Abstract
Objective
The loss of neural ability leading to subsequent diminishing of motor function and the impairment below the location of the injury is a result of the SCI (Spinal Cord Injury). Among the many therapeutic agents for SCI, the exosomes considered as extracellular vesicles seem to be the most promising. Sonic Hedgehog (Shh) is an exosome-carrying protein. This Study's purpose was to identify whether Shh is required for exosomes from BMSCs (mesenchymal stem cells of the bone) and plays a protective effect on SCI.
Methods
Spinal cord injection with shRNA Shh-adeno associated virus (sh-Shh-AAV) were used to silence Shh. Exosomes were extracted from BMSCs. Rats that had suffered SCI were given intravenous injections of exosomes through the veins of the tail. Immunohistochemistry was used to identify the expression of Shh glycoprotein molecule as well as the expression of Gli-1 (glioma-associated oncogene homolog 1) in the rat spinal cord tissues. Western blot was performed to measure the levels of growth associated protein-43 (GAP-43). The BBB (Basso Beattie Bresnahan) score was used to assess the motor functions of the hind legs. In the same manner, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling or TUNEL and Nissl Staining was deployed to assess the level of regeneration of neurons and assess the level of histopathological damage in the tissues of the Spinal Cord.
Results
In the case of the rats with SCI, the levels of display of Gli-1 and Shh showed dramatic improvement after the BMSCs exosome injections. In comparison to rats with SCI, the subjects of BMSCs exosomes group showed an improvement in their SCI, including a higher BBB score and Nissl body count, increasing GAP-43 expression, along with a much-decreased number of cells that suffered apoptosis. While the exosome effect on Spinal Cord Injury was completely ineffective in rats that had Shh silencing.
Conclusions
Exosomes secreted from BMSCs showed great effectiveness in the SCI healing with a vital involvement of Shh in this repair.
Keywords: Sonic hedgehog, Exosomes, Spinal cord injury, BMSCs
1. Introduction
Sensory discernment and neurological abilities are compromised due to axon demyelination and neural cell apoptosis on account of mechanical damage of the spinal cord [1]. Compared to individuals who have moderate injuries, patients with severe Spinal Cord Injuries (SCI) suffer from total paraplegia that is irreversible and become a severe economic as well as a psychological burden [2]. Clinically, the treatment of SCI is an extremely uphill task owing mainly to the poor regenerative capacity of neurons. Hence, we see a number of studies being devoted to the replacement and regeneration of neurons [3,4].
Recently, the research of stem cell transplantation in SCI therapies has been increasingly deepened, showing a good application prospect [5,6]. The mesenchymal stem cells of the bone marrow (BMSCs) are a kind of multi-potential stem cells with self-renewal ability [7]. It was previously assumed that the system of transplantation of stem cells to promote repair lies in homing, and in finding the replacement for tissue that has been damaged after identifying the differentiating factors. But the current premise is that the transplanted cells are more important for repair, specifically, their secretion of vesicles of an extracellular nature [8,9]. Exosomes are a kind of extracellular vesicles with a diameter of 40–100 nm. Various cells can secrete exosomes, and the exosomes that carry the lipids and the nucleic acids through the medium of proteins help regulate the biological activities [10]. BMSCs-derived exosomes can not only simulate most biological functions, but also have the advantages of small size, less ability to block microvessels, no proliferation ability, and lower risk of tumor induction compared with BMSCs [11]. Gu et al. [12] showed that BMSC exosomes can weaken the apoptosis of the neurons and, therefore, enhance the recovery of functional behavior in SCI. Therefore, exosomes have more advantage therapeutically and are going to witness these as a replacement for stem cell treatment for SCI.
When it comes to post-surgery regeneration of nerve endings, Shh or Sonic Hedgehog signaling pathway is recommended to play an important role [13,14]. When Ptch (Patched) and Shh are bound at the receptor, relieving its repressive activity on the Smo (Smoothened G-Protein), thereby propagating the commencement of Gli-1 (Giloma based oncogene homolog 1). The target gene expression vital for cell differentiation, cell survival and growth involves Gli-1 activation [15]. Earlier work showed that Shh, in the case of patients who are diabetic and in the case of prostatectomy patients, has an impressive clinical application potential [16]. Besides, activated Shh signaling pathway was found to promote recovery from SCI in a rat model [17]. Our prior study found that Shh-overexpressing BMSC-derived exosomes can facilitate SCI repair in rats [18]. However, whether Shh is necessary for BMSC-derived exosomes to augment SCI repair is yet to be studied. Shh effects and the secreted exosomes from the BMSCs’ impact on SCI were studied and evaluated in this paper. The results of this study will lay forth a valuable theoretical clinical foundation to help in the treatment and the guidance of SCI.
2. Materials and methods
2.1. Animal details
Fifty male rats of Sprague–Dawley variety with weights ranging from 230 to 250 g were procured from Jiesijie experimental animal company (Shanghai, China). Pathogen-free animal rooms at 22–26 °C with 40–60% humidity was made available specifically for these rats and they were normally housed here. All rats were given free access towater and feed.
The Guizhou Provincial People's Hospital (Guiyang, China) governed protocols as derived from their ethics committee were implemented for the conduction of all animal experiments, and the approval number was 2019–016.
2.2. Isolation and culture of BMSCs
As per the earlier assessment and explanation, the Bone marrow Stromal Cells were kept isolated and culture was performed [19]. Pentobarbital (1%) with a set dosage of eighty milligrams per kilogram was deployed as an intraperitoneal injection as anesthesia in a set of 3-week-old rats composed of a weight range of eighty to a hundred grams. The tibia and the femur of the rats were exposed by separating the surface muscles and the fascia, in the rats, subsequent to being disinfected for 10 min with 75% alcohol. Hanks solution was used to rinse thereby obtaining the bone marrow. One seventy grams of BMSCs was centrifuged at 4 °C for 5 min and the Hanks solution was used to mix evenly the BMSCs at the end of the centrifugation. This was then succeeded by a further centrifuge at 4 °C with 1050 g for 5 min. Dulbecco's Modified Eagle's Medium from Gibco (USA) was used to suspend the BMSCs. The modified Eagle's Medium contained 1% penicillin/streptomycin form Gibco and ten percent fetal bovine serum Hyclone (GE Healthcare Sciences). Using a sterile culture flask, the BMSCs were then seeded in an incubator maintained at 37 °C at 5% CO2 levels. Every two days the medium was replaced. After progressing at the logarithmic growth phase for 3 generations, the subsequent assays entailed the use of these BMSCs.
2.3. Surface marker discovery in BMSCs
The surface markers of BMSCs were analyzed employing FACS (fluorescence-activated cell sorting) analysis [20]. The cells were suspended again in phosphate buffered saline (PBS) at 105 cells/mL after Tripsinizing (in 0.25% Trypsin) and rinsing. Incubation of these cells with 5 mL of antibodies of fluorescein isothiocyanate-labeled CD34, CD44, CD45, and CD90 was done in the dark at 4 °C for 30 min. All antibodies were purchased from Invitrogen (USA). A flow cytometer (FACScan, BD Biosciences) was employed for analysis following a double washing of the cells in PBS after which data analyses were done by FlowJo (BD Biosciences).
2.4. Isolation and identification of exosomes
Extraction of the exosomes from BMSCs culture media was performed using ExoQuick-TC kit (SBI, USA) by following the prescribed protocol. A transmission electron microscope (Libra 120; Zeiss, Oberkochen, Germany) was employed to observe the exosomal ultrastructure. The antibodies against CD9, TSG101 and CD63 (Abcam, UK) are the representative exosome markers that were identified using western blotting.
2.5. Generation of adeno associated virus (AAV)-expressing shh shRNA
The plasmid with predesigned shRNA sequence that targets the rat Shh gene (sh-Shh: 5′-CCCGACATCATATTTAAGGAT-3′), along with a plasmid containing nonspecific shRNA sequences (sh-NC: 5′-GACGACAGTGTCACTCTTAGG-3′) were constructed by Vigenebio (Shandong, China). The sh-Shh plasmid was packaged into an AAV9 (adeno-associated virus serotype 9) vector to create shRNA Shh-AAV (1 × 1012 pfu/mL). sh–NC–AAV (1 × 1012 pfu/mL) served as the control.
2.6. Spinal cord injection of AAV
An injection was administered using a Hamilton syringe of 30 gauge. Ten microliters sh–NC–AAV or sh-Shh-AAV at rate of 2 μL per min were injected into the exposed area of the spinal cord. The wound was sutured after slowly disengaging the injection from the site of the wound. After 72 h, western blotting was done using the resected spinal cord from the euthanized rats to find out if sh-Shh-AAV injection had the desired silencing effect.
2.7. Rat model of spinal cord injury
The rats were first anesthetized using a pentobarbital sodium dosage of 50 mg per kg of a 1 percentile strength. An incision of 2–3 cm was made in the midline of the back after positioning the disinfected rats on the operating table. After the incisions were made and the muscles were separated, the vertebrae were exposed. The T10 spinal cord was exposed after detaching the lamina and the spinous processes while at the same time totally preserving the dura mater. A standard striking object was used with 2 N force to strike a blow, and the wound was washed and then stitched up using penicillin saline. Blood stasis forming rapidly at the site of the wound on the spinal cord along with fast contractions and lower limb tremors indicated that the model was successfully established. In the case of sham-operated controls, anesthetized rats were given a neural plate fracture with an openly viewable spinal cord with no treatment given.
2.8. Groups and spinal cord tissue collection
A 4-group random division was conducted among the Spinal Cord Injury rats (n = 10/group): SCI, SCI + BMSCs exosomes (SCI + Exo), SCI + BMSCs exosomes + sh–NC–AAV (SCI + Exo/sh-NC), and SCI + BMSCs exosomes + sh-Shh-AAV (SCI + Exo/sh-Shh). After spinal cord injection of sh–NC–AAV or sh-Shh-AAV for 72 h, SCI modeling was conducted. Sixty min post-SCI, an intravenous administration of 200 μg/mL of BMSCs exosomes (200 μL) was performed through the tail vein. A total of three injections were given once every two days. Physiological saline injected rats formed the SCI control group. A penicillin dosage of 2 × 105 U/kg once a day for three days was given as an injection, following the saline injection; urinary assistance was facilitated three times a day until normal urination was restored. On the 28th day following an evaluation of the motor functions, the rats were euthanized, followed by dissection of spinal cord tissue samples of sham group and the four groups and subsequent processing for either liquid nitrogen-based storage or preparation of paraffin-embedded section.
2.9. Motor function assessment
The locomotor ability of the rats was assessed using the BBB (The Basso Beanie Bresnahan) score [21]. Independent researchers (n = 2) blinded to the study analyzed the data. Testing was done on 1, 3, 7, 14, and 28 days post-surgery and the BBB scores were recorded.
2.10. Nissl body staining
The sections were infiltrated with a 1:1 solution of anhydrous ethanol: chloroform overnight at room temperature followed by subsequent treatments with 100% absolute ethanol and 95% alcohol, and rinsing with thrice distilled water. Pre-warmed tar purple was used to stain the separated sections (0.1%, pH = 3) at 37 °C for 10 min followed by a rinse in thrice distilled water. Subsequently, differentiated was done with 95% alcohol for 5 min followed by dehydration in 100% anhydrous ethanol and xylene for 5 min. In the end, observation was conducted after the various sections were mounted under a microscope. The impact of each of the treatment factor on the motor function was analyzed by randomly selecting motor neurons from the anterior horn of the five sites where the staining was conducted.
2.11. TUNEL staining
Sections (10 μm) of the rat spinal cords were utilized for TUNEL assays in accordance with the prescribed protocol of the manufacturer (Roche molecular Biochemicals Inc., Mannheim, Germany). Proteinase K and 0.3% H2O2 were used to pretreat the sections briefly, following which, incubation was done with terminal deoxynucleotidyl transferase for 1 h at 37 °C. Subsequently, peroxidase-conjugated antibody was added for 30 min after which the DAB substrate kit (Vector Laboratories, INC, Burlingame, CA) was employed for = color development. TUNEL positive cells displayed a brown staining in the nucleus. An observer (blinded to the work) enumerated the TUNEL-positive cells in 3 non-overlapping microscopic fields, and results are expressed as a percentage of total cells.
2.12. Immunohistochemistry
A four-percentile methyl alcohol solution was used to fix the spinal cords and they were embedded in paraffin, then dewaxed and washed in PBS. Subsequent to the dewaxing, the sections were placed in a boiling buffer (citrate buffer; pH = 6.0) for 10 min. This was followed by a 15-min treatment with 3% H2O2 and subsequent blocking of each section in 10% (v/v) bovine serum albumin in PBS for 1 h. Following this, an overnight incubation with a 1: 500 dilution of the primary antibody against Shh or Gli-1 (ProteinTech, Wuhan, China) was done at 4 °C. Washing followed by a 60-min incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody in PBS was done the next day followed by development in DAB and counterstaining with hematoxylin. A light microscope (Olympus, Tokyo, Japan) was employed for imaging followed by assessment by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.13. Western blot analysis
Proteins from exosomes and rat spinal cords tissues were extracted using lysates. The proteins were quantitated in each sample employing the bicinchoninic acid (BCA) assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). Following the resolution of forty micrograms of total protein on 10% SDS-PAGE, transfer was done to nitrocellulose membranes (Millipore, Jaffrey, NH, USA). This was followed by a 60-min blocking treatment in 5% nonfat milk and subsequent overnight incubation in the following Abcam primary antibodies (1:500) of CD9, CD63, TSG (1:2000) of β-actin and 1:1000 of Shh and growth associated protein-43 (GAP-43) (ProteinTech) at 4 °C. This was followed by a 60-min incubation with anti-rabbit IgG at room temperature post-washing the membranes. Signal detection was done employing an enhanced chemiluminescence (ECL) detection system (Thermo Scientific, Rockford, IL, USA). β-actin was utilized to normalize Shh and GAP-43 relative protein levels and ImageJ software (National Institutes of Health) was employed for densitometric analysis of the bands.
2.14. Statistical analysis
The Graph Prism Program, Version 7.0 (GraphPad, San Diego, CA, USA) was employed for all analyses. The data were expressed as mean ± standard deviation (SD). The t-test was employed for comparing two groups, while the analysis was corrected for multiple comparisons between more than two groups. The Mann–Whitney U test was employed to quantify the BBB scores. Statistical significance was at P < 0.05.
3. Results
3.1. Preparation of BMSCs-derived exosomes
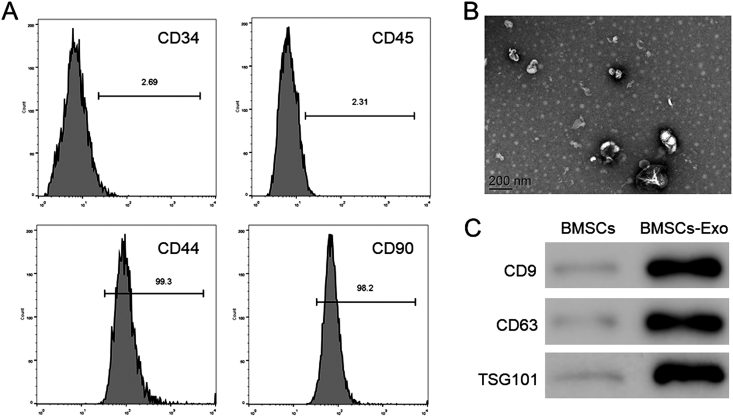
Following density gradient centrifugation-based isolation of BMSCs, the identity of third passage cells was corroborated by FACS. CD44, and CD90 was detected in more than 95% of BMSCs while the cells were negative for CD34 or CD45 (Fig. 1A), that is in line with the features of these cells. Subsequently, exosomes were isolated from BMSCs and assessed by transmission electron microscopy and western blotting. Electron micrographs showed a spheroid morphology of the collected products (Fig. 1B). The expression of CD9, CD63, and TSG101 in exosomes that are specific exosomal markers was revealed by western blotting, while they were almost undetectable in BMSCs (Fig. 1C).
Fig. 1.
Identification of bone marrow mesenchymal stem cells (BMSCs) and BMSCs exosomes. (A) Analyses of CD34, CD45, CD44, and CD90 markers by fluorescence-activated cell sorting (FACS) analysis of cell surface (B) Transmission electron microscopy of exosomes from BMSCs. Scale bars = 200 nm (C) Western blotting for the exosomal markers CD9, CD63, and TSG101.
3.2. Effect of shh expression on BMSCs exosomes in SCI treatment
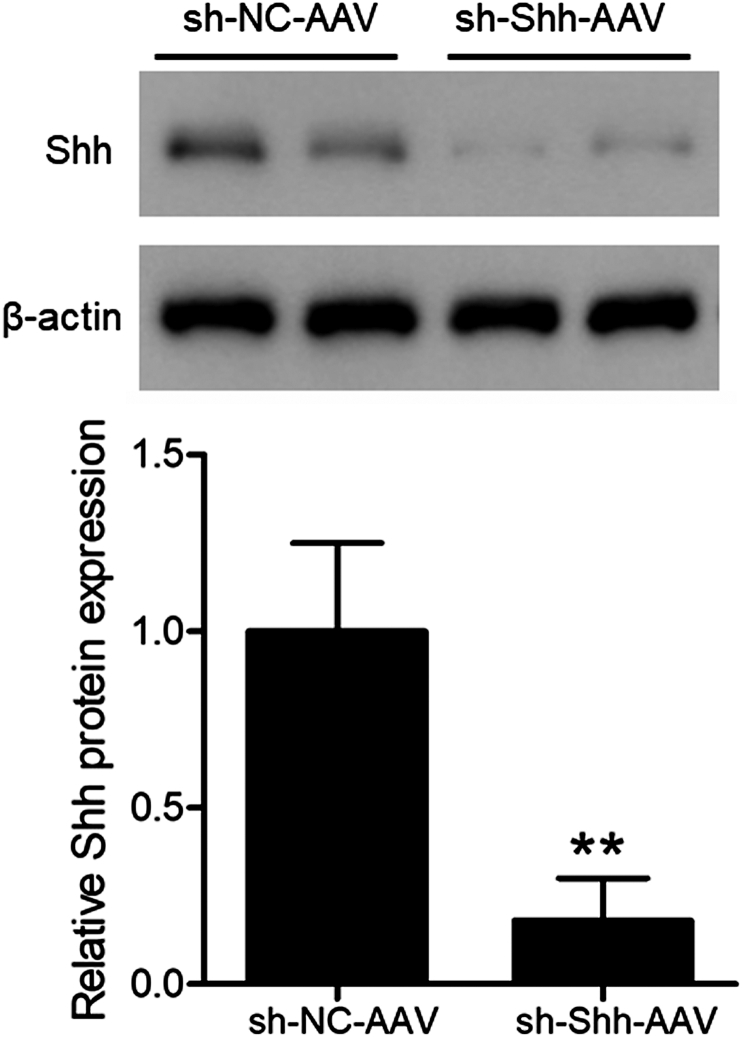
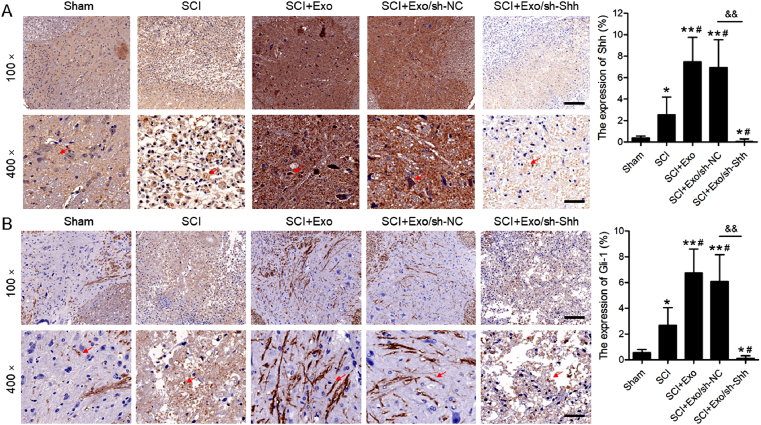
To determine the effectiveness of shRNA Shh-AAV in the spinal cord tissues, western blotting was performed. The shRNA group exhibited evidently lower levels of Shh against the control (Fig. 2). Therefore, the efficacy of shRNA Shh-AAV was validated, and this provided a strong foundation for the following experiments. To determine the Shh role to BMSCs Exo protective effects against SCI, exosomes derived from BMSCs were injected into SCI model rats injected with sh–NC–AAV or sh-Shh-AAV. Twenty-eight days post-operation, the immunohistochemical analyses of Shh and Gli-1 expression in spinal cord tissues was done. The SCI group displayed a conspicuously elevated level of Shh vs. that of the sham group (Fig. 3A; P < 0.05). Moreover, the Shh protein levels were relatively elevated in the SCI rats following the BMSC-derived exosomal injection (SCI + Exo, P < 0.05) group compared to the SCI control group. However, the Shh levels exhibited lower in shRNA Shh-AAV injection SCI rats with exosomes treatment (SCI + Exo/sh-Shh) than that in shRNA NC-AAV injected SCI rats (SCI + Exo/sh-NC). Moreover, the expression trend of Gli-1 (Fig. 3B) was consistent with that of Shh across all specimens.
Fig. 2.
Identification of silencing effect after sh-Shh-AAV injection on Shh Expression in spinal cord tissues. Western blots for Shh in specimens after spinal cord injection of sh–NC–AAV or sh-Shh-AAV for three days, and quantitative analysis of Shh by t-test. ∗∗P < 0.01 versus sh–NC–AAV group.
Fig. 3.
Effects of shRNA-Shh-AAV on Shh and Gli-1 Expression in spinal cord tissues. Immunohistochemical analyses of Shh (A) and Gli-1 (B) expression in specimens of sham and SCI rats 28 days post-operation. Red arrows indicate positive expression. Scale bars = 200 μm or 50 μm. N = 5/group. ∗P < 0.05, ∗∗P < 0.01 versus sham group. #P < 0.05 versus SCI group. &&P < 0.01 versus as indicated.
3.3. Effects of Shh-knockdown on BMSCs exosomes promoted neuronal regeneration in SCI rats
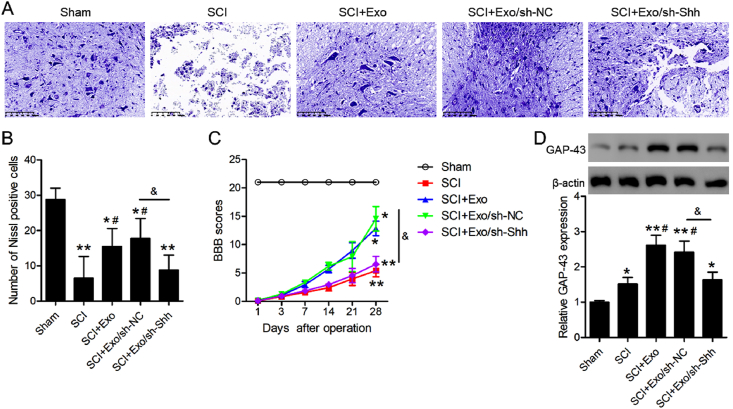
To further investigate Shh-knockdown to the neuroprotective effects of BMSCs exosomes in the five groups, the Nissl bodies in the spinal cord anterior horn were observed by Nissl staining (Fig. 4A). The SCI group demonstrated a conspicuously lower number of these bodies vs. that of the sham group. Conversely, this number increased significantly after BMSCs exosomes injection in SCI rats with or without shRNA NC-AAV vs. the SCI group, while the BMSCs exosomes did not work in the SCI rats injected with shRNA Shh-AAV (Fig. 4B). Furthermore, the SCI group demonstrated a conspicuous lowering of the BBB score vs. the sham group and BMSCs exosomes injection partly upregulated the BBB score. Importantly, the BBB scores were significantly lower in the sh-Shh AAV injected SCI rats injected with BMSCs exosomes than that in the sh-NC injected SCI rats 28-days post-operation (Fig. 4C). We also measured the levels of GAP-43, which is related to neurogenesis. As shown in Fig. 4D, the GAP-43 expression in the spinal cord tissue of the SCI rats was obviously higher compared to that in the sham group. The administration of BMSCs exosomes increased GAP-43 protein expression when compared to that in SCI rats (P < 0.05), while Shh-knockdown inhibited the increase in GAP-43 protein expression. This result indicated that BMSCs exosomes induce GAP-43 protein levels, thus promoting neuronal growth in SCI rats depending on the presence of Shh.
Fig. 4.
Effects of shRNA-Shh-AAV on BMSCs exosomes promoted neuronal regeneration of SCI rats. (A) Nissl staining to detect Nissl bodies in specimens of sham and SCI rats at 28 days post-operation. Scale bars = 100 μm (B) Nissl positive cells in the samples are quantified in the bar graph (C) The BBB scores at the indicated time points following the operation (D) Western blot analysis of GAP-43 protein and its expression relative to β-actin is presented by histogram. N = 5/group. ∗P < 0.05, ∗∗P < 0.01 versus sham group. #P < 0.05 versus SCI group. &P < 0.05 versus as indicated.
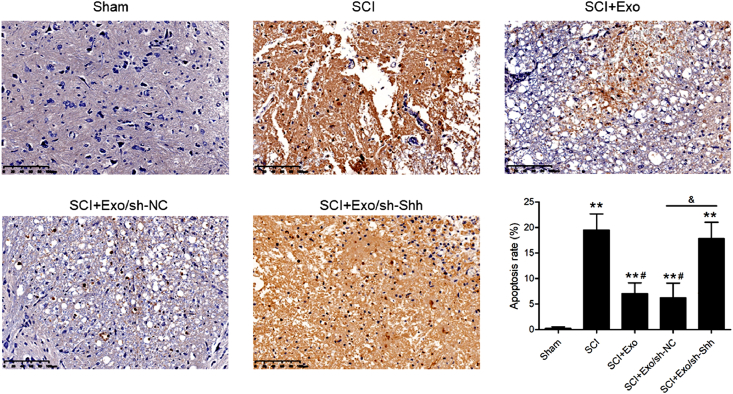
3.4. Shh-knockdown on BMSCs exosomes inhibited apoptosis in SCI rat spinal cord tissues
The SCI group demonstrated a conspicuously higher number of TUNEL-positive cells vs. the sham (Fig. 5), and the BMSCs exosomes administration in SCI rats with or without shRNA NC-AAV injection significantly decreased this number compared to the SCI group. Whereas, the decrease in TUNEL-positive cells no longer appeared in the SCI rats injected with shRNA Shh-AAV although BMSCs exosomes injections was also used.
Fig. 5.
Effects of Shh-knockdown on BMSCs exosome-inhibited apoptosis in spinal cord tissues of SCI rats. Representative images of TUNEL staining in sham and SCI rats at 28 days post-operation. Scale bars = 100 μm. Quantification of TUNEL-positive cells. N = 5/group. ∗∗P < 0.01 versus sham group. #P < 0.05 versus SCI group. &P < 0.05 versus as indicated.
4. Discussion
Globally, SCI has at all times presented a challenge frequently leading to calamitous limb dysfunction and paralysis. This majorly entails the following two pathological processes, primary injury and secondary injury. The biggest obstacle to repair after SCI is the neuronal death caused by secondary injury [22]. In this study, we demonstrated that BMSCs-derived exosomes had protective effects in rat SCI models, and their mechanisms included Shh signaling activation, that improved neural function recovery, and promoted neuronal survival.
Shh is a member of the Hedgehog signaling family, and the Hedgehog signaling pathway also includes transmembrane protein receptor Ptch, G-protein coupled receptor Smo, nuclear transcription factor Gli, etc., are vitally involved in mammalian embryonic development and organ formation, such as cell phenotypic induction, limb growth and development, and neural tube directional differentiation [23]. Zhang et al. [17] found that lentivirus-mediated PTC1 and PTC2 gene silencing promoted the recovery of SCI in rats by increasing Shh expression and activating the Hedgehog signaling pathway. Our earlier report showed that Shh can promote neuronal survival [24]. Many diseases including SCI have been targeted using the novel exosomes that serve in communication across cells to deliver nucleic acids or proteins as systemic or local vehicles [25]. Zhang et al. [26] found that human placenta-derived mesenchymal stem cell derived exosomes support angiogenesis in SCI to augment neurologic function. These studies suggested that Shh upregulation or mesenchymal stem cell-derived exosome treatment is the hope for SCI repair.
In this work, we probed how transferring BMSC-derived exosomes influence a rat-SCI model with or without Shh-knockdown. Our current study indicates that Shh expression in spinalcord tissues was observably upregulated in SCI rats compared to the sham-operated rats that is in line with an earlier study [27]. Post-exosome administration, the levels of Shh and Gli-1 in spinal cord tissues were further significantly increased at the 28th post-SCI against that in only SCI rats. However, Shh-knockdown inhibited the Shh and Gli-1 expression although exosome therapy was also used. These findings are demonstrative of the putative vital involvement of Shh and Gli-1 in BMSCs therapy after SCI in rats.
Nissl bodies can be used as a marker of the functional state of neurons [28]. We observed that most Nissl bodies could be found in the sham rats and significantly reduced in the SCI rats. However, SCI rats with BMSCs exosomes injection could partly increase the number of Nissl bodies, while BMSCs exosomes did not work in the Shh-knockdown SCI rats while the BBB score changed in rats post-SCI thus corroborating the role of these exosomes in augmenting neural regeneration with a critical role of Shh in SCI. GAP-43, a neuronal phosphoprotein, plays an essential role in nerve growth [29]. In the current study, we identified up-regulation of GAP-43 after SCI as a response by spinal cord tissue for neuro-protection. Further, BMSCs exosomes treatment further enhanced the GAP-43 expression, while Shh-knockdown abolished this enhanced effect of BMSCs exosomes for GAP-43, indicating the indispensable role of Shh in the treatment for SCI using exosomes. Consistent with our results, a previous study revealed elevated GAP-43 in the spinal cord after SCI injury, and magnetic stimulation treatment further increased GAP43 level, which is beneficial to motor function recovery after acute SCI [30].
Neural cell apoptosis is often reported in central nervous system pathologies with post-SCI apoptosis often exacerbated to cause additional tissue damage [31]. The effects of BMSCs exosomes on neural apoptosis of injured spinal cords were assessed using TUNEL staining. The findings revealed that BMSCs exosomes significantly decreased the apoptotic neural cell in spinal cord tissues in SCI rats with or without sh-NC AAV injection, rather than in the SCI rats with sh-Shh AAV injection. Shen et al. [32] indicated that Shh pathway activation can inhibit neural apoptosis and neurodegeneration in rats after SCI. Combined with our results, it is suggested that the presence of Shh is a necessary factor for exosomes to treat SCI.
5. Conclusions
In the rats, the spinal cord injuries were repaired by the bone marrow cells secreted exosome injection. Arelevant mechanism might be associated with the activation of Shh signaling pathway to putatively protect neurons. In other words, exosomes cannot be used to treat SCI without Shh. Thus, the combined treatment of exosomes and Shh may achieve a better therapeutic effect in SCI. However, the specific mechanism of BMSCs exosomes for regulating Shh expression to cure spinal cord injury in rats remains to be further confirmed. Future relevant assays are warranted for further corroboration of these observations.
Funding
This study was supported by funding from Guiyang Science and Technology Project Zhuke contract (No[2019]9-5-3).
Authors' contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
None.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Zhang Q., Xiong Y., Li B., Deng G.Y., Fu W.W., Cao B.C., et al. Total flavonoids of hawthorn leaves promote motor function recovery via inhibition of apoptosis after spinal cord injury. Neural regeneration research. 2021;16:350–356. doi: 10.4103/1673-5374.286975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephan K., Huber S., Haberle S., Kanz K.G., Buhren V., van Griensven M., et al. Spinal cord injury--incidence, prognosis, and outcome: an analysis of the TraumaRegister DGU. Spine J. 2015;15:1994–2001. doi: 10.1016/j.spinee.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 3.Sun X., Zhang C., Xu J., Zhai H., Liu S., Xu Y., et al. Neurotrophin-3-Loaded multichannel nanofibrous scaffolds promoted anti-inflammation, neuronal differentiation, and functional recovery after spinal cord injury. ACS Biomater Sci Eng. 2020;6:1228–1238. doi: 10.1021/acsbiomaterials.0c00023. [DOI] [PubMed] [Google Scholar]
- 4.Yao S., He F., Cao Z., Sun Z., Chen Y., Zhao H., et al. Mesenchymal stem cell-laden hydrogel microfibers for promoting nerve fiber regeneration in long-distance spinal cord transection injury. ACS Biomater Sci Eng. 2020;6:1165–1175. doi: 10.1021/acsbiomaterials.9b01557. [DOI] [PubMed] [Google Scholar]
- 5.Zou Y., Zhao Y., Xiao Z., Chen B., Ma D., Shen H., et al. Comparison of regenerative effects of transplanting three-dimensional longitudinal scaffold loaded-human mesenchymal stem cells and human neural stem cells on spinal cord completely transected rats. ACS Biomater Sci Eng. 2020;6:1671–1680. doi: 10.1021/acsbiomaterials.9b01790. [DOI] [PubMed] [Google Scholar]
- 6.Gong Z., Lei D., Wang C., Yu C., Xia K., Shu J., et al. Bioactive elastic scaffolds loaded with neural stem cells promote rapid spinal cord regeneration. ACS Biomater Sci Eng. 2020;6:6331–6343. doi: 10.1021/acsbiomaterials.0c01057. [DOI] [PubMed] [Google Scholar]
- 7.Ukeba D., Yamada K., Tsujimoto T., Ura K., Nonoyama T., Iwasaki N., et al. Bone marrow aspirate concentrate combined with in situ forming bioresorbable gel enhances intervertebral disc regeneration in rabbits. J bone and joint surgery American. 2021;103:e31. doi: 10.2106/JBJS.20.00606. [DOI] [PubMed] [Google Scholar]
- 8.Li F., Zhang J., Chen A., Liao R., Duan Y., Xu Y., et al. Combined transplantation of neural stem cells and bone marrow mesenchymal stem cells promotes neuronal cell survival to alleviate brain damage after cardiac arrest via microRNA-133b incorporated in extracellular vesicles. Aging. 2021;13:262–278. doi: 10.18632/aging.103920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S., Stöckl S., Lukas C., Götz J., Herrmann M., Federlin M., et al. hBMSC-derived extracellular vesicles attenuate IL-1β-induced catabolic effects on OA-chondrocytes by regulating pro-inflammatory signaling pathways. Frontiers in bioengineering and biotechnology. 2020;8:603598. doi: 10.3389/fbioe.2020.603598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Chen W., Zhao L., Li Y., Liu Z., Gao H., et al. Obesity regulates miR-467/HoxA10 axis on osteogenic differentiation and fracture healing by BMSC-derived exosome LncRNA H19. J Cell Mol Med. 2021;25:1712–1724. doi: 10.1111/jcmm.16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu G.D., Cheng P., Liu T., Wang Z. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Frontiers in cell and developmental biology. 2020;8:608521. doi: 10.3389/fcell.2020.608521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J., Jin Z.S., Wang C.M., Yan X.F., Mao Y.Q., Chen S. Bone marrow mesenchymal stem cell-derived exosomes improves spinal cord function after injury in rats by activating autophagy. Drug Des Dev Ther. 2020;14:1621–1631. doi: 10.2147/DDDT.S237502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreau N., Boucher Y. Hedging against neuropathic pain: role of hedgehog signaling in pathological nerve healing. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21239115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan B., Jing L., Cao M., Hu Y., Gao X., Bu X., et al. Melatonin promotes Schwann cell proliferation and migration via the shh signalling pathway after peripheral nerve injury. Eur J Neurosci. 2020;53:720–731. doi: 10.1111/ejn.14998. [DOI] [PubMed] [Google Scholar]
- 15.Yin S., Bai X., Xin D., Li T., Chu X., Ke H., et al. Neuroprotective effects of the sonic hedgehog signaling pathway in ischemic injury through promotion of synaptic and neuronal Health. Neural Plast. 2020;2020:8815195. doi: 10.1155/2020/8815195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angeloni N.L., Bond C.W., Tang Y., Harrington D.A., Zhang S., Stupp S.I., et al. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials. 2011;32:1091–1101. doi: 10.1016/j.biomaterials.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y.D., Zhu Z.S., Zhang D., Zhang Z., Ma B., Zhao S.C., et al. Lentivirus-mediated silencing of the PTC1 and PTC2 genes promotes recovery from spinal cord injury by activating the Hedgehog signaling pathway in a rat model. Exp Mol Med. 2017;49:e412. doi: 10.1038/emm.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia Y., Lu T., Chen Q., Pu X., Ji L., Yang J., et al. Exosomes secreted from sonic hedgehog-modified bone mesenchymal stem cells facilitate the repair of rat spinal cord injuries. Acta Neurochir. 2021;163:2297–2306. doi: 10.1007/s00701-021-04829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L., Fan C., Zhang Y., Yang Y., Wang D., Deng C., et al. Adiponectin enhances bone marrow mesenchymal stem cell resistance to flow shear stress through AMP-activated protein kinase signaling. Sci Rep. 2016;6:28752. doi: 10.1038/srep28752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammadian M., Boskabady M.H., Kashani I.R., Jahromi G.P., Omidi A., Nejad A.K., et al. Effect of bone marrow derived mesenchymal stem cells on lung pathology and inflammation in ovalbumin-induced asthma in mouse. Iranian journal of basic medical sciences. 2016;19:55–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng H., Liu N., Yang Y.Y., Xing H.Y., Liu X.X., Li F., et al. Lentivirus-mediated downregulation of α-synuclein reduces neuroinflammation and promotes functional recovery in rats with spinal cord injury. J Neuroinflammation. 2019;16:283. doi: 10.1186/s12974-019-1658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vafaei-Nezhad S., Pour Hassan M., Noroozian M., Aliaghaei A., Shirazi Tehrani A., Abbaszadeh H.A., et al. A review of low-level laser therapy for spinal cord injury: challenges and safety. J Laser Med Sci. 2020;11:363–368. doi: 10.34172/jlms.2020.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Li J., Feng L. Hedgehog signaling pathway as a therapeutic target for ovarian cancer. Cancer epidemiology. 2016;40:152–157. doi: 10.1016/j.canep.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Jia Y., Wu D., Zhang R., Shuang W., Sun J., Hao H., et al. Bone marrow-derived mesenchymal stem cells expressing the Shh transgene promotes functional recovery after spinal cord injury in rats. Neurosci Lett. 2014;573:46–51. doi: 10.1016/j.neulet.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Ren Z., Qi Y., Sun S., Tao Y., Shi R. Mesenchymal stem cell-derived exosomes: hope for spinal cord injury repair. Stem Cell Dev. 2020;29:1467–1478. doi: 10.1089/scd.2020.0133. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C., Zhang C., Xu Y., Li C., Cao Y., Li P. Exosomes derived from human placenta-derived mesenchymal stem cells improve neurologic function by promoting angiogenesis after spinal cord injury. Neurosci Lett. 2020;739:135399. doi: 10.1016/j.neulet.2020.135399. [DOI] [PubMed] [Google Scholar]
- 27.Kong Y.L., Wang Y.F., Zhu Z.S., Deng Z.W., Chen J., Zhang D., et al. Silencing of the MEKK2/MEKK3 pathway protects against spinal cord injury via the hedgehog pathway and the JNK pathway. Mol Ther Nucleic Acids. 2019;17:578–589. doi: 10.1016/j.omtn.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Gao Z., Zhang Z., Bian Q., Li Y., Ma D., Liu Z., et al. Mild hypothermia protects rat cortical neurons against oxygen-glucose deprivation/reoxygenation injury via the PI3K/Akt pathway. Neuroreport. 2021;32:312–320. doi: 10.1097/WNR.0000000000001593. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya S., Dinda A., Vishnubhatla S., Anwar M.F., Jain S. A combinatorial approach to modulate microenvironment toward regeneration and repair after spinal cord injury in rats. Neurosci Lett. 2021;741:135500. doi: 10.1016/j.neulet.2020.135500. [DOI] [PubMed] [Google Scholar]
- 30.Liu H., Xiong D., Pang R., Deng Q., Sun N., Zheng J., et al. Effects of repetitive magnetic stimulation on motor function and GAP43 and 5-HT expression in rats with spinal cord injury. J Int Med Res. 2020;48 doi: 10.1177/0300060520970765. 300060520970765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao X., Sun C., Fan B., Zhao C., Zhang Y., Duan H., et al. Neurotropin exerts neuroprotective effects after spinal cord injury by inhibiting apoptosis and modulating cytokines. J orthopaedic translation. 2021;26:74–83. doi: 10.1016/j.jot.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding L.L., Hu S.F., He X.W., Zhang P., Zhao F.F., Liu T.P., et al. Acupuncture combined with moxibustion promote the recovery of spinal cord injury in correlation with Shh/Gli-1 signaling pathway. J spinal cord medicine. 2020:1–11. doi: 10.1080/10790268.2020.1766900. [DOI] [PMC free article] [PubMed] [Google Scholar]