Abstract
The Hartmann's procedure first described in 1920 is a gold standard for a variety of emergent procedures of the sigmoid colon. A standardized approach to a robotic reversal of a Hartmann's procedure is described to reestablish bowel continuity.
Keywords: colorectal surgery, robotic surgery, Hartmann's reversal
Throughout history, bowel surgery has been fraught with difficulty due to complex pathophysiology of disease, lack of empiric data identifying the best operative approaches as well as the available technology to affect repair.
Alexis Littre performed the first known colostomy in 1710 after observing a child with rectal atresia. 1 In 1793, Durret performed a sigmoid colostomy on a female infant; her survival to adulthood records the first known successful diversion. In 1920, Hartmann performed a descending colostomy with closure of the distal rectal stump after rectal cancer resection, as a means to avoid the excessive mortality of an abdominoperineal resection (38%); this success gave his name to the operation still performed today. 2 The reversal of the colostomy and re-establishment of continuity was then described by Boyden, who reversed six patients, some after only 2 weeks with an ostomy. 3
The Hartmanǹs procedure (and staged closure after 3–6 months) is the gold standard for a variety of emergent procedures involving the sigmoid colon including diverticulitis, obstructing colonic Crohn's disease, colon cancer with perforation, and trauma in the setting of feculent peritonitis, when creating an anastomosis is not deemed prudent. Traditionally performed in an open fashion, colostomy reversal has benefitted from the technological advances in minimally invasive surgery. Anderson et al published the first report of laparoscopic Hartmann's reversal (HR) in 1993. 4 de'Angelis et al followed with the first description of a robotic reversal of an end colostomy, finding the platform to be safe, feasible, and valuable. 5
Robotic technology offers various benefits to surgeons performing a minimal invasive approach to HR. One of the main difficulties in a laparoscopic approach is the extensive adhesiolysis often needed to dissect out the colostomy and the rectal stump. The use of straight laparoscopic instruments creates difficulty reaching and then lysing adhesions in the deep pelvis, toward the abdominal wall, and colostomy site. A stable 3D camera platform and various wristed instruments lend themselves well to the adhesiolysis, which is a common cause of conversion in laparoscopy.
Steps of the Robotic Procedure
Access and Port Placement
An end colostomy reversal is always at least the patient's second abdominal surgery. Anticipating extensive adhesions from a prior midline incision, our preferred method of establishing pneumoperitoneum is using an optical port at Palmer̀s point in the left upper quadrant. It is therefore important to plan the robotic port placement prior to insertion of this initial port.
When utilizing the DaVinci Si system, we prefer to place a 12-mm laparoscopic trocar as the camera port above the umbilicus or at the midpoint between xyphoid and symphysis pubis in patients with a larger pannus. This port can be offset slightly toward the right, as it otherwise can be too close to the colostomy site. Arm one is placed in the right lower quadrant. We prefer to use scissors with monopolar energy through this port. Arm 2 is placed in the right upper quadrant, midway between the camera port and the optical entry port. We typically utilize a fenestrated bipolar dissector in port 2. Arm three can then replace the original optical entry trocar. An atraumatic Cadiere forceps is commonly used here to assist with the dissection. Virtual lines from all robotic trocars to the left lower quadrant should have equal distance between each other to avoid instrument collision, ideally separated from each other by 8 cm. An accessory 5-mm laparoscopic port can be placed in the right flank for additional retraction by an assistant, as needed.
The DaVinci Xi system has significant design improvements to allow for a broader reach into multiple quadrants with less instrument collision. The trocars are typically placed in an oblique line from the left upper quadrant to the right lower quadrant. This line is again moved slightly to the right away from the colostomy site on the left. The distance between all four robotic trocars is typically 8 to 10 cm. Again, an additional laparoscopic 5-mm flank port can be placed as needed for additional retraction ( Fig. 1 ).
Fig. 1.
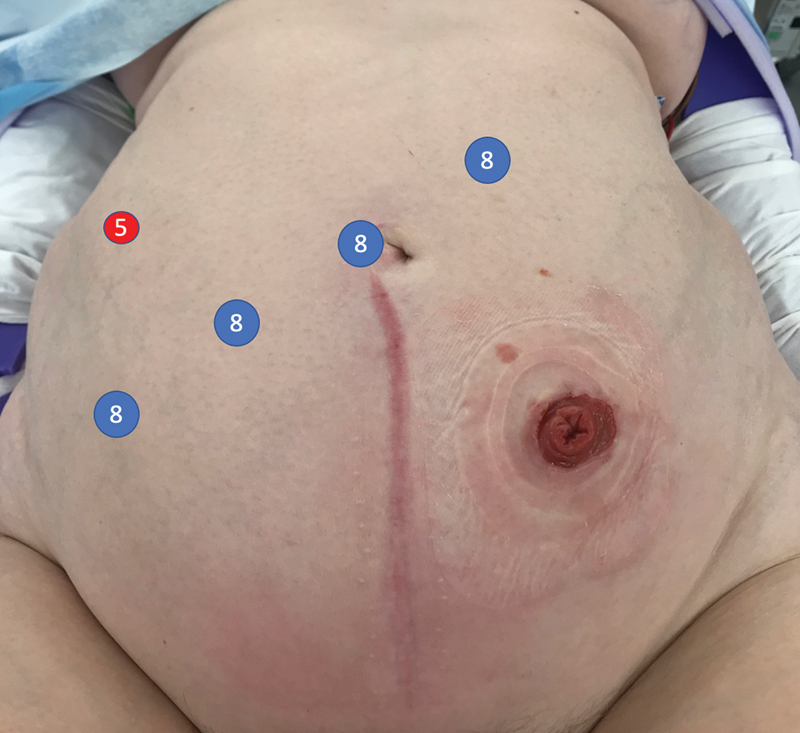
Robotic trocar placement.
Procedure
After establishing pneumoperitoneum, the patient is placed in lithotomy with the left side up to facilitate the small bowel moving out of the pelvis. Using sharp dissection, small bowel adhesions and omentum are lysed from the descending colon toward the colostomy. The colostomy is then circumferentially dissected off the fascial edges ( Fig. 2 ). The flexibility of the robotic instruments can allow dissection above the level of the fascia toward the skin. External pressure can be given by the bedside assistant to reduce the hernia sac into the peritoneal cavity, as needed. The descending colon and splenic flexure are then mobilized from medial to lateral, and any remaining peritoneal attachments, the splenocolic ligament and omentum are divided. The colostomy limb can be stapled off using the DaVinci stapler in arm one. A circumferential stomal dissection at the skin level is then performed. We prefer to then place a GelPOINT Mini Advanced Access Platform (Applied Medical, Rancho Santa Margarita, CA) through the ostomy site. This can serve as an extraction site and an additional trocar site while maintaining pneumoperitoneum. The proximal colon is mobilized until it falls into the pelvis with no tension. Once adequate mobilization of the proximal colon is achieved, the pelvic portion of the operation begins. Dissection commences to identify the rectal stump, and adhesions are lysed between the rectum and surrounding structures, such as small bowel, uterus, ovaries, adnexae, and peritoneum. It is common that the rectal stump needs to be restapled to completely resect residual sigmoid colon ( Fig. 3 ). The extent and anatomy of the adhesions can be unique to every patient. The rectum can be mobilized posteriorly in the bloodless total mesorectal excision plane to facilitate reach and advancement of the EEA stapler. Optimal visualization and instrument reach into the pelvis are essential benefits of the robotic approach.
Fig. 2.
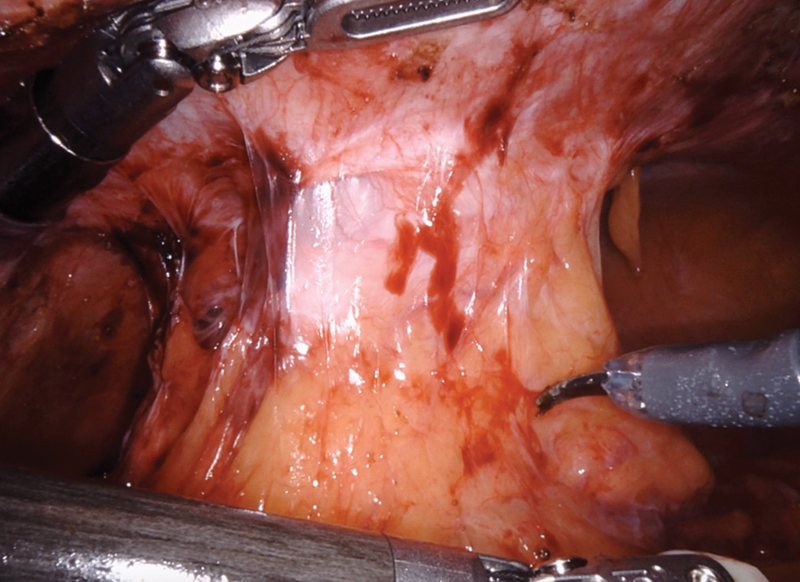
Intracorporeal mobilization of the end colostomy.
Fig. 3.
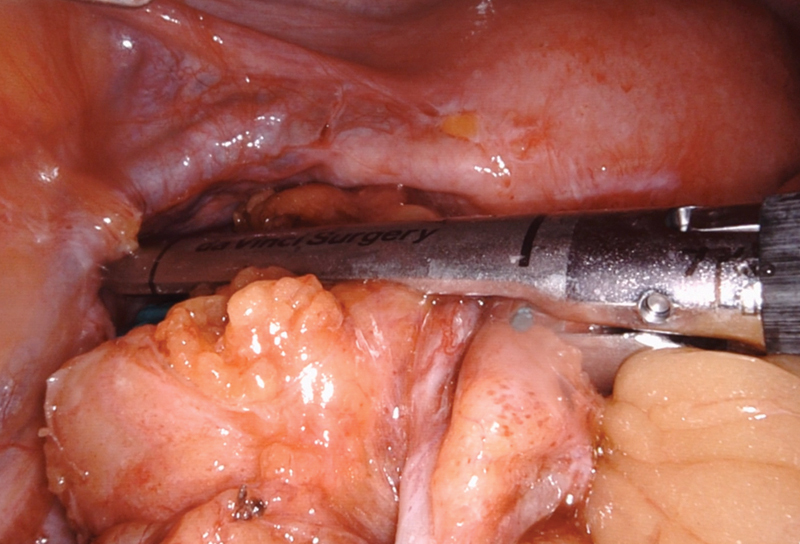
Stapling at the rectosigmoid junction.
Typically, the anastomosis is created using an EEA stapler. The simplest way to secure the anvil is by bringing the proximal bowel through the gel platform. It can then be reduced into the abdomen and pneumoperitoneum can be reestablished. Then, utilizing the robotic arms, the anvil can be mated to the EEA stapler passed through the anorectum ( Fig. 4 ). Regaining pneumoperitoneum and reinstating robotic 3D imaging allows for excellent visualization of the creation of the anastomosis, as well as a subsequent leak test. One caveat is that the leak test may be challenging if the patient is in steep Trendelenburg position, as the saline tends to flow out of the pelvis. The Xi system, when synched with the Table Motion technology, allows for easy repositioning of the table when performing an anastomotic leak test. In addition, Firefly immunofluorescent technology can also be employed to assess the vascular supply of the anastomosis at this point utilizing either the Si or Xi systems ( Fig. 5 ).
Fig. 4.
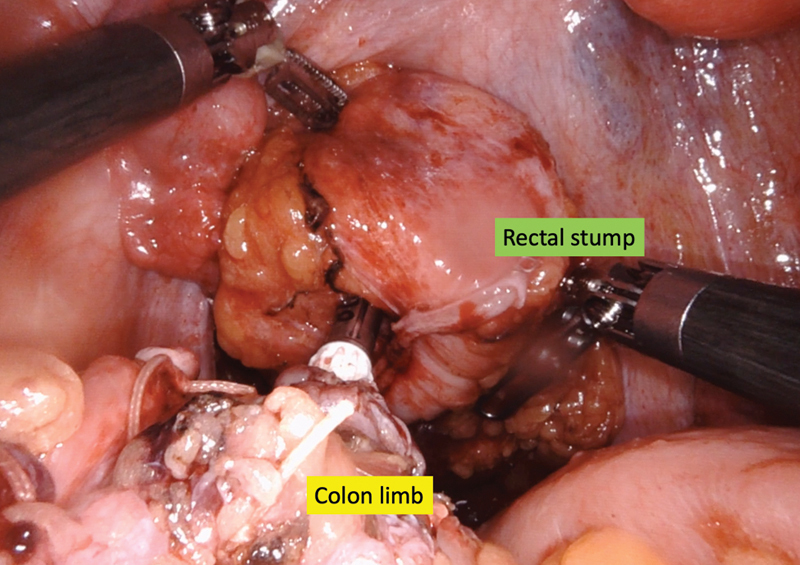
Colorectal anastomosis utilizing an EEA stapler.
Fig. 5.
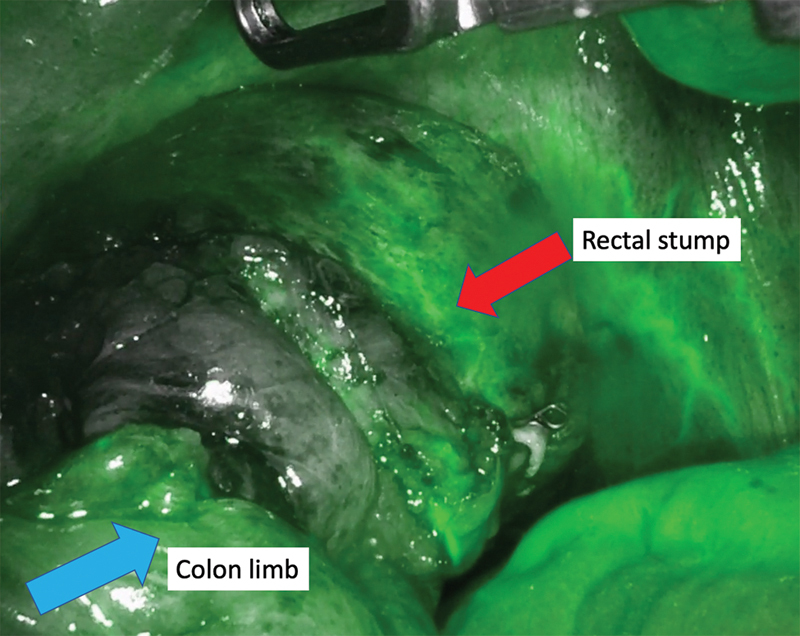
Perfusion assessment utilizing indocyanine green.
Review of the Literature
Over 20 years of published data supports laparoscopic HR. However, the literature on the robotic approach is scant. Details of the learning curve for robotic colorectal surgery, in general, and reports of the safety and feasibility in simple and complex cases exist in a limited fashion. 6 7 8 9 10 11 To date, just four case reports and one case series of robotic HR have been published. 5 Guiliani et al 12 described the technical aspects of robotic HR and their preliminary experience with 24 patients. The mean operating room time was 240 minutes and there were no conversions to open surgery. While they had three minor complications, they reported no major complications or 30-day readmissions. The mean length of stay was 6 days. There are neither comparative studies nor randomized trials evaluating the perceived advantages of the robotic approach over laparoscopic or open HR.
Conclusion
The introduction of robotic technology facilitates the minimal invasive approach to an HR by improving visualization and dissection capabilities in the tight spaces of the pelvis and around the colostomy aperture. There may be advantages to the robotic approach over traditional laparoscopy, resulting in fewer conversions to an open approach. Given growing evidence of improved postoperative outcomes, robotic HR should be considered as an alternative approach to the open technique.
References
- 1.Holschneider A M, Hutson J M. Berlin: Springer; 2006. Anorectal Malformations in Children: Embryology, Diagnosis, Surgical Treatment, Follow-up; p. 3. [Google Scholar]
- 2.Sanderson E R. Henri Hartmann and the Hartmann operation. Arch Surg. 1980;115(06):792–793. doi: 10.1001/archsurg.1980.01380060088026. [DOI] [PubMed] [Google Scholar]
- 3.Boyden A M. The surgical treatment of diverticulitis of the colon. Ann Surg. 1950;132(01):94–109. [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson C A, Fowler D L, White S, Wintz N. Laparoscopic colostomy closure. Surg Laparosc Endosc. 1993;3(01):69–72. [PubMed] [Google Scholar]
- 5.de'Angelis N, Felli E, Azoulay D, Brunetti F. Robotic-assisted reversal of Hartmann's procedure for diverticulitis. J Robot Surg. 2014;8(04):381–383. doi: 10.1007/s11701-014-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abodeely A, Lagares-Garcia J A, Duron V, Vrees M. Safety and learning curve in robotic colorectal surgery. J Robot Surg. 2010;4(03):161–165. doi: 10.1007/s11701-010-0204-0. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings A L, Woodland J H, Crawford D L. Telerobotic surgery for right and sigmoid colectomies: 30 consecutive cases. Surg Endosc. 2006;20(11):1713–1718. doi: 10.1007/s00464-005-0771-8. [DOI] [PubMed] [Google Scholar]
- 8.DeNoto G, Rubach E, Ravikumar T S. A standardized technique for robotically performed sigmoid colectomy. J Laparoendosc Adv Surg Tech A. 2006;16(06):551–556. doi: 10.1089/lap.2006.16.551. [DOI] [PubMed] [Google Scholar]
- 9.Maciel V, Lujan H J, Plasencia G. Diverticular disease complicated with colovesical fistula: laparoscopic versus robotic management. Int Surg. 2014;99(03):203–210. doi: 10.9738/INTSURG-D-13-00201.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juo Y Y, Agarwal S, Luka S, Satey S, Obias V. Single-Incision Robotic Colectomy (SIRC) case series: initial experience at a single center. Surg Endosc. 2015;29(07):1976–1981. doi: 10.1007/s00464-014-3896-9. [DOI] [PubMed] [Google Scholar]
- 11.Elliott P A, McLemore E C, Abbass M A, Abbas M A. Robotic versus laparoscopic resection for sigmoid diverticulitis with fistula. J Robot Surg. 2015;9(02):137–142. doi: 10.1007/s11701-015-0503-6. [DOI] [PubMed] [Google Scholar]
- 12.Giuliani G, Formisano G, Milone M, Salaj A, Salvischiani L, Bianchi P P. Full robotic Hartmann's reversal: technical aspects and preliminary experience. Colorectal Dis. 2020;22(11):1734–1740. doi: 10.1111/codi.15249. [DOI] [PubMed] [Google Scholar]


