Abstract
Background and Objectives:
Carbapenems have been the choice of antibiotics for the treatment of infections caused by multidrug-resistant bacteria. The main objective of this study was to determine the prevalence of carbapenemase (bla VIM and bla IMP ) producing isolates among Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii.
Materials and Methods:
A total of 1,151 clinical samples were collected from the patients visiting Annapurna Neurological Institute and Allied Science and Annapurna Research Centre, Kathmandu, between June 2017 and January 2018. Antibiotic susceptibility testing (AST) was performed on the Enterobacteriaceae, P. aeruginosa and A. baumannii isolates using the Kirby-Bauer disk diffusion method. The modified Hodge test (MHT) was performed on the carbapenem-resistant isolates to confirm carbapenemase production. DNA was extracted and then screened for bla VIM and bla IMP genes by multiplex PCR.
Results:
Of the total 1,151 clinical samples, 253 (22.0%) showed positive growth. Of them, 226 (89.3%) were identified as Enterobacteriaceae, P. aeruginosa, and A. baumannii. Among the 226 isolates, 106 (46.9%) were multidrug-resistant. Out of the 106, 97 (91.5%) isolates showed resistance to at least one of the carbapenem used. Among the 97 carbapenem-resistant isolates, 67 (69.1%) showed the modified Hodge test (MHT) positive results. bla VIM and bla IMP were detected in 40 and 38 isolates respectively using multiplex PCR assay.
Conclusion:
This study determined a high prevalence of MDR and carbapenem resistance among Enterobacteriaceae, P. aeruginosa, and A. baumannii as detected by the presence of bla VIM and bla IMP genes. This study recommends the use of rapid and advanced diagnostic tools along with conventional phenotypic detection methods in the clinical settings for early detection and management of drug-resistant pathogens to improve treatment strategies.
Keywords: Carbapenems, Carbapenemase, Multidrug resistance, bla VIM , bla IMP
INTRODUCTION
Carbapenems are versatile β-lactam antibiotics having a broad-spectrum antibacterial activity. Carbapenems have been considered to be the most reliable last-resort treatment for infections caused by multidrug-resistant (MDR) pathogens (1). However, the rapid emergence of carbapenem-resistant Gram-negative bacteria pose a significant threat to public health (2) and has become a major public health concern worldwide over the last decade (3–5). WHO (2017) ranks carbapenem-resistant Enterobacteriaceae (CRE), carbapenem-resistant P. aeruginosa (CRPsA), and carbapenem-resistant A. baumannii (CRAB) in the highest priority category (i.e., critical) in the global priority list of pathogens. These bacteria are difficult to treat due to increased antibiotic resistance levels and high mortality rates (2). They have the potential for widespread transmission of resistance via mobile genetic elements (2). Carbapenem resistance in Gram-negative bacteria is the major contributing factor for multidrug-resistance (1). Most importantly, various mechanisms are predicted to continue the evolution of carbapenem-resistant Gram-negative nosocomial pathogens in the future (1, 6). If it continues at this pace, untreatable infections could emerge on a large scale, and the world may experience dramatic situations of the pre-antibiotic era (1).
The early recognition of CRE-CRAB-CRPsA and specifically required infection prevention and control (IPC) practices and procedures to prevent their occurrence effectively, appropriate antimicrobial therapy, and control their spread in acute health care facilities is much needed (2, 7). Identification of such carbapenemase producers can be made by both phenotypic and molecular-based techniques (8). Various non-molecular-based tests have been proposed to detect carbapenemase activity; however, each of them has certain limitations, and none of them is 100% sensitive or 100% specific (8). Modified Hodge test (MHT) is a simple and economical tool for the phenotypic detection of carbapenemase activity (9). MHT is first Clinical and Laboratory Standards Institute (CLSI) recommended growth-based and a conventional gold standard phenotypic biochemical testing method for carbapenemase detection (10). The MHT relies on the ability of carbapenemase producers to decrease the local concentration of carbapenem antibiotics, which enables the carbapenem-susceptible E. coli isolate to grow uninhibited around the streak line drawn from the carbapenem disk to the edge of the MHA plate, producing a cloverleaf appearance (11). The MHT is also sensitive to examining several carbapenemase activities, including VIM, IMP, and OXA-48-like enzymes (12). On the other hand, molecular techniques like RT-PCR remain the reference standard for the identification and differentiation of a specified set of carbapenemase genes with excellent sensitivity and specificity (8).
Variable prevalence of carbapenemase genes (from as low as 0.5% to as high as 100%) has been documented worldwide (13–20). In Nepal, studies show the prevalence of carbapenem resistance between 5% to 25% (21–24). In Nepal, the prevalence of carbapenemase genes among clinical bacterial isolates is largely under-reported as there are limited studies related to the detection and characterization of carbapenemases using both phenotypic and genotypic methods. It is essential to set up standard phenotypic and genotypic methods on antibiotic susceptibility tests to effectively screen drug-resistant pathogens in hospitals (25). Therefore, this paper reports the prevalence of carbapenemase genes among clinical bacterial isolates (Enterobacteriaceae, A. baumannii, and P. aeruginosa) in Nepal using both phenotypic and genotypic methods.
MATERIALS AND METHODS
Study design.
A cross-sectional study was conducted during a period of six months from June 2017 to January 2018. The sample size was calculated using Fischer’s formula. All patients (in-patients and outpatients) attending Annapurna Neurological Institute of Health and Allied Sciences who submitted properly collected and labelled clinical specimens were included, and improperly collected samples or those lacking proper labelling were excluded from the study. The samples included in this study were urine (n=384), stool (n=121), pus (n=152), blood (n=224), CSF (n=53), vaginal swab (n=19), wound swab (n=38), and catheter tip (n=160).
Isolation and identification of the bacterial isolates.
A total of 1,151 samples were collected and were processed following the standard microbiological techniques (26). Briefly, the specimens were cultured on nutrient agar, brain heart infusion (BHI) broth (blood samples), MacConkey agar, and blood agar. The isolates were identified based on colony morphology, Gram staining result, and conventional biochemical methods (27). Only the isolates belonging to Enterobacteriaceae, P. aeruginosa, and A. baumannii were further processed for antibiotic susceptibility testing (AST), MHT, and molecular typing.
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing (AST) was performed using the Kirby-Bauer disk diffusion technique (28). The antibiotics used included amikacin (30 μg), piperacillin/tazobactam (100/10 μg), nitrofurantoin (300 μg), ciprofloxacin (5 μg), azithromycin (15 μg), ceftazidime (30 μg), aztreonam (30 ug), amoxicillin (20 ug), imipenem (10 μg), meropenem (10 μg), ertapenem (10 ug), cotrimoxazole, cefoxitin (30 ug), polymyxin B (300 units), colistin (10 μg) and cefepime (30 μg). Ertapenem was used only for Enterobacteriaceae isolates. Nitrofurantoin was used only for isoaltes from urine, catheter tips and vaginal swabs. Colistin sulphate was used only for Pseudomonas isolates whereas polymyxin B was used only for the isolates which showed resistance to all the antibiotics used in the primary AST. The bacterial isolates showing resistance towards three or more different classes of antibiotics were reported as multidrug-resistant (MDR). Escherichia coli (ATCC 25922) was used as the control of the antimicrobial susceptibility testing.
Screening of carbapenemase producers.
For screening of possible carbapenemase producers, imipenem, meropenem, and ertapenem disks were incorporated in the primary in AST plate. The organisms resistant to any of these antibiotics were screened as potential carbapenemase producers and labelled as carbapenem-resistant isolates. The carbapenem-resistant isolates were subjected to further confirmatory tests.
Confirmation of carbapenemase activity.
MHT was used for the confirmation of carbapenemase activity (29). An overnight suspension of E. coli (ATCC 25922) adjusted to the turbidity of the 0.5 McFarland standard was inoculated evenly on the surface of the MHA plate containing 70 μg per ml of ZnSO4. ZnSO4 was used to increase the sensitivity of the test (29). After brief drying at room temperature, imipenem was placed on the center of the plate. The organisms that showed the carbapenem resistance previously during AST were stroked from the edge of the disk to the periphery of the plate and incubated overnight at 37°C aerobically. The presence of a clover leaf-shaped inhibition zone indicated the production of carbapenemase.
Molecular characterization of bla VIM and bla IMP producers.
All the MHT positive isolates were preserved in tryptic soy broth (TSB) containing 20% glycerol and stored at −20ºC until used. DNA was extracted by phenol-chloroform extraction technique. The DNA extracts were resuspended in Tris-EDTA (10 mM Tris-HCL, 0.10 mM EDTA, (pH 8.0) buffer and stored at 4°C for further analysis. Amplification of the DNA extracts was performed by multiplex PCR. The final volume (25 μl) of PCR mixture consisting of master mix (12.5 μl), forward primer (10 pM) (0.5 μl), reverse primer (10 pM) (0.5 μl), DNA template (4 μl), and ddH O (7.5 μl) was prepared. The master mix solution used for PCR was manufactured by Thermo Fisher Scientific (V.A. Graiciuno g.8 Vilnius, LT-02241 Lithuania). The primers used were: VIM-F, 5′-GATGGTGTTTGGTCGCATA-3′; VIM-R, 5′-CGAATGCGCAGCACCAG-3′ and IMP-F, 5′-GGAATAGAGTGGCTTAAYTC; IMP-R, 5′-GGTTTAAYAAAACAACAACC-3′. The PCR was done as described by Poirel et al. (30) using a thermocycler (Proflex, Thermo Fisher, USA) (initial denaturation at 94°C for 5 minutes, denaturation at 94°C for 45 seconds, annealing at 55°C for 30 seconds and extension at 72°C for 45 seconds with a final extension at 72°C for 10 minutes at the end of 35 cycles, followed by maintenance at 4°C). The amplified DNA was purified by the ethanol precipitation method.
Visualization of the PCR products.
The amplified PCR products were separated by agarose gel electrophoresis (1.5%) in 1×TAE buffer (0.04 Tris-acetate. 0.001 M EDTA, pH 8.0), stained with ethidium bromide, and visualized using the gel-doc system.
Quality control.
A standard aseptic procedure was adopted in this study. All batches of the culture media and chemical reagents were processed with aseptic techniques following CLSI guidelines. In AST and MHT, quality control was maintained using the control strains of E. coli ATCC 25922. During PCR, quality control was assured by E. coli isolates carrying both the genes under question. Blank (negative controls) were prepared without the DNA. All these controls were used in each batch of the PCR assay.
Data analysis.
SPSS v16.0 was used for statistical analysis. Chi-square test was applied at 95% CI among demographic variables.
RESULTS
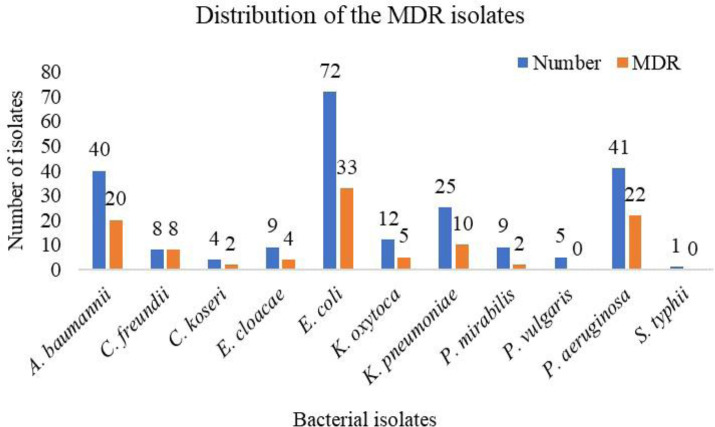
Out of 1,151 samples, 253 (22.0%) were culture positive and 226 (89.3%) were identified as members of Enterobacteriaceae, P. aeruginosa or A. baumannii (EPA). Of the 226 isolates, 116 (53.1%) were isolated from the male patients and 110 (48.7%) from the female patients. Highest culture positivity was seen in vaginal swab followed by wound swab. The highest number of isolates were obtained from the urine sample owing to the highest sample count of the urine sample. Among the total culture-positive isolates, E. coli (n=72, 28.5%) was the predominant organism followed by P. aeruginosa (n=41, 16.2%), A. baumannii (n=40, 15.8%), and others (Fig. 1).
Fig. 1.
Distribution of the MDR isolates
AST and carbapenem resistance.
The highest resistance was found against cefoxitin 66.8% (151/226), and the least resistance was observed against polymyxin B (19.5%; 8/41). Meropenem was found to be the most effective and ertapenem was found to be the least effective among the carbapenems. Ertapenem was used only against Enterobacteriaceae isolates, of which 35.2% (51/145) showed resistance to ertapenem. Besides the carbapenems, amikacin was found to be the most effective antibiotic. (Table 1). A total of 97 isolates were carbapenem resistant i.e. resistant to at least one of the crabapenem antibiotic used.
Table 1.
Antibiotic susceptibility pattern of growth positive organisms
| S No. | Antibiotics used | Resistant N (%) | Susceptible N (%) |
|---|---|---|---|
| 1 | Ciprofloxacin (n = 226) | 121 (53.5%) | 105 (46.5%) |
| 2 | Azithromycin (n =226) | 106 (46.9%) | 120 (53.1%) |
| 3 | Cotrimoxazole (n =226) | 109 (48.2%) | 117 (51.8%) |
| 4 | Amoxycillin (n =226) | 108 (47.8%) | 118 (52.2%) |
| 5 | Amikacin (n =226) | 88 (38.9%) | 138 (61.1%) |
| 6 | Cefoxitin (n =226) | 151 (66.8%) | 75 (33.2%) |
| 7 | Ceftazidime (n =226) | 132 (58.4%) | 94 (33.6%) |
| 8 | Cefepime (n =226) | 117 (51.8%) | 109 (48.2%) |
| 9 | Meropenem (n =226) | 70 (31.0%) | 156 (69.0%) |
| 10 | Imipenem (n =226) | 75 (33.2%) | 151 (66.8%) |
| 11 | Ertapenem (n =145) | 51 (35.17%) | 94 (64.83%) |
| 12 | Piperacillin/Tazobactam (n =226) | 98 (43.40%) | 128 (56.60%) |
| 13 | Aztreonam (n =226) | 108 (47.8%) | 118 (52.2%) |
| 14 | Nitrofurantoin (n =119) | 26 (21.85%) | 93 (78.15%) |
| 15 | Colistin (n =41) | 12 (29.27%) | 29 (70.63%) |
| 16 | Polymyxin B (n =41) | 8 (19.51%) | 33 (80.49%) |
Out of 226 isolates, 106 isolates were found to be MDR. Highest MDR was seen in C. freundii isolates (100.0%) and the least in P. mirabilis (Fig. 1). Among the carbapenem resistant isolates, 67 (69.1%) showed MHT positive results. MHT positivity was predominant in Citrobacter spp. (50.0%) and the least in Proteus spp. (7.14%) (Fig. 2). Among the 97 carbapenem resistant isolates, 88 (90.7%) were MDR, whereas 9 (9.3%) isolates were non-MDR. Among the MHT positive isolates, 63 (94.0%) isolates were MDR, and 4 isolates were non-MDR (Table 2). In this study, we found that MDR phenotype was significantly associated with MHT positive phenotype (p<0.001).
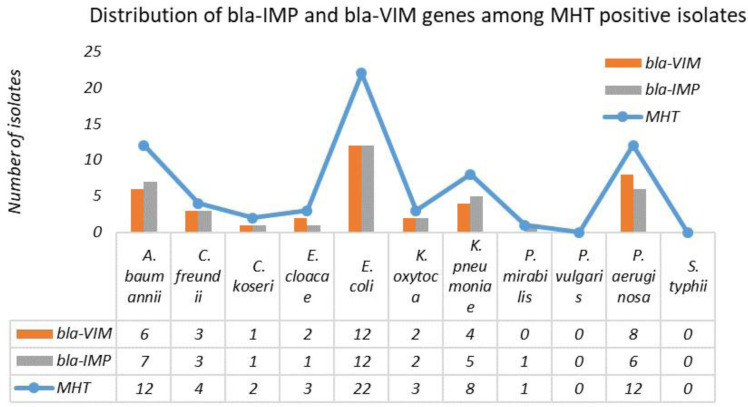
Fig. 2.
Distribution of bla -VIM and bla -IMP genes among MHT positive isolates
Table 2.
MDR and MHT positivity among carbapenem resistant isolates (n=97)
| MDR | MHT | Total | p-value | |
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| MDR | 25 (28.4%) | 63 (71.6%) | 88 | 0.001 |
| non-MDR | 5 (55.5%) | 4 (44.5%) | 9 | |
| Total | 30 (30.9%) | 67 (69.1%) | 97 | |
bla VIM and bla IMP genes.
Among the MHT positive isolates, 63 (88.1%) isolates showed the presence of either or both the genes. bla VIM gene was present in 40 (59.7%) and bla IMP gene was present in 38 (56.7)% of the MHT positive isolates. Among these, 19 isolates showed the presence of both genes. MDR phenotype was also significantly associated with the presence of bla VIM and bla IMP genes (p=0.005). All the isolates harboring either of both genes were MDR (Table 3, Figs. 3 and 4).
Table 3.
Relation between MHT, MDR and carbapenemase gene
| MHT | MDR | Carbapenemase gene | p-value | |
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| MHT positive (n = 67) | MDR (n = 63) non-MDR (n = 4) | 59 | 4 | 0.005 |
| 0 | 4 | |||
Fig. 3.
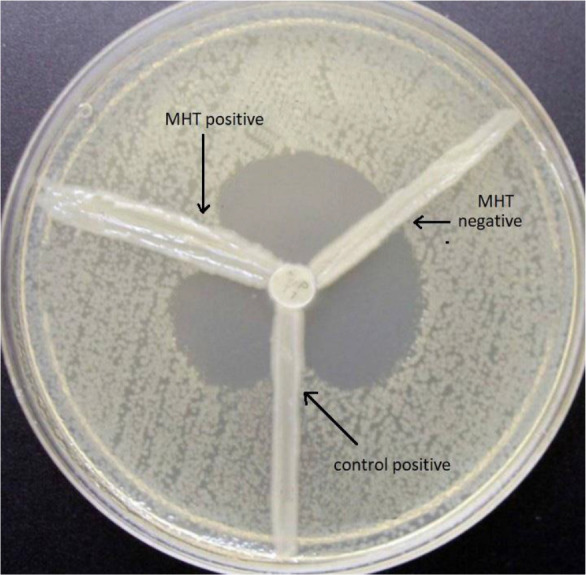
MHT test showing positive isolate, negative control and positive control (K. pneumoniae ATCC1705)
Fig. 4.
Agarose gel electrophoresis (1.5%) used for separation of PCR products. Lane 1: DNA ladder (1kb), Lane 2: positive control, Lane 3 and 6: VIM positive, Lane 5: IMP positive, Lane 4 and 7: both VIM and IMP positive, Lane 8: negative control
DISCUSSION
Carbapenems are β-lactam antibiotics with broad-spectrum activity and are used to treat infections known or suspected to be caused by MDR bacteria (1). The emergence and rapid spread of carbapenemases in Enterobacteriaceae, Pseudomonas, and Acinetobacter (EPA) species are becoming a significant public health crisis worldwide (2). Studies have shown an increasing trend of carbapenem resistance over the past decade (3).
In this study, a total of 1,151 samples were processed, out of which 253 (22.0%) samples were found to be culture positive. A slightly higher rate of growth positivity (27.4%) in the clinical samples was reported by Karn et al. (21). The prevalence of Gram-negative isolates in this study was 19.6% (n=226), while the one reported by Karn et al. (21) was 23.7%. In this study, the major isolates were E. coli, followed by P. aeruginosa, A. baumannii, and K. pneumoniae. Karn et al. (21) reported a much higher prevalence of E. coli (81.0%) among the Gram-negative isolates, followed by K. pneumoniae, A. baumannii, and C. freundii being the major isolates. In another similar type of study, Thapa et al. (31) reported 54.5% culture positivity for Gram-negative isoaltes, which included E. coli (54.9%), followed by Acinetobacter (16.0%) and P. aeruginosa (14.9%). The high prevalence of E. coli in these clinical specimens is not surprising as it is a normal flora of the human body and is highly opportunistic in immunocompromised patients (32).
Two-third of the isolates showed resistance to cefoxitin, whereas nearly one-fifth showed resistance to polymyxin B. Carbapenems and amikacin were were found to be effective antibiotics against the Gram-negative isolates. A total of 38.4% of Enterobacteriaceae isolates were found to be resistant to at least one of the carbapenem antibiotics used. Among the Enterobacteriaceae isolates, the resistance against carbapenem antibiotics was found to be 32.41% (imipenem), 28.96% (meropenem), and 35.17% (ertapenem). Slightly lower rates of resistance in Enterobacteriaceae have been reported worldwide (33–35). Higher resistance in our study might be related to the frequent irrational use of antibiotics in the country. Gupta et al. (23) reported 22.16% resistance to meropenem and 17.3% to imipenem. Karn et al. (21) reported the prevalence of carbapenem resistance among the bacteria belonging to Enterobacteriaceae ranging from 4.5% to 20.0%. Similarly, Gupta et al. (23) reported the prevalence of carbapenem resistance among Enterobacteriaceae varying from 17% to 22%. In this study, 67.5% A. baumannii isolates and 70.7% P. aeruginosa isolates were resistant to carbapenem antibiotics. For A. baumannii, 0–100% resistance has been reported in the South Asian region, 32.0–36.5% in North America, and 58.1–60.1% in Europe (36). For P. aeruginosa, 17.0–50.0% resistance rates have been reported in Asia-Pacific region (34), 0%–35.6% in Europe (35) and 10.3–19.4% in North America (33).
In this study, two-fifth of the isolates were found to be MDR. It is similar to the multidrug-resistance rate reported by Aryal et al. and Cai et al. (25, 33). Thapa et al. (31) reported slightly higher MDR than ours. However Shilpakar et al. (37) and Manandhar et al. (38) reported a much higher MDR than ours; 91.3% and 90.1% respectively. In this study, 44.1% of the members of Enterobacteriaceae were found to be MDR, which is slightly lower than that reported by Manandhar et al. (38). The highest MDR rate was seen in Citrobacter spp. (83.3%) followed by E. coli (45.8%) and others. Thapa et al. (31) reported a slightly lower MDR in Enterobactericeae (37.6%) and P. aeruginosa (44.4%) than ours. In a similar study, Mishra et al. (39) recorded MDR in 53.7% of total isolates. Mishra et al. (31) reported MDR in 95.0% of A. baumannii and 65.9% of P. aeruginosa, which is higher than our findings. Manandhar et al. (38) and Thapa et al. (31) reported a much higher prevalence of MDR in Acinetobacter than ours; 90.1% and 82.8% respectively. This study indicated a slighy increase in the level of MDR bacteria in Nepal.
In this study, 69.1% isolates were found to be MHT positive. The highest MHT positivity was observed in Citrobacter spp. (50.0%). A similar rate of MHT positivity was reported by Amjad et al. (9). A slightly lower MHT positive rate (63.6%) has been reported by Gurung et al. (40). We found a significant association between MDR and MHT positivity and between MDR and the presence of bla VIM and bla IMP genes. Manandhar et al. (39) also reported a significant association between MDR and the presence of ESBL genes. In this study, the carbapenemase genes were detected in 88% of the phenotypically confirmed isolates. The bla VIM was detected in 59.7%, and bla IMP was detected 56.7% of the total MHT positive isolates which is similar to the findings of Satir et al. (18). However, these findings were higher than those reported by Adam and Wafa (19). Kazemine-zhad et al. (20) reported a much lower prevalence of the bla VIM and bla IMP genes than ours. These differences in the prevalence of the carbapenemase genes might be due to the difference in distribution and dissemination in different geographical regions and the pattern of antibiotics used. This study showed a high prevalence of carbapenemase genes in the clinical isolates.
Only MHT was used as the phenotypic method for the detection of carbapenemase activity in this study. Although the MHT is inexpensive, relatively straightforward to perform, and uses readily available reagents (41), it has various limitations like the requirement of an overnight incubation, difficulty in interpretation, low specificity (approximately 91%) (10), and chances of false positivity in isolates producing ESBLs or AmpC cephalosporins in conjunction with porin mutations (42, 43). Genotypic detection of the carbapenemase gene is the gold standard, although it only detects a prespecified set of known carbapenemase genes and seems less suitable for the epidemiology of ESBL genes (44). Furthermore, other disadvantages like the high cost, the requirement for trained technicians, and the inability to detect novel carbapenemase genes (44) limit the use of molecular-based technologies for the detection of carbapenemase genes, especially in low-income countries like Nepal. Hence, the combination of phenotypic tests like MHT and genotypic detection by PCR can be a rapid and cost-effective solution for screening and detecting carbapenemase producers, developing antibiotic strategies, and preventing the dissemination of MDR pathogens (8, 9).
CONCLUSION
Our findings show a high prevalence of resistant genes (bla IMP and bla VIM ) and MDR among Gram-negative isolates, which is an alarming sign, calling for urgent intervention measures to control the growth and spread of these isolates. As there is a lack of a single definitive method, it is suggested to utilize several phenotypic detection methods aided with molecular methods to detect resistant isolates. In this study, PCR detected carbapenemase genes (bla IMP and bla VIM ) in about 88% of the phenotypically confirmed isolates. MHT positive or bla VIM and bla IMP positive isolates are likely to be multidrug-resistant.
This is among the limited study exploring bla VIM and bla IMP genes using both phenotypic and molecular tests among patients attending a tertiary health care center in Nepal. The findings of this study can inform the antimicrobial policy for tertiary care centers, including preparing the management of hospital infections, treatment, and diagnostic procedures. There are a few limitations of this study that includes investigation of limited carbapenemase genes, use of MHT only for confirmation of carbapenemase production, short duration of the study, and being conducted at a single tertiary care center. This study will be a valuable reference for future studies at multiple tertiary care centers exploring other carbapenemase genes in different settings/hospitals of Nepal.
ACKNOWLEDGEMENTS
We would like to acknowledge the laboratory staff and research participants of ANIAS for their help and support. We are equally grateful to all the colleagues to all the people who directly or indirectly helped in completing this work.
REFERENCES
- 1.Meletis G. Carbapenem resistance: an overview of the problem and future perspectives. Ther Adv Infect Dis 2016;3:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in health care facilities. World Health Organization. 2017. https://apps.who.int/iris/handle/10665/259462. Accessed 19 Aug 2019. [PubMed] [Google Scholar]
- 3.Nordmann P, Naas T, Poirel L. Global spread of carbap-enemase-producing Enterobacteriaceae. Emerg Infect Dis 2011;17:1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 2007;20:440–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornaglia G, Giamarellou H, Rossolini GM. Metallo-β-lactamases: a last frontier for beta-lactams? Lancet Infect Dis 2011;11:381–393. [DOI] [PubMed] [Google Scholar]
- 6.Meletis G, Chatzidimitriou D, Malisiovas N. Double-and multi-carbapenemase-producers: the excessively armored bacilli of the current decade. Eur J Clin Microbiol Infect Dis 2015;34:1487–1493. [DOI] [PubMed] [Google Scholar]
- 7.Birgy A, Bidet P, Genel N, Doit C, Decre D, Arlet G, et al. Phenotypic screening of carbapenemases and associated β-lactamases in carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 2012;50:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordmann P, Gniadkowski M, Gisk CG, Poirel L, Woodford N, Miriagou V, et al. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2012;18:432–438. [DOI] [PubMed] [Google Scholar]
- 9.Amjad A, Mirza IA, Abbasi SA, Farwa U, Malik N, Zia F. Modified Hodge test: a simple and effective test for detection of carbapenemase production. Iran J Microbiol 2011;3:189–193. [PMC free article] [PubMed] [Google Scholar]
- 10.Wayne PA. (2010). Clinical and Laboratory Standards Institute (CLSI). performance standards for antimicrobial susceptibility testing: 19th informational supplement. M100–S29.
- 11.Tamma PD, Simner PJ. Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J Clin Microbiol 2018;56(11):e01140–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutgring JD, Limbago BM. The problem of carbapenemase-producing-carbapenem-resistant-Enterobacteriaceae detection. J Clin Microbiol 2016;54:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control . Carbapenemase-producing bacteria in Europe: interim results from the European survey on carbapenemase-producing Enterobacteriaceae (EuSCAPE) project. Stockholm: ECDC; 2013. [Google Scholar]
- 14.Bahmani N. Detection of VIM-1, VIM-2 and IMP-1 metallo-β-lactamase genes in Klebsiella pneumoniae isolated from clinical samples in Sanandaj, Kurdistan, west of Iran. Iran J Microbiol 2019;11:225–231. [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail SJ, Mahmoud SS. First detection of New Delhi metallo-β-lactamases variants (NDM-1, NDM-2) among Pseudomonas aeruginosa isolated from Iraqi hospitals. Iran J Microbiol 2018;10:98–103. [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Sun QL, Shen Y, Zhang Y, Yang JW, Shu LB, et al. Rapid increase in prevalence of carbapenem-resistant Enterobacteriaceae (CRE) and emergence of colistin resistance gene mcr-1 in CRE in a hospital in Henan, China. J Clin Microbiol 2018;56(4):e01932–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savard P, Perl TM. A call for action: managing the emergence of multidrug-resistant Enterobacteriaceae in the acute care settings. Curr Opin Infect Dis 2012;25:371–377. [DOI] [PubMed] [Google Scholar]
- 18.Mirbagheri SZ, Meshkat Z, Naderinasab M, Rostami S, Nabavinia MS, Rahmati M. Study on imipenem resistance and prevalence of blaVIM1 and blaVIM2 metallo-beta lactamases among clinical isolates of Pseudomonas aeruginosa from Mashhad, northeast of Iran. Iran J Microbiol 2015;7:72–78. [PMC free article] [PubMed] [Google Scholar]
- 19.Adam MA, Elhag WI. Prevalence of metallo-β-lactamase acquired genes among carbapenems susceptible and resistant gram-negative clinical isolates using multiplex PCR, Khartoum hospitals, Khartoum Sudan. BMC Infect Dis 2018;18:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazeminezhad B, Rad AB, Gharib A, Zahedifard S. BlaVIM and blaIMP genes detection in isolates of carbapenem-resistant P. aeruginosa of hospitalized patients in two hospitals in Iran. Iran J Pathol 2017;12:392–396. [PMC free article] [PubMed] [Google Scholar]
- 21.Pokharel K, Dawadi BR, Bhatt CP, Gupte S, Jha B. Resistance pattern of carbapenem on Enterobacteriaceae. JNMA J Nepal Med Assoc 2018;56:931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bora A, Sanjana R, Jha BK, Mahaseth SN, Pokharel K. Incidence of metallo-beta-lactamase producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in central Nepal. BMC Res Notes 2014;7:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta E, Mohanty S, Sood S, Dhawan B, Das BK, Kapil A. Emerging resistance to carbapenems in a tertiary care hospital in north India. Indian J Med Res 2006;124:95–98. [PubMed] [Google Scholar]
- 24.Devkota SP, Paudel A, Bhatta DR, Gurung K. Carbapenemase among clinical bacterial isolates in Nepal. J Nepal Health Res Counc 2020;18:159–165. [DOI] [PubMed] [Google Scholar]
- 25.Aryal SC, Upreti MK, Sah AK, Ansari M, Nepal K, Dhungel B, et al. Plasmid-mediated AmpC β-lactamase CITM and DHAM genes among gram-negative clinical isolates. Infect Drug Resist 2020;13:4249–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheesbrough M. (2006).District laboratory practice in tropical countries, part II. 2nd ed. Cambridge University Press. Cambridge. pp. 35–60. [Google Scholar]
- 27.Cheesbrough M. (2006). District laboratory practice in tropical countries, part II. 2nd ed. Cambridge University Press. Cambridge. pp. 62–70. [Google Scholar]
- 28.Wayne PA. (2013). Clinical and Laboratory Standards Institute (CLSI). performance standards for antimicrobial susceptibility testing: 23rd informational supplement. M100–S23. [Google Scholar]
- 29.Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol 2003;41:4623–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for the detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 2011;70:119–123. [DOI] [PubMed] [Google Scholar]
- 31.Thapa P, Bhandari D, Shrestha D, Parajuili H, Chaudhary P, Amatya J, et al. A hospital based surveillance of metallo-beta-lactamase producing gram negative bacteria in Nepal by imipenem-EDTA disk method. BMC Res Notes 2017;10:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price LB, Hungate BA, Koch BJ, Davis GS, Liu CM. Colonizing opportunistic pathogens (COPs): the beasts in all of us. PLoS Pathog 2017;13(8):e1006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai B, Echols R, Magee G, Ferreira JCA, Morgan G, Ariyasu M, et al. Prevalence of carbapenem-resistant gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 2017;4:ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendes RE, Mendoza M, Banga Singh KK, Castanheira M, Bell JM, Turnidge JD, et al. Regional resistance surveillance program results for 12 Asia-Pacific nations (2011). Antimicrob Agents Chemother 2013;57:5721–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostyanev T, Vilken T, Lammens C, Timbermont L, Van’t Veen A, Goossens H. Detection and prevalence of carbapenem-resistant gram-negative bacteria among European laboratories in the COMBACTE network: a COMBACTE lab-net survey. Int J Antimicrob Agents 2019;53:268–274. [DOI] [PubMed] [Google Scholar]
- 36.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. Antimicrobial susceptibility of Acinetobacter calcoaceticus–Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY antimicrobial surveillance program (1997–2016). Open Forum Infect Dis 2019;6(Suppl 1):S34–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shilpakar A, Ansari M, Rai KR, Rai G, Rai SK. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase producing gram-negative isolates from clinical samples in a tertiary care hospital of Nepal. Trop Med Health 2021;49:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manandhar S, Zellweger RM, Maharjan N, Dangol S, Prajapati KG, Thwaites G, et al. A high prevalence of multi-drug resistant ram-negative bacilli in a Nepali tertiary care hospital and associated widespread distribution of extended-spectrum beta-lactamase (ESBL) and carbapenemase-encoding genes. Ann Clin Microbiol Antimicrob 2020;19:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra SK, Acharya J, Kattel HP, Koirala J, Rijal BP, Pokhrel BM. Metallo-beta-lactamase producing gram-negative bacterial isolates. J Nepal Health Res Counc 2012;10:208–213. [PubMed] [Google Scholar]
- 40.Gurung S, Kafle S, Dhungel B, Adhikari N, Thapa Shrestha U, Adhikari B, et al. Detection of OXA-48 gene in carbapenem-resistant Escherichia coli and Klebsiella pneumoniae from urine samples. Infect Drug Resist 2020;13:2311–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasteran F, Mendez T, Rapoport M, Guerriero L, Corso A. Controlling false-positive results obtained with the Hodge and Masuda assays for detection of class a carbapenemase in species of Enterobacteriaceae by incorporating boronic acid. J Clin Microbiol 2010;48:1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilrich D, Poirel L, Nordmann P. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol 2012;50:477–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carvalhaes CG, Picao RC, Nicoletti AG, Xavier DE, Gales AC. Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: be aware of false positive results. J Antimicrob Chemother 2010;65:249–251. [DOI] [PubMed] [Google Scholar]
- 44.Stuart JC, Voets G, Scharringa J, Fluit AC, Leverstein-Van Hall MA. Detection of carbapenemase-producing Enterobacteriaceae with a commercial DNA microarray. J Med Microbiol 2012;61:809–812. [DOI] [PubMed] [Google Scholar]