Abstract
Background and aims
We aim to cover most of the current evidence on the mutual effect of diabetes & COVID-19 infection on each other and the management of the COVID-19 patients with diabetes.
Methods
We utilized databases to review the current evidence related to diabetes mellitus and COVID-19.
Results
We discussed the most recent evidence of diabetes milieus and COVID-19 regarding risk factors, management, complications, and telemedicine.
Conclusion
Diabetes mellitus is associated with a significant risk of complications, extended hospital stays, and mortality in COVID-19 infected patients.
Keywords: Diabetes mellitus, COVID-19, Management, Pathophysiology, Risk factor, Type 1, Typ 2
1. Introduction
Diabetes mellitus is a well-known risk factor for worse clinical outcomes in patients with Coronavirus Disease 2019 (COVID-19). However, the relationship between these two entities seems to be bidirectional [1]. The ongoing pandemic of COVID-19 has significantly affected blood glucose control in patients with diabetes mellitus. The results of this effects can be classified into direct effects (those directly related to the viral infection) and indirect effects (those related to the impact of the pandemic on the management of blood glucose or the use of proposed treatments for the infection that also affect glucose homeostasis).
As a direct effect, the COVID-19 infection has resulted in striking changes in patients' metabolism with significant elevations in blood glucose. It is attributed to the increased release of cytokines and inflammatory mediators, which led to increased insulin resistance and the associated hyperglycemia [2]. In addition, it has been suggested that COVID-19 might be involved in developing acute diabetes mellitus in certain patients by targeting ACE2 receptors located in pancreatic islets resulting in pancreatic injury [3].
Many therapies have been repurposed for the management of COVID-19. For instance, glucocorticoids have a remarkable impact on blood glucose. The use of glucocorticoids has been associated with a decreased mortality in critically ill patients infected with COVID-19 [4]. It is well known that glucocorticoid use results in a significant change in glucose homeostasis due to increased gluconeogenesis and a simultaneous increase in insulin resistance in various tissues [5].
Several studies have described the indirect effects of the COVID-19 pandemic on diverse populations during the lockdown. Some studies indicated that there is an improvement in glycemic control, while others state there is no significant change [6] or that the glycemic control in this population has worsened [7]. Herein we will review the available literature related to COVID-19 and Diabetes Mellitus.
1.1. Pathophysiology of diabetes mellitus and COVID-19
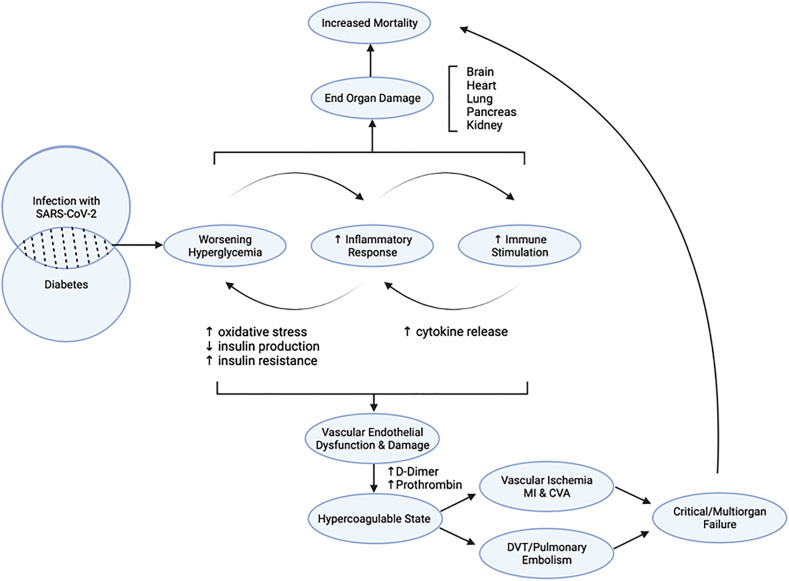
Infection with SARS-CoV-2 in the setting of Diabetes mellitus (DM) initiates a flywheel of cascading effects that result in increased mortality. Infection with COVID-19 predisposes infected individuals to hyperglycemia, leading to hyper glycosylation of ACE2 and increased viral proliferation (Fig. 1 ) [8]. Worsening of hyperglycemia induces inflammation, endothelial dysfunction, and thrombosis via the generation of oxidative stress driving the dysregulation of glucose metabolism and hypercoagulability further [9]. Severe infection in the individuals predisposed to vasculopathy and impaired immunity may accentuate thrombotic and ischemic complications associated with multiorgan failure and increased mortality rates.
Fig. 1.
Pathophysiology of diabetes mellitus and COVID-19.
All the forms of DM can be characterized by abnormal signaling along pathways involved in glucose metabolism. DM is considered a vasculopathy equivalent as the progression of the disease is associated with both macrovascular and microvascular damage. Chronic hyperglycemia and insulin resistance contribute to vasculopathy through various mechanisms. These include abnormal signaling along the AGE-RAGE and oxidative damage. Increased formation of advanced glycation end products (AGEs) and activation of the receptors for advanced glycation end products (RAGE) in the AGE-RAGE axis can accelerate vascular damage [10]. In addition, metabolic abnormalities associated with oxidative damage causes changes in mitochondrial expression of superoxide in endothelial cells of both large and small vessels. Over time, increased superoxide production mediates a cascade of epigenetic alterations that result in persistent expression of proinflammatory pathways even after the normalization of blood glucose levels [11].
Alterations in innate and adaptive immunity, including abnormal cytokine responses, inhibition of leukocyte recruitment, and neutrophil dysfunction, are also driven by states of chronic hyperglycemia [12]. The complications of chronic hyperglycemia are compounded during acute viral infections since activated immune responses can promote systemic insulin resistance and worsening of hyperglycemia [13]. Severe COVID-19 progression is significantly associated with increased blood glucose levels [14]. In the setting of concomitant SARS-CoV-2 infection and DM, monitoring blood glucose levels to track worsening hyperglycemia has more prognostic value than hemoglobin A1c (HbA1c) in these patients. These findings suggest that worsening hyperglycemia occurs acutely and may drive rapid clinical deterioration in patients with pre-existing vasculopathy and endothelial dysfunction.
While the relationship between DM and COVID-19 remains complex, and aberrant immune responses may contribute to poor disease progression by accelerating thrombotic and ischemic complications associated with multiorgan failure and increased mortality.
1.2. Type 2 diabetes mellitus as a risk factor for COVID-19
Type 2 Diabetes Mellitus (T2DM) is considered a risk factor for a poor prognosis in COVID-19 [1]. Many mechanisms have been described to explain the poorer prognosis of COVID-19 in diabetics, but these remain hypothetical at the time of this article. Some of these mechanisms include impaired neutrophil degranulation and complement activation, increased glucose concentration in airway secretion, which significantly increases viral replication, exaggerated proinflammatory cytokine response in diabetes, decreased viral clearance, and a more significant presence associated comorbidities [15].
One of the essential aspects of the relationship between COVID-19 and T2DM is that the information on the condition of hyperglycemia at the time of hospital admission is more relevant for prognostic purposes than the HbA1c. It is thought that COVID-19 predisposes infected individuals to hyperglycemia, leading to hyperglycosylation of the angiotensin-converting enzyme 2 (ACE2), the natural viral receptor on the host cell surface [16]. The acute hyperglycemia in these patients induces inflammation, endothelial dysfunction, and thrombosis via the generation of oxidative stress [2]. This may also enhance tissue tropism and viral penetration into the cells leading to increased virulence, pathogenicity, and susceptibility to severe infections. It has been hypothesized that the COVID-19 might affect pancreatic B-cells to produce insulin, which would aggravate underlying lack of glycemic control in the setting of T2DM [17].
Regardless of the mechanisms, when comparing COVID-19 patients with and without T2DM, the patients with T2DM tend to develop more severe forms of the disease and have a significant increase in inflammatory markers compared to non-diabetics (i.e., higher levels of C-reactive protein, procalcitonin, ferritin, lactate dehydrogenase, and d-dimer) [18]. Furthermore, the prevalence of diabetes among the patients admitted to intensive care units for COVID-19 is two-to threefold higher, along with the mortality rate which is twice that of non-diabetic patients [17]. There is limited evidence suggesting that newly diagnosed diabetes might be associated with a higher admission rate to ICU, invasive mechanical ventilation, and mortality risk than pre-existing diabetes [19].
Studies have shown that a family history of T2DM might also be a significant risk factor for this infection. Healthy individuals with a family history of T2DM tend to develop early endothelial dysfunction. Endothelial dysfunction has been reported to be a critical event in the pathophysiology of COVID-19, which has been associated with pulmonary microvascular thrombosis. Hyperinsulinemia and hyperglycemia observed in this population could promote the expression of ACE2 in various tissues, including microvascular endothelial cells known to act as a receptor for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) [20].
Based on these findings, T2DM affects the disease severity and is one of the independent risk factors for inadequate therapeutic response in patients with COVID-19 [18]. Understanding the interactions between these two diseases is crucial in developing appropriate therapeutic approaches. Further studies are warranted to elucidate the mechanisms by which diabetes affects these patients' prognosis and how these pathways can be modified to generate better outcomes in COVID-19 patients with diabetes.
1.3. COVID-19 and type 1 diabetes mellitus
The prevalence of type 1 diabetes mellitus (T1DM) in COVID-19 patients ranges between 0.15% to 28.98% [21]. Hyperglycemia has been observed to be a risk factor for COVID-19-related complications. According to preliminary findings from a multicenter surveillance study in the United States that assessed clinical outcomes of COVID-19 in patients with T1DM, a total of 48.5% of patients had high blood glucose levels; 45.5%, elevated temperature; 39.4%, dry cough; 33.3%, excess fatigue, and vomiting; 30.3%, shortness of breath; 27.3%, nausea; and 21.2%, body/headaches [22]. Additionally, abdominal and chest pain, chills, loose stools, and loss of taste and smell were reported by <15% of patients [22]. In these patients with COVID-19 and T1DM, obesity (39.4%) and hypertension or cardiovascular disease (12.1%) were the most prevalent comorbidities, while diabetic ketoacidosis was the most common adverse outcome [22].
In a whole-population study that assessed the independent effects of diabetic status and its type on in-hospital death in English patients with COVID-19, 1.5% of in-hospital COVID-19-related deaths were occurred in patients with T1DM versus 31.4% in those with T2DM [23]. Over the 72 days of the study, unadjusted mortality rates per 100,000 individuals were 138 for those with T1DM versus 27 for those without diabetes. Interestingly, when adjusted for age, sex, deprivation, ethnicity, and geographical region, the odds ratio (OR) for in-hospital COVID-19-related death was 3.51 (95% CI = 3.16–3.90) in the patients with T1DM [23].
A population-based cohort study assessed the associations between risk factors and mortality due to COVID-19 in patients with T1DM [24]. Older age and male sex were found to be closely associated with increased COVID-19-related mortality [24]. COVID-19-related mortality was found to be significantly higher among the individuals from black and Asian ethnicities [24]. Additionally, mortality was significantly higher among the socioeconomically deprived individuals (Hazard ratio [HR] 1.93 [95% CI = 1.36–2.72], P = 0.0002) [24]. Preceding HbA1c (HbA1c > 86 mmol/mol; HR 2.23 [95% CI 1.50–3.30, p < 0.0001] was also strongly associated with COVID-19-related mortality [24]. A body mass index (BMI) of <20.0 kg/m2 (HR 2.45 [95% CI 1.60–3.75, p < 0.0001]), 35.0–39.9 kg/m2 (1.72 [1.21–2.46, p = 0.0028]), and >40.0 kg/m2 (2.33 [1.53–3.56, p < 0.0001]) was seen to be associated with significantly increased COVID-19-related deaths [24]. Impaired renal function and previous hospital admissions with stroke or heart failure were closely associated with COVID-19-related mortality (p < 0.0001) [24].
A cross-sectional, web-based survey involved all Italian pediatric diabetes centers in determining if the diagnosis of pediatric T1DM or its acute complications were altered during the early phase of COVID-19 [25]. Compared with the data in 2019, a 23% reduction was observed in new diabetes cases in 2020 [25]. Among newly diagnosed patients with diabetic ketoacidosis (DKA), the proportion of patients with severe DKA in 2020 was 44.3% compared to 36.1% in 2019 (p = 0.03) [25]. Hence, the COVID-19 pandemic might have changed the presentation of diabetes and the severity of DKA [25].
2. Drug therapy of DM among COVID-19 patients
Herein we discuss both oral and injectable non-insulin antidiabetics [summarized in Table 1 ] as well as insulin and their relation to COVID-19 management and outcomes.
Table 1.
Antidiabetics and COVID-19 adapted from singh et al. [26].
| Anti-diabetic drug | Potential benefit | Final results |
|---|---|---|
| Metformin | It has been described as a potent drug to reduce mortality with its use in patients with type 2 diabetes and covid-19 infection. | Its use should be continued in patients with type 2 diabetes mellitus until hospitalized or unless contraindicated. It is unclear at this point whether its potential mortality benefit extends to inpatient use or in non-diabetic covid-19 patients. |
| Pioglitazone | Possible reduction in inflammatory markers. | There is no significant evidence to promote its use in patients with diabetes mellitus and covid-19 infection. Further studies are needed to elucidate its potential benefit. |
| Sulfonylureas | Potential reduction in disease severity. | Studies have not demonstrated a significant difference in the rate of ICU admissions or disease severity with its use. |
| DPP-4 inhibitors | It has been proposed that DPP-4 could be involved with the receptor-binding domain of SARS-CoV-2. Furthermore, it could offer anti-inflammatory, anti-fibrotic and immunomodulatory effects. Several researchers have proposed its use as a repurposed agent for COVID-19. | Several studies found no significant difference in rate mortality rate, poor prognosis, or rate of ICU admission. However, others did find some benefit in the rate of intubation, the severity of disease, and discharge rate. Further studies are needed to elucidate its potential benefit. |
| SLGT-2 inhibitors | Anti-inflammatory properties; decrease in lactic acidosis of dapagliflozin, which could potentially reverse acid-base balance in hypoxia. | Studies have not demonstrated a significant difference in disease severity or mortality rates. |
| GLP-1 receptor agonists | Improvement in right ventricular function and anti-inflammatory effects during acute lung injury. | There is limited data available. Some studies have shown no significant difference in the rate of ICU admissions or mortality. |
3. Metformin
The majority of the T2DM patients on oral hypoglycemics take Metformin, either alone or with other drugs. Metformin is the preferred initial drug to treat type 2 DM. Metformin therapy may be associated with lactic acidosis. This is rare in the absence of other factors predisposing to lactic acidosis but could happen in patients suffering renal impairment, severe infections, or sepsis [27]. Metformin can be continued in asymptomatic COVID-19 patients or patients with mild symptoms. However, Metformin should be stopped in patients hospitalized with severe COVID-19 infection due to the risk of lactic acidosis [28].
It is worth mentioning that better health outcomes have been reported in COVID-19 patients receiving Metformin [29]. It is hypothesized that Metformin may inhibit virus entry into cells through adenosine monophosphate (AMP)-activated protein kinase activation and the B–mammalian target of rapamycin (m TOR) signaling pathway [30].
4. Sulfonylureas
Sulfonylureas may induce hypoglycemia, so in the patients with severe COVID-19 with inadequate oral intake, it is safer to restrict their usage. In addition, simultaneous use of hydroxychloroquine may increase the risk of hypoglycemia [31].
5. Dipeptidyl peptidase 4 (DPP-4) inhibitors
DPP-4 inhibitors are well tolerated and can be continued safely in COVID-19 patients [29]. They are associated with a low risk of hypoglycemia. DPP-4 may act as a receptor for some coronaviruses, and hence, DPP-4 inhibitors might inhibit such binding and mitigate COVID-19 infection [32]. However, this anticipated advantage has not been proven in clinical trials [29].
5.1. Glucagon-like peptide-1 receptor agonists (GLP-1 agonists)
GLP-1 agonists may decrease water intake [15], and they may also cause nausea and vomiting in the patients. Thus, GLP-1 agonist therapy is associated with an increased risk of dehydration and aspiration pneumonia [33]. GLP-1 agonists should be restricted in patients with severe COVID-19 [31]. If GLP-1 agonists are administered to COVID-19 patients, they should be closely monitored, and fluid intake should be adequate [28]. GLP-1 agonists may have an anti-inflammatory effect and may attenuate pulmonary inflammation in rats with a respiratory syncytial virus (RSV) infection and experimental lung injury [34]. Whether GLP-1 agonists may have an anti-inflammatory effect in patients with COVID-19 or not needs further evaluation and assessment.
5.2. Pioglitazone
Pioglitazone upregulates Angiotensin converting-2 enzyme (ACE-2) in rat tissues [35], leading to concerns that it may increase COVID-19 severity. Since ACE2 acts as a receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enter cells [36].
Wu et al. showed that using virtual computer screening, Pioglitazone may be a potent inhibitor of 3 chymotrypsin-like protease, which is necessary for RNA synthesis and replication of SARS CoV-2 [37]. However, this claim requires both in-vitro and in-vivo validation. COVID-19 infected diabetic patients have a higher risk of developing a cytokine storm. Pioglitazone inhibits the secretion of proinflammatory cytokines, and so it may mitigate the cytokine storm [38].
5.3. Sodium-glucose cotransporter 2 (SGLT2) inhibitors
SGLT2-inhibitors may have a potent antiviral effect by increasing lactate concentration and simultaneously decreasing the intracellular pH, thereby potentially lowering the viral load [39]. Canagliflozin therapy, an SGLT2-inhibitor, could induce a reduction in interleukin-6 (IL-6) levels, which plays an essential role in triggering the cytokine release syndrome (CRS) in COVID-19 patients [40].
SGLT2 inhibitors increase the excretion of sodium and glucose through urine, leading to osmotic diuresis and possibly dehydration. Additionally, SGLT2 inhibitors can cause euglycemic ketosis. SGLT2 inhibitors should not be given for critically ill patients with severe COVID-19. However, there is no reason to limit their use in patients with a mild form of the disease [28].
The DARE-19 trial is a recent study that aims to evaluate the role of SGLT-2 inhibitors in non-critically ill patients hospitalized due to COVID-19 infection. The investigation is a double-blinded, placebo-controlled, phase III study centered on evaluating the impact of dapagliflozin on the incidence of complications and all-cause mortality in COVID-19 infected patients with respiratory failure. Patients with and without diabetes were included in the study. Primarily the aim was to assess organ failure or death in patients treated with this SGLT-2 inhibitor versus placebo. Secondary outcomes included the evaluation of composite kidney endpoint and all-cause mortality [41]. The patients were randomized to receive either dapagliflozin 10 mg daily or a placebo for 30 days. A total of 1250 patients were enrolled and followed up for a total of 90 days. Those who were critically ill at the time of presentation, with significantly impaired kidney function (GFR <25 ml/min/1.73m2), T1DM, or prior history of diabetic ketoacidosis were excluded. The study results have shown no significant reduction in organ dysfunction, death, or improvement in patients treated with dapagliflozin compared to placebo [42].
5.4. Insulin
Insulin therapy is preferred for hospitalized patients, including moderate to severe COVID-19 disease. For non-critical hospitalized patients, a basal plus bolus correction regimen is recommended with a target blood sugar range of 140–180 mg/dl [43]. Critically ill COVID-19 diabetic patients in ICU are managed by intravenous insulin infusion. Intensive insulin therapy was found to exert an anti-inflammatory effect in critically ill patients and reduce the levels of inflammatory markers (C-reactive protein (CRP) and mannose-binding lectin (MBL)) compared to conventional insulin therapy [44]. Whether the anti-inflammatory effect of insulin is beneficial in COVID-19 patients or not needs further assessment and evaluation.
A possible therapeutic regimen in non-critically ill COVID-19 diabetic patients is a combination of basal Insulin with GLP-1 agonist, given as a single injection. The rationale beyond this therapeutic strategy is giving a single daily injection minimizing exposure to COVID-19 patients. Also, both insulin and GLP-1 agonists have a glucose-lowering effect and a possible anti-inflammatory effect [45].
5.5. Effects of hyperglycemia on complications of COVID-19
Hyperglycemia is a well-known, established risk factor for mortality due to an increased susceptibility to infections, mainly due to pneumonia. COVID-19 is characterized by pneumonia that has led to the death of over 1.65 million individuals worldwide [46]. According to a retrospective, observational study involving adult patients (n = 184) with laboratory-confirmed COVID-19 and uncontrolled hyperglycemia (blood glucose [BG] >180 mg/dL within any 24 h), the mortality rate was 28.8% versus 6.2% in patients without diabetes or hyperglycemia (n = 386, p < 0.001). Additionally, 41.7% of patients with uncontrolled hyperglycemia died versus 14.8% of patients with diabetes (p < 0.001) [47]. Furthermore, the median length of stay in patients with diabetes and/or uncontrolled hyperglycemia was more prolonged than in patients without diabetes or hyperglycemia (p < 0.001) [47].
Another retrospective study involving consecutive patients with COVID-19 (n = 605) reported that fasting BG (FBG ≥7.0 mmol/l) was an independent predictor for 28-day mortality in patients with COVID-19, who were not previously diagnosed with diabetes (hazard ratio [H.R.] 2.30 [95% CI = 1.49–3.55) [48]. In a retrospective, multicenter study involving patients (n = 952) with COVID-19 and pre-existing T2DM, patients with T2DM were found to need more medical interventions and significantly higher mortality and multiple organ injury versus patients without T2DM (7.8% vs. 2.7%) [49].
A retrospective single-center case series involving consecutive Chinese patients (n = 138) hospitalized with COVID-19-associated pneumonia reported a history of diabetes to be associated with 22.2% of intensive care unit (ICU) admissions compared with 5.9% of non-ICU admissions (p = 0.009) [50].
A recent meta-analysis that assessed the outcomes of COVID-19 in patients with hyperglycemia reported death in 17% (0.10–0.25), 11% to have been admitted to the ICU, and 23% to have required mechanical or non-mechanical ventilation [51]. Additionally, severe or critical COVID-19 was seen to develop in 72% of patients, acute cardiac injury in 14%, acute respiratory distress syndrome in 15%, and acute kidney injury in 5% [51]. It is worth mentioning that acute kidney injury, whether associated with hyperglycemia or not, is a predictor of a poor outcome in COVID-19 patients. This meta-analysis confirms that hyperglycemia is a predisposing factor for the death and progression of non-critical to critical diseases in patients with COVID-19, both in diabetic and non-diabetic [51].
5.6. DKA and COVID-19
Hyperglycemic complications of diabetes mellitus include Diabetic Ketoacidosis (DKA), hyperosmolar hyperglycemic syndrome (HHS). DKA is the commonest of these complications, with the highest hospitalizations rates that also increased in the last decade in developed countries, but positively, mortality decreased to less than 0.5% [52].
Similar to a case of any severe infections, DKA can occur in diabetic patients with COVID-19. Severe Acute Respiratory Syndrome Coronavirus-1 (SARS-CoV-1) was found to bind to the ACE2 receptor in the pancreatic islets, which causes cellular damage leading to acute onset of diabetes DKA [53]; hence COVID-19 was expected to have a similar mechanism.
One study in Wuhan, China, reported the prevalence of ketonemia or ketonuria among hospitalized COVID-19 patients to be 6.4%, with 7% of them meeting American Diabetes Association (ADA) DKA criteria and all of them had a prior diagnosis of diabetes mellitus [54].
Many studies and case reports later reported new-onset diabetes with DKA as the presenting symptom in COVID-19 or DKA without respiratory symptoms, and even euglycemic DKA was reported [55]. COVID-19 has also been linked to new-onset T1DM with DKA and pancreatic autoantibodies [56].
Data were presented from many countries with variable presentations, In a study done in Peru, 14 patients were admitted with DKA, and four of them were diagnosed with COVID-19, and two of them had associated findings suggestive of pancreatitis [57]. The number of DKA diagnoses in an Australian hospital during the COVID-19 pandemic, increased but comparable to before the pandemic, while the number of severe DKA was significantly higher (45% vs. 5%; p < 0.003) [58]. In the UK, it was noticed that diabetes mellitus was overrepresented in hospitalized COVID-19 patients (31%) while its national prevalence is 7% and data of T2DM hospital bed occupancy was 18% before the pandemic, with DKA present in 2% of hospitalized COVID-19 patients [59].
A recent systematic review evaluated 110 cases of DKA with COVID-19, with the majority (91/110) presenting with DKA and (19/110) had DKA/HHS; males represented 63% of the cohort, and 77% had a prior diagnosis of T2DM. Mortality reached 45%, and it was higher in the DKA/HHS group than the DKA group (67% vs. 29%). This high mortality rate could be related to COVID-19 or combining both factors. A single study conducted at Jacobi center in New York had DKA mortality of 50% when patients had concomitant COVID-19 [60], likely due to the severity of their respiratory illness and the overwhelming pandemic on the healthcare system in New York.
Management of DKA with COVID-19 follows the standard protocol, although it was noted that patients required higher than usual insulin doses [61]. However, due to limited resources and personal protective equipment (PPE), hospitals initiated different protocols utilizing fewer infusion pumps to use them for pressors and started using subcutaneous insulin analogs.
DKA has always been famous for thrombotic complications, and was found to be increased when combined with COVID-19 due to its potential thrombotic complications [62], highlighting the role of prophylactic anticoagulation in patients with DKA and COVID-19.
5.7. Corticosteroids and COVID-19
Steroids show severe negative effects on diabetics. They are a part of many medicines used for both short-term and long-term treatment. Primarily they are used for their anti-inflammatory and immunosuppressive actions with subsequent undesirable side effects such as osteoporosis, hypertension, hyperglycemia, and steroid-induced diabetes [63].
Many factors contribute to the degree of hyperglycemia, including duration of treatment, dosage, relative potency, and associated infection. The higher the dose of steroid therapy, the higher the risk of developing steroid-induced diabetes. The use of steroids in diabetic patients increases the risk of hospitalization due to uncontrolled blood sugar [64].
In the first few months of the pandemic, World Health Organization (WHO) recommended against corticosteroid treatment, based on historical evidence in clinical studies of Middle East Respiratory Syndrome-corona Virus (MERS-CoV) and SARS-CoV [65] that showed treatment complications such as delayed viral clearance, opportunistic infections, and hyperglycemia.
Following the warning, many institutions initiated observational studies and randomized controlled trials (RCT) on steroid therapy for COVID-19, with the most extensive study in the UK, also known as The RECOVERY trial, which showed dexamethasone as opposed to usual care reduced 28-day mortality in patients requiring oxygen therapy or mechanical ventilation [66].
Following this study and as of September 2, 2020, the WHO changed the previous recommendations, and steroids remained as the only drug of proven benefit for Covid-19 patients.
Steroids have beneficial effects in the states of hyper inflammation, which is the hallmark of Covid-19 but at the expense of worsening hyperglycemia, new-onset diabetes, and hyperglycemic complications.
5.8. Co-infections in diabetic patients with COVID 19
DM is an important prognostic factor, as studies have shown a 10-fold higher risk of death in this patient population [67]. In addition, a disturbing glucolipid metabolism undoubtedly leads to immunosuppression by decreasing the innate immune response, and COVID 19 itself leads to a weakening of the immune system by lowering the T-lymphocyte count [68]. As a result, these patients are more susceptible to other bacterial, viral, and fungal infections.
Candida species (Albicans and non-Albicans) are among the co-infections observed in COVID-19 patients and presented as invasive candidiasis. It can also cause fungal pneumonia. Another severe fungal superinfection is invasive pulmonary aspergillosis which is associated with high mortality rates. The diagnosis implies microbiological and/or histopathological evidence using special fungal staining [69]. Cryptococcal disease and pneumocystis jirovecii have also been detected in COVID-19 patients, particularly those treated with immunosuppressants [68]. Patients with diabetes mellitus, especially those with poorly blood glucose control, and COVID-19 were noticed to have an increased risk of coccidioidomycosis. Patients at risk lived in endemic areas (California, Arizona) and had occupational exposure. Diabetes potentiated the likelihood of disseminated disease, relapsing coccidioidomycosis, and cavitary lung disease [70]. Fungal infections can be missed, as many of them manifest similar to COVID-19.
According to one case series in Washington, the rate of bacterial co-infection in COVID-19 patients was 4.8%. A study from China revealed Mycoplasma pneumoniae and Legionella pneumophila are among the most frequently isolated organisms causing respiratory illness in patients with COVID 19. The prevalence of diabetes was 54%, and a strong correlation was shown between hyperglycemia and co-infection [71]. Patients with bacterial co-infections were found to have higher mortality and higher rates of steroid use.
Mucormycosis is an emerging fungal infection globally, with severe and often fatal disease symptoms and varied clinical manifestations. The outbreak of cases reported in the wake of the second wave of COVID-19 in India has brought unprecedented focus to this disease worldwide [72]. A total of 71% of COVID-19 associated mucormycosis (CAM) cases are reported from India. This is explained by the fact that India has the highest burden of mucormycosis in the world and has the second-largest number of adults with diabetes [72]. This otherwise innocuous organism in an immunocompetent host can become aggressive and highly destructive in patients with an impaired immune system, such as those with hematological malignancies, immunosuppression, poorly controlled diabetes, iron overload, and significant trauma. DM was present in 94% of CAM patients, highlighting the significant role of diabetes in the development of such complications.
5.9. Telemedicine and diabetes during COVID-19 pandemic
Telemedicine delivers healthcare services from a distance by using information and communication technology to exchange valid information to manage the patients. It is part of e-health, which also includes mobile health and electronic medical record. Phone calls, video conferences, and social media apps facilitate communication between healthcare providers and diabetic patients [73]. Also, blood glucose readings using various devices such as meter, pump, and sensor facilitate diabetic management by e-health. However, this approach faced several obstacles such as regulations, payment, privacy, confidentiality, and prescription. Several developed and developing countries started to create telemedicine laws and regulations during the COVID-19 pandemic [74].
Lockdown during the COVID-19 pandemic has a negative impact on blood glucose levels by changing food habits, decreasing physical activity, and running out of medications. Telemedicine could help mitigate the effects of lockdown by providing education, adjusting medications, decreasing visits to healthcare facilities, and reducing the risk of unnecessary direct exposure and disease transmission [75]. Moreover, diabetic patients need regular foot checkups. Using video calling as triage for the diabetic foot could prevent catastrophic complications. A meta-analysis of 42 randomized control trials showed that telemedicine has a better impact on the control of hemoglobin A1C than standard care, especially in more than 40 years old and T1DM patients [76]. E-health should be part of the standard of care during and after the pandemic.
6. Conclusion
Diabetes mellitus carries a significant risk of complications, extended hospital stays, and mortality in COVID-19 infected patients. Therefore, insulin is preferred to oral hypoglycemic medications in the management of hospitalized COVID-19 infected diabetic subjects. This cohort has recommended frequent blood sugar checks and prompt management of hypoglycemia, hyperglycemia, and DKA.
Funding
None.
Conflict of interest
The authors of these paper certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Declaration of competing interest
The authors confirm the absence of personal and financial interests impacting the outcomes of this research study.
Acknowledgments
None.
References
- 1.Elamari S., et al. Characteristics and outcomes of diabetic patients infected by the SARS-CoV-2. The Pan African medical journal. 2020;37 doi: 10.11604/pamj.2020.37.32.25192. 32-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceriello A. Hyperglycemia and COVID-19: what was known and what is really new? Diabetes Res Clin Pract. 2020;167 doi: 10.1016/j.diabres.2020.108383. 108383-108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nassar M., et al. Diabetes & metabolic syndrome. Clinical Research & Reviews; 2021. COVID-19 vaccine-induced myocarditis case report with literature review; p. 102205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterne J.A.C., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. Jama. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alessi J., et al. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndrome. 2020;12:80. doi: 10.1186/s13098-020-00583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Dalmazi G., et al. Comparison of the effects of lockdown due to COVID-19 on glucose patterns among children, adolescents, and adults with type 1 diabetes: CGM study. BMJ open diabetes research & care. 2020;8(2) doi: 10.1136/bmjdrc-2020-001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biancalana E., et al. Short-term impact of COVID-19 lockdown on metabolic control of patients with well-controlled type 2 diabetes: a single-centre observational study. Acta Diabetol. 2020:1–6. doi: 10.1007/s00592-020-01637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim S., et al. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceriello A. Hyperglycemia and COVID-19: what was known and what is really new? Diabetes Res Clin Pract. 2020;167:108383. doi: 10.1016/j.diabres.2020.108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrie J.R., Guzik T.J., Touyz R.M. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berbudi A., et al. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16(5):442–449. doi: 10.2174/1573399815666191024085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Šestan M., et al. Virus-induced interferon-γ causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity. 2018;49(1):164–177.e6. doi: 10.1016/j.immuni.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Chen J., et al. The impact of COVID-19 on blood glucose: a systematic review and meta-analysis. Front Endocrinol. 2020;11(732) doi: 10.3389/fendo.2020.574541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee S., et al. COVID-19: the endocrine opportunity in a pandemic. Minerva Endocrinol. 2020;45(3):204–227. doi: 10.23736/S0391-1977.20.03216-2. [DOI] [PubMed] [Google Scholar]
- 16.Lippi G., Sanchis-Gomar F., Henry B.M. Response to: is newly diagnosed diabetes a stronger risk factor than pre-existing diabetes for COVID-19 severity? J Diabetes. 2021;13(2):179–180. doi: 10.1111/1753-0407.13127. [DOI] [PubMed] [Google Scholar]
- 17.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46(4):265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., et al. Impacts of type 2 diabetes on disease severity, therapeutic effect, and mortality of patients with COVID-19. J Clin Endocrinol Metab. 2020;105(12) doi: 10.1210/clinem/dgaa535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon S.J., et al. Independent impact of diabetes on the severity of coronavirus disease 2019 in 5,307 patients in South Korea: a nationwide cohort study. Diabetes & metabolism journal. 2020;44(5):737–746. doi: 10.4093/dmj.2020.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarado-Vasquez N. Could a family history of type 2 diabetes be a risk factor to the endothelial damage in the patient with COVID-19? Med Hypotheses. 2020;146 doi: 10.1016/j.mehy.2020.110378. 110378-110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassar M., et al. The association between COVID-19 and type 1 diabetes mellitus: a systematic review. Diabetes Metab Syndr. 2021;15(1):447–454. doi: 10.1016/j.dsx.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebekozien O.A., et al. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83–e85. doi: 10.2337/dc20-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barron E., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holman N., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. The Lancet Diabetes & Endocrinology. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabbone I., et al. Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020;43(11):2870–2872. doi: 10.2337/dc20-1321. [DOI] [PubMed] [Google Scholar]
- 26.Singh A.K., et al. Non-insulin anti-diabetic agents in patients with type 2 diabetes and COVID-19: a Critical Appraisal of Literature. Diabetes Metab Syndr. 2021;15(1):159–167. doi: 10.1016/j.dsx.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeFronzo R., et al. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016;65(2):20–29. doi: 10.1016/j.metabol.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Bornstein S.R., et al. Practical recommendations for the management of diabetes in patients with COVID-19. The Lancet Diabetes & Endocrinology. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108183. 108183-108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apicella M., et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. The Lancet Diabetes & Endocrinology. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettge K., et al. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metabol. 2017;19(3):336–347. doi: 10.1111/dom.12824. [DOI] [PubMed] [Google Scholar]
- 34.Bloodworth M.H., et al. Glucagon-like peptide 1 receptor signaling attenuates respiratory syncytial virus-induced type 2 responses and immunopathology. J Allergy Clin Immunol. 2018;142(2):683–687. doi: 10.1016/j.jaci.2018.01.053. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viby N.E., et al. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154(12):4503–4511. doi: 10.1210/en.2013-1666. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W., et al. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. ScientificWorldJournal. 2014 doi: 10.1155/2014/603409. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni W., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W.Y., et al. Pioglitazone inhibits the expression of inflammatory cytokines from both monocytes and lymphocytes in patients with impaired glucose tolerance. Arterioscler Thromb Vasc Biol. 2008;28(12):2312–2318. doi: 10.1161/ATVBAHA.108.175687. [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee J.J., Gangopadhyay K.K., Ray S. Management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):666. doi: 10.1016/S2213-8587(20)30226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garvey W.T., et al. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32–37. doi: 10.1016/j.metabol.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Kosiborod M., et al. Effects of dapagliflozin on prevention of major clinical events and recovery in patients with respiratory failure because of COVID-19: design and rationale for the DARE-19 study. Diabetes Obes Metabol. 2021;23(4):886–896. doi: 10.1111/dom.14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dharam J., Kumbhani M., SM, FACC Dapagliflozin in respiratory Failure in patients with COVID-19 - DARE-19. 2021 05/16/2021. https://www.acc.org/latest-in-cardiology/clinical-trials/2021/05/14/02/40/dare-19 [cited 2021 July 22th]; Available from:
- 43.Lim S., et al. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen T.K., et al. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88(3):1082–1088. doi: 10.1210/jc.2002-021478. [DOI] [PubMed] [Google Scholar]
- 45.Longo M., et al. Treating type 2 diabetes in COVID-19 patients: the potential benefits of injective therapies. Cardiovasc Diabetol. 2020;19(1):115. doi: 10.1186/s12933-020-01090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Organization, W.H. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/. 2020 [cited 2020 12/12]; Available from: https://covid19.who.int/.
- 47.Bode B., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S., et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020;63(10):2102–2111. doi: 10.1007/s00125-020-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu L., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metabol. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee M.H., et al. Effects of hyperglycaemia on complications of COVID-19: a meta-analysis of observational studies. Diabetes Obes Metabol. 2021;23(1):287–289. doi: 10.1111/dom.14184. [DOI] [PubMed] [Google Scholar]
- 52.Palermo N.E., Sadhu A.R., McDonnell M.E. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metab. 2020;105(8) doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang J.K., et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J., et al. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metabol. 2020;22(10):1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oriot P., Hermans M.P. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: case report and review of the literature. Acta Clin Belg. 2020:1–5. doi: 10.1080/17843286.2020.1780390. [DOI] [PubMed] [Google Scholar]
- 56.Alfishawy M., et al. New-onset type 1 diabetes mellitus with diabetic ketoacidosis and pancreatitis in a patient with COVID-19. Sci Afr. 2021;13 doi: 10.1016/j.sciaf.2021.e00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Concepcion Zavaleta M.J., et al. Diabetic ketoacidosis during COVID-19 pandemic in a developing country. Diabetes Res Clin Pract. 2020;168 doi: 10.1016/j.diabres.2020.108391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawrence C., et al. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet Med. 2021;38(1) doi: 10.1111/dme.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman N., et al. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108291. 108291-108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chamorro-Pareja N., et al. Letter to the editor: unexpected high mortality in COVID-19 and diabetic ketoacidosis. Metab Clin Exp. 2020;110 doi: 10.1016/j.metabol.2020.154301. 154301-154301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korytkowski M., et al. A pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. J Clin Endocrinol Metab. 2020;105(9):dgaa342. doi: 10.1210/clinem/dgaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The Lancet H. COVID-19 coagulopathy: an evolving story. Lancet Haematol. 2020;7(6) doi: 10.1016/S2352-3026(20)30151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang J.L., Weiss R.E. Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev. 2014;30(2):96–102. doi: 10.1002/dmrr.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nassar M., et al. Current systematic reviews and meta-analyses of COVID-19. World J Virol. 2021;10(4):182–208. doi: 10.5501/wjv.v10.i4.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arabi Y.M., et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 66.Horby P., et al. Effect of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y., et al. Clinical characteristics and outcomes of type 2 diabetes patients infected with COVID-19: a retrospective study. Engineering. 2020;6(10):1170–1177. doi: 10.1016/j.eng.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhatt K., et al. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9(1) doi: 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song G., Liang G., Liu W. Fungal Co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185(4):599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heaney A.K., et al. Coccidioidomycosis and COVID-19 Co-infection, United States, 2020. Emerg Infect Dis. 2021;27(5):1266–1273. doi: 10.3201/eid2705.204661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alosaimi B., et al. Influenza co-infection associated with severity and mortality in COVID-19 patients. Virol J. 2021;18(1):127. doi: 10.1186/s12985-021-01594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh A.K., et al. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4) doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh A., Gupta R., Misra A. Telemedicine for diabetes care in India during COVID19 pandemic and national lockdown period: guidelines for physicians. Diabetes Metab Syndr. 2020;14(4):273–276. doi: 10.1016/j.dsx.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Services., U.S.D.o.H.H . 2020. OCR announces notification of enforcement discretion for telehealth remote communications during the COVID-19 Nationwide Public Health Emergency.https://www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcementdiscretion-for-telehealth-remote-communications-during-thecovid-19.html 2020]; Available from: [Google Scholar]
- 75.Charles B.L. Telemedicine can lower costs and improve access. Healthc Financ Manag. 2000;54(4):66–69. [PubMed] [Google Scholar]
- 76.Tchero H., et al. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J e Health. 2019;25(7):569–583. doi: 10.1089/tmj.2018.0128. [DOI] [PubMed] [Google Scholar]