Abstract
Aims
Our observational study aimed to evaluate the impact of the lockdown period due to 2019 Coronavirus disease pandemic on glycaemic control in a cohort of paediatric patients with type 1 diabetes (T1D).
Methods
Eighty-five patients with T1D aged 5–18 years using continuous glucose monitoring (CGM) systems were enrolled. Demographic and clinical data, including glucose metrics generated by CGM-specific web-based cloud platforms, were collected in three different periods (pre-lockdown phase, lockdown phase, and post-lockdown phase) of 90 days each and were statistically analysed.
Results
During the lockdown period, a clear improvement in almost all CGM metrics (time in range, time above range, coefficient of variation, and glucose management indicator) was observed in our study population, regardless of age and insulin type treatment. In the months following lockdown, maintaining satisfactory diabetes outcomes was confirmed only in younger patients (aged 5–9 years) and in those individuals on hybrid closed loop therapy.
Conclusions
The increasing use of innovative technological devices together with data sharing systems and interaction with multidisciplinary diabetes team through telemedicine allowed paediatric patients with T1D to improve glucose metrics during the lockdown period. However, our findings showed that the achievement of better glycaemic control was transient for most patients.
Keywords: Adolescence, Ambulatory glucose profile, Children, Hybrid closed loop, Pandemic, Time in range
1. Introduction
Since the beginning of the last year, global health authorities have been facing a deadly foe called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of 30 March 2021, more than 128,000,000 people have been infected and more than 2,800,000 deaths have been reported (https://www.worldometers.info/coronavirus/). The 2019 Coronavirus disease (COVID-19) pandemic represents an unprecedented challenge from a public health standpoint, not only for the very high number of cases and deaths but also due to a wide variety of indirect damage. Above all, the development of feelings of fear, discouragement, anxiety and the onset or worsening of mental health conditions such as depression or obsessive–compulsive disorder have all been reported to be associated with the COVID-19 pandemic [1], [2]. These adverse effects were mainly noticed in some risk groups such as patients with pre-existing psychiatric disorders, people with chronic disease, children, and adolescents [3], [4], [5], [6], [7]. To minimize community-based viral transmission, almost all countries introduced extremely restrictive measures on people’s daily activities and movements across the country, known as ‘lockdown’. During the first pandemic wave, the Italian Government imposed a rigorous lockdown which lasted from March 9 to May 3, 2020. Schools and universities suspended didactic activities, non-essential businesses were closed, outdoors sports, leisure activities, and travel between cities were all severely limited. Hospitals shut their outpatient departments, deferring all “non-urgent” healthcare activities [8]. During this period, children and adolescents were forced to spend most of their time at home [9], and those with type 1 diabetes (T1D) had to modify their approach to the disease mainly due to the suspension of outdoor physical and leisure activities and the related increase of sedentary behaviour. They were also unable to comply with scheduled outpatient follow-up visits. Instead, telemedicine and virtual care offered important, alternative approaches to improve access, efficacy, efficiency, and cost-effectiveness of medical care [10]. Although Italy was more unprepared than other countries in managing patients with chronic diseases due to limited availability and diffusion of large-scale telemedicine solutions [11], our paediatric patients and their families were able to regularly contact our paediatric diabetes team for discussion or advice on managing their disease.
2. Aim of the study
Aim of the present study was to evaluate glycaemic control during the lockdown period compared to the previous and the following months, in a cohort of paediatric patients with T1D on intensive insulin therapy (i.e. continuous subcutaneous insulin infusion or multiple daily injections) who regularly use continuous glucose monitoring (CGM) systems. Secondary outcome was to assess the influence of age or treatment type on the changes of diabetes outcomes before, during, and after the COVID-19 lockdown.
3. Material and methods
We performed an observational, retrospective study that involved reviewing the electronic medical records of children and adolescents with T1D followed-up at the Paediatric Diabetes Centre at the University Hospital of Messina. The study was conducted in accordance with the Helsinki Declaration, good clinical practice, and all applicable laws and regulations.
Inclusion criteria were as follows: age between 5 and 18 years, duration of disease ≥ 1 year, current insulin type treatment >3 months, daily sensor use >75%, informed consent from patients and their parents to access the CGM data remotely. Exclusion criteria were changes in insulin type treatment, use of corticosteroids or drugs known to have a relevant impact on glycaemic control, and hospitalizations during the entire study period. We collected clinical data in three different periods of 90 days each: Period 1 (pre-lockdown phase) from December 10, 2019 to March 8, 2020, Period 2 (lockdown phase) from March 9 to June 6, 2020, and Period 3 (post-lockdown phase) from June 7 to September 4, 2020.
Demographic and clinical data (e.g. duration of diabetes, auxological parameters, presence of other autoimmune diseases, type of insulin treatment, brand and model of glucose sensor, last year glycated haemoglobin mean value) were collected from data recorded in the computerized clinical registry. Anthropometric parameters were missing from the lockdown phase (Period 2) due to the closure of hospital outpatient services. Glucose data were extracted from the ambulatory glucose profile generated by CGM-specific web-based cloud platforms. The following CGM metrics were considered: time in range (TIR – time expressed in percentage in the ideal range of glucose between 70 and 180 mg/dl), time above range (TAR - time expressed in percentage above 180 mg/dl), time below range (TBR – time expressed in percentage below 70 mg/dl), coefficient of variation expressed in percentage (%CV), and glucose management indicator (GMI).
To evaluate the role of age on glycaemic control during the study period, we considered three age classes: pre-pubertal age (5–9 years) that is usually characterized by close parental care of diabetes, pubertal age (10–14 years), and adolescence (15–18 years) that is a well-known period of life at high-risk of poor clinical outcomes [12]. To assess the potential relationship between insulin treatment type and glucose levels, we also divided patients into three groups: those who practiced multiple daily injections of insulin (MDI group), those who wore insulin pumps with non-automated delivery systems (CSII group), and those who used hybrid closed loop insulin pumps (hybrid close loop - HCL group).
3.1. Statistical analysis
The numerical data were expressed as mean ± standard deviations, and median with interquartile ranges. Categorical variables were described as absolute frequencies and percentages. The non-parametric approach was used since most numerical variables were not normally distributed, as verified by the Kolmogorov Smirnov test.
To evaluate variation of the auxological parameters over time, the Wilcoxon test was applied. The same test was applied to perform all two-by-two comparisons between three time points (pre-lockdown phase, during lockdown and post-lockdown phase) for glycometabolic parameters (TIR, TAR, TBR, %CV, and GMI). For these multiple comparisons, we applied Bonferroni’s correction, for which the significance alpha level 0.050 was divided by the number of possible comparisons (equal to three); thus, the “adjusted” significance level for this analysis is equal to 0.050/3 = 0.017. This analysis was performed both for all casuistry and stratifying for age classes (5–9 years, 10–14 years and 15–18 years) and for insulin treatment type (MDI, CSII, and HCL therapy).
A chi-square test was applied to evaluate association between categorical variables such as gender, age classes, and insulin treatment type.
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22 (Armonk, NY, IBM Corp.). A p-value < 0.05 was considered statistically significant.
4. Results
Our study population consisted of a cohort of 85 patients with an equal distribution among males and females. Mean age of the study participants was 11.5 ± 3.7 years. Mean duration of diabetes was 5.2 ± 3.3 years. Of the 85 patients, 22.4% were on MDI therapy, 29.4% used HCL systems, and the remaining 48.2% belonged to the CSII group. Study participants had a mean HbA1c value of 6.9 ± 0.8% (52.4 ± 8.6 mmol/mol) in the year preceding the start of the study. Of the 85 patients, 12 (14.1%) had at least one other autoimmune disease. Details of demographic and clinical data of our study population are described in Table 1 .
Table 1.
Descriptive statistics for categorical (percentages) and numerical (mean ± SDS and interquartile ranges) variables of 85 patients included in study.
| Variables | Percentages and mean ± SDS | Median (IQR) |
|---|---|---|
| Age (years) | 11.5 ± 3.7 | 12 (9; 14.5) |
|
Age classes 5–9 years 10–14 years 15–18 years |
27 (31.8%) 37 (43.5%) 21 (24.7%) |
|
|
Gender Male Female |
43 (51.6%) 42 (49.4%) |
|
| Age at diagnosis (years) | 6.4 ± 3.5 | 6.2 (3.4; 8.9) |
| Duration of diabetes (years) | 5.2 ± 3.3 | 4.8 (2.8; 7.6) |
| Weight Z score | 0.25 ± 0.79 | 0.20 (-0.10; 0.80) |
| BMI Z score | 0.39 ± 0.81 | 0.39 (-0.19; 0.94) |
| Last year mean value HbA1c (%) | 6.9 ± 0.8 | 6.8 (6.4; 7.3) |
| Last year mean value HbA1c (mmol/mol) | 52.4 ± 8.6 | 51.2 (47; 56.2) |
|
Insulin treatment type Multiple daily injections Sensor augmented pump Hybrid closed loop |
19 (22.4%) 25 (29.4%) 41 (48.2%) |
|
|
Autoimmune comorbidities Hashimoto’s thyroiditis Celiac disease Hashimoto’s thyroiditis + celiac disease Celiac disease + autoimmune hepatitis None |
6 (7.0%) 4 (4.7%) 1 (1.2%) 1 (1.2%) 73 (85.9%) |
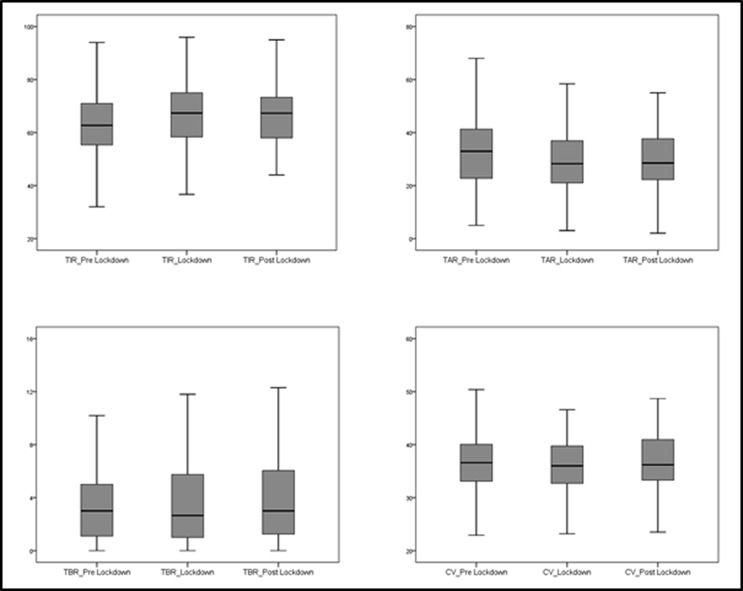
Fig. 1 and Table 2 summarize the main findings during the three periods analysed. No significant changes were found in anthropometric parameters between Period 1 and Period 3. There was a significant increase of TIR at Period 2 and Period 3 compared to Period 1 (p < 0.001 both). Similarly, across the different time points, there was a significant reduction of TAR (p < 0.001 both) and GMI (p < 0.001 and p = 0.015). %CV was significantly lower in Period 2 than in Period 1 (p = 0.003), whereas %CV in Period 3 significantly increased compared with Period 2 (p < 0.001). TBR remained unchanged between Period 1 and Period 2, whereas TBR in Period 3 was significantly higher than in Period 2 (p = 0.004).
Fig. 1.
Boxplots illustrating the distribution of CGM metrics in three timepoints (pre-lockdown, lockdown, post lockdown).
Table 2.
Comparison of anthropometric parameters and CGM data between different time points of observation.
| Period 1 (Pre-lockdown) | p value1 | Period 2 (Lockdown) | p value2 | Period 3 (Post-lockdown) | p value3 | |
|---|---|---|---|---|---|---|
| Weight Z score | 0.25 ± 0.8 | / | / | / | 0.3 ± 0.8 | 0.979 |
| BMI Z score | 0.39 ± 0.81 | / | / | / | 0.41 ± 0.86 | 0.597 |
| TIR | 62.7 ± 13 | <0.001 | 66.6 ± 12.9 | 0.426 | 66.4 ± 11.7 | <0.001 |
| TAR | 33.5 ± 13.4 | <0.001 | 29.6 ± 13.3 | 0.773 | 29.4 ± 12.1 | <0.001 |
| TBR | 3.8 ± 3.5 | 0.814 | 3.8 ± 3.4 | 0.004 | 4.3 ± 3.6 | 0.026 |
| CV | 36.9 ± 6.2 | 0.003 | 36 ± 5.8 | <0.001 | 37.1 ± 6.2 | 0.633 |
| GMI (%) | 7.1 ± 0.6 | <0.001 | 7 ± 0.6 | 0.488 | 7 ± 0.6 | 0.015 |
Statistical comparison between pre-lockdown and lockdown phase.
Statistical comparison between lockdown phase and post-lockdown.
Statistical comparison between pre-lockdown and post-lockdown.
When dividing the study population according to age, we found that TIR in Period 2 was higher than TIR in Period 1 in the three groups (p < 0.001 for 5–9 years, p = 0.004 for 10–14 years, p = 0.001 for 15–18 years), but this improvement was confirmed only in younger patients (aged 5 – 9 years) in Period 3 (p = 0.005). TAR was significantly lower in Period 2 compared to Period 1 in all the age groups (p < 0.001 for 5–9 years, p = 0.003 for 10–14 years, p = 0.007 for 15–18 years). This finding was also seen both in younger patients and in those aged 10–14 years (p = 0.001 and p = 0.007, respectively) by comparing Period 3 with Period 1, whereas no changes were seen in adolescents. In Period 2, GMI levels significantly decreased in patients aged 5–9 years and in those aged 10–14 years (p = 0.008 and p = 0.016, respectively) in comparison with Period 1. No significant differences in the other metrics of glucose control were observed (Table 3 ). Interestingly, TBR levels remained stable across the different periods of study in all the age classes.
Table 3.
Changes in CGM metrics from pre-lockdown phase to the following months according to age of study participants.
| Variables | 5–9 years |
10–14 years |
15–18 years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Period 1 (Pre-lockdown) | Period 2 (Lockdown period) | p | Period 1 (Pre-lockdown) | Period 2 (Lockdown period) | p | Period 1 (Pre-lockdown) | Period 2 (Lockdown period) | p | |
| TIR | 59.7 ± 13.4 | 64.3 ± 13.3 | <0.001 | 63.5 ± 11.2 | 66.7 ± 11 | 0.004 | 64.9 ± 15.2 | 69.4 ± 15.4 | 0.001 |
| TAR | 35.9 ± 14.4 | 31.4 ± 14.9 | <0.001 | 33.1 ± 11.8 | 29.6 ± 11 | 0.003 | 31.1 ± 14.9 | 27.5 ± 15.2 | 0.007 |
| TBR | 4.2 ± 3.1 | 4.3 ± 3.1 | 0.423 | 3.4 ± 3.3 | 3.5 ± 3.3 | 0.893 | 4 ± 4.5 | 3.6 ± 3.9 | 0.264 |
| CV | 38.2 ± 5 | 37.9 ± 5.2 | 0.275 | 36.7 ± 5.4 | 35.8 ± 4.9 | 0.169 | 35.5 ± 8.3 | 34.8 ± 7.2 | 0.041 |
| GMI (%) | 7.2 ± 0.6 | 6.9 ± 0.6 | 0.008 | 7.2 ± 0.6 | 7.1 ± 0.6 | 0.016 | 7 ± 0.7 | 7 ± 0.7 | 0.560 |
| Period 1 (Pre-lockdown) | Period 3 (Post-lockdown) | p | Period 1 (Pre-lockdown) | Period 3 (Post-lockdown) | p | Period 1 (Pre-lockdown) | Period 3 (Post-lockdown) | p | |
| TIR | 59.7 ± 13.4 | 64.2 ± 9.6 | 0.005 | 63.5 ± 11.2 | 67.4 ± 10.8 | 0.021 | 64.9 ± 15.2 | 67.2 ± 15.4 | 0.135 |
| TAR | 35.9 ± 14.4 | 31.2 ± 10.7 | 0.001 | 33.1 ± 11.8 | 28.6 ± 11.1 | 0.007 | 31.1 ± 14.9 | 28.7 ± 15.2 | 0.096 |
| TBR | 4.2 ± 3.1 | 4.6 ± 3.3 | 0.250 | 3.4 ± 3.3 | 4.2 ± 3.4 | 0.026 | 4 ± 4.5 | 4.1 ± 4.3 | 0.791 |
| CV | 38.2 ± 5 | 38.9 ± 5.3 | 0.367 | 36.7 ± 5.4 | 36.8 ± 5.3 | 0.700 | 35.5 ± 8.3 | 35.4 ± 8.4 | 0.467 |
| GMI (%) | 7.2 ± 0.6 | 7 ± 0.5 | 0.083 | 7.2 ± 0.6 | 7 ± 0.6 | 0.067 | 7 ± 0.7 | 7 ± 0.7 | 0.590 |
When considering different insulin treatment types used by the study participants, we found that patients belonging to the HCL group significantly improved glucose metrics, particularly in terms of TIR (p < 0.001), TAR (p = 0.001), and %CV (p = 0.005) in Period 2 compared to Period 1. Better TIR and TAR levels were also observed in Period 3 in comparison with Period 1 (p < 0.001 both). By comparing Period 1 and Period 2, in the CSII group, TIR, TAR, and GMI significantly improved in Period 2 (p = 0.002 for TIR, p = 0.002 for TAR, p = 0.003 for GMI), and patients on MDI therapy also showed better TIR and TAR levels (p = 0.004 both). Interestingly, in patients on MDI or CSII therapy, none of the metrics of glucose control significantly changed between Period 1 and Period 3 (Table 4 ).
Table 4.
Changes in CGM metrics from pre-lockdown phase to the following months according to insulin treatment type used by study participants.
| Variables | MDI group |
CSII group |
HCL group |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Period 1 (Pre-lockdown) | Period 2 (Lockdown period) | p | Period 1 (Pre-lockdown) | Period 2 (Lockdown period) | P | Period 1 (Pre-lockdown) | Period 2 (Lockdown period) | p | |
| TIR | 58 ± 15.6 | 63.7 ± 15.1 | 0.004 | 63.2 ± 10.9 | 65.9 ± 10.3 | 0.002 | 64.6 ± 12.6 | 68.5 ± 13.2 | <0.001 |
| TAR | 39.2 ± 17 | 33 ± 16.8 | 0.004 | 32.5 ± 12.2 | 29.6 ± 11.2 | 0.002 | 31.5 ± 11.8 | 28.1 ± 12.8 | 0.001 |
| TBR | 2.8 ± 3.2 | 3.3 ± 3.6 | 0.122 | 4.3 ± 3.3 | 4.5 ± 3.2 | 0.808 | 4 ± 3.8 | 3.5 ± 3.4 | 0.148 |
| CV | 35.4 ± 5.4 | 35.4 ± 5.7 | 0.711 | 38 ± 4 | 37.4 ± 4.4 | 0.139 | 36.9 ± 7.5 | 35.4 ± 6.6 | 0.005 |
| GMI (%) | 7.3 ± 0.7 | 7 ± 0.7 | 0.024 | 7.1 ± 0.7 | 7 ± 0.6 | 0.003 | 7 ± 0.5 | 7 ± 0.6 | 0.065 |
| Period 1 (Pre-lockdown) | Period 3 (Post-lockdown) | p | Period 1 (Pre-lockdown) | Period 3 (Post-lockdown) | p | Period 1 (Pre-lockdown) | Period 3 (Post-lockdown) | p | |
| TIR | 58 ± 15.6 | 63.3 ± 12.2 | 0.085 | 63.2 ± 10.9 | 64.7 ± 10.3 | 0.383 | 64.6 ± 12.6 | 68.9 ± 12.1 | <0.001 |
| TAR | 39.2 ± 17 | 33.5 ± 12.6 | 0.070 | 32.5 ± 12.2 | 30.3 ± 11.7 | 0.211 | 31.5 ± 11.8 | 30 ± 11.7 | <0.001 |
| TBR | 2.8 ± 3.2 | 3.1 ± 2.9 | 0.348 | 4.3 ± 3.3 | 5.1 ± 3.2 | 0.038 | 4 ± 3.8 | 4.3 ± 4 | 0.436 |
| CV | 35.4 ± 5.4 | 35.8 ± 5.4 | 0.523 | 38 ± 4 | 39.3 ± 4.5 | 0.025 | 36.9 ± 7.5 | 36.5 ± 7.3 | 0.153 |
| GMI (%) | 7.3 ± 0.7 | 7.1 ± 0.5 | 0.120 | 7.1 ± 0.7 | 7 ± 0.7 | 0.168 | 7 ± 0.5 | 7 ± 0.6 | 0.107 |
5. Discussion
The COVID-19 pandemic has dramatically changed the approach to patients with chronic diseases. Telemedicine services have been strengthened to minimize hospitalizations, outpatient visits, and, consequently, SARS-CoV-2 exposure risk [13]. Technology has played a crucial role during this pandemic, particularly for children and adolescents who have been able to continue school lessons and maintain socialization with their peers [14]. The increasing use of innovative technological devices (i.e. CSII and CGM) together with data sharing systems and the interaction with multidisciplinary diabetes team through telemedicine allowed patients not to worsen glycaemic control during the lockdown period. Rachmiel et al. reported their experience on a large cohort of paediatric and young adult patients who greatly benefited from telehealth visits during the COVID-19 lockdown [15]. Our results showed that there was a significant improvement of almost all the glucose control metrics, as suggested by the increase of TIR levels and the reduction of TAR values. Although TBR values remained unchanged, glycaemic variability improved as demonstrated by the reduction of %CV. Finally, GMI levels also decreased. The improvement of glycaemic control during the lock-down period in our study population was absolute, regardless of age and insulin type treatment.
Despite the conclusions of a simulation model that predicted a direct association between duration of lockdown and worsening of glycaemic control and increase in diabetes-related complications [16], our findings, as well as those of other studies, demonstrate the opposite [17], [18], [19], [20], [21]. Aragona et al. [22] introduced the term “lockdown effect” to define the surprising, beneficial impact among patients with T1D on glycaemic control. The reasons that could explain this effect are various. The family environment certainly had a relevant impact, especially for younger T1D patients as they spent most of their time at home with their parents. Closer attention to the management of diabetes by children’ parents facilitated the achievement of better glycaemic outcomes. It has been demonstrated that children attending school are often not adequately managed due to several causes such as limited availability of glucagon kits and the shortage of trained personnel able to manage daily diabetes-specific emergencies [23]. In addition, abstention from school and the “stay at home” rule may have contributed to maintaining a healthy diet and more regular distribution of meals. This hypothesis is consistent with the results on the comparison of anthropometric parameters between pre- and post-lockdown in our study population. The lack of difference in weight and BMI are suggestive of a regular dietary regimen in our patients during the lockdown period. These results are contrary with those of an Arabian study that showed an association between the lockdown and a significant increase in paediatric patients' weight and BMI [24]. We suppose that the online consultant schedule with our diabetes team’s dietician might have significantly helped patients and their parents to avoid overeating and consume the correct amount of carbohydrates. Despite Governmental decrees banning outdoor sports and activities, several reports have shown that more than half children and adolescents with T1D regularly exercise at home (e.g. spin bike, treadmill) several times a week [18], [19], [25]. Maintaining regular physical activity in a safe home environment has been demonstrated as an essential strategy to allow young individuals with T1D to further improve their glycaemic control during the COVID-19 crisis [26]. Of note, unlike other studies [18], [19], adolescent patients also obtained satisfactory diabetes outcomes during the lockdown period. We can speculate that the obligation to stay at home has allowed adolescents to have more time to manage their disease and to make appropriate treatment adjustments. Furthermore, the exclusion of the influence of some school and extra-curricular activities might have reduced stress levels and unpredictability caused by multiple and overlapping commitments [26]. Brener et al. also found an improvement in CGM metrics among adolescent patients, particularly in terms of glycaemic variability [27].
The only CGM metric that remained unchanged during the lockdown period was TBR. This could be influenced by satisfactory values at the start of the study since they were already below the target of 4–5% established by recommendations from the International Consensus on Time in Range [28]. However, clear improvements of TIR and TAR allowed reducing the glycaemic variability assessed by %CV. A %CV ≤ 36% appears to be compatible with the definition of stable glycaemic control in diabetes [29]. The achievement of %CV levels below the established threshold is currently considered a relevant therapeutic target since glycaemic variability is recognized as a possible independent risk factor for the development of diabetic macrovascular and microvascular complications [30].
To the best of our knowledge, there are few published data on the evolution of glycaemic control in T1D patients after the end of lockdown. Some studies described that the first weeks after lockdown had no negative impact on glycaemic control [22], [31]. Unlike these studies, we used a longer follow-up observation period to evaluate if these results were confirmed also after three months. In our study population, some glycometabolic parameters such as TIR, TAR, and GMI were better in the post-lockdown if compared to pre-lockdown data. However, when analysing changes in glycaemic control based on the age of our patients, we found that the most relevant results were present in younger patients (pre-pubertal age class). On the contrary, there were no significant changes in glucose metrics in adolescent patients between pre-lockdown and post-lockdown. This interesting finding revealed that adolescents with T1D have been reluctant to persist in the proper approach to their disease. Although the lockdown period and Governmental restrictive laws allowed them indirectly to better manage their diabetes, returning to normal daily activities caused an expected worsening of their glycaemic control. It is well known that adolescence is a tricky transition phase during which individuals often want to feel free from parental control and they probably chose to prioritize something else over proper disease management when the lockdown was lifted [32]. This aspect may further explain the different responses in glycaemic control between younger patients and teenagers.
Finally, our results showed that improvements related to the lockdown phase were confirmed in the following months only in patients using the most innovative technological devices available for disease management (i.e. HCL and advanced HCL). The relationship between the HCL insulin therapy and improvements in CGM metrics during the lockdown had already been described among both paediatric and adult patients with T1D [26], [31]. Some studies also reported benefits on glycaemic control in patients who were updating to HCL system through virtual training programs during the COVID-19 pandemic [33], [34]. Based on our findings, we can suppose that eating every meal at home might allow a more precise carbohydrate count. At the same time, spending more time than usual evaluating their own ambulatory glucose profile might allow to make adjustments in pre-programmed settings such as insulin active time, blood glucose target, and insulin to carbohydrate ratio(s). Therefore, we can hypothesise that the lockdown might have represented an opportunity for patients on HCL therapy and their families to carry out a sort of auto-training, so as to ensure better therapeutic targets also in the following months. Previous studies, including real-life experiences, have already demonstrated that HCL use is associated with improved glycaemic outcomes [35], [36], [37]. HCL systems are considered to be related to a new era of diabetes management [38] and it would be fair to suppose that HCL will soon become prevalent in the treatment choice rather than MDI therapy or insulin pumps that are not capable of talking to CGM systems.
6. Conclusions
On the basis of our experience, the improvement in glycaemic control during the lockdown period was transient for most paediatric patients with T1D. In the months following the lockdown, the achievement of satisfactory diabetes outcomes was mainly confirmed in younger patients and in those individuals using HCL systems. These results confirm the crucial aspect of parental control in the management of diabetes during childhood, as well as the increasing benefits from the most innovative technological devices that currently represent the best treatment choice for people with diabetes.
Authors’ contributions
SP drafted and wrote the paper; PB, BB and GL collected data; AA realized statistical analysis; FL and GS contributed to discussion and reviewed the paper. The paper has been read and approved by all the authors and each author considers that the paper represents their honest work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
None.
Funding
The authors received no funding from an external source.
References
- 1.Mansfield K.E., Mathur R., Tazare J., Henderson A.D., Mulick A.R., Carreira H., et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digit Health. 2021;3(4):e217–e230. doi: 10.1016/S2589-7500(21)00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lange KW. Coronavirus disease 2019 (COVID-19) and global mental health. Glob Health J. 2021. http://doi.org/10.1016/j.glohj.2021.02.004. [DOI] [PMC free article] [PubMed]
- 3.Czeisler MÉ, Howard ME, Rajaratnam SMW. Mental Health During the COVID-19 Pandemic: Challenges, Populations at Risk, Implications, and Opportunities. Am J Health Promot. 2021;35:301–11. [DOI] [PubMed]
- 4.Kang Chuanyuan, Yang Shuran, Yuan Jing, Xu Li, Zhao Xudong, Yang Jianzhong. Patients with chronic illness urgently need integrated physical and psychological care during the COVID-19 outbreak. Asian J Psychiatry. 2020;51:102081. doi: 10.1016/j.ajp.2020.102081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Yan, Yue Song, Hu Xiaoran, Zhu Jin, Wu Zifan, Wang JianLi, et al. Associations between feelings/behaviors during COVID-19 pandemic lockdown and depression/anxiety after lockdown in a sample of Chinese children and adolescents. J Affect Disord. 2021;284:98–103. doi: 10.1016/j.jad.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito Susanna, Giannitto Nino, Squarcia Antonella, Neglia Cosimo, Argentiero Alberto, Minichetti Paola, et al. Development of Psychological Problems Among Adolescents During School Closures Because of the COVID-19 Lockdown Phase in Italy: A Cross-Sectional Survey. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.628072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dipasquale Valeria, Passanisi Stefano, Cucinotta Ugo, Cascio Antonio, Romano Claudio. Implications of SARS-COV-2 infection in the diagnosis and management of the pediatric gastrointestinal disease. Ital J Pediatr. 2021;47(1) doi: 10.1186/s13052-021-01020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buonsenso D, Onesimo R, Valentini P, et al. Children’s Healthcare During Corona Virus Disease 19 Pandemic: the Italian Experience. Pediatr Infect Dis J. 2020;39:e137–40. [DOI] [PMC free article] [PubMed]
- 9.Passanisi S, Lombardo F, Salzano G, et al. Are Children Most of the Submerged Part of SARS-CoV-2 Iceberg? Front Pediatr. 2020;8:213. [DOI] [PMC free article] [PubMed]
- 10.Garg Satish K., Rodbard David, Hirsch Irl B., Forlenza Gregory P. Managing New-Onset Type 1 Diabetes During the COVID-19 Pandemic: Challenges and Opportunities. Diabetes Technol Ther. 2020;22(6):431–439. doi: 10.1089/dia.2020.0161. [DOI] [PubMed] [Google Scholar]
- 11.Omboni Stefano. Telemedicine During the COVID-19 in Italy: A Missed Opportunity? Telemed J E-Health. 2020;26(8):973–975. doi: 10.1089/tmj.2020.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raymond Jennifer. Updates in behavioural and psychosocial literature in adolescents with type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2015;22(4):265–269. doi: 10.1097/MED.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 13.Fung Alex, Irvine Mike, Ayub Aysha, Ziabakhsh Shabnam, Amed Shazhan, Hursh Brenden E. Evaluation of telephone and virtual visits for routine pediatric diabetes care during the COVID-19 pandemic. J Clin Transl Endocrinol. 2020;22:100238. doi: 10.1016/j.jcte.2020.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salzano Giuseppina, Passanisi Stefano, Pira Francesco, Sorrenti Lacrima, La Monica Giuseppa, Pajno Giovanni Battista, et al. Quarantine due to the COVID-19 pandemic from the perspective of adolescents: the crucial role of technology. Ital J Pediatr. 2021;47(1) doi: 10.1186/s13052-021-00997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachmiel Marianna, Lebenthal Yael, Mazor-Aronovitch Kineret, Brener Avivit, Levek Noa, Levran Neria, et al. Glycaemic control in the paediatric and young adult population with type 1 diabetes following a single telehealth visit - what have we learned from the COVID-19 lockdown? Acta Diabetol. 2021;58(6):697–705. doi: 10.1007/s00592-021-01673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosal Samit, Sinha Binayak, Majumder Milan, Misra Anoop. Estimation of effects of nationwide lockdown for containing coronavirus infection on worsening of glycosylated haemoglobin and increase in diabetes-related complications: A simulation model using multivariate regression analysis. Diabetes Metab Syndr. 2020;14(4):319–323. doi: 10.1016/j.dsx.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesa A, Viñals C, Pueyo I, et al. The impact of strict COVID-19 lockdown in Spain on glycemic profiles in patients with type 1 Diabetes prone to hypoglycemia using standalone continuous glucose monitoring. Diabetes Res Clin Pract. 2020;167:108354. [DOI] [PMC free article] [PubMed]
- 18.Predieri Barbara, Leo Francesco, Candia Francesco, Lucaccioni Laura, Madeo Simona F., Pugliese Marisa, et al. Glycemic Control Improvement in Italian Children and Adolescents With Type 1 Diabetes Followed Through Telemedicine During Lockdown Due to the COVID-19 Pandemic. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.59573510.3389/fendo.2020.595735.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Dalmazi Guido, Maltoni Giulio, Bongiorno Claudio, Tucci Lorenzo, Di Natale Valeria, Moscatiello Simona, et al. Comparison of the effects of lockdown due to COVID-19 on glucose patterns among children, adolescents, and adults with type 1 diabetes: CGM study. BMJ Open Diabetes Res Care. 2020;8(2):e001664. doi: 10.1136/bmjdrc-2020-001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández Elsa, Cortazar Alicia, Bellido Virginia. Impact of COVID-19 lockdown on glycemic control in patients with type 1 diabetes. Diabetes Res Clin Pract. 2020;166:108348. doi: 10.1016/j.diabres.2020.108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pla B, Arranz A, Knott C, Sampedro M, Jiménez S, Hernando I, et al. Impact of COVID-19 Lockdown on Glycemic Control in Adults with Type 1 Diabetes Mellitus. J Endocr Soc. 2020;4:bvaa149. [DOI] [PMC free article] [PubMed]
- 22.Aragona M, Rodia C, Bertolotto A, et al. Type 1 diabetes and COVID-19: The «lockdown effect». Diabetes Res Clin Pract. 2020;170:108468. [DOI] [PMC free article] [PubMed]
- 23.Alaqeel Aqeel A. Are children and adolescents with type 1 diabetes in Saudi Arabia safe at school? Saudi Med J. 2019;40(10):1019–1026. doi: 10.15537/smj.2019.10.24582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agha Abdulmoein E. Al, Alharbi Razan S., Almohammadi Omar A., Yousef Sondos Y., Sulimani Ahad E., Alaama Rawan A. Impact of COVID-19 lockdown on glycemic control in children and adolescents. Saudi Med J. 2021;42(1):44–48. doi: 10.15537/smj.2021.1.25620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passanisi Stefano, Pecoraro Maria, Pira Francesco, Alibrandi Angela, Donia Vittoria, Lonia Paola, et al. Quarantine Due to the COVID-19 Pandemic From the Perspective of Pediatric Patients With Type 1 Diabetes: A Web-Based Survey. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.0049110.3389/fped.2020.00491.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tornese Gianluca, Ceconi Viola, Monasta Lorenzo, Carletti Claudia, Faleschini Elena, Barbi Egidio. Glycemic Control in Type 1 Diabetes Mellitus During COVID-19 Quarantine and the Role of In-Home Physical Activity. Diabetes Technol Ther. 2020;22(6):462–467. doi: 10.1089/dia.2020.0169. [DOI] [PubMed] [Google Scholar]
- 27.Brener Avivit, Mazor-Aronovitch Kineret, Rachmiel Marianna, Levek Noa, Barash Galia, Pinhas-Hamiel Orit, et al. Lessons learned from the continuous glucose monitoring metrics in pediatric patients with type 1 diabetes under COVID-19 lockdown. Acta Diabetol. 2020;57(12):1511–1517. doi: 10.1007/s00592-020-01596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battelino Tadej, Danne Thomas, Bergenstal Richard M., Amiel Stephanie A., Beck Roy, Biester Torben, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnier Louis, Colette Claude, Wojtusciszyn Anne, Dejager Sylvie, Renard Eric, Molinari Nicolas, et al. Toward Defining the Threshold Between Low and High Glucose Variability in Diabetes. Diabetes Care. 2017;40(7):832–838. doi: 10.2337/dc16-1769. [DOI] [PubMed] [Google Scholar]
- 30.Sun B., Luo Z., Zhou J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc Diabetol. 2021;20:9. doi: 10.1186/s12933-020-01200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longo Miriam, Caruso Paola, Petrizzo Michela, Castaldo Filomena, Sarnataro Annalisa, Gicchino Maurizio, et al. Glycemic control in people with type 1 diabetes using a hybrid closed loop system and followed by telemedicine during the COVID-19 pandemic in Italy. Diabetes Res Clin Pract. 2020;169:108440. doi: 10.1016/j.diabres.2020.108440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velez Gabriel, Hahn Madeline, Recchia Holly, Wainryb Cecilia. Rethinking Responses to Youth Rebellion: Recent Growth and Development of Restorative Practices in Schools. Curr Opin Psychol. 2020;35:36–40. doi: 10.1016/j.copsyc.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Gómez Ana M., Henao Diana, Parra Darío, Kerguelen Alfonso, Pinilla Marisol Vergara, Muñoz Oscar Mauricio, et al. Virtual training on the hybrid close loop system in people with type 1 diabetes (T1D) during the COVID-19 pandemic. Diabetes Metab Syndr. 2021;15(1):243–247. doi: 10.1016/j.dsx.2020.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigersky R.A., Velado K., Zhong A., et al. The Effectiveness of Virtual Training on the MiniMedTM 670G System in People with Type 1 Diabetes During the COVID-19 Pandemic. Diabetes Technol Ther. 2021;23:104–109. doi: 10.1089/dia.2020.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berget Cari, Messer Laurel H., Vigers Tim, Frohnert Brigitte I., Pyle Laura, Wadwa R. Paul, et al. Six months of hybrid closed loop in the real-world: An evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21(2):310–318. doi: 10.1111/pedi.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanapka Lauren G., Wadwa R. Paul, Breton Marc D., Ruedy Katrina J., Ekhlaspour Laya, Forlenza Gregory P., et al. Extended Use of the Control-IQ Closed-Loop Control System in Children With Type 1 Diabetes. Diabetes Care. 2021;44(2):473–478. doi: 10.2337/dc20-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isganaitis Elvira, Raghinaru Dan, Ambler-Osborn Louise, Pinsker Jordan E., Buckingham Bruce A., Wadwa R. Paul, et al. Closed-Loop Insulin Therapy Improves Glycemic Control in Adolescents and Young Adults: Outcomes from the International Diabetes Closed-Loop Trial. Diabetes Technol Ther. 2021;23(5):342–349. doi: 10.1089/dia.2020.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleppo G., Webb K.M. Integrated Insulin Pump and Continuous Glucose Monitoring Technology in Diabetes Care Today: A Perspective of Real-Life Experience With the MinimedTM 670G Hybrid Closed-Loop System. Endocr Pract. 2018;24:684–692. doi: 10.4158/EP-2018-0097. [DOI] [PubMed] [Google Scholar]