Abstract
Background:
The use of bone-anchored osseointegration implants for amputation reconstruction continues to expand throughout the world. Benefits are thought to include the elimination of socket-related problems and improved control and proprioception of the prosthetic limb. Reported outcomes have been positive, but skepticism remains with regard to the risk of infection and implant failure. Further results from early adopters are therefore needed prior to widespread acceptance and regulatory approval.
Methods:
A retrospective review of the first 31 consecutive patients who underwent implantation of a press-fit osseointegration implant of the femur or tibia with follow-up of at least 6 months was performed. The primary outcome was the patient-reported Questionnaire for persons with a Transfemoral Amputation (Q-TFA) measured preoperatively and 6 to 12 months postoperatively. Patient-Reported Outcomes Measurement Information System (PROMIS) and Limb Deformity-Scoliosis Research Society (LD-SRS) scores, 2-minute and 6-minute walk tests, and complications were also recorded.
Results:
In this study, 18 femoral reconstructions and 13 tibial reconstructions were performed, with a mean follow-up (and standard deviation) of 21.1 ± 9.2 months. Twenty-eight reconstructions were single-stage implantations. All Q-TFA domains improved significantly (p < 0.001) from preoperatively to postoperatively, including the global score (25.0 ± 17.4 to 81.2 ± 17.6 points), prosthetic use (50.2 ± 39.9 to 91.2 ± 18.7 points), prosthetic mobility (49.7 ± 26.9 to 81.4 ± 21.5 points), and prosthetic problems (46.4 ± 17.5 to 9.1 ± 6.6 points). The overall and functional outcome domains of the PROMIS and LD-SRS and the 2-minute walk test (243 ± 107 to 369 ± 151 ft [74 ± 33 to 112 ± 46 m]; p = 0.022) and 6-minute walk test (609 ± 323 to 1,054 ± 555 ft [186 ± 98 to 321 ± 169 m]; p = 0.016) also improved significantly. Serious adverse events included 2 periprosthetic hip fractures, 1 explantation for septic loosening, and 1 explantation for aseptic loosening, with an overall implant retention of 93%. The most common complication was low-grade, soft-tissue infection requiring oral antibiotics.
Conclusions:
Similar to the early experience of other international centers, osseointegration implants improved the overall and functional experience of patients compared with socket prosthetics. Complications were present but manageable and were not a deterrent to ongoing support of the technology.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Bone-anchored osseointegration prostheses made from porous titanium have been an emerging treatment in the field of amputation reconstruction for the last 2 decades. The patient-reported and functional results have been overwhelmingly positive when compared with those from the traditional socket prosthesis1-6, which is plagued by numerous difficulties for the patient, including poor fit, skin irritation and ulceration, excessive sweating, aggravating and time-consuming donning and doffing, range-of-motion limitations, and, most importantly, poor energy transfer from the bone to the prosthesis7,8. Patients utilizing osseointegration prostheses tend to wear their prosthesis more and achieve higher function than is possible with a socket prosthesis, especially for above-the-knee amputees2,3,5,9-11. Although the concern for infection with a transcutaneous metal implant remains a valid concern, data have indicated that most are superficial and successfully treated without requiring explantation of the prosthesis1,4,5,10,12,13.
Familiarity with this approach and most of the relevant literature stems from a few high-volume centers around the world1,2,5,6, and the experience in the United States has been limited by regulatory restrictions until recently, when the U.S. Food and Drug Administration (FDA) allowed limited use of certain implants. This study evaluates our early experience with lower-extremity osseointegration prosthesis implantation using a porous-coated titanium stem axially impacted into position following a reaming and broaching technique at a tertiary academic center. The purpose of this study was to compare results, using the same widely used outcome metric, the Questionnaire for persons with a Transfemoral Amputation (Q-TFA), with other early international results and to provide advice for what other early adopters of the technique may expect to encounter.
Materials and Methods
A retrospective review of prospectively gathered data was approved by the institutional review board and patients gave informed consent for the study. All 31 patients who underwent an osseointegration prosthesis implantation in the femur or tibia beginning in October 2017 with follow-up of at least 6 months were included. Inclusion criteria were the presence of an above-the-knee or below-the-knee amputation with any difficulty using a traditional socket prosthesis. Also included were patients with chronic pain or extremity dysfunction electing to undergo amputation with primary osseointegration reconstruction. Known or suspected infection based on magnetic resonance imaging (MRI) examination was treated with surgical debridement and placement of a cement antibiotic spacer for at least 6 weeks prior to implantation with an osseointegration prosthesis. Exclusion criteria included patients with opioid dependence not responsive to treatment, psychiatric conditions not amenable to a permanent implant, prior radiation to the residual bone, severely osteoporotic bone, and bone segments too short to support an implant.
Surgical procedures were performed by a single surgeon at an academic tertiary referral hospital. The first 2 implants were custom implants manufactured by Signature Orthopaedics, and the remainder utilized the Osseointegrated Prosthetic Limb (OPL) system (Permedica Manufacturing). Computed tomographic (CT) scans were used preoperatively to plan the appropriate implant size that is customized in shape, length, and diameter. These implants are press-fit impacted into the residual bone after appropriate reaming and have a 0.5-mm macroporous titanium ingrowth surface coating. The stem ends in a transcutaneous collar that is coated and polished titanium-niobium oxynitride ceramic to permit skin gliding14. A dual-cone adaptor screws into the collar along 1 side and attaches to the prosthesis abutment on the other side (Figs. 1-A through 1-D). Routine culture specimens were taken of the residual bone in all cases with a prior amputation or prior debridement for infection. Patients who had positive cultures were treated with 6 weeks of bacteria-specific antibiotics but retention of the implant. For patients with symptoms of phantom limb pain, painful neuroma, or complex regional pain syndrome, targeted muscle reinnervation was performed at the time of the surgical procedure by a plastic surgeon.
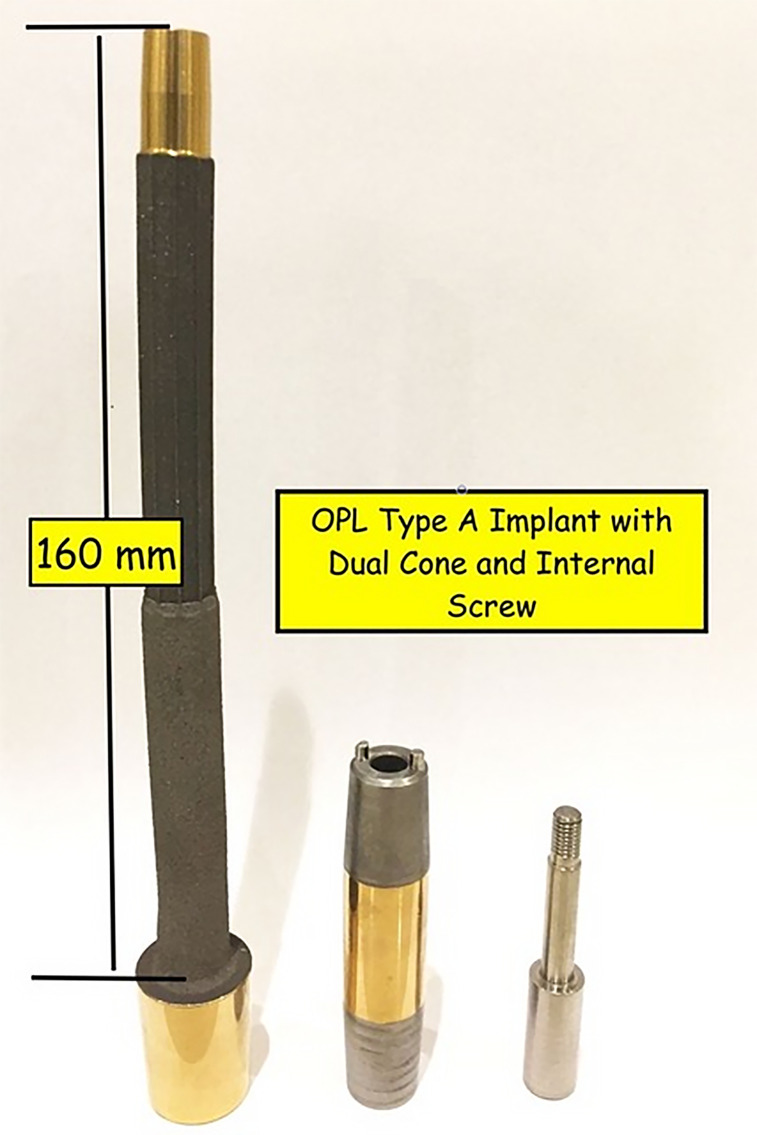
Fig. 1-A.
The components of the OPL include the main implant with the transcutaneous collar and porous-coated stem, the dual cone adaptor, and the screw that seats the dual cone in the stem. The standard length for the femur and tibia with sufficient residual bone is 160 mm.
Fig. 1-B.
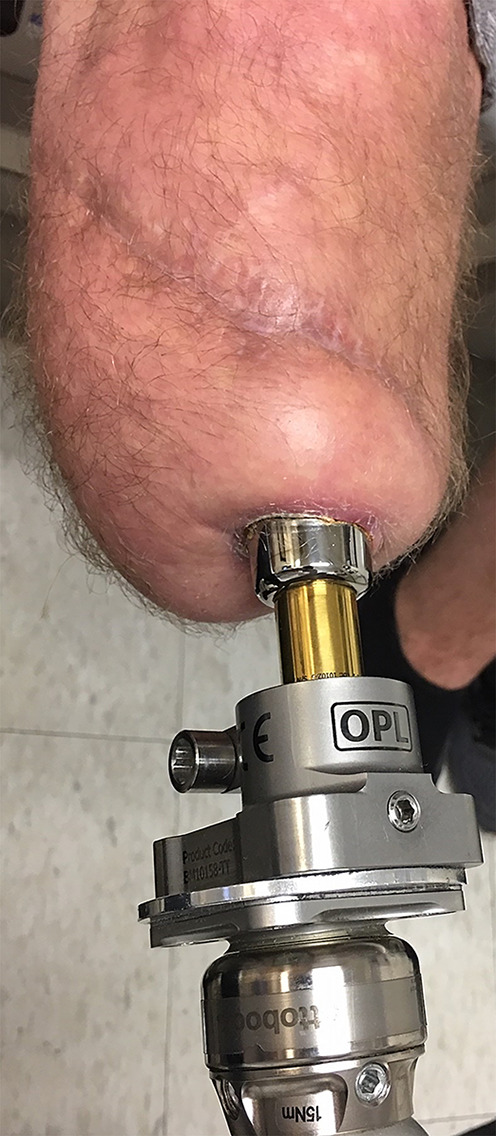
The dual cone (gold) is attached to the bushing and prosthetic adaptor (labeled OPL), which supports standard prosthetic components.
Fig. 1-C.
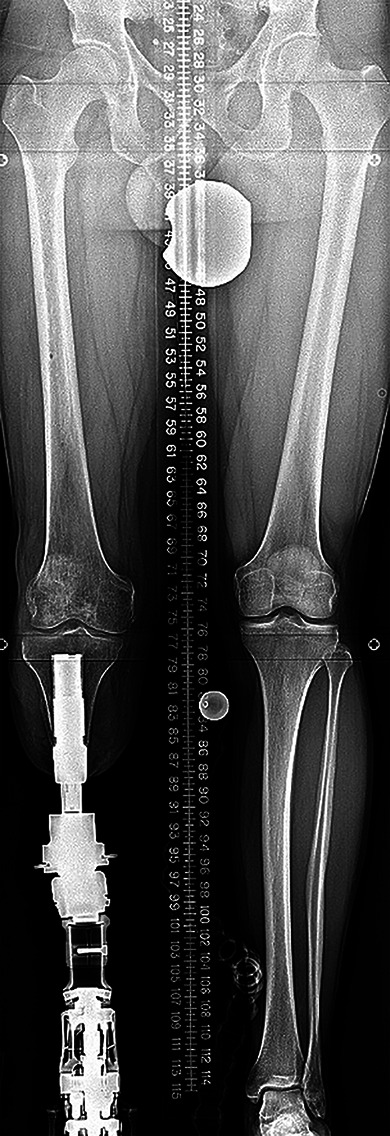
Standing radiograph showing assembly for the tibial prosthesis.
Fig. 1-D.
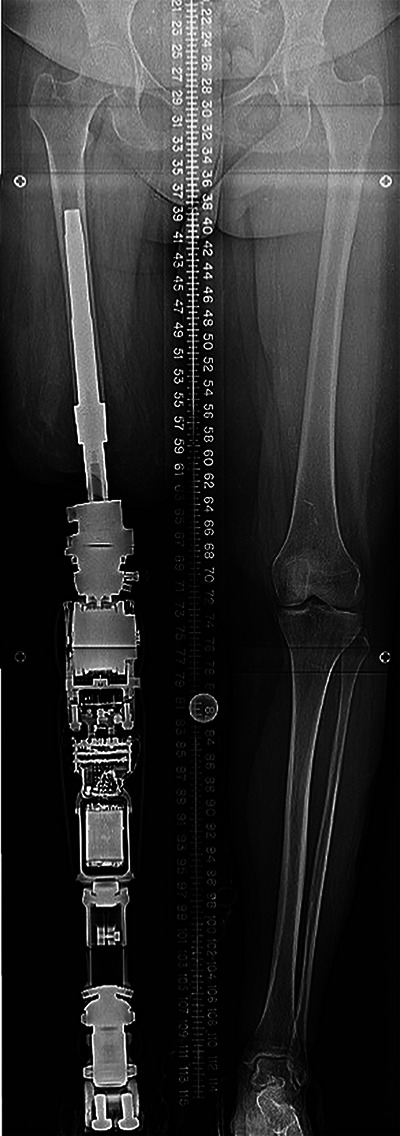
Standing radiograph showing assembly for the femoral prosthesis.
Three early patients had a 2-stage implantation consisting of 8 weeks of closed soft tissue over the implant to allow for bone ingrowth. Based on international surgeon experience, the remainder were implanted in a single stage with immediate creation of the skin stoma. Progressive loading of the implant using a rubber cap over the exposed implant was initiated the day after the stoma creation unless the bone was a short segment or osteopenic or fracture occurred during implantation, in which case 4 to 6 weeks of non-weight-bearing were prescribed to allow ingrowth of bone. Loading started at 20 lb (9 kg) and progressed 5 lb (2.3 kg) every other day for 6 to 8 weeks. Once full weight-bearing on the cap was achieved, a prosthetic leg was attached to the implant and protected weight-bearing under the guidance of a physical therapist was initiated for 6 weeks.
The primary outcome measure was the Q-TFA, which was also administered to patients with a transtibial amputation. The Q-TFA has 4 domains to measure patient experience with a prosthesis: prosthetic use, prosthetic mobility, prosthetic problems, and a global score. The questionnaire was administered preoperatively and between 6 months and 1 year postoperatively. The Q-TFA is not applicable to some patients preoperatively, including those who do not wear a prosthesis at all and those undergoing primary amputation. We also collected the Limb Deformity-Scoliosis Research Society (LD-SRS) score, a validated outcome score15 that includes patient-reported functional activity, mental health, pain, and self-image domains, and the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function, Pain Intensity, Pain Interference, EuroQol 5 Dimensions (EQ-5D), Global Mental Health, and Global Physical Health scores. Patients also performed 2-minute and 6-minute walk tests preoperatively, if they were ambulatory on the involved limb with a socket prosthesis, and at 6 months to 1 year postoperatively.
Complication data were extracted and compiled from the medical record. Infectious complications were considered low-grade soft-tissue infection if they required oral antibiotics, high-grade soft-tissue infection if they required intravenous antibiotics, and deep infection or osteomyelitis if bone changes were evident on radiographs. Simple mechanical complications included screw, dual cone, or abutment failures requiring replacement parts, and severe complications involved fracture of the implanted stem. Overall, adverse events were categorized as severe (deep infection requiring explantation, implant fracture, periprosthetic fracture requiring operative fixation, aseptic loosening leading to loss of the implant), moderate (high-grade soft tissue and deep infection resolving with surgical debridement and/or intravenous antibiotics with a retained implant, soft tissue and/or stoma revision), or minor (low-grade soft-tissue infection, mechanical complications treated in an office setting).
Demographic data were analyzed using descriptive statistics. Statistical analysis comparing preoperative and postoperative functional scores was performed using the 2-sample t test with equal variances. When analyzing the 2 interval variables of Q-TFA score and time to amputation, the Pearson correlation coefficient (r) was calculated. Coefficient values of 0 to 0.29 indicated a weak correlation, 0.3 to 0.49 indicated a moderate correlation, and 0.5 to 1.0 indicated a strong correlation. For all tests, significance was set at p ≤ 0.05. The software used to perform the analysis was Stata/IC 13.1 for Mac (64-bit Intel) (StataCorp).
Results
Thirty-one patients who underwent implantation of an osseointegration prosthesis (18 femoral and 13 tibial) and had follow-up of at least 6 months (mean follow-up [and standard deviation], 21.1 ± 9.2 months) were included in the analysis. There were 19 male patients and 12 female patients (Table I). The mean age was 49.6 ± 12.0 years in the femoral reconstruction group and 51.3 ± 14.1 years in the tibial reconstruction group. The primary reason for amputation was trauma in 22 cases; other causes included necrotizing fasciitis (1 patient), chronic periprosthetic knee infection (2 patients), vascular injury with ischemia (2 patients), neurologic injury or complex regional pain syndrome (3 patients), and bone deformity with pain or arthritis (1 patient). Two patients with complex regional pain syndrome underwent primary amputations with immediate bone-anchored prosthesis placement. Six patients (3 in the femoral reconstruction group and 3 in the tibial reconstruction group) underwent bone debridement and placement of an antibiotic cement spacer based on a known infection distal to the amputation (e.g., in a total knee prosthesis) or MRI evidence of osteomyelitis of the residual bone (Table II). Eight patients in the femoral reconstruction group and 7 patients in the tibial reconstruction group had concurrent targeted muscle reinnervation.
TABLE I.
Patient Demographic Characteristics
| Femoral Reconstruction Group (N = 18) | Tibial Reconstruction Group (N = 13) | |
|---|---|---|
| Patient characteristics | ||
| Sex* | ||
| Male | 11 | 8 |
| Female | 7 | 5 |
| Age† (yr) | 49.6 ± 12.0 | 51.3 ± 14.1 |
| Time since amputation† (yr) | 7.8 ± 8.8 | 9.4 ± 12.5 |
| Amputation etiology* | ||
| Trauma | 13 | 9 |
| Necrotizing fasciitis | 1 | — |
| Chronic periprosthetic infection | 2 | — |
| Vascular injury | 2 | — |
| Neurologic injury or complex regional pain syndrome | — | 3 |
| Deformity | — | 1 |
| Residual bone length† (mm) | 222 ± 94 | 119 ± 34 |
The values are given as the number of patients.
The values are given as the mean and the standard deviation.
TABLE II.
Surgical Details, Implants, and Rehabilitation
| Femoral Reconstruction Group (N = 18) | Tibial Reconstruction Group (N = 13) | |
|---|---|---|
| Surgical details* | ||
| Antibiotic cement spacer before osseointegration | 3 | 3 |
| Two-stage implantation | 3 | 0 |
| Single-stage implantation | 15 | 13 |
| Targeted muscle reinnervation | 8 | 7 |
| Implant characteristics† | ||
| Diameter (mm) | 17 (14 to 25) | 21 (14 to 31) |
| Length (mm) | 148 (80 to 160) | 109 (65 to 160) |
| Time until prosthetic leg was attached‡ (wk) | 8.6 ± 3.4 | 10.0 ± 3.0 |
The values are given as the number of patients.
The values are given as the mean, with the range in parentheses.
The values are given as the mean and the standard deviation.
The shape and length of the residual femora were fairly consistent. The implant length was 160 mm (full length in the implant system) in 13 of the 18 patients in the femoral reconstruction group, and, in the shorter residual bones, it was 140 mm in 2 patients and 120 mm, 100 mm, and 80 mm in the 3 other patients. The diameter was 14 to 18 mm in 14 of 18 patients and 19 mm, 20 mm, 21 mm, and 25 mm in the other 4 patients. The residual tibiae were more variable, and the implants ranged from 65 to 160 mm in length and 14 mm to 31 mm in diameter (Table II). The mean time to attachment of a prosthetic leg was shorter in the femoral reconstruction group at 8.6 ± 3.4 weeks compared with the tibial reconstruction group at 10.0 ± 3.0 weeks.
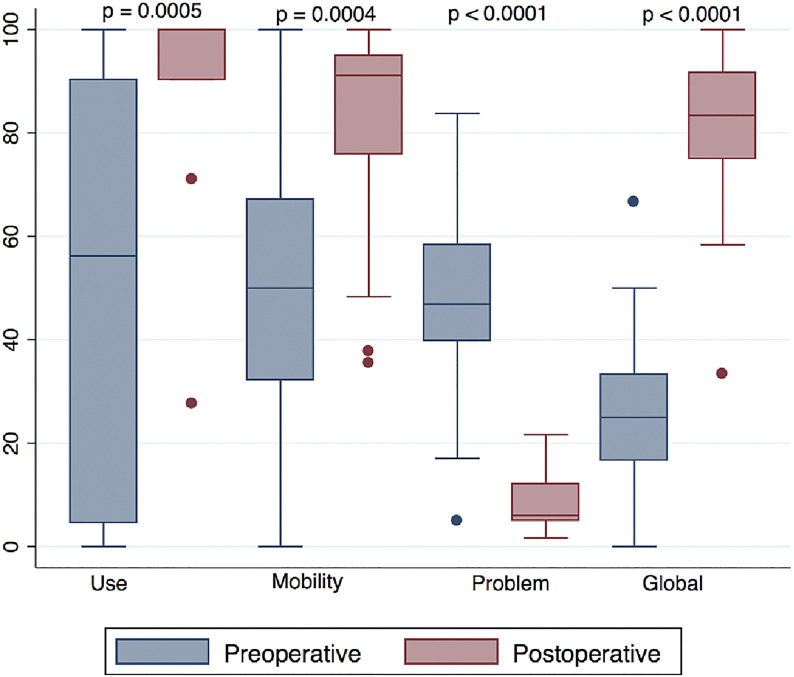
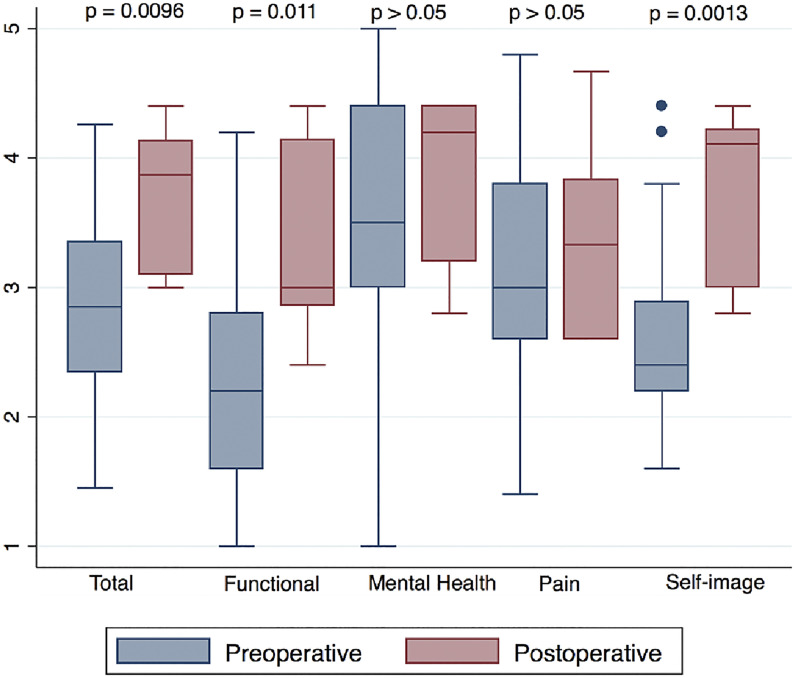
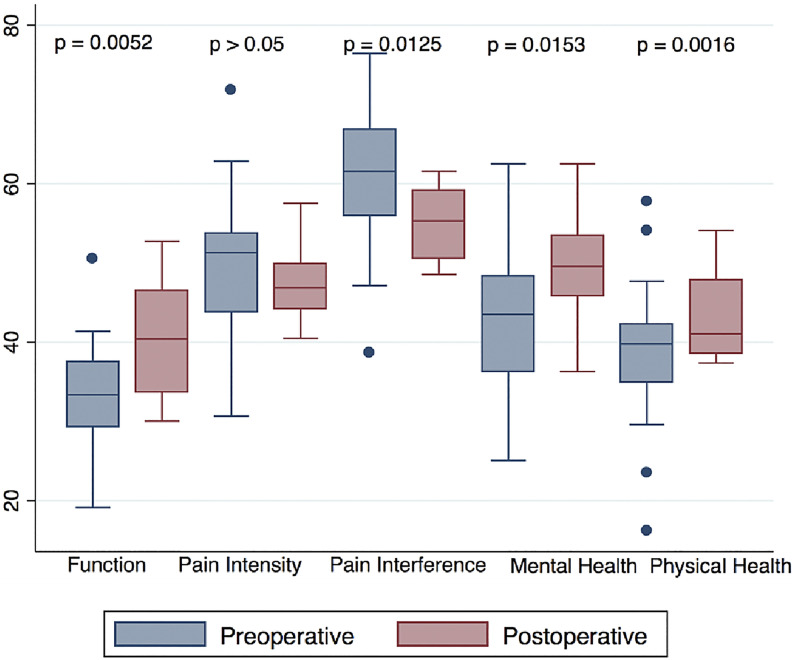
The primary outcome measure (Q-TFA) was completed by 23 patients preoperatively, 5 patients never wore a prosthesis or were undergoing primary amputation, and 3 patients were treated before the beginning of Q-TFA questionnaire use. Sixteen patients completed the questionnaire between 6 months and 1 year postoperatively. There were clinically important and significant improvements in all domains of the Q-TFA (Fig. 2, Table III). There was also an association between the time since amputation and higher postoperative Q-TFA global scores (r = 0.577; p = 0.039). The LD-SRS demonstrated significant improvement in the total score, functional activity domain, and self-image or appearance, as well as improvements in the mental health and pain scores that were not significant (Fig. 3). The PROMIS scores demonstrated significant improvements in the overall health (EQ-5D), global mental health, global physical health, pain interference, and functional domains and improvement in pain intensity that was not significant (Fig. 4).
Fig. 2.
Box plots of the preoperative (blue) and postoperative (red) Q-TFA domains. The top and bottom of the box indicate the interquartile range (IQR), the line within the box indicates the median, the whiskers indicate the minimum and maximum values, and the circles indicate outliers (values less than the first quartile – 1.5 × IQR or greater than the third quartile + 1.5 × IQR).
Fig. 3.
Box plots of the preoperative (blue) and postoperative (red) LD-SRS domains. The top and bottom of the box indicate the IQR, the line within the box indicates the median, the whiskers indicate the minimum and maximum values, and the circles indicate outliers (values less than the first quartile – 1.5 × IQR or greater than the third quartile + 1.5 × IQR).
Fig. 4.
Box plots of the preoperative (blue) and postoperative (red) PROMIS domains. The top and bottom of the box indicate the IQR, the line within the box indicates the median, the whiskers indicate the minimum and maximum values, and the circles indicate outliers (values less than the first quartile – 1.5 × IQR or greater than the third quartile + 1.5 × IQR).
TABLE III.
Results of the Q-TFA, Patient-Reported Outcome Measures, and Functional Tests
| Preoperative Score* | Postoperative Score* | P Value | |
|---|---|---|---|
| Q-TFA score | |||
| Global | 25.0 ± 17.4 | 81.2 ± 17.6 | <0.001† |
| Prosthetic use | 50.2 ± 39.9 | 91.2 ± 18.7 | <0.001† |
| Prosthetic mobility | 49.7 ± 26.9 | 81.4 ± 21.5 | <0.001† |
| Prosthetic problem | 46.4 ±17.5 | 9.1 ± 6.6 | <0.001† |
| LD-SRS | |||
| Total | 2.87 ± 0.68 | 3.66 ± 0.55 | 0.01† |
| Functional activity | 2.33 ± 0.90 | 3.36 ± 0.76 | 0.01† |
| Mental health | 3.45 ± 0.98 | 3.91 ± 0.65 | 0.25 |
| Pain | 3.13 ± 0.97 | 3.36 ± 0.76 | 0.58 |
| Self-image or appearance | 2.60 ± 0.77 | 3.76 ± 0.64 | 0.001† |
| PROMIS | |||
| Function | 33.0 ± 7.7 | 42.9 ± 6.9 | 0.005† |
| Pain intensity | 49.1 ± 9.9 | 42.3 ± 6.9 | 0.098 |
| Pain interference | 59.8 ± 10.8 | 47.8 ± 8.5 | 0.01† |
| EQ-5D | 0.59 ± 0.11 | 0.76 ± 0.09 | 0.001† |
| Global mental | 42.3 ± 10.3 | 53.5 ± 9.4 | 0.015† |
| Global physical | 39.1 ± 9.4 | 53.2 ± 9.1 | 0.002† |
| Functional tests | |||
| 2-minute walk test (ft) | 243 ± 107 (74 ± 33 m) | 369 ± 151 (112 ± 46 m) | 0.022† |
| 6-minute walk test (ft) | 609 ± 323 (186 ± 98 m) | 1,054 ± 555 (321 ± 169 m) | 0.016† |
The values are given as the mean and the standard deviation.
Significant.
The 2-minute and 6-minute walk tests were completed by 17 patients preoperatively; in the rest of the patients, 6 were not able to wear a prosthesis and walk on the affected extremity, 2 were undergoing elective amputation, and 6 patients were treated before the beginning of Q-TFA questionnaire use. Nine patients completed the functional tests postoperatively, and the remainder did not follow up in person because of travel restrictions at the time of study. There were significant improvements in ambulatory distance at 2 and 6 minutes (Table III). Of the 6 patients unable to use a prosthesis prior to the surgical procedure, all were walking with a prosthesis postoperatively. In the femoral reconstruction group, 4 patients were using 2 crutches, 4 patients were using a cane or 1 crutch, 6 patients used no assistive device, and walking aid use by 4 patients was unknown. In the tibial reconstruction group, of the 11 patients with a retained implant, all 11 used no assistive device.
There were 6 positive cultures obtained from the routine specimens taken at the time of implantation (Table IV). None appeared grossly infected and none were from the 6 patients treated with an antibiotic spacer. Two patients were treated with oral antibiotics and 4 patients were treated with intravenous antibiotics for 6 weeks. Only 1 patient developed 3 recurrent (rather than persistent) infections following treatment and was treated with a surgical debridement of the distal bone and prolonged intravenous antibiotics. The patient had no signs of infection when the antibiotics were discontinued and retained the original implant. Over the course of follow-up, 23 other soft-tissue infections occurred in 15 patients; 20 of these infections were treated with oral antibiotics and 3 were treated with intravenous antibiotics. Two of the 3 intravenous antibiotics were used in 1 patient who later underwent surgical debridement, but the implant was retained. One patient required explantation due to septic loosening of the implant. After a 6-week course of antibiotics, the patient underwent reimplantation 5 months later and had experienced no further complications at the most recent follow-up.
TABLE IV.
Summary of Complications and Treatment
| Complications | Femoral Reconstruction Group (N = 18) | Tibial Reconstruction Group (N = 13) |
|---|---|---|
| Follow-up* (mo) | 23.1 ± 10.4 | 18.3 ± 6.8 |
| Infection† | ||
| Positive cultures at implantation | 3 | 3 |
| 6-week antibiotic treatment | ||
| Oral | 2 | 0 |
| Intravenous | 1 | 3 |
| Recurrent infections | 0 | 3 (1 patient) |
| Surgical debridements | 0 | 1 (same patient) |
| Infections during follow-up† | ||
| No. of infections | 15 | 8 |
| No. of patients | 9 | 6 |
| Antibiotics | ||
| Oral | 15 | 6 |
| Intravenous | 0 | 2 |
| Surgical debridements | 0 | 1 |
| Explantation | 0 | 1 |
| Taking suppressive antibiotics | 0 | 0 |
| Mechanical† | ||
| Broken attachments | 6 | 2 |
| Broken implants | 0 | 0 |
| Periprosthetic fracture requiring open reduction and internal fixation† | 2 | 0 |
| Soft tissue† | ||
| Longer dual cone needed | 3 | 0 |
| Stoma revision | 1 | 0 |
| Aseptic loosening† | 0 | 1 |
| Overall adverse events† | ||
| Severe | 2 | 2 |
| Moderate | 2 | 8 |
| Minor | 26 | 8 |
The values are given as the mean and the standard deviation.
The values are given as the number of infections or patients.
At the most recent follow-up, none of the base or intramedullary portions of the implants had fractured. Eight attachment pieces broke and required replacement. Two of these were managed in the operating room, but these occurred early in the study and would have been managed in the clinic setting if they had happened later in the study. There were 2 displaced proximal femoral fractures that required open reduction and internal fixation. Both implants were well fixed in the femoral diaphysis and were retained, and both fractures healed uneventfully in satisfactory alignment. Three dual cones were upsized to manage soft-tissue impingement on the prosthetic leg. One patient underwent subsequent revision of the distal soft tissue and stoma due to impingement. One patient with osteoporotic bone had spontaneous aseptic loosening of the implant that dislodged from the bone. Nine months later, the patient underwent reimplantation with a larger implant and had experienced no further complications at the most recent follow-up.
Discussion
The results of this study demonstrate significant improvements in overall and functional patient-reported outcomes and ambulatory distance using a bone-anchored osseointegration prosthesis compared with a traditional socket prosthesis. Although overall pain improved on average, pain interference improved significantly, suggesting that activity is less hampered by painful stimuli. A bone-anchored osteointegration prosthesis also allowed all patients who could not use any prosthesis preoperatively to begin ambulation postoperatively. Early complications were present but manageable without explantation in 93% of cases. The most common adverse events were low-grade, soft-tissue infections and simple mechanical failures. These results are consistent with the early experience of other international centers with osseointegration prostheses.
In a 2-year prospective Swedish study, 48 patients underwent above-the-knee amputation reconstructions with 6-month latency prior to stoma creation and abutment attachment using the Osseointegrated Prosthesis for the Rehabilitation for Amputees (OPRA), which utilizes a screw-thread-bone interface instead of macroporous coating14. Four patients had implants removed during the study period (1 for infection and 3 for loosening, with an overall survival rate of 92%), and there were 3 ipsilateral hip fractures, but 89% of patients used the prosthesis and Q-TFA scores improved significantly across domains9,16. Some of the patients who underwent failed treatment requested reimplantation, similar to the 2 patients who underwent explantation in the current study, who still believed in the technique despite complications. A German study evaluated 37 patients using the Integral-Leg-Prosthesis (ILP); all patients had above-the knee amputations but with 6-week latency to stoma creation and abutment attachment. There were 1 explantation for deep infection, 1 explantation for implant failure, and 2 explantations for chronic soft-tissue irritation (later solved with a smooth coupler), and there were 2 later reimplantations. Overall, 35 of 37 patients stated that they would undergo the procedure again10. After modifications to the initial ILP design, no implants were explanted after a mean follow-up of 32 months, although there were 2 periprosthetic hip fractures1. The results from 22 patients in the Netherlands using the ILP at 1 year after osseointegration demonstrated significant improvements in the Q-TFA prosthetic use (56 to 101 hr/wk) and global score (39 to 63), along with significant improvement in the 6-minute walk test (321 to 423 ft [98 m to 129 m]) and the Timed Up and Go (TUG) score (15.1 to 8.1 sec)2. They also demonstrated a decrease in oxygen consumption from 1,330 to 1,093 mL/min. In an Australian study of 50 above-the-knee amputations with a mean follow-up of 21.5 months using the ILP or OPL with 4 to 8-week latency, 21 patients had infection (none requiring an explantation), 4 had periprosthetic fracture, 1 had implant fracture requiring revision, and 1 underwent explantation for pain due to an undersized implant; however, all of the outcome scores, including the Q-TFA, Short Form-36 (SF-36), 6-minute walk test, and TUG, improved significantly17. More recently, the early results for 22 tibial osseointegration prostheses were published and showed a similar increase in Q-TFA use and global scores but a higher low-grade infection rate compared with femoral prostheses at 1 year; however, this led to explantation in only 1 patient with a femoral artery occlusion18.
Whether the early results of osseointegration are durable is an ongoing question because most centers do not have long-term follow-up to date. The 5-year results of the prospective OPRA study demonstrated 92% implant survival, consistently improved outcome scores, and mechanical complications with the abutment that were more common in those with the highest mobility scores, which could be viewed as success of the prosthesis as much as an indictment of it11. In a follow-up study specifically addressing osteomyelitis, the 10-year cumulative risk of explantation for infection was 9% and resolution of infection with antibiotics was obtained without explantation in 25% of osteomyelitis diagnoses19. At 15 years, >64% of patients continued to state that their overall situation as an amputee was better as a result of osseointegration5.
Although the data certainly capture some level of the patient experience, it is worth emphasizing the overall satisfaction, deemed revolutionary by many, that patients subjectively report while using a bone-anchored prosthesis instead of a socket20. The osseoperception attained via the prosthesis is a powerful sensation that enhances the function of the prosthesis21. Although the experience of a socket prosthesis could be characterized as an endless war with constant battling, a bone-anchored prosthesis with a simple mechanical attachment improves prosthesis handling, limb control, and range of motion. This may explain why the self-image or appearance domain of the LD-SRS improved significantly. Although the stoma needs daily maintenance for optimum health, this is no different from hundreds of our other daily routines.
Early reports such as this are important to provide further evidence to lift the regulatory barriers currently in place for the more widespread adoption of bone-anchored osseointegrated implants. Only with more data will the implants, techniques, and protocols undergo the scrutiny and challenge needed to refine this procedure further. Mechanical complications and infection will never be eliminated entirely, but this is broadly true of orthopaedic surgery and the functional improvements of many overshadow the occasional failures. There is a large community of amputees in the United States22, and it will be important to answer how broadly this procedure may be applied to them. Patients undergoing amputation for complications of diabetes or vasculopathy may seem to be poor candidates for a bone-anchored osseointegration prosthesis, but it is possible that the more proximal tissue retains enough health to sustain a prosthesis and prevent other complications that would result from perpetual recumbency.
Financially, amputees routinely undergo socket changes every few years, and those with difficulties can expect even more costly socket revisions. Future study comparing the cost of the socket and osseointegrated prosthetics will be important. Other comparative outcomes to consider studying include psychology, productivity, and return to work.
The limitations of this study included its small number of patients and short-term follow-up. The procedures were performed by a single surgeon with predominantly 1 implant system and technique, which limited direct comparison with those discussed in other publications. The study was underpowered to analyze many interesting variables with regard to osseointegration that will be the focus of future studies, including the comparison of femoral and tibial reconstructions, the impact of implant length and diameter, the latency between amputation and osseointegration, the impact of bacterial colonization in routine cultures on the ultimate infectious outcome, and the effect of targeted muscle reinnervation on patient-reported outcomes. Not all comparisons of the primary outcome could be paired because of the lack of applicability of the outcome measure to those without a prior prosthesis. The loss of in-person follow-up during a period of societal quarantine and travel restrictions also impacted the functional outcomes available for analysis. Given the ongoing rise in telemedicine as a tool for patient follow-up, especially for patients distant from their surgical center, new ways will need to be devised to obtain these data. However, a strength of this study is the minimal bias resulting from loss to follow-up due to the lack of availability of other treatment centers familiar with the implant or capable of supplying requisite parts when complications arise. Our practice setting offers numerous ways for patients to communicate with the physician directly, which helps to capture adverse events.
In conclusion, this study of early patient-reported and functional outcomes of patients with lower-extremity amputations who underwent reconstruction with a bone-anchored osseointegration prosthesis is consistent with other published literature demonstrating improvement across all Q-TFA domains compared with patients who undergo traditional socket prostheses. Complications leading to the failure of the prosthesis do occur, but at an acceptable rate for a new implant that improves the patient experience of living with a prosthesis. More widespread adoption of these implants will aid multi-institutional collaboration and refinement of the procedure.
Source of Funding
There was no specific funding for the completion of this research.
Footnotes
Investigation performed at the Hospital for Special Surgery, New York, NY
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A315).
References
- 1.Juhnke DL, Beck JP, Jeyapalina S, Aschoff HH. Fifteen years of experience with Integral-Leg-Prosthesis: cohort study of artificial limb attachment system. J Rehabil Res Dev. 2015;52(4):407-20. [DOI] [PubMed] [Google Scholar]
- 2.Van de Meent H, Hopman MT, Frölke JP. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013November;94(11):2174-8. Epub 2013 Jun 14. [DOI] [PubMed] [Google Scholar]
- 3.Hagberg K, Hansson E, Brånemark R. Outcome of percutaneous osseointegrated prostheses for patients with unilateral transfemoral amputation at two-year follow-up. Arch Phys Med Rehabil. 2014November;95(11):2120-7. Epub 2014 Jul 24. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg K, Brånemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses—rehabilitation perspective. J Rehabil Res Dev. 2009;46(3):331-44. [PubMed] [Google Scholar]
- 5.Hagberg K, Ghassemi Jahani SA, Kulbacka-Ortiz K, Thomsen P, Malchau H, Reinholdt C. A 15-year follow-up of transfemoral amputees with bone-anchored transcutaneous prostheses. Bone Joint J. 2020January;102-B(1):55-63. [DOI] [PubMed] [Google Scholar]
- 6.Al Muderis M, Lu W, Tetsworth K, Bosley B, Li JJ. Single-stage osseointegrated reconstruction and rehabilitation of lower limb amputees: the Osseointegration Group of Australia Accelerated Protocol-2 (OGAAP-2) for a prospective cohort study. BMJ Open. 2017March22;7(3):e013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001December;25(3):186-94. [DOI] [PubMed] [Google Scholar]
- 8.Pezzin LE, Dillingham TR, Mackenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004May;85(5):723-9. [DOI] [PubMed] [Google Scholar]
- 9.Hagberg K, Brånemark R, Gunterberg B, Rydevik B. Osseointegrated trans-femoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet Orthot Int. 2008March;32(1):29-41. [DOI] [PubMed] [Google Scholar]
- 10.Aschoff HH, Kennon RE, Keggi JM, Rubin LE. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endo-exo device. J Bone Joint Surg Am. 2010December;92(Suppl 2):180-6. [DOI] [PubMed] [Google Scholar]
- 11.Brånemark RP, Hagberg K, Kulbacka-Ortiz K, Berlin Ö, Rydevik B. Osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective five-year follow-up of patient-reported outcomes and complications. J Am Acad Orthop Surg. 2019August15;27(16):e743-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Muderis M, Khemka A, Lord SJ, Van de Meent H, Frölke JP. Safety of osseointegrated implants for transfemoral amputees: a two-center prospective cohort study. J Bone Joint Surg Am. 2016June1;98(11):900-9. [DOI] [PubMed] [Google Scholar]
- 13.Marano AA, Modiri O, Rozbruch SR, Otterburn DM. Soft tissue contouring at the time of osseointegrated implant reconstruction for lower extremity amputation. Ann Plast Surg. 2020July;85(S1)(Suppl 1):S33-6. [DOI] [PubMed] [Google Scholar]
- 14.Hoellwarth JS, Tetsworth K, Rozbruch SR, Handal MB, Coughlan A, Al Muderis M. Osseointegration for amputees: current implants, techniques, and future directions. JBJS Rev. 2020March;8(3):e0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabricant PD, Borst EW, Green SA, Marx RG, Fragomen AT, Rozbruch SR. Validation of a modified Scoliosis Research Society instrument for patients with limb deformity: the Limb Deformity-Scoliosis Research Society (LD-SRS) score. J Limb Lengthening Reconstr. 2016;2(2):86-93. [Google Scholar]
- 16.Brånemark R, Berlin O, Hagberg K, Bergh P, Gunterberg B, Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective study of 51 patients. Bone Joint J. 2014January;96-B(1):106-13. [DOI] [PubMed] [Google Scholar]
- 17.Muderis MA, Tetsworth K, Khemka A, Wilmot S, Bosley B, Lord SJ, Glatt V. The Osseointegration Group of Australia Accelerated Protocol (OGAAP-1) for two-stage osseointegrated reconstruction of amputated limbs. Bone Joint J. 2016July;98-B(7):952-60. [DOI] [PubMed] [Google Scholar]
- 18.Atallah R, van de Meent H, Verhamme L, Frölke JP, Leijendekkers RA. Safety, prosthesis wearing time and health-related quality of life of lower extremity bone-anchored prostheses using a press-fit titanium osseointegration implant: a prospective one-year follow-up cohort study. PLoS One. 2020March9;15(3):e0230027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tillander J, Hagberg K, Berlin Ö, Hagberg L, Brånemark R. Osteomyelitis risk in patients with transfemoral amputations treated with osseointegration prostheses. Clin Orthop Relat Res. 2017December;475(12):3100-8. Epub 2017 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundberg M, Hagberg K, Bullington J. My prosthesis as a part of me: a qualitative analysis of living with an osseointegrated prosthetic limb. Prosthet Orthot Int. 2011June;35(2):207-14. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs R, Brånemark R, Olmarker K, Rydevik B, Van Steenberghe D, Brånemark PI. Evaluation of the psychophysical detection threshold level for vibrotactile and pressure stimulation of prosthetic limbs using bone anchorage or soft tissue support. Prosthet Orthot Int. 2000August;24(2):133-42. [DOI] [PubMed] [Google Scholar]
- 22.Varma P, Stineman MG, Dillingham TR. Epidemiology of limb loss. Phys Med Rehabil Clin N Am. 2014February;25(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]