Abstract
Objective:
Blood cultures are fundamental in evaluating for sepsis, but excessive cultures can lead to false positive results and unnecessary antibiotics. Our objective was to create consensus recommendations focusing on when to safely avoid blood cultures in pediatric intensive care unit (PICU) patients.
Design:
A panel of 29 multidisciplinary experts engaged in a two-part modified Delphi process. Round 1 consisted of a literature summary and an electronic survey sent to invited participants. In the survey, participants rated a series of recommendations about when to avoid blood cultures on 5 point Likert scale. Consensus was achieved for the recommendation(s) if 75% of respondents chose a score of 4 or 5, and these were included in the final recommendations. Any recommendations that did not meet these a priori criteria for consensus were discussed during the in-person expert panel review (Round 2). Round 2 was facilitated by an independent expert in consensus methodology. After a review of the survey results, comments from round 1, and group discussion, the panelists voted on these recommendations in real-time.
Setting:
Experts’ institutions; in-person discussion in Baltimore, Maryland
Subjects:
Experts in pediatric critical care, infectious diseases, nephrology, oncology, and laboratory medicine
Interventions:
None
Measurements and Main Results:
Of the 27 original recommendations, 18 met criteria for achieving consensus in Round 1; some were modified for clarity or condensed from multiple into single recommendations during Round 2. The remaining 9 recommendations were discussed and modified until consensus was achieved during Round 2, which had 26 real-time voting participants. The final document contains 19 recommendations.
Conclusions:
Using a modified Delphi process, we created consensus recommendations on when to avoid blood cultures and prevent overuse in the PICU. These recommendations are a critical step in disseminating diagnostic stewardship on a wider scale in critically ill children.
Indexing: blood culture, bacteremia, sepsis, clinical decision-making, infection, quality improvement
Introduction
In hospitalized children, severe sepsis is common (8.2% prevalence) and deadly (25% mortality rate) [1, 2]. Bacterial bloodstream infections are an important cause of sepsis, and blood cultures are the gold standard for diagnosing bacteremia. Blood cultures are typically coupled with empiric antibiotics, and results guide subsequent therapy. Delayed initiation of broad-spectrum antibiotics increases morbidity and mortality [3]. Accordingly, pediatric hospitals now prioritize early sepsis recognition and rapid antibiotic administration as key performance metrics [4]. Rapid diagnosis of bacterial sepsis is clearly beneficial, but in practice, PICU clinicians are faced with two important challenges: the non-specific nature of presenting symptoms of sepsis in children, and the limitations of blood cultures as a diagnostic test. In children, clinical symptoms like fever or leukocytosis are neither sensitive nor specific for infection, and no single biomarker or decision rule can perfectly identify patients likely to have bacteremia [5–7]. Blood cultures, perceived as a low-risk test for a disease with potentially fatal outcomes, are subject to excessive use with a low threshold in PICUs, as well as in other healthcare settings (e.g., emergency department, outpatient clinics) [8]. In fact, blood cultures have an overall yield of only 5–15% and up to a 50% false positive rate [9–11]. Indiscriminate use of blood cultures leads to avoidable false positive results, unnecessary antibiotics, increased lengths of stay, and increased costs [9,12].
Diagnostic stewardship, which refines the use of diagnostic tools in order to improve treatment decisions, is thus critically needed for blood cultures [13–15]. Preliminary work has shown that reduction in unnecessary blood cultures in pediatric intensive care unit (PICU) patients is feasible and safe [16–18]. Presently, however, no widely accepted guidelines or recommendations for the optimal use of blood cultures in PICU patients exists, and blood culture practices vary widely in the PICU setting [19]. In recognition of this knowledge gap, we now present the first-ever consensus recommendations on blood cultures in critically ill children, developed via a 14-site PICU collaborative named Bright STAR (Blood culture improvement guidelines and diagnostic stewardship for antibiotic reduction in critically ill children), and using a modified Delphi consensus method.
Methods
Brief description of Bright STAR Collaborative
Bright STAR (NCT03441126) is a quality improvement collaborative of 14 PICUs across the United States that started in 2018, and is led by a multidisciplinary coordinating team with expertise in pediatric infectious diseases, pediatric critical care, patient safety and quality, and human factors engineering. The goals of Bright STAR are to improve the use of blood cultures in critically ill children, by standardizing practices and safely reducing unnecessary cultures and their downstream consequences, such as unnecessary antibiotics. Each enrolled site identified project champions, evaluated current blood culture practices, engaged key stakeholders, created a new clinical tool for changing blood culture practice with stakeholder support, implemented this tool, monitored for safety and balancing metrics of the new blood culture practices (such as delayed diagnosis of bacteremia), and shared blood culture rates, antibiotics use, and a variety of other data elements with the coordinating team. Analysis of these primary outcomes is currently underway. The consensus recommendation work described below was a distinct project within Bright STAR, in which content experts came together to establish consensus-based recommendations for safe reduction of blood cultures in PICU patients.
Selection of modified Delphi method and regulatory oversight
The Delphi process is a validated method of achieving consensus that has been widely used in health care [20]. We chose to use a 2-round modified Delphi method with electronic (email) adaptations, a process effectively used in pediatric critical care and other clinical settings to establish consensus when data and strong evidence are limited and clinical practice is highly variable [21–22]. This work was deemed exempt from Institutional Review Board (IRB) oversight at Johns Hopkins University, the main study site.
Expert Panel
The coordinating team convened a multidisciplinary, multi-institution physician expert panel (Supplement 1). Out of 34 total invited experts, 29 people completed Round 1, and 26 people completed both Round 1 and Round 2. Of the final 26 participants, 20 were subject matter experts in pediatric critical care and/or pediatric infectious diseases from 12 Bright STAR sites or the Bright STAR study team with experience implementing programs to optimize blood culture use (Supplement 1). The 6 non-Bright STAR participants were subject matter experts in pediatric critical care, oncology, nephrology, and microbiology. Four national societies were represented, including the Pediatric Acute Lung Injury and Sepsis Investigator Network (PALISI), Society of Critical Care Medicine (SCCM), Society for Healthcare Epidemiology of America (SHEA), and the Pediatric Infectious Diseases Society (PIDS).
Literature Review and Development of Recommendations
This document is not based on a systematic literature search; instead the coordinating team conducted a scoping review on blood culture use in pediatric patients and compiled a summary of existing pertinent literature [23]. Based on the literature review and specific evidence to date for safe blood culture reduction in PICU patients from the study team’s previous work [16, 18], the coordinating team developed 27 recommendations about blood culture practices and when to avoid blood cultures. These recommendations were grouped in three core domains: 1) general practices and decision-making for blood cultures in PICU patients 2) specific clinical scenarios in which blood cultures can be safely deferred in immunocompetent PICU patients and 3) specific clinical scenarios in which blood cultures can be safely deferred in immunocompromised PICU patients. The coordinating team circulated the literature summary to participants.
Delphi Process
Round 1: Survey
An electronic survey including the 27 recommendations and scenarios was created and distributed via email to each of the participants between July to August 2019. Participants rated the importance, feasibility, and overall level of agreement with the proposed recommendations on a 5-point Likert Scale, from lowest (1) to highest (5) importance, feasibility, and overall level of agreement with the recommendation, respectively (Qualtrics, Provo, UT). The coordinating team defined, a priori, that “achieving consensus” for a recommendation required 75% of respondents to give the recommendation a score of 4 or 5 for overall level of agreement [24–25]. We combined the scores of 4 and 5 for each item into a percentage agreement, separating those recommendations that scored ≥75% from those that scored <75%. Recommendations meeting this criterion were included as final recommendations, and items not meeting this criterion were discussed during Round 2.
Round 2: Panel Discussion
Three months after completing the Round 1 surveys, we held a two-day in person session, where an expert in Delphi consensus methodology shared the Round 1 survey results and facilitated in-person discussion. Participants were asked to review each recommendation that had not achieved consensus in Round 1 and share reasons for agreement or disagreement with the recommendation. Participants proposed modifications or revisions and participants voted in real time on revised recommendations using blinded paper ballots. The same criteria (≥75% agreement) was used during Round 2 to include a recommendation in the final recommendations.
Additionally, participants reviewed and modified the recommendations that had already achieved consensus in Round 1 to clarify or condense multiple recommendations into single recommendations.
Intended use of these recommendations
Safe blood culture reduction in children is a topic that lacks the level of evidence required for a formal guideline using the GRADE or a similar systematic methodology. These consensus recommendations are based on the subject matter experts’ synthesis of evidence, current practices, practical considerations, and consideration of potential harm where applicable. These recommendations do not encompass all clinical situations and are not meant to be a substitute for individual clinical judgment by qualified professionals. Please also note that every recommendation is based on the absence of any signs or symptoms concerning for sepsis, as determined by the judgment of the clinicians caring for the patient and current national guidelines [26].
Review and Endorsement
This document was reviewed and endorsed by PALISI, PIDS, SHEA, and SCCM [Supplement 2].
Results
In Round 1, we received 29 completed surveys from 34 invited participants for an 85% response rate. A survey was considered complete if ≥90% of items were answered. Of the 27 original proposed recommendations, 18 met pre-determined criteria for consensus based on survey results. These 18 were condensed into 12 recommendations during Round 2 discussions. Of the 9 recommendations that did not meet consensus criteria based on the Round 1 survey results, 3 achieved consensus after Round 2 discussion and voting; 2 were merged into other recommendations; 4 were not included as consensus was not achieved (Supplement 3). In addition, 2 new recommendations were proposed during the Round 2 meeting and were included after achieving consensus.
Overall, participating subject matter experts developed 19 consensus recommendations that provide guidance on how to safely reduce blood cultures in critically ill children (see Figure 1, Tables 1–3, and Executive Summary in Supplement 4). Recommendations were organized in the areas of general clinical practice for blood cultures, cultures in immunocompetent patients with and without a central venous catheter (CVC), and cultures in immunocompromised patients (Tables 1–3). There were 8 recommendations (either from Round 1 or new proposals during Round 2) that were discussed but did not reach consensus for inclusion (Supplement 5, Non-Consensus “NC” recommendations). Supplements 6–9 detail all of the individual recommendations with important points or commentary that were raised during the Round 2 discussions related to their inclusion or exclusion from the final recommendations.
Figure 1.
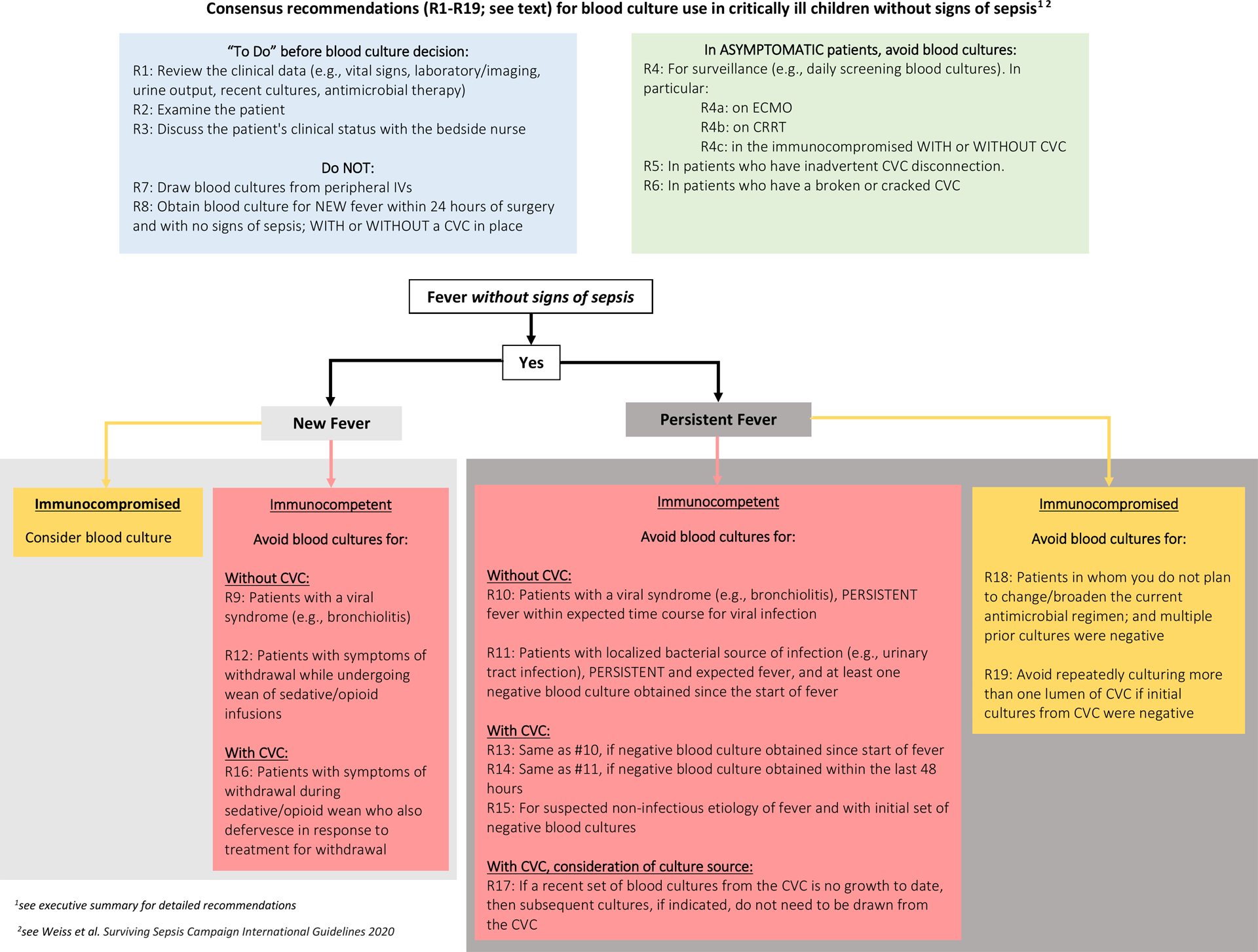
Delphi consensus recommendations for blood culture use in critically ill children without signs of sepsis
Table 1.
Consensus recommendations on general clinical practices for blood cultures in critically ill children
| Recommendation | % In Agreement with recommendation* | |
|---|---|---|
| 1 | Clinicians should review a patient’s clinical data (such as vital signs, existing laboratory/imaging data, urine output, recent cultures, current antimicrobial therapy) prior to making the decision to order or not order a blood culture. | 97% (27/28) |
| 2 | Clinicians should perform a physical exam prior to making the decision to order or not order a blood culture. | 89% (25/28) |
| 3 | Clinicians should discuss a patient’s clinical status with the bedside nurse to inform the decision to order or not order a blood culture. | 96% (25/26) |
| 4 | Avoid surveillance blood cultures (e.g. daily screening blood cultures) in all patients. 4a Avoid surveillance blood cultures (e.g. daily screening blood cultures) for patients on extracorporeal membrane oxygenation (ECMO). 4b Avoid surveillance blood cultures (e.g. daily screening blood cultures) for patients on continuous renal replacement therapy (CRRT). 4c Avoid surveillance blood cultures (e.g. daily screening blood cultures) in immunocompromised patients WITH or WITHOUT central venous catheters. |
96% (24/25) |
| 5 | Avoid blood cultures in asymptomatic patients who experience an inadvertent central venous catheter disconnection. | 89% (26/29) |
| 6 | Avoid blood cultures in asymptomatic patients who have a broken or cracked central venous catheter. | 83% (24/29) |
| 7 | Avoid drawing blood cultures from peripheral IVs. | 100% (26/26) |
| 8 | Avoid blood culture in patients with NEW fever within 24 hours after surgery, with no signs of sepsis, WITH or WITHOUT a central venous catheter in place. | 96% (25/26) |
Denominator varies slightly due to unanswered items on a small number of surveys analyzed, with a possible total of 29 responses
Table 3.
Consensus recommendations on blood culture practices in symptomatic immunocompromised critically ill children, WITH or WITHOUT a central venous catheter
| Recommendation | % In Agreement with recommendation* | |
|---|---|---|
| 18 | After repeated negative-to-date blood cultures, avoid additional blood cultures in immunocompromised patients with PERSISTENT fever, but without signs of sepsis or infection, in whom you do not plan to change/broaden the current antimicrobial regimen. | 89% (24/27) |
| 19 | For PERSISTENT fever in immunocompromised patients without signs of sepsis, if initial set of blood cultures from all lumens of central venous catheters were negative, avoid repeatedly culturing more than one lumen of that central venous catheter. | 85% (23/27) |
Denominator varies slightly due to unanswered items on a small number of surveys analyzed, with a possible total of 29 responses
Discussion
Below, we have summarized key discussion points, challenges, and opportunities raised regarding the proposed recommendations, including those recommendations that did and did not reach consensus. Five important themes emerged from our panel’s discussion: pre-culture decision making for clinicians, surveillance cultures, cultures in the setting of a central venous catheter, culture source, and cultures in immunocompromised PICU patients.
1. Pre-culture decision making for clinicians (recommendations 1–3; Table 1):
There was strong agreement within our expert panel that specific review of a patient’s clinical status (e.g., vital signs, laboratory results), along with a physical exam and a discussion with the patient’s nurse, should occur before a clinician orders a blood culture. These recommendations may seem “obvious”, but our prior data found that pre-culture data review and physical exams are not frequently done by PICU providers before ordering blood cultures [24]. In contrast, a proposed recommendation that discussion with a PICU patient’s consulting services should occur prior to blood culture decision did not reach consensus, as there was concern among our panel that this could lead to delay in patient evaluation.
2. Surveillance blood cultures (recommendations 4a, 4b, 4c; Table 1):
The expert panel’s recommendation to avoid blood cultures in asymptomatic patients, including patients whose temperature regulation may be impacted by support devices [i.e., ECMO (extracorporeal membrane oxygenation) or CRRT (continuous renal replacement therapy)], is consistent with existing guidance, such as the Extracorporeal Life Support Organization’s (ELSO) recommendation to avoid surveillance blood cultures [27]. However, surveillance blood culture practices for ECMO patients are variable despite these existing specific recommendations: for example, 4/14 Bright STAR sites initially reported routine surveillance blood cultures for ECMO patients. Prior literature suggests variability in practice regarding surveillance blood cultures for ECMO or CRRT patients [28–30]. Our consensus recommendations represent an opportunity to better standardize practices across institutions.
3. The impact of a CVC on blood culture practices (recommendations 13–17; Table 2).
Table 2.
Consensus recommendations for blood culture practices in symptomatic immunocompetent critically ill children, WITH and WITHOUT a central venous catheter
| Recommendation | % In Agreement with recommendation* | |
|---|---|---|
| 9 | Avoid blood culture in patients with a viral syndrome (such as bronchiolitis), NEW fever, no signs of sepsis, and WITHOUT central venous catheter in place. | 85% (23/27) |
| 10 | Avoid blood culture in patients with a viral syndrome (such as bronchiolitis), PERSISTENT fever within expected time course for viral infection, no signs of sepsis, and WITHOUT central venous catheter in place. | 89% (24/27) |
| 11 | Avoid blood culture in patients with a localized bacterial source of infection (e.g., urinary tract infection or focal pneumonia), PERSISTENT and expected fever, no signs of sepsis, at least one negative blood culture obtained since the start of fever, and WITHOUT a central venous catheter. | 81% (22/27) |
| 12 | Avoid blood culture in patients with NEW fever, no signs of sepsis, and with symptoms of withdrawal while undergoing wean of sedative/opioid infusions, and WITHOUT a central venous catheter in place. | 88% (23/26) |
| 13 | Avoid repeat blood cultures in patients with a symptomatic viral infection (such as bronchiolitis), PERSISTENT fever within expected time course for this viral infection, no signs of sepsis, and who has already had at least one negative blood culture obtained since the start of fever, WITH central venous catheter in place. | 100% (27/27) |
| 14 | Avoid blood culture in patients with a documented localized bacterial infection (e.g., urinary tract infection or focal pneumonia), PERSISTENT and expected fever, no signs of sepsis, and who has a blood culture that is negative to date obtained within the last 48 hours, and WITH a central venous catheter. | 100% (27/27) |
| 15 | For PERSISTENT fever in immunocompetent patients WITH a central venous catheter, suspected non-infectious etiology of fever and no documented source of infection, without signs of sepsis, and with initial set of negative blood cultures, avoid additional blood cultures. | 78% (21/27) |
| 16 | Avoid blood culture in patients with NEW fever, no signs of sepsis, and with symptoms of withdrawal while undergoing wean of sedative/opioid infusions, WITH a central venous catheter in place, who defervesces in response to treatment for withdrawal. | 100% (26/26) |
| 17 | For PERSISTENT fever in patients WITH central venous catheter and without signs of sepsis, if a recent set of blood cultures from the catheter is no growth to date, then subsequent cultures, if indicated, do not need to be drawn from the catheter. | 96% (25/26) |
Denominator varies slightly due to unanswered items on a small number of surveys analyzed, with a possible total of 29 responses
Because CVCs are a known risk factor for the development of bacteremia [31], our panel gave strong weight to the presence of a CVC when considering how to approach critically ill patients with new or persistent fevers. Several times, consensus about a recommendation hinged on documenting negative blood cultures at least once for patients with a CVC before deferring additional cultures. Overall, for patients with CVCs, there are two subtle but important scenarios where blood cultures can be avoided: 1) after an initial set of negative blood cultures in patients with CVCs with ongoing fever in the absence of other new symptoms or plans to change antimicrobials; and 2) when non-infectious etiologies are identified in patients with CVCs and fever such as a recent surgery or sedative wean. Agreement on these points may spare patients unnecessary blood cultures and their associated negative consequences (risk of false positives, excessive CVC entry, phlebotomy for patients with marginal hemoglobin levels during critical illness, and strain on laboratory resources).
4. Blood culture sources (recommendation 7, Table 1; recommendation 17, Table 2; recommendation 19, Table 3).
Evidence for best practice for blood culture source in critically ill children is limited. In the survey work that predated this consensus conference, we described that culture source was subject to notable variation across provider type and institutions [19]. Only 3 recommendations related to culture source reached consensus (recommendations 7, 17, 19), with several focusing on the avoidance of blood cultures from specific sources, such as from every lumen of a CVC or from a PIV.
One important issue (and subject of much debate during our Round 2 conference) is the utility of blood cultures drawn from CVCs. A positive blood culture from a CVC means one of three scenarios is present: bacteremia, catheter colonization, or culture contamination. Colonization (growth of microorganisms on the surface of a catheter such that the colonizing organisms would be expected to grow in blood samples obtained from the catheter) is quite different from contamination (when organisms that are not actually present in a blood sample are grown in culture). In practice, it can be challenging to distinguish between these three scenarios, and the clinical uncertainty can have significant consequences for patients (either overtreatment of false positives or under-treatment of bacteremia) [32]. Many studies have investigated the utility of cultures from CVCs instead of or in addition to peripheral venipuncture samples. Conclusions of these studies vary, but overall, it appears that CVC cultures have increased sensitivity and negative predictive value compared to peripheral specimens, but at the cost of lower specificity and higher rates of false positive results [9]. Multiple guidelines call for blood culture samples to come preferentially from peripheral venipuncture, either alone or in combination with CVC blood cultures, but this practice is not consistently used in pediatrics when a CVC is present [19, 33–34]. Discussions on this topic among our expert panel during Round 2 cited perceived logistical barriers (difficult venous access in children), desire to limit painful procedures for ill children, and strong belief that clinically meaningful bacterial infections would be missed if CVCs were not sampled for culture in the proposed scenarios. Acknowledging these concerns, and that existing data to drive blood culture source practices for PICU patients are limited, the clinicians in our panel did not reach consensus on several proposed recommendations about blood culture source during our Delphi process (Supplement 5).
5. Blood cultures in immunocompromised PICU patients (recommendations 18–19, Table 3).
Immunocompromised critically ill children are a patient population that presents unique challenges with regard to blood culture diagnostic stewardship. Given this population’s high-risk of morbidity and mortality from bloodstream infections, clinicians are understandably hesitant to reduce diagnostic testing for bacteremia [35–37]. However, immunocompromised PICU patients are also at high risk of poor outcomes associated with overtesting, such as excessive entry into CVCs, adverse effects of unnecessary antibiotics such as kidney injury, and antimicrobial resistance [38–39]. Although not the focus of this work, we presented a limited number of candidate recommendations to our expert panel, knowing that an in-depth exploration of blood culture diagnostic stewardship in this patient population was beyond the scope of our consensus conference.
Implementation considerations
Specific attention to implementation strategies for consensus recommendations facilitates their successful translation into clinical practice [40]. An in-depth exploration of implementation strategies for blood culture improvement is currently underway as part of the Bright STAR collaborative, and implementation of these recommendations is an important future direction. During our in-person meeting, expert panelists discussed potential implementation strategies for these consensus recommendations, drawing on their collective experience with changing blood culture practices. We have summarized this material in Table 4. In general, PICUs should have a policy to guide the use of blood cultures, embracing the role of diagnostic stewardship in reducing unintended patient harm [13]. Such policies can include these 19 recommendations and units could develop an accompanying institution-specific algorithm or tool to support implementation (see Figure 1; though note that this particular algorithm is provided as an example and has not yet been used in any sites). Striking a balance between these recommendations and the needs of local stakeholders and local patient populations is critically important in fostering successful clinical practice change, and we encourage institutions to take these 19 recommendations and our example algorithm as an adaptable starting point for launching their own diagnostic stewardship programs.
Table 4.
Suggested implementation strategies for consensus recommendations from Delphi panel participants
| 1. Secure buy-in from leaders and key stakeholders (e.g., PICU clinicians, consultants, patient/families) by providing literature supporting recommended practices |
| 2. Assess local practices and engage stakeholders to adapt the recommendations for local guidelines |
| 3. Provide education (e.g., orientation, presentations, reminders) to PICU clinicians |
| 4. Prepare materials (e.g., scripts) to explain recommended practices (e.g., peripheral culture) to patients and families |
| 5. Apply a phased implementation strategy [(e.g., starting with a subset of PICU patients and growing/spreading to other PICU populations (patients “outside” of algorithm)] |
| 6. Use the electronic medical record and other tools/technologies (e.g., text messages) to integrate recommended practices into daily workflow (e.g., order sets/best practice alerts) and address other system challenges (e.g., unit layout and spatial distance between physician workroom and patient rooms) |
| 7. Collect data on compliance with, and impacts of, recommended practices and provide feedback to leaders and key stakeholders continuously |
Limitations
There are several important limitations to this work. The majority of the expert panelists participated in the Bright STAR collaborative to improve blood culture stewardship, likely impacting their evaluation of proposed recommendations. The panel was not balanced with respect to race or gender, though sites self-selected their team leads and this was not a recruitment strategy by the coordinating team. Generalizability of these recommendations is limited owing to the nature of modified Delphi work and our sample size of participating experts, though the number of participants here is within the range of typical panels for the Delphi process. Finally, safe use of these recommendations requires clinician expertise in managing critically ill children and the ability to frequently re-evaluate patients in the highly monitored setting of the PICU, and these recommendations should not be used in the absence of either of these important elements of PICU care.
Conclusions
A multidisciplinary expert group created 19 recommendations to reduce unnecessary blood cultures in critically ill children while maintaining an appropriately high index of suspicion for patients with potential infection who need blood cultures. These recommendations represent the first guidance of their kind in pediatric critical care, and may allow standardization of a highly variable clinical practice and reduction in unintended harm from unnecessary testing and treatment. Evidence to direct many of the recommendations remains limited in this patient population. Our inability to achieve consensus in some areas underscores that further work is needed to better inform clinical use of diagnostic testing in the PICU setting.
Supplementary Material
Research in Context.
Blood culture practices in critically ill children vary widely, and overuse of cultures can lead to false positive results, unnecessary antibiotics, and patient harm.
Diagnostic stewardship efforts can safely reduce blood culture overuse, but no standards or guidelines currently exist to guide clinicians in specific scenarios.
To meet this need, a multi-center collaborative called Bright STAR used a modified Delphi method to develop the first-ever consensus recommendations for reducing blood culture overuse in the pediatric intensive care unit.
At the bedside.
We recommend that every PICU consider implementing diagnostic stewardship for blood cultures to avoid unnecessary testing and excess antibiotics in critically ill children.
A multidisciplinary expert panel developed 19 recommendations for blood cultures that can be avoided in critically ill children.
Additional study is needed to determine optimal implementation strategies.
Acknowledgements
The authors wish to thank the participants at each Bright STAR study site and the leadership of the enrolled critical care units for supporting this diagnostic stewardship work. We also wish to thank PIDS, SCCM, SHEA, and PALISI for their review and endorsement of this work. Finally, the authors would like to thank Valerie Deloney, MBA for her editorial assistance and organizational expertise in the development of this manuscript.
Financial support:
The study was funded by the Agency for Healthcare Research and Quality (grant 1R18HS025642). Dr. Milstone also receives support from K24AI141580. Dr. Woods-Hill also receives support from the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL151381. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Copyright Form Disclosure:
Drs. Woods-Hill, Koontz, Voskertchian, Xie, and Milstone’s institutions received funding from the Agency for Healthcare Research and Quality (AHRQ). Dr. Woods-Hill’s institution received funding from the National Institutes of Health (NIH). Drs. Woods-Hill and Xie received support for article research from the NIH. Drs. Woods-Hill and Milstone received support for article research from the AHRQ. Dr. Milstone’s institution received funding from Merck. Drs. Shea, Miller, and Fackler have disclosed that they do not have any potential conflicts of interest.
Footnotes
Reprints: No reprints are requested.
The coordinating center for the study was the Johns Hopkins Children’s Center in Baltimore, Maryland.
References
- 1.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015;191(10):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013;14(7):686–693. [DOI] [PubMed] [Google Scholar]
- 3.Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014;42(11):2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Children’s Hospital Association. Improving Pediatric Sepsis Outcomes (IPSO) is successfully challenging sepsis. Available at: https://www.childrenshospitals.org/sepsiscollaborative.AccessedApril 30, 2020.
- 5.Hsiao A and Baker M. Fever in the new millennium: a review of recent studies of markers of serious bacterial infection in febrile children. Current Opin Pediatr 2005; 17(1), 56–61. [DOI] [PubMed] [Google Scholar]
- 6.Nijman RG, Moll HA, Vergouwe Y, et al. C-reactive protein bedside testing in febrile children lowers length of stay at the emergency department. Pediatr Emerg Care 2015;31:633–9. [DOI] [PubMed] [Google Scholar]
- 7.Lautz AJ, Dziorny AC, Denson AR, et al. Value of Procalcitonin Measurement for Early Evidence of Severe Bacterial Infections in the Pediatric Intensive Care Unit. J Pediatr 2016; 179: 74–81.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiragu AW, Zier J, Cornfield DN. Utility of blood cultures in postoperative pediatric intensive care unit patients. Pediatr Crit Care Med 2009; 10:364–8. [DOI] [PubMed] [Google Scholar]
- 9.Doern GV, Carroll KC, Diekema DJ, et al. A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem. Clin Microbiol Rev. 2019; 30:33(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamy B, Dargère S, Arendrup MC, et al. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol 2016;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bone RC, Fisher CJ Jr, Clemmer TP, et al. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med 1989;17:389–393. [PubMed] [Google Scholar]
- 12.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect 2011;77(3):233–236. [DOI] [PubMed] [Google Scholar]
- 13.Morgan DJ, Malani P, Diekema DJ. Diagnostic stewardship – leveraging the laboratory to improve antimicrobial use. JAMA 2017; 318: 607–608 [DOI] [PubMed] [Google Scholar]
- 14.Klompas M, Calandra T, Singer M. Antibiotics for Sepsis—Finding the Equilibrium. JAMA 2018;320(14):1433–1434. [DOI] [PubMed] [Google Scholar]
- 15.Coon ER, Quinonez RA, Moyer VA, et al. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics 2014;134:1013–23. [DOI] [PubMed] [Google Scholar]
- 16.Woods-Hill CZ, Fackler J, Nelson McMillan K, et al. Association of a Clinical Practice Guideline With Blood Culture Use in Critically Ill Children. JAMA Pediatr 2017; 171(2):157–164. [DOI] [PubMed] [Google Scholar]
- 17.Xie A, Woods-Hill CZ, King AF, et al. Work system assessment to facilitate the dissemination of a quality improvement program for optimizing blood culture use: a case study using a human factors engineering approach. J Pediatric Infect Dis Soc 2019; 28;8(1):39–45. [DOI] [PubMed] [Google Scholar]
- 18.Woods-Hill CZ, Lee L, Xie A, et al. Dissemination of a novel framework to improve blood culture use in three pediatric intensive care units. Pediatr Qual Saf 2018;3:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods-Hill CZ, Xie A, King A, et al. Practices, perceptions, and attitudes in the evaluation of critically ill children for bacteremia a national survey. Pediatr Crit Care Med 2020; 21(1): e23–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landeta J. Current validity of the Delphi method in social sciences. Technol Forecast Soc Change 2006; 73(5): 467–482. [Google Scholar]
- 21.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16(5):428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakai A, O’Sullivan R, Staunton P, et al. Development of key performance indicators for emergency departments in Ireland using an electronic modified-Delphi consensus approach. Eur J Emerg Med 2013;20(2):109–114 [DOI] [PubMed] [Google Scholar]
- 23.Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018; 18, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulkedid R, Abdoul H, Loustau M, et al. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 2011; 6(6):e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 201467(4):401–9. [DOI] [PubMed] [Google Scholar]
- 26.Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med 2020; 21(2):e52–e106. [DOI] [PubMed] [Google Scholar]
- 27. https://www.elso.org/Portals/0/Files/Infection-Control-and-Extracorporeal-Life-Support.pdf.
- 28.Glater-Welt LB, Schneider JB, Zinger MM, et al. Nosocomial Bloodstream Infections in Patients Receiving Extracorporeal Life Support: Variability in Prevention Practices: A Survey of the Extracorporeal Life Support Organization Members. J Intensive Care Med 2016; 31(10) 654–659. [DOI] [PubMed] [Google Scholar]
- 29.Le Blanc L, Lesur O, Valiquette L, et al. Role of routine blood cultures in detecting unapparent infections during continuous renal replacement therapy. Intensive Care Med 2006;32:1802–1807. [DOI] [PubMed] [Google Scholar]
- 30.Santiago MJ, López-Herce J, Vierge E, et al. Infection in critically ill pediatric patients on continuous renal replacement therapy. Int J Artif Organs 2017; 40(5):224–229. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central-line associated bloodstream infection: systematic review and meta-analysis. Infection 2015; 43: 29–36. [DOI] [PubMed] [Google Scholar]
- 32.Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev 2006;19(4):788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JM, Binnicker MJ, Campbell S, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 2018; 67(6): e1–e94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Septimus E. Collecting cultures: a clinician guide. Available from: https://www.cdc.gov/antibiotic-use/core-elements/collecting-cultures.html.AccessedApril 30, 2020.
- 35.Handrup MM, Møller JK, Rutkjaer C, et al. Importance of blood cultures from peripheral veins in pediatric patients with cancer and a central venous line. Pediatr Blood Cancer 2015; 62(1):99–102. [DOI] [PubMed] [Google Scholar]
- 36.Kelly M, Conway M, Wirth K, et al. Moving CLABSI Prevention Beyond the ICU: Risk Factors in Pediatric Oncology Patients. Infect Control Hosp Epidemiol 2011; 32(11): 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon A, Ammann RA, Bode U, et al. Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC Infect Dis 2008; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods-Hill CZ, Srinivasan L, Schriver E, et al. Novel risk factors for central-line associated bloodstream infections in critically ill children. Infect Control Hosp Epidemiol 2020; 41(1):67–72. [DOI] [PubMed] [Google Scholar]
- 39.Karandikar MV, Milliren C, Zaboulian R, et al. Limiting Vancomycin Exposure in Pediatric Oncology Patients With Febrile Neutropenia May Be Associated With Decreased Vancomycin-Resistant Enterococcus Incidence. J Pediatric Infect Dis Soc 2019; pii: piz064. doi: 10.1093/jpids/piz064. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagliardi AR, Marshall C, Huckson S, et al. Developing a checklist for guideline implementation planning: Review and synthesis of guideline development and implementation advice. Implement Sci 2015; 10(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


