 134 |
Thick blood film sensitivity was 60.7%.
DISCUSSION
To date PCR has been a powerful tool for malaria diagnosis. Many studies have demonstrated the greater sensitivity and specificity of PCR than of the thick blood film. However, microscopic detection remains the most reliable standard. The techniques for detecting specific PCR products (gel electrophoresis with ethidium bromide staining and dot blot hybridization) are not compatible with those used in routine diagnostic laboratories which require a prompt diagnosis for initiating treatment of severe or acute malaria in patients with clinical symptoms. Other applications have been proposed: the detection of asymptomatic carriers in areas of endemicity (2) or during epidemiological studies (17, 29), the monitoring of patient responses to antimalarial drugs, and genotype characterization (16, 28).
We wanted to develop a fast, sensitive PCR method for detecting all four human malaria species to screen blood donors in areas of endemicity. Most PCR diagnostic assays use organic extraction with phenol-chloroform and ethanol precipitation of DNA from blood samples. This method is not practical for large numbers of samples: it is time-consuming and requires the use of hazardous chemical products. To overcome this problem, several methods of sample preparation have been developed: saponin lysis and filtration or centrifugation, direct spotting on a membrane with lysis with distilled water, and boiling in the presence of a chelating resin (Chelex 100). All these procedures are simple and give similar results. We therefore selected the method proposed by Vu et al. (25), which requires only erythrocyte lysis followed by centrifugation.
Almost all PCR methods reported detect only P. falciparum, which is the most virulent species, or use one set of primers for each human malaria species (20) or species-specific probe. Because of our concern with screening blood donors, we considered a method which allows the detection of all human parasite species with a single probe preferable to those detecting only one species, considering the morbidity associated with other types of malaria. We were able to detect the four human malaria species with a single set of primers. The chosen amplified target was a region of the SSU rRNA gene conserved within every member of the genus Plasmodium. This method is also very sensitive, as we were able to detect a single parasite in 50 μl of whole blood. Since the thick blood film detection limit corresponds to five parasites/μl, the sensitivity of PCR detection is, on average, 250 times greater. The PCR diagnosis method therefore permits the detection of more cases of low-level parasitemia than thick blood film examination (5), and its use for blood screening can minimize the risk of malaria transmission by blood transfusion in areas of endemicity (25).
PCR diagnosis has also been used for monitoring antimalarial therapeutic responses and chemoresistance. Previous studies showed that if PCR yields positive results for 5 to 8 days after treatment, therapeutic failure possibly due to parasite resistance may be predicted (11). The World Health Organization has defined four degrees of resistance (S, RI, RII, and RIII) based on the evolution of parasitemia detected by microscopic examination of thick blood films. It is likely that the greater sensitivity of PCR modifies the classification criteria, as there is the possibility that an RI resistance pattern as defined by microscopy could be reclassified as an RII pattern when PCR is employed. Hence, early identification of the type of parasite resistance would permit an appropriate therapy change. In conclusion, this rapid and accurate test for P. falciparum and other plasmodial species may facilitate early diagnosis and initiation of appropriate antimalarial therapy.
The PCR method has been previously demonstrated to be sensitive and specific for diagnosis of malaria and may be preferable to microscopy as the reference standard for evaluating new diagnostic tests. On the basis of the first step of our study, which has demonstrated the 100% specificity and the high absolute sensitivity of the PCR compared to the Giemsa-stained thick smear based on a serial dilution, we have chosen PCR as our standard. Therefore, we classified every negative thick smear corresponding to a positive PCR result as a false negative, and this classification is corroborated by the early PCR detection of treatment failure.
ACKNOWLEDGMENTS
We are grateful to Antoine Minaret and Marie-Paule Nivez for technical assistance and to Caroline Doerig and Jacques Breton for reviewing the manuscript.
This study was supported by a grant from the Raoul Follereau Association.
REFERENCES
- 1.Abdullah N R, Furuta T, Taib R, Kita K, Kojima S, Wah M J. Short report: development of a new diagnostic method for Plasmodium falciparum infection using a reverse transcriptase-polymerase chain reaction. Am J Trop Med Hyg. 1996;54:162–163. doi: 10.4269/ajtmh.1996.54.162. [DOI] [PubMed] [Google Scholar]
- 2.Arai M, Mizukoshi C, Kubochi F, Kakutani T, Wataya Y. Detection of Plasmodium falciparum in human blood by a nested polymerase chain reaction. Am J Trop Med Hyg. 1994;51:617–626. doi: 10.4269/ajtmh.1994.51.617. [DOI] [PubMed] [Google Scholar]
- 3.Barker R J, Banchongaksorn T, Courval J M, Suwonkerd W, Rimwungtragoon K, Wirth D F. A simple method to detect Plasmodium falciparum directly from blood samples using the polymerase chain reaction. Am J Trop Med Hyg. 1992;46:416–426. doi: 10.4269/ajtmh.1992.46.416. [DOI] [PubMed] [Google Scholar]
- 4.Beadle C, Long G W, Weiss W R, McElroy P D, Maret S M, Oloo A J, Hoffman S L. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–568. doi: 10.1016/s0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 5.Bottius E, Guanzirolli A, Trape J, Rogier C, Konate L, Druilhe P. Malaria: even more chronic in nature than previously thought; evidence for subpatient parasitaemia detectable by the polymerase chain reaction. Trans R Soc Trop Med Hyg. 1996;90:15–19. doi: 10.1016/s0035-9203(96)90463-0. [DOI] [PubMed] [Google Scholar]
- 6.Craig M H, Sharp B L. Comparative evaluation of four techniques for the diagnosis of Plasmodium falciparum infections. Trans R Soc Trop Med Hyg. 1997;91:279–282. doi: 10.1016/s0035-9203(97)90074-2. [DOI] [PubMed] [Google Scholar]
- 7.Gay F, Traore B, Zanoni J, Danis M, Fribourg-Blanc A. Direct acridine orange fluorescence examination of blood slides compared to current techniques for malaria diagnosis. Trans R Soc Trop Med Hyg. 1996;90:516–518. doi: 10.1016/s0035-9203(96)90300-4. [DOI] [PubMed] [Google Scholar]
- 8.Goman M, Langsley G, Hyde J E, Yankovsky N K, Zolg J W, Scaife J G. The establishment of genomic DNA libraries for the human malaria parasite Plasmodium falciparum and identification of individual clones by hybridization. Mol Biochem Parasitol. 1982;5:391–400. doi: 10.1016/0166-6851(82)90012-3. [DOI] [PubMed] [Google Scholar]
- 9.Goman M, Mons B, Scaife J. The complete sequence of a Plasmodium malariae SSUrRNA gene and its comparison to other plasmodial SSUrRNA genes. Mol Biochem Parasitol. 1991;45:281–288. doi: 10.1016/0166-6851(91)90096-o. [DOI] [PubMed] [Google Scholar]
- 10.Jaureguiberry G, Hatin I, d’Auriol L, Galibert G. PCR detection of Plasmodium falciparum by oligonucleotide probes. Mol Cell Probes. 1990;4:409–414. doi: 10.1016/0890-8508(90)90031-t. [DOI] [PubMed] [Google Scholar]
- 11.Kain K C, Kyle D E, Wongsrichanalai C, Brown A E. Qualitative and semiquantitative polymerase chain reaction to predict Plasmodium falciparum treatment failure. J Infect Dis. 1994;170:1626–1630. doi: 10.1093/infdis/170.6.1626. [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto F. Rapid diagnosis of malaria by fluorescence microscopy with light microscope and interference filter. Lancet. 1991;337:200–202. doi: 10.1016/0140-6736(91)92159-y. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Wirtz R A, McConkey G A, Sattabongkot J, Waters A P, Rogers M J, McCutchan T F. Plasmodium: genus-conserved primers for species identification and quantification. Exp Parasitol. 1995;81:182–190. doi: 10.1006/expr.1995.1107. [DOI] [PubMed] [Google Scholar]
- 14.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 15.McCutchan T F, De La Cruz V F, Lal A A, Gunderson J H, Elwood H J, Sogin M L. Primary sequences of two small subunit ribosomal RNA genes from P. falciparum. Mol Biochem Parasitol. 1988;28:63–68. doi: 10.1016/0166-6851(88)90181-8. [DOI] [PubMed] [Google Scholar]
- 16.Mercereau-Puijalon O, Fandeur T, Bonnefoy S, Jacquemot C, Sarthou J-L. A study of the genomic diversity of Plasmodium falciparum in Senegal 2. Typing by the use of the polymerase chain reaction. Acta Trop. 1991;49:293–304. doi: 10.1016/0001-706x(91)90080-4. [DOI] [PubMed] [Google Scholar]
- 17.Roper C, Elhassan I M, Hviid L, Giha H, Richardson W, Babiker H, Satti G M H, Theander T G, Arnot D E. Detection of very low level Plasmodium falciparum infections using the nested polymerase chain reaction and a reassessment of the epidemiology of unstable malaria in Sudan. Am J Trop Med Hyg. 1996;54:325–331. doi: 10.4269/ajtmh.1996.54.325. [DOI] [PubMed] [Google Scholar]
- 18.Saiki R K, Scharf S, Faloona F, Mullis K B, Horn G T, Erlich H A, Arnheim N. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 19.Sethabutr O, Brown A E, Panyim S, Kain K C, Webster K, Echeverria P. Detection of Plasmodium falciparum by polymease chain reaction in a field study. J Infect Dis. 1992;166:145–148. doi: 10.1093/infdis/166.1.145. [DOI] [PubMed] [Google Scholar]
- 20.Snounou G, Viriyakosol S, Zhu X P, Jarra W, Pinheiro L, do Rosario V E, Thaithong S, Brown K N. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 21.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 22.Tirasophon W, Rajkulchai P, Ponglikitmongkol M, Wilairat P, Boonsaeng V, Panyin S. A highly sensitive, rapid, and simple polymerase chain reaction-based method to detect human malaria (Plasmodium falciparum and Plasmodium vivax) in blood samples. Am J Trop Med Hyg. 1994;51:308–313. doi: 10.4269/ajtmh.1994.51.308. [DOI] [PubMed] [Google Scholar]
- 23.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 24.Viriyakosol S, Siripon N, Zhu X P, Jarra W, Seugorn A, Brown K N, Snounou G. Plasmodium falciparum: selective growth of subpopulations from field samples following in vitro culture, as detected by the polymerase chain reaction. Exp Parasitol. 1994;79:517–525. doi: 10.1006/expr.1994.1112. [DOI] [PubMed] [Google Scholar]
- 25.Vu T T, Tran V B, Phan N T, Le T T, Luong V H, O’Brien E, Morris G E. Screening donor blood for malaria by polymerase chain reaction. Trans R Soc Trop Med Hyg. 1995;89:44–47. doi: 10.1016/0035-9203(95)90652-5. [DOI] [PubMed] [Google Scholar]
- 26.Wataya Y, Arai M, Kubochi F, Mizukoshi C, Kakutani T, Ohta N, Ishii A. DNA diagnosis of falciparum malaria using a double PCR technique: a filed trial in the Solomon Islands. Mol Biochem Parasitol. 1993;58:165–168. doi: 10.1016/0166-6851(93)90101-3. [DOI] [PubMed] [Google Scholar]
- 27.Waters A P, McCutchan T F. Rapid, sensitive diagnosis of malaria based on ribosomal RNA. Lancet. 1989;i:1343–1346. doi: 10.1016/s0140-6736(89)92800-6. [DOI] [PubMed] [Google Scholar]
- 28.Wooden J, Gould E E, Paull A T, Sibley C H. Plasmodium falciparum: a simple polymerase chain reaction method for differentiating strains. Exp Parasitol. 1992;75:207–212. doi: 10.1016/0014-4894(92)90180-i. [DOI] [PubMed] [Google Scholar]
- 29.Zalis M G, Ferreira da Cruz M F, Balthazar-Guedes H C, Banic D M, Alecrim W, Souza J M, Druilhe P, Daniel-Ribeiro C T. Malaria diagnosis: standardization of a polymerase chain reaction for the detection of Plasmodium falciparum parasites in individuals with low-grade parasitemia. Parasitol Res. 1996;82:612–616. doi: 10.1007/s004360050173. [DOI] [PubMed] [Google Scholar]