Sir,
At the time of writing, Australia has one of the lowest SARS-CoV-2 infection rates globally due mostly to closure of the Australian border, and non-pharmaceutical interventions such as physical distancing in conjunction with high rates of diagnostic testing and isolation of positive cases and their contacts.1 To date, highly sensitive reverse-transcription PCR (RT-PCR) assays performed in clinical laboratories have been the cornerstone of diagnostic testing for SARS-CoV-2. However, depending on the setting, RT-PCR results have taken approximately 24–48 hours to return: this has led to delays in contact tracing and therefore preventable transmission of disease.2 Rapid point of care (POC) antigen and molecular tests are generally less sensitive than RT-PCR assays but it has been suggested that increasing test frequency and decreasing test turnaround time can offset lower test sensitivity for effective COVID-19 control.3 , 4 To date, few studies have assessed the performance and implementation of rapid molecular POC SARS-CoV-2 testing in a setting with a low prevalence of SARS-CoV-2.
At the time of study initiation, the Xpert Xpress (Cepheid, USA) was the only Therapeutic Goods Administration-listed rapid POC molecular assay in use in Australia. However, there were severe constraints on supply of tests, especially to Australia, necessitating evaluation of other assays. The ID NOW COVID-19 assay (Abbott, USA) is a rapid, instrument-based molecular isothermal amplification test for the detection of SARS-CoV-2 from oropharyngeal, nasal and nasopharyngeal swabs (NPS). The ID NOW turnaround time for positive results can be as little as 5 minutes with negative results in 13 minutes.
A March 2021 Cochrane review of rapid SARS-CoV-2 tests found that studies were mainly from Europe or North America. Using data from evaluations following the manufacturer's instructions for use (IFU), the average sensitivity of ID NOW was 73.0% [95% confidence interval (CI) 66.8–78.4%] and average specificity 99.7% (95% CI 98.7–99.9%) with a total of only four evaluations with 812 samples and 222 cases. This Cochrane review found that studies of antigen tests were of higher methodological quality compared to studies of molecular tests.5
To date, nine peer-reviewed published studies have evaluated performance of ID NOW with positive agreement/sensitivity varying between 48–94% compared to RT-PCR. However, only four of these studies have used dry swabs as recommended in the current manufacturer's IFU. The variable performance of the ID NOW may be attributed to the type of swab used, the time of testing post-onset of symptoms and the reference assay used.5 , 6 Knowledge of test specificity is especially important in low-prevalence use; of the eight studies that assessed specificity of the ID NOW, only one tested more than 80 negative samples.7
We undertook a prospective study of participants presenting for SARS-CoV-2 testing across two academic hospitals located in Victoria, Australia, during a period of low SARS-CoV-2 prevalence. In Victoria, the COVID-19 pandemic has been characterised by two peaks of transmission: the first occurring between March and April 2020 (maximum 622 active cases) and the second between July and September (maximum 7880 active cases). This study commenced on 2 November 2020 and continued until 4 December 2020 after significant public health interventions had controlled transmission, during which time the 14-day average in metropolitan Melbourne decreased from 20.3 to zero new cases per day. The two participating hospital networks were Monash Health (Clayton and Casey screening clinics, located in Melbourne's south-eastern suburbs) and Austin Hospital screening clinic (located in Melbourne's north-eastern suburbs).
A standard-of-care (SOC) swab for RT-PCR was collected according to local hospital procedure; all sites performed a single combined throat and deep nasal swab, of either one or both nasal cavities. A second-deep nasal swab was collected at the same time using the dry foam swab provided in the ID NOW COVID-19 kit and tested according to the IFU. All swabs were collected by trained healthcare professionals. The Abbott ID NOW COVID-19 test was performed at the point of care.
SOC swabs were collected using either a flocked ESwab (Copan, USA) in 1 mL liquid amies or flocked swab (Kang Jian, China) in 3 mL universal transport media and tested using the preferred RT-PCR assay at each of the pilot sites: at Monash Health using the Respiratory Pathogens 12-well assay (AusDiagnostics, Australia)8 or Xpert Xpress SARS-CoV-2 assay (Cepheid, USA);5 at Austin Health using the Coronavirus Typing (8-well) panel (AusDiagnostics)9 or the Xpert Xpress SARS-CoV-2 assay.
Statistical analyses were performed using SPSS statistical software package version 27 (SPSS, USA), or GraphPad Prism, version 9.0 (GraphPad, USA).
Ethics review and study approval was provided by Monash Health Human Research and Ethics Committee (RES-20-0000-678A).
In total, 1044 paired swabs were performed for both ID NOW and RT-PCR testing (634 at Monash Health; 410 at Austin Health) from 1037 participants. One swab was excluded due to missing RT-PCR data leaving 1043 paired swabs for evaluation. Median duration of symptoms was 1 day (range 0–60 days) and median age of participants was 36 years (range 4–91 years); 44.1% were male and gender information was not reported for three patients. Fifty-five participants were asymptomatic, two had previously confirmed COVID-19, and 986 participants were symptomatic with suspected COVID-19 symptoms.
In this prospective arm of the study the specificity of the Abbott ID NOW test using the kit's dry swab was 99.9% when tested on 1043 prospectively collected paired swabs (95% CI 99.47–100%). The rate of invalid results was 1.9% on initial testing: four from Monash (where there were 6 users) and 16 from Austin (16 users), all of which were resolved by reinserting the test cartridge. One participant had a positive ID NOW result with negative RT-PCR and 1042 participants had negative ID NOW results. Our study includes over double the number of RT-PCR-negative patients included in all currently published studies combined (following the manufacturer's IFU). There were no positive RT-PCR results during the study period (Table 1 ).
Table 1.
Results for clinical evaluations of ID NOW COVID-19 assay
| Retrospective clinical evaluation | Days since symptom onseta |
|
|---|---|---|
| 1–21 days (median 3) | 1–7 days (median 2) | |
| Number of positive casesb | 21 | 14 |
| Number of negative casesc | 316 | 314 |
| Number of positive SARS-CoV-2 samples by SOC RT-PCR | 19 | 13 |
| Ct range of positive SARS-CoV-2 samples by SOC RT-PCR | 17.1–37.3 | 17.1–31.8 |
| Number of positive SARS-CoV-2 samples by ID NOW | 17 | 11 |
| Number of false-positive SARS-CoV-2 samples by ID NOW | 1 | 1 |
| Number of negative SARS-CoV-2 samples by SOC RT-PCR | 318 | 316 |
| Number of negative SARS-CoV-2 samples by ID NOW | 320 | 317 |
| Number of false-negative SARS-CoV-2 samples by ID NOW |
3 |
2 |
| Prospective hospital pilot sites |
Monash |
Austin |
| Staff using test, n | 6 | 16 |
| Total participants, n | 634 | 410 |
| Excluded, n | 0 | 1 |
| Total included, N | 634 | 409 |
| Median age, years (range) | 35 (8–83) | 36 (4–90) |
| Male gender, n (%) | 290 (45.7%) | 167 (40.8%) |
| Asymptomatic, n (%) | 48 (7.6%) | 7 (1.7%) |
| Median days of symptoms (range) | 2 (1–60) | 1 (0–14) |
| Positive RT-PCR result, n (%) | 0 (0%) | 0 (0%) |
| Positive Abbott ID NOW result, n (%) | 1 (0.2%) | 0 (0%) |
Where known for symptomatic cases. Individuals who were 1–7 days since symptom onset are a subset of the group of individuals who are 1–21 days since symptom onset.
Individuals with known COVID-19 infection notified to Department of Health and Human Services (DHHS): only 19 of 21 of these individuals were RT-PCR positive at the time of recruitment to this study.
Individuals who tested negative for SARS-CoV-2 and were not considered COVID-19 cases by DHHS at the time.
Additionally, patients with recent RT-PCR confirmed SARS-CoV-2 infection (identified through State Health Department notifications) were invited to provide a deep nasal swab collected by a trained healthcare professional using the foam swab provided in the ID NOW COVID-19 kit. A SOC swab was also collected and tested at the Microbiological Diagnostic Unit Public Health Laboratory on the Aptima SARS-CoV-2 assay (Hologic, USA) according to the manufacturer's instructions. For validation purposes, additional PCR-negative patient samples were obtained from testing sites at the University of Melbourne and Monash Health.
Positive agreement amongst the 19 participants with recent RT-PCR-confirmed infection was 76.9% (10/13) for those with less than 7 days since symptom onset compared to Aptima SARS-CoV-2 assay. NPA was 99.7% (317/318, 95% CI 98.26–99.99%) for 318 samples that were negative on Aptima SARS-CoV-2 assay (Table 1). Of the four previous studies that used dry swabs, the sensitivity of the ID NOW was between 48–75%, but these studies were small; the only one of these studies that included more than 31 positive patients found a sensitivity of 75% in 187 RT-PCR positive patients.6 In our study, due to the low prevalence of infection during the study period, assessment of clinical sensitivity was limited but consistent with the Cochrane review and with this only other reasonably-sized peer-reviewed publication that followed the manufacturer's IFU. However, further studies are required to assess the effect on sensitivity of using anterior nasal versus deep nasal/NPS.
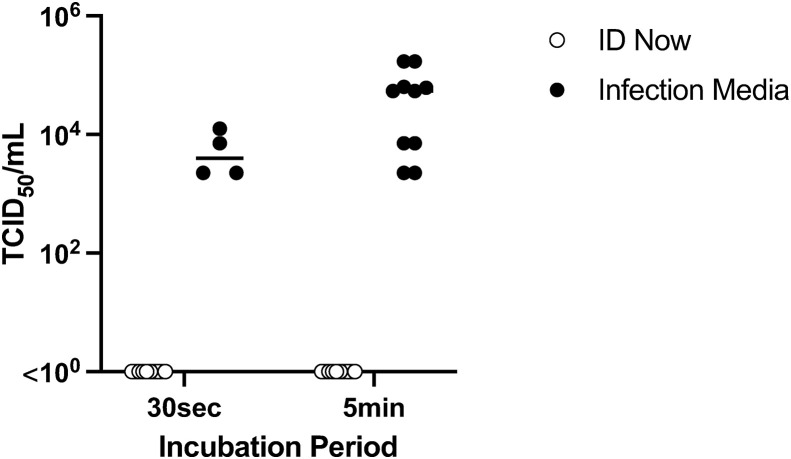
To assess safety for testing staff, the ability of the Abbott ID NOW sample receiver buffer to inactivate SARS-CoV-2 was studied in three independent experiments. According to the manufacturer's directions, the sample receiver capsules (total n=10) were pre-heated to 56°C for 3 minutes, then 200 μL of undiluted SARS-CoV-2 human/Victoria/01/2020 (concentration 105.3TCID50/mL) was pipetted into the 2.5 mL buffer. As a control, the equivalent volume of room temperature infection media (Minimal Essential Media supplemented with 10 μM HEPES, 2 mM glutamine and antibiotics) was also spiked with 200 μL SARS-CoV-2. The presence of infectious virus was assessed using a 50% Tissue Culture Infectious Dose (TCID50) assay.10 The preheated buffer was able to reduce the SARS-CoV-2 infectious titre to non-detectable levels (<10 TCID50/mL) within 30 seconds of spiking, while infectious virus was recoverable from the spiked infection media control after 5 minutes of incubation (Fig. 1 ).
Fig. 1.
Results of virucidal study. When pre-heated to 56°C the ID NOW elution/lysis buffer was able to reduce the SARS-CoV-2 infectious titre to non-detectable levels (<100 TCID50/mL) within 30 seconds of spiking, while recovery of infectious virus from the spiked infection media control was between 103.6 TCID50/mL and 104.92 TCID50/mL after 5 minutes of incubation (∗∗∗p<0.001, two-way ANOVA, Sidak's multiple comparisons test). Data represents pooled results from three independent experiments: Experiment 1 tested n=3 ID NOW buffer samples at 30 seconds and at 5 minutes, with n=3 spiked infection media at 5 minutes; Experiment 2 was an exact repeat of Experiment 1; Experiment 3 tested n=4 ID NOW buffer samples at 30 seconds and at 5 minutes and n=4 spiked infection media at 30 seconds and 5 minutes.
One advantage of POC NAT assays over POC antigen tests is result management including objective result interpretation, result recording and potential for automated result communication (such as cloud-based interfacing with laboratory information systems) to enable results communication to patients, their health care providers and public health officials.11 We did observe a greater number of invalid results in sites that employed more users, suggesting that experience in running the assay may affect performance. Guidelines for POC testing in Australia recommend training and competency assessments for staff performing testing, in addition to an overarching quality framework to ensure quality control and quality assurance.12
In summary, we found this rapid NAT assay to have a high specificity when evaluated prospectively in a real-life low-prevalence POC setting. In this low-prevalence context, due to the lower sensitivity when compared to laboratory RT-PCR, this and similar rapid POC NAT assays may be most useful in enabling the rapid triage of public health and hospital resources while expediting confirmatory PCR testing.
Acknowledgements
We thank the COVID-19 Diagnostics Research Group, which in addition to the listed authors, also comprises Nick Tayler, Jason A. Trubiano, Olivia Smibert, George Drewett, Fiona James, Socheata Chea, Steven Edwards, Nicole Isles, Michelle Sait and Beau Carr, for their laboratory and clinical support for this study. We also thank Kirsten Holden and staff at Clayton and Casey Screening Clinic, as well as Grace Gibney and staff at Austin Health Screening Clinic, for collecting the extra study swabs from patients.
Conflicts of interest and sources of funding
This research was funded by the Medical Research Future Fund (MRFF) 2020 COVID-19 Diagnostics Grant Opportunity as part of the COVID-19 Strategic Planning and Delivery of Testing program and the Victorian Government Department of Health as part of the Doherty Institute Innovative Testing Program. The funders were not involved in data analysis or manuscript preparation. The authors state that there are no conflicts of interest to disclose.
References
- 1.Chang S.L., Harding N., Zachreson C., et al. Modelling transmission and control of the COVID-19 pandemic in Australia. Nat Commun. 2020;11:5710. doi: 10.1038/s41467-020-19393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kretzschmar M.E., Rozhnova G., Bootsma M.C., van Boven M., van de Wijgert J.H., Bonten M.J. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020;5:e452–e459. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mina M.J., Parker R., Larremore D.B. Rethinking COVID-19 test sensitivity - a strategy for containment. N Engl J Med. 2020;383 doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 4.Larremore D.B., Wilder B., Lester E., et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. medRxiv. 2020:8 Sep. doi: 10.1101/2020.06.22.20136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinnes J., Deeks J.J., Berhane S., et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serei V.D., Cristelli R., Joho K., et al. Comparison of Abbott ID NOW COVID-19 rapid molecular assay to Cepheid Xpert Xpress SARS-CoV-2 assay in dry nasal swabs. Diagn Microbiol Infect Dis. 2020;99:115208. doi: 10.1016/j.diagmicrobio.2020.115208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington A., Cox B., Snowdon J., et al. Comparison of Abbott ID Now and Abbott m2000 methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from symptomatic patients. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00798-20. e00798-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attwood L.O., Francis M.J., Hamblin J., Korman T.M., Druce J., Graham M. Clinical evaluation of AusDiagnostics SARS-CoV-2 multiplex tandem PCR assay. J Clin Virol. 2020;128:104448. doi: 10.1016/j.jcv.2020.104448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams E., Bond K., Chong B., et al. Implementation and evaluation of a novel real-time multiplex assay for SARS-CoV-2: in-field learnings from a clinical microbiology laboratory. Pathology. 2020;52:754–759. doi: 10.1016/j.pathol.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.Y.H., Best N., McAuley J., et al. Validation of a single-step, single-tube reverse transcription loop-mediated isothermal amplification assay for rapid detection of SARS-CoV-2 RNA. J Med Microbiol. 2020;69:1169–1178. doi: 10.1099/jmm.0.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhi S., Tayler N., Hoang T., et al. Multi-site assessment of rapid, point-of-care antigen testing for the diagnosis of SARS-CoV-2 infection in a low-prevalence setting: a validation and implementation study. Lancet Reg Health West Pac. 2021;9:100115. doi: 10.1016/j.lanwpc.2021.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Pathology Accreditation Advisory Council . Australian Government Department of Health; Canberra: 2015. Guidelines for Point of Care Testing (PoCT): (First Edition 2015)https://www1.health.gov.au/internet/main/publishing.nsf/Content/health-npaac-poctguid [Google Scholar]