Abstract
Since December 2019, severe acute respiratory syndrome coronavirus 2 has been found to be the culprit in the coronavirus disease 2019 (COVID-19), causing a global pandemic. Despite the existence of many vaccine programs, the number of confirmed cases and fatalities due to COVID-19 is still increasing. Furthermore, a number of variants have been reported. Because of the absence of approved anti-coronavirus drugs, the treatment and management of COVID-19 has become a global challenge. Under these circumstances, drug repurposing is an effective method to identify candidate drugs with a shorter cycle of clinical trials. Here, we summarize the current status of the application of drug repurposing in COVID-19, including drug repurposing based on virtual computer screening, network pharmacology, and bioactivity, which may be a beneficial COVID-19 treatment.
Keywords: Drug repurposing, COVID-19, SARS-CoV-2, Virtual screening, 3C-like protease
Graphical abstract
Highlights
-
•
Mechanism of SARS-CoV-2 infection and drug targets were reviewed.
-
•
Drug repurposing against COVID-19 based on computer virtual screening, network pharmacology, bioactivity were summarized.
-
•
The use of drug repurposing in COVID-19 was addressed.
1. Introduction
The coronavirus disease 2019 (COVID-19) has spread almost globally, with 201 million confirmed cases and over 4.2 million cumulative deaths as of August 6, 2021 [1,2]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of this disease, poses a great crisis to global human health and economic development [3,4]. Unfortunately, the global epidemic situation of COVID-19 continues to spread, but no approved specific drugs have been developed. Therefore, there is an urgent need to explore effective therapeutic agents.
Traditional drug discovery is a high-input, long-cycle (10–15 years), and high-risk process, with low success rates [5,6]. Drug repurposing is an effective way to solve this problem. Drug repurposing is used to explore new applications of approved or investigational drugs beyond the scope of the original medical, which consist of four steps: compound identification, compound acquisition, development, and Food and Drug Administration (FDA) post-marketing safety monitoring [7,8]. Compared to traditional drug discovery, drug repurposing has the advantages of shorter research and development time, less development cost, and lower risk, and may reveal novel targets and pathways for approved drugs [9,10]. For example, sildenafil, originally developed as an antihypertensive drug, has been used to treat erectile dysfunction and pulmonary hypertension [11]. In addition, thalidomide was first developed as a sedative, but was discontinued after it exhibited serious side effects, resulting in severe skeletal congenital disabilities in neonates; however, it was later serendipitously discovered to be effective in treating erythema nodosum leprosum and multiple myeloma [12,13].
However, at present, accident-based drug repurposing plays an inferior role. With continuous advances in bioinformatics and chemoinformatics, drug repurposing has gradually developed into a data-driven innovative drug development strategy. Many approaches have been used for drug repurposing, including molecular dynamics, molecular docking, network pharmacology, and retrospective clinical analysis [9]. There is often insufficient time to develop drugs against emergent diseases, and in this context drug repurposing can help to identify drugs which can be quickly applied for clinical purposes. In this review, we focus on in silico, network-based, and activity-based drug repurposing approaches, in the hope of providing some reference information for COVID-19 therapy.
2. The cell entry process and key targets of SARS-CoV-2
SARS-CoV-2 is an enveloped RNA virus, whose source has not been conclusively clarified, but evidence suggests that it may originate from bats or bat droppings [[14], [15], [16]]. SARS-CoV-2 encodes four major structural proteins: the spike (S) glycoprotein (cleaved into the S1 and S2 subunits by the transmembrane serine protease 2 (TMPRSS2)), membrane (M) glycoprotein, envelope (E) protein, and nucleocapsid (N) protein (containing the structural component of the helical nucleocapsid) [[17], [18], [19]]. It encodes several non-structural proteins, including 3C-like protease (3CLpro), RNA-dependent RNA polymerase (RdRp), and papain-like protease (PLpro) [20]. 3CLpro is responsible for viral replication and RdRp for coronavirus replication and transcription [12,21]. PLpro, a multifunctional enzyme with the ability to antagonize host innate immune responses and process viral polyproteins, is essential for coronavirus replication [22,23].
Coronaviruses can enter host cells via endosomal and non-endosomal pathways [24]. When the cell surface is devoid of extracellular proteases, coronaviruses use the endosomal pathway to enter cells. The energetically unfavorable membrane fusion reaction can be overcome with the assistance of pH-dependent endosomal cysteine protease cathepsins, thereby promoting coronavirus entry into host cells [25]. The cysteine protease cathepsin activates the S glycoprotein, allowing the S glycoprotein to recognize the host cell cluster differentiation 147 or angiotensin-converting enzyme 2 (ACE2), a membrane receptor of SARS-CoV-2 [[26], [27], [28]]. When the endosomal pH is reduced, the viral membrane fuses with the endocytic vesicle membrane, releasing viral RNA into the cytosol [29]. Conversely, in the presence of host proteases, such as TMPRSS2, non-endosomal viruses can enter the plasma membrane [25,30].
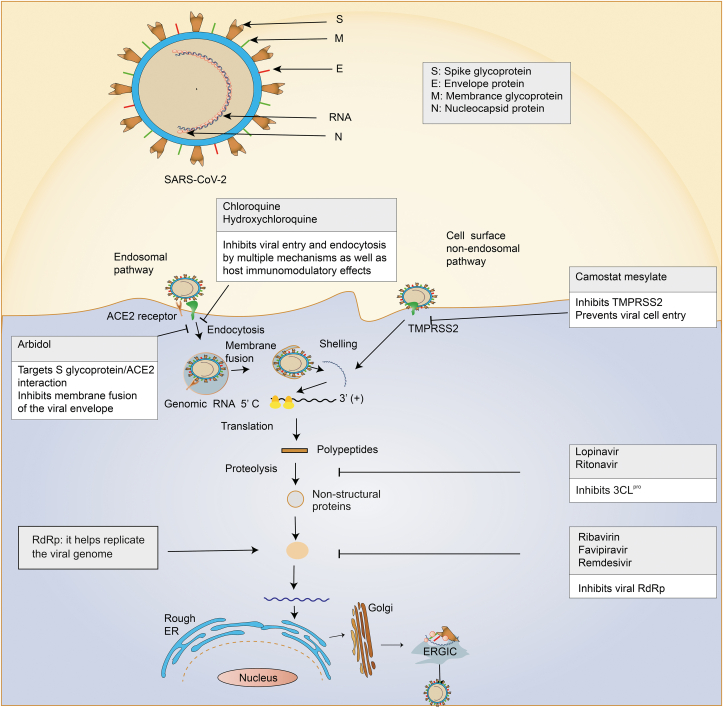
After the virus enters the cell, essential viral polyproteins are translated and hydrolyzed into effector proteins [31,32]. Nucleocapsids, composed of genomic RNA and N protein, enter the endoplasmic reticulum (ER) and Golgi apparatus, and are further transported to the budding zone through the ER and Golgi apparatus [32,33]. The M glycoprotein interacts with the nucleocapsid to form the basic structure of the virus. Moreover, the S and E glycoproteins combine to form viral vesicles that fuse with the cell membrane and are freed by exocytosis [34]. Non-structural proteins, S glycoprotein, TMPRSS2, and ACE2 are important drug targets for anti-COVID-19 therapies (Fig. 1).
Fig. 1.
The mechanisms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and drug targets are reviewed. SARS-CoV-2 consists of RNA and four main structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N), which encodes several non-structural proteins, including 3C-like protease (3CLpro), RNA-dependent RNA polymerase (RdRp), and papain-like protease (PLpro). Entry of SARS-CoV-2 into host cells includes both endosomal and non-endosomal pathways. The effect of anti-SARS-CoV-2 can be exerted by the inhibitors of corresponding target proteins. ACE2: angiotensin-converting enzyme 2; TMPRSS2: transmembrane serine protease 2; ER: endoplasmic reticulum.
3. Application of drug repurposing in COVID-19
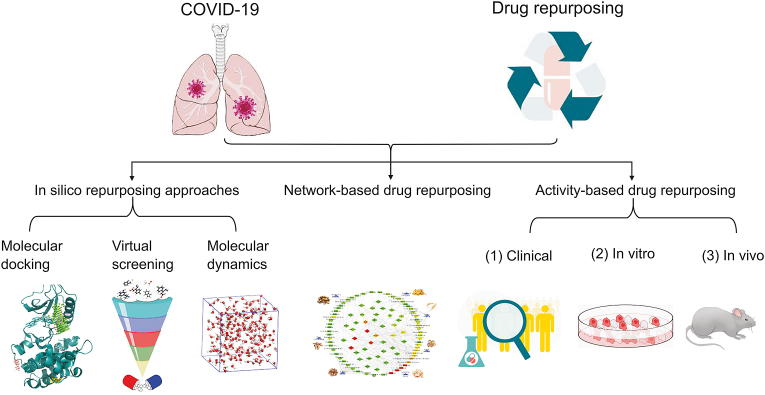
James Whyte Black, a British scientist and Nobel Prize winner in Physiological Medicine, pointed out “the most fruitful basis for new drug discovery is to start with old drugs” [35]. In the current COVID-19 pandemic, the drug repurposing strategy has played a crucial role. Here, we review potential anti-COVID-19 drugs selected by computer technology, networks, and drug activity [36,37] (Fig. 2).
Fig. 2.
Drug repurposing against coronavirus disease 2019 (COVID-19) based on computer virtual screening, network pharmacology, and bioactivity were summarized. It mainly includes three kinds of methods, including in silico repurposing approaches, network-based drug repurposing, and activity-based drug repurposing. Among them, in silico repurposing approaches mainly exerts molecular docking, virtual screening and molecular dynamics, and activity-based drug repurposing is mainly related to in vivo, in vitro, and clinical trials.
3.1. In silico repurposing approaches
In silico repurposing is a method based on computer technology to accelerate the discovery of new uses of drugs, which can be used to develop virtual screening platforms, predict the binding affinity between protein targets and ligands, and accelerate and promote the process of drug-target recognition, binding, and successful localization [9,38]. In recent years, there have been many successful cases of drug repurposing using computer technology. Several important drug targets have been identified in previous studies of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), such as S glycoprotein, ACE2, TMPRSS2, 3CLpro, RdRp, and PLpro [30]. The gene and invasion mechanisms of SARS-CoV-2 are similar to those of SARS-CoV [39]. As a matter of fact, in silico repurposing approaches have been increasingly used during the COVID-19 epidemic, especially using 3CLpro as a target to screen drugs.
3CLpro, a cysteine protease with high homology and structural conservation, is critical for viral replication [40]. Recently, various drugs with 3CLpro inhibitory activity have been screened by molecular docking and molecular dynamics simulations. Viomycin was discovered to combine firmly with SARS-CoV-2 3CLpro by deeply embedding itself inside the binding pocket, and showed a higher CDOCKER energy value than ritonavir and lopinavir did [41]. Conivaptan and azelastine maintained proximity to the SARS-CoV-2 3CLpro binding pocket by participating in hydrophobic interactions with active site residues [42]. Oolonghomobisflavan-A showed many hydrogen bonds with the 3CLpro and high molecular mechanics-Poisson–Boltzmann surface area binding energy [43]. In addition, leupeptin hemisulfate, pepstatin A, nelfinavir, birinapant, lypression, and octreotide have received attention as potential inhibitors of 3CLpro, because they show reasonably well molecular mechanics-generalized born surface area score and possess drug-like properties [44]. On the other hand, other in silico repurposing approaches are also widely used. In a virtual screening based on electronic pharmacophores, binifibrate, bamifylline, ezetimibe and others were found to be potential inhibitors of 3CLpro [45]. The Ro5 bioactivity model was used to demonstrate the inhibitory capacity of small molecules against protein targets. In this model, nelfinavir and itacitinib showed the best inhibitory activity and affinity against 3CLpro with favorable pharmacokinetics, and inhibited viral replication and excessive inflammation [46]. On the basis of virtual screening of ChEMBL database, the results showed that the 28 bioactive compounds targeting SARS-CoV-2 3CLpro, such as sepimostat and curcumin, had considerable affinity for SARS-CoV-2 3CLpro [47]. Moreover, by molecular modeling and virtual screening of SARS-CoV-2 3CLpro, ledipasvir and velpatasvir could relieve fatigue and headache in COVID-19 patients, with minimal side effects [48], and the combination of ribavirin, telbivudine, vitamin B12, and nicotinamide has been made available as a treatment for COVID-19 [49].
Besides 3CLpro, other targets have also been studied extensively. Many approved antiviral drugs, such as sofosbuvir, favipiravir, cefuroxime, hydroxychloroquine, and IDX-184, have shown significant binding interactions with RdRp in molecular docking experiments [[50], [51], [52], [53], [54]]. In addition, casopitant, an neurokinin-1 receptor antagonist for the treatment of chemotherapy-induced nausea was shown to inhibit RdRp by virtual screening [55]. By molecular modeling and molecular docking of SARS-CoV-2 PLpro, some anti-viral, anti-bacterial, muscle relaxant, and anti-tussive drugs, such as valganciclovir, thymidine, cefamandole, ecycline, chlorphenesin carbamate, and levodropropizine, have been selected to inhibit PLpro [56]. In addition, virtual screening and molecular docking of S glycoprotein showed that ivermectin could be successfully used to control SARS-CoV-2 replication in vitro [57]. A total of 7173 clinically approved drugs were screened with ACE2 as a target, and the results showed that lividomycin, burixafor, quisinostat, fluylline, pemetrexed, proffylline, edotecarin, and diniylline had inhibitory effects on ACE2 [58]. Lucas et al. [59] screened more than 70,000 compounds using a chemical library and concluded that bromhexine hydrochloride had a stimulating inhibitory effect on TMPRSS2. In addition, benzquercin, a TMPRSS2 inhibitor, has been found to affect cellular entry of SARS-CoV-2 [60].
In summary, the application of in silico repurposing approaches in COVID-19 therapy is mainly related to key protein targets (Table 1) [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60]]. The rapid development of in silico repurposing approaches provides new methods for new drug research, and is continuously applied in drug repurposing research. At present, in silico repurposing approaches have made a significant contribution in the COVID-19 pandemic. Nevertheless, further in vivo and in vitro clinical trials are needed to verify this hypothesis.
Table 1.
In silico repurposing approaches to screening drug candidates for the treatment of COVID-19.
| Target | Initial indications | Drug name | Stage | Screening method | Refs. |
|---|---|---|---|---|---|
| 3CLpro | Anti-microbial drug | Viomycin | FDA approved | Molecular docking and molecular dynamics simulation | [41] |
| Hyponatremia | Conivaptan | FDA approved | Molecular docking and molecular dynamics simulation | [42] | |
| Allergy | Azelastine | FDA approved | Molecular docking and molecular dynamics simulation | [42] | |
| Plant | Oolonghomoisflavan-A | NA | Molecular docking and molecular dynamics simulation | [43] | |
| NA | Leupeptin, hemisulphate, pepstatin A, nelfinavir, lypression, birinapant, and octreotide | Approved | Molecular dynamics simulation and virtual screening | [44] | |
| NA | Binifibrate, bamifylline, rilmazafon, afatinib, ezetimibe, macimorelin, and acetate | Approved | E-pharmacophore based virtual screening, structure based virtual screening, and estimation of dinding free energy | [45] | |
| NA | Nelfinavir and itacitinib | FDA approved | Molecular docking and bioactivity model | [46] | |
| NA | Sepimostat and curcumin | Approved | Structure-based virtual screenings | [47] | |
| HCV drugs | Velpatasvir and ledipasvir | Approved | Virtual screening | [48] | |
| Vitamin | Vitamin B12 and nicotinamide | FDA approved | Schrodinger glide docking module | [49] | |
| Antiviral drug | Telbivudine | FDA approved | Schrodinger glide docking module | [49] | |
| RdRp | Antiviral drug | Ribavirin, remdesivir, sofosbuvir, galidesivir, tenofovir, favipiravir, cefuroxime, hydroxychloroquine, and IDX-184 | Approved | Molecular docking | [[50], [51], [52], [53], [54]] |
| Neurokinin-1 receptor antagonist | Casopitant | Developent | Virtual screening | [55] | |
| PLpro | Antiviral drug | Valganciclovir and thymidine | Approved | Homology modeling and molecular docking | [56] |
| Anti-bacterial drug | Cefamandole and tigecycline | Approved | Homology modeling and molecular docking | [56] | |
| Muscle relaxant drug | Chlorphenesin carbamate | Approved | Homology modeling and molecular docking | [56] | |
| Anti-tussive drug | Levodropropizine | Approved | Homology modeling and molecular docking | [56] | |
| S glycoprotein | NA | Ivermectin | FDA approved | Molecular dynamics simulation | [57] |
| ACE2 | NA | Lividomycin, burixafor, quisinostat, fluprofylline, pemetrexed, spirofylline, edotecarin, and diniprofylline | Approved | Molecular docking | [58] |
| TMPRSS2 | Respiratory diseases and pneumonia | Bromhexine hydrochloride | Approved | Virtual screening | [59] |
| NA | Benzquercin | FDA approved | Virtual screening | [60] |
NA: not available.
3.2. Network-based drug repurposing
Network pharmacology refers to the science of integrating, comparing, and analyzing drug action and biological networks, and exploring the interactions between drugs and specific nodes or modules in the integrated network in order to more deeply understand the laws of interaction between medications and the body [61,62]. With the progress of network biology research, network pharmacology technology provides a new means for drug research and development, which has become an important technology in drug repurposing research. At present, network-based drug repurposing for COVID-19 has been carried out to discover drugs that can effectively treat COVID-19.
Based on the design principle of network-based in vitro study, melatonin and toremifene have synergistic effects in reducing viral infection and replication and abnormal inflammatory response, and show strong biological feasibility [63]. Zhou et al. [64] systematically predicted over 2,000 FDA-approved or experimental drugs based on systems pharmacology and network methods. They also screened 16 drug candidates (e.g., mercaptopurine) and 3 potential drug combinations (e.g., sirolimus plus dactinomycin) for SARS-CoV-2 and these results have some guiding value for preclinical studies and drug combination discovery of old drugs.
Analysis of protein-protein interactions (PPI) is an important approach to identifying common targets. A study constructed and combined PPI and chemo-protein interaction (CPI) networks using differentially expressed genes (DEGs), which were recognized from the microarray data repository of blood samples of SARS-CoV patients, and found that melatonin could better resist immune damage in COVID-19 compared with chloroquine [65]. Gordon et al. [66] determined 332 high-confidence SARS-CoV-2-human PPIs which are related to various biological processes, such as transcription and ubiquitin regulation, and found 69 promising compounds, including 29 FDA-approved drugs. Moreover, Cava et al. [67] used gene expression profiles from public databases to study the functions of ACE2-related genes, and built a PPI network containing genes coexpressed with ACE2. Finally, nimesulide, fluticasone propionate, and thiabendazole were screened out as treatments for COVID-19. To speed up the discovery of potential therapeutic drugs for COVID-19, the COVID Experiences Surveys, a network-based platform, integrated public and latest data on the effects of SARS-CoV-2 and the host [68]. This allows visual exploration of the virus-host interaction set and implements a systemic medical algorithm.
It is worth pointing out that network-based drug repurposing provides a new research method for scientific research into traditional Chinese medicine (TCM). The active targets and ingredients of TCM prescriptions were acquired from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform, and a PPI core network was acquired, showing connections between the active ingredients of TCM and disease-related proteins. Through this process, two Chinese herbal medicine formulas acquired from the Hubei Province Diagnosis and Treatment Protocol for COVID-19 and ephedra-glycyrrhiza drug pair were found to have positive effects on COVID-19, which is worthy of further promotion and application [69,70]. In summary, in the absence of a specific drug for COVID-19 treatment, network-based drug repurposing is a feasible strategy for discovering effective therapeutics for COVID-19.
3.3. Activity-based drug repurposing
Activity-based drug repurposing can be understood as repurposing based on similarities in drug actions (biological effects). For example, some drugs with therapeutic effects on fever, diarrhea, and cough could be studied to determine whether they may have therapeutic effects on these symptoms in COVID-19 by experimental and clinical trials.
In this outbreak, many active compounds of TCM and TCM herbal formulas have been discovered to have potential activity against COVID-19 [71]. The Lianhuaqingwen capsule (LH) is a finished product of a Chinese herbal formula that exerts broad-spectrum antiviral and immunomodulatory effects against various influenza viruses [72]. LH has been approved by the National Health Commission of the People's Republic of China for COVID-19 treatment, with the impacts of inhibiting the release of inflammatory factors, anti-SARS-CoV-2 replication, and reducing the transcriptional expression of proinflammatory factors [73]. A clinical trial of 284 patients showed that LH could be considered to improve the clinical symptoms of COVID-19 [74]. Fritillaria, a herbal medicine with multiple active components such as alkaloids and terpenoids, which have anti-inflammatory, anti-cholinesterase, and antiviral activities and anti-tussive, expectorant, anti-asthmatic effects, has been widely applied to treat respiratory diseases, including COVID-19 [75]. In addition, Luo et al. [76] found that Qingfei Paidu decoction, used to treat non-infectious bronchitis, can aid recovery from COVID-19 and is beneficial for regulating intestinal function and maintaining the balance of the microenvironment.
Many types of Western medicines have also been suggested to be effective. Various drugs with antiviral activity are widely used for treating COVID-19. Lopinavir-ritonavir (kaletra), a drug for the treatment of HIV, may act against SARS-CoV-2 by inhibiting the coronavirus endopeptidase C30 [77]. Remdesivir is a broad-spectrum antiviral drug with high selectivity for viral polymerases and low toxicity in humans. It binds to RdRp in the form of nucleoside triphosphates, thereby counteracting the Ebola virus [78]. And remdesivir prevents coronavirus replication by interfering with the viral polymerase activity [79]. The results of preliminary clinical trials showed that patients treated with remdesivir had a 31% faster recovery time than those who received a placebo [80]. Arbidol is a powerful broad-spectrum antiviral drug, whose unique mechanism involves targeting the S glycoprotein/ACE2 interaction, thus inhibiting the fusion of the virus envelope and host cell membrane [81,82]. Ivermectin, an antiparasitic drug with antiviral effects in vitro, is related to the suppression of the host importin α/β1, thereby disrupting the immune escape mechanism [83]. Single use of ivermectin in COVID-19 patients could reduce viral RNA by approximately 5,000 fold within 48 h [84].
In addition, some Western medicines used to treat inflammatory diseases have been discovered to treat COVID-19. Camostat mesylate, an effective serine protease inhibitor approved in the treatment of pancreatic inflammation, could stop SARS-CoV-2 from entering bronchial epithelial cells by restraining TMPRSS2, significantly decreasing the expression of coronavirus S glycoprotein, and reducing the infection of SARS-CoV-2 [85,86]. A case of acute eosinophilic pneumonia induced by camostat mesylate was reported because of anaphylaxis in 2016 [87]. Chloroquine and hydroxychloroquine are commonly used to treat malaria and many autoimmune diseases [[88], [89], [90], [91]]. Chloroquine can disturb the binding of SARS-COV-2 and ACE2 receptors by reducing the terminal glycosylation of ACE2 receptors, as well as blocking the fusion process of virus with host cells and subsequent viral replication, by increasing the pH of endosomes and lysosomes [[92], [93], [94]]. Clinical trials have shown that chloroquine effectively treats COVID-19, including improving pulmonary symptoms and shortening the course of disease [92,95]. However, chloroquine has many side effects, such as diarrhea, rashes, weakness, headache, and muscle pain, and only shows an inhibitory effect against SARS-CoV-2 in vitro at a maximal dose, which could cause serious toxicity [96]. Hydroxychloroquine is also a research hotspot. Hydroxychloroquine inhibits SARS-CoV-2 more effectively than chloroquine in in vitro study [97], while Tufan et al. [98] pointed out that hydroxychloroquine may be considered as a treatment option for patients with severe COVID-19. Moreover, the time to SARS-CoV-2 clearance can be shortened by the combination of hydroxychloroquine and streptomycin [97]. However, a multicenter, blinded, placebo-controlled randomized trial proved that hydroxychloroquine had no therapeutic benefit in adults hospitalized with COVID-19 [99].
Severe COVID-19 is characterized by a hyperinflammatory state or even a cytokine storm, resulting in the elevation of a large number of cytokines, such as interleukin 1 (IL-1) and IL-6 [100,101]. Therefore, some receptor blockers have received much attention. Anakinra is an anti-IL-1 receptor blocker approved for the treatment of rheumatoid arthritis, which has been shown to improve respiratory function and may be beneficial for severe COVID-19 [102,103]. Tocilizumab, a humanized anti-IL-6 monoclonal antibody, inhibits membrane-bound and soluble IL-6 receptors, which is commonly used in rheumatology [78]. At present, tocilizumab has been successfully applied to a few severe cases of COVID-19 without adverse events [81,104]. Sarilumab, another IL-6 receptor antagonist, is also available as a treatment for severe COVID-19 [81]. Convalescent plasma (CP) therapy refers to the therapeutic process involving the collection of plasma components from patients who have recovered from specific infections, and infusing them into critically ill patients to provide passive immunization; this method was applied in multiple viral outbreaks including SARS, MERS, Spanish flu, and Ebola [105,106]. A controlled trial including ten patients with severe COVID-19 confirmed that CP could significantly improve oxyhemoglobin saturation, increase lymphocyte counts, and decrease C-reactive protein [107]. Furthermore, CP could promote virus-negative transformation at 72 h and improve adverse events, but did not result in clinical improvement within 28 days, as reported in an open-label, multicenter, randomized clinical trial [108].
We can thus conclude that activity-based drug repurposing is an effective and feasible strategy. In this epidemic, various types of drugs have been screened and verified by in vivo and in vitro preclinical research, and clinical trials, resulting in the identification of many valuable drugs for COVID-19 treatment.
4. Conclusion and future prospectives
COVID-19 has been the most widespread global pandemic in the past hundred years. Drug research and development in response to this sudden outbreak of a pathogenic virus is a major problem that needs to be addressed, and drug repurposing has provided valuable information in this regard.
Here, we summarize several commonly used techniques for drug repurposing and discuss the main contributions of each. First, through virtual screening in multiple databases, in silico repurposing approaches have predicted a variety of drugs acting on key SARS-CoV-2 protein targets such as 3CLpro and RdRp. Second, network pharmacology has been used to identify and screen various candidate drugs for COVID-19, such as mercaptopurine and sirolimus. Finally, through experimental verification of drugs with multiple activities, such as improving respiratory symptoms, antiviral activity, and immunomodulatory activity, activity-based drug repurposing has found a series of drugs with therapeutic effects on COVID-19, such as LH and kaletra. Among them, in silico repurposing approaches are likely to be more effective and quicker to work in COVID-19 treatment.
Although a vast number of coronavirus-based or host-based trials for drugs with in vivo or in vitro activities against SARS-CoV-2 have been carried, only a few of these drugs can be applied in clinical practice. Drug repurposing provides a personal reference basis for rapid and efficient drug discovery for COVID-19 treatment. However, as the accuracy of results in drug repurposing is related to the sufficient degree of data mastered by researchers, the application of this method in practice is limited to a certain extent [105]. Further clinical trials of the screened drugs still have the possibility of limited efficacy and potential side effects. Developing more accurate and efficient drug repurposing technologies could potentially reduce the risk of and increase the effectiveness of drug repurposing, thus better finding effective drugs against new infectious diseases [106].
Acknowledgments
This project was supported by the PhD Start-up Fund of Guangdong Medical University (Grant No.: B2019016), Administration of Traditional Chinese Medicine of Guangdong Province (Grant No.: 20201180), Science and Technology Special Project of Zhanjiang (Project No.: 2019A01009), Natural Science Foundation of Guangdong Province (Grant No.: 2016B030309002), Basic and Applied Basic Research Program of Guangdong Province (Grant No.: 2019A1515110201), Educational Commission of Guangdong Province (Grant No.: 4SG20138G), and Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (Grant No.: ZJW-2019-007).
Footnotes
Peer review under responsibility of Xi’an Jiaotong University.
Declaration of competing interests
The author declared that there are no conflicts of interest.
References
- 1.Lu H., Stratton C.W., Tang Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y., Wang G., Cai X.P., et al. An overview of COVID-19. J. Zhejiang Univ.-SC. 2020;21:343–360. doi: 10.1631/jzus.B2000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A.K., Jneid H., Addison D., et al. Current perspectives on coronavirus disease 2019 and cardiovascular disease: A white paper by the JAHA editors. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeu Y., Yoon Y., Park S. Protein localization vector propagation: a method for improving the accuracy of drug repositioning. Mol. Biosyst. 2015;11:2096–2102. doi: 10.1039/c5mb00306g. [DOI] [PubMed] [Google Scholar]
- 6.DiMasi J.A., Hansen R.W., Grabowski H.G. The price of innovation: new estimates of drug development costs. J. Health Econ. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 7.Xue H., Li J., Xie H., et al. Review of drug repositioning approaches and resources. Int. J. Biol. Sci. 2018;14:1232–1244. doi: 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 9.Pushpakom S., Iorio F., Eyers P.A., et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 10.Papapetropoulos A., Szabo C. Inventing new therapies without reinventing the wheel: the power of drug repurposing. Br. J. Pharmacol. 2018;175:165–167. doi: 10.1111/bph.14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sardana D., Zhu C., Zhang M., et al. Drug repositioning for orphan diseases. Brief. Bioinform. 2011;12:346–356. doi: 10.1093/bib/bbr021. [DOI] [PubMed] [Google Scholar]
- 12.Singhal S., Mehta J., Desikan R., et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 13.Chen S., Tian J., Li Z., et al. Feline infectious peritonitis virus Nsp5 inhibits type I interferon production by cleaving NEMO at multiple sites. Viruses. 2019;12:43. doi: 10.3390/v12010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sah R., Rodriguez-Morales A.J., Jha R., et al. Complete genome sequence of a 2019 novel coronavirus (SARS-CoV-2) strain isolated in Nepal. Microbiol. Resour. Announc. 2020;9 doi: 10.1128/MRA.00169-20. e00169-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutard B., Valle C., de Lamballerie X., et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C., Zhou Q., Li Y., et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorbalenya A.E., Pringle F.M., Zeddam J.-L., et al. The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J. Mol. Biol. 2002;324:47–62. doi: 10.1016/S0022-2836(02)01033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X., Chou C.-Y., Chang G.-G. Thiopurine analogue inhibitors of severe acute respiratory syndrome-coronavirus papain-like protease, a deubiquitinating and deISGylating enzyme. Antivir. Chem. Chemother. 2009;19:151–156. doi: 10.1177/095632020901900402. [DOI] [PubMed] [Google Scholar]
- 23.Cheng K.-W., Cheng S.-C., Chen W.-Y., et al. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antivir. Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan J.F., Lau S.K., To K.K., et al. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirato K., Kawase M., Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J. Virol. 2013;87:12552–12561. doi: 10.1128/JVI.01890-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walls A.C., Park Y.-J., Tortorici M.A., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H., Penninger J.M., Li Y., et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch B.J., van der Zee R., de Haan C.A., et al. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Z., Xu Y., Bao L., et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zumla A., Chan J.F., Azhar E.I., et al. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., et al. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80:5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fehr A.R., Perlman S. Coronaviruses: Methods and Protocols, Humana Press. 2015. Coronaviruses: An Overview of Their Replication and Pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knoops K., Kikkert M., van den Worm S.H., et al. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadler K., Masignani V., Eickmann M., et al. SARS--beginning to understand a new virus. Nat. Rev. Microbiol. 2003;1:209–218. doi: 10.1038/nrmicro775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raju T.N. The Nobel chronicles. 1988: James Whyte Black, (b 1924), Gertrude Elion (1918-99), and George H Hitchings (1905-98) Lancet. 2000;355:1022. doi: 10.1016/s0140-6736(05)74775-9. [DOI] [PubMed] [Google Scholar]
- 36.Yu H., Chen J., Xu X., et al. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng F., Liu C., Jiang J., et al. Prediction of drug-target interactions and drug repositioning via network-based inference. PLoS Comput. Biol. 2012;8 doi: 10.1371/journal.pcbi.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodos R.A., Kidd B.A., Shameer K., et al. In silico methods for drug repurposing and pharmacology. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016;8:186–210. doi: 10.1002/wsbm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morse J.S., Lalonde T., Xu S., et al. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry M., Fielding B.C., Gamieldien J. Potential broad spectrum inhibitors of the coronavirus 3CLpro: a virtual screening and structure-based drug design study. Viruses. 2015;7:6642–6660. doi: 10.3390/v7122963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahanta S., Chowdhury P., Gogoi N., et al. Potential anti-viral activity of approved repurposed drug against main protease of SARS-CoV-2: an in silico based approach. J. Biomol. Struct. Dyn. 2021;39:3802–3811. doi: 10.1080/07391102.2020.1768902. [DOI] [PubMed] [Google Scholar]
- 42.Odhar H.A., Ahjel S.W., Albeer A., et al. Molecular docking and dynamics simulation of FDA approved drugs with the main protease from 2019 novel coronavirus. Bioinformation. 2020;16:236–244. doi: 10.6026/97320630016236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhardwaj V.K., Singh R., Sharma J., et al. Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2021;39:3449–3458. doi: 10.1080/07391102.2020.1766572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittal L., Kumari A., Srivastava M., et al. Identification of potential molecules against COVID-19 main protease through structure-guided virtual screening approach. J. Biomol. Struct. Dyn. 2021;39:3662–3680. doi: 10.1080/07391102.2020.1768151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arun K.G., Sharanya C.S., Abhithaj J., et al. Drug repurposing against SARS-CoV-2 using E-pharmacophore based virtual screening, molecular docking and molecular dynamics with main protease as the target. J. Biomol. Struct. Dyn. 2021;39:4647–4658. doi: 10.1080/07391102.2020.1779819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Z., Bastas O., Demtchenko M., et al. Ro5 Bioactivity Lab: Identification of Drug Candidates for COVID-19, ChemRxiv. https://chemrxiv.org/engage/chemrxiv/article-details/60c74af40f50db3836396b4e
- 47.Tsuji M. Potential anti-SARS-CoV-2 drug candidates identified through virtual screening of the ChEMBL database for compounds that target the main coronavirus protease. FEBS Open Bio. 2020;10:995–1004. doi: 10.1002/2211-5463.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y.W., Yiu C.-B., Wong K.-Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL (pro)) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates, F1000Res. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kandeel M., Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251:117627. doi: 10.1016/j.lfs.2020.117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aftab S.O., Ghouri M.Z., Masood M.U., et al. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020;18:275. doi: 10.1186/s12967-020-02439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bibi N., Gul S., Ali J., et al. Viroinformatics approach to explore the inhibitory mechanism of existing drugs repurposed to fight against COVID-19. Eur. J. Pharmacol. 2020;885:173496. doi: 10.1016/j.ejphar.2020.173496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J. Biomol. Struct. Dyn. 2021;39:3204–3212. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iftikhar H., Ali H.N., Farooq S., et al. Identification of potential inhibitors of three key enzymes of SARS-CoV2 using computational approach. Comput. Biol. Med. 2020;122:103848. doi: 10.1016/j.compbiomed.2020.103848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu C., Liu Y., Yang Y., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Oliveira O.V., Rocha G.B., Paluch A.S., et al. Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening. J. Biomol. Struct. Dyn. 2021;39:3924–3933. doi: 10.1080/07391102.2020.1772885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teralı K., Baddal B., Gülcan H.O. Prioritizing potential ACE2 inhibitors in the COVID-19 pandemic: insights from a molecular mechanics-assisted structure-based virtual screening experiment. J. Mol. Graph. Model. 2020;100:107697. doi: 10.1016/j.jmgm.2020.107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucas J.M., Heinlein C., Kim T., et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DurdaĞi S. Virtual drug repurposing study against SARS-CoV-2 TMPRSS2 target. Turk. J. Biol. 2020;44:185–191. doi: 10.3906/biy-2005-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hopkins A.L. Network pharmacology. Nat. Biotechnol. 2007;25:1110–1111. doi: 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- 62.Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 63.Cheng F., Rao S., Mehra R. COVID-19 treatment: combining anti-inflammatory and antiviral therapeutics using a network-based approach. Cleve. Clin. J. Med. 2020 doi: 10.3949/ccjm.87a.ccc037. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y., Hou Y., Shen J., et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hazra S., Chaudhuri A.G., Tiwary B.K., et al. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: a network-based meta-analysis. Life Sci. 2020;257:118096. doi: 10.1016/j.lfs.2020.118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cava C., Bertoli G., Castiglioni I. In silico discovery of candidate drugs against Covid-19. Viruses. 2020;12:404. doi: 10.3390/v12040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadegh S., Matschinske J., Blumenthal D.B., et al. Exploring the SARS-CoV-2 virus-host-drug interactome for drug repurposing. Nat. Commun. 2020;11:3518. doi: 10.1038/s41467-020-17189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu S., Wang J., Shen H. Network pharmacology-based analysis of the role of traditional Chinese herbal medicines in the treatment of COVID-19, Ann. Palliat. Med. 2020;9:437–446. doi: 10.21037/apm.2020.03.27. [DOI] [PubMed] [Google Scholar]
- 70.Li X., Qiu Q., Li M., et al. Chemical composition and pharmacological mechanism of ephedra-glycyrrhiza drug pair against coronavirus disease 2019 (COVID-19) Aging (Albany NY) 2021;13:4811–4830. doi: 10.18632/aging.202622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu Q., Huang Y., Liu X., et al. Potential therapeutic effect of traditional Chinese medicine on coronavirus disease 2019: A review. Front. Pharmacol. 2020;11:570893. doi: 10.3389/fphar.2020.570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding Y., Zeng L., Li R., et al. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement Altern. Med. 2017;17:130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li R., Hou Y., Huang J., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu K., Guan W.-J., Bi Y., et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y., Hou H., Ren Q., et al. Natural drug sources for respiratory diseases from Fritillaria: chemical and biological analyses. Chin. Med. 2021;16:40. doi: 10.1186/s13020-021-00450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo E., Zhang D., Luo H., et al. Treatment efficacy analysis of traditional Chinese medicine for novel coronavirus pneumonia (COVID-19): an empirical study from Wuhan, Hubei Province, China. Chin. Med. 2020;15:34. doi: 10.1186/s13020-020-00317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao X., Jiang Y., Zhao Y., et al. Analysis of the susceptibility to COVID-19 in pregnancy and recommendations on potential drug screening. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1209–1220. doi: 10.1007/s10096-020-03897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCreary E.K., Pogue J.M. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa105. ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Wit E., Feldmann F., Cronin J., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U.S.A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanders J.M., Monogue M.L., Jodlowski T.Z., et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 82.Boriskin Y.S., Leneva I.A., Pécheur E.-I., et al. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 83.Yavuz S.S., Ünal S. Antiviral treatment of COVID-19. Turk. J. Med. Sci. 2020;50:611–619. doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caly L., Druce J.D., Catton M.G., et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawase M., Shirato K., van der Hoek L., et al. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeda K., Takada T., Kawarada Y., et al. JPN Guidelines for the management of acute pancreatitis: medical management of acute pancreatitis. J. Hepatobiliary Pancreat. Surg. 2006;13:42–47. doi: 10.1007/s00534-005-1050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ota S., Hara Y., Kanoh S., et al. Acute eosinophilic pneumonia caused by camostat mesilate: the first case report. Respir. Med. Case Rep. 2016;19:21–23. doi: 10.1016/j.rmcr.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grigg M.J., William T., Dhanaraj P., et al. A study protocol for a randomised open-label clinical trial of artesunate-mefloquine versus chloroquine in patients with non-severe Plasmodium knowlesi malaria in Sabah, Malaysia (ACT KNOW trial) BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Savarino A., Boelaert J.R., Cassone A., et al. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu C., Lu L., Wan J.-P., et al. The pharmacological mechanisms and therapeutic activities of hydroxychloroquine in rheumatic and related diseases. Curr. Med. Chem. 2017;24:2241–2249. doi: 10.2174/0929867324666170316115938. [DOI] [PubMed] [Google Scholar]
- 91.Schlagenhauf P., Grobusch M.P., Maier J.D., et al. Repurposing antimalarials and other drugs for COVID-19, Travel. Med. Infect. Dis. 2020;34:101658. doi: 10.1016/j.tmaid.2020.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahraei Z., Shabani M., Shokouhi S., et al. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int. J. Antimicrob. Agents. 2020;55:105945. doi: 10.1016/j.ijantimicag.2020.105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jean S.S., Lee P.-I., Hsueh P.-R. Treatment options for COVID-19: the reality and challenges. J. Microbiol. Immunol. Infect. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 95.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 96.Shah N., Davariya V., Gupta S.K., et al. Review: An insight into coronaviruses: Challenges, security and scope. Rev. Med. Virol. 2020;30:1–6. doi: 10.1002/rmv.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao X., Ye F., Zhang M., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Self W.H., Semler M.W., Leither L.M., et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: A randomized clinical trial. J. Am. Med. Assoc. 2020;324:2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou Y., Fu B., Zheng X., et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dimopoulos G., de Mast Q., Markou N., et al. Favorable anakinra responses in severe covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117–123.e1. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramírez J., Cañete J.D. Anakinra for the treatment of rheumatoid arthritis: a safety evaluation. Expet Opin. Drug Saf. 2018;17:727–732. doi: 10.1080/14740338.2018.1486819. [DOI] [PubMed] [Google Scholar]
- 104.Le R.Q., Li L., Yuan W., et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bloch E.M., Shoham S., Casadevall A., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar P., Sah A.K., Tripathi G., et al. Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in COVID-19. Mol. Cell. Biochem. 2021;476:553–574. doi: 10.1007/s11010-020-03924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U.S.A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li L., Zhang W., Hu Y., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]