Abstract
The Food and Drug Administration (FDA) approved pembrolizumab on June 29, 2020, for the treatment of patients with unresectable or metastatic microsatellite instability-high (MSI-H) colorectal cancer (CRC) with no prior systemic treatment for advanced disease. The approval was based on data from Study Keynote-177, which randomly allocated patients to receive either pembrolizumab or standard of care (SOC) with chemotherapy. Overall survival (OS) and independently-assessed progression free survival (PFS) were the primary endpoints. At the time of the final PFS analysis and second pre-specified interim OS analysis, the estimated median PFS was 16.5 months (95% CI: 5.4, 32.4) vs. 8.2 months (95% CI: 6.1, 10.2) in the pembrolizumab and SOC arms, respectively (Hazard Ratio [HR]: 0.60 (95% CI: 0.45, 0.80; two-sided p-value= 0.0004)). FDA assessed unblinded OS data during the review of the application and identified no safety concerns that would preclude approval of this supplement. Adverse reactions occurring in >30% of patients receiving pembrolizumab were diarrhea, fatigue/asthenia, and nausea. Adverse reactions occurring in >30% of patients receiving SOC were diarrhea, nausea, fatigue/asthenia, neutropenia, decreased appetite, peripheral neuropathy (high-level term), vomiting, abdominal pain, constipation, and stomatitis. Duration of treatment in the pembrolizumab arm was almost double (median 11.1 months, range 0-30.6 months) than the duration of treatment in patients receiving SOC (median 5.7 months). Approval of pembrolizumab is likely to change the treatment paradigm for 1st line treatment with MSI-H advanced CRC given the study results and different safety profile.
Introduction
Pembrolizumab (Keytruda, Merck) is a humanized monoclonal IgG1 antibody that binds to programmed death receptor-1 (PD-1) blocking its interactions with the PD-1 and 2 ligands, and releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response. Pembrolizumab is approved for the treatment of multiple solid tumors, classical Hodgkin lymphoma, and primary mediastinal large B-cell lymphoma (1).
Microsatellite instability (MSI) results from the accumulation of errors in DNA microsatellites (short repetitive sequences in DNA) due to mutations or silencing (e.g., i.e., via promotor hypermethylation) of genes coding for mismatch repair (MMR) proteins (MLH1, MSH2, MSH6, and PMS2) that are responsible for recognizing and correcting errors in mismatched nucleotides (2). This increased rate of mutations in MSI-high (MSI-H) tumors increases the probability of neoantigen formation (3) and treatment with immunotherapy has been shown to be effective independently of tumor histology; this led to FDA’s approval of pembrolizumab for a tissue-agnostic indication in patients with unresectable or metastatic, MSI-H, or deficient MMR solid tumors after prior therapy and for patients with MSI-H/dMMR metastatic colorectal cancer (mCRC) who received at least 2 prior lines of therapy (4). Although prior therapies in the two groups were described separately in labeling, FDA considers pembrolizumab to have a single tissue agnostic indication.
Colorectal cancer (CRC) is the 4th most common cancer in the U.S. (5), and the MSI-H/dMMR phenotype occurs in approximately 4% of patients with metastatic disease (6). Nivolumab and ipilimumab, also checkpoint inhibitors, are approved for the treatment of patients with MSI-H/dMMR metastatic CRC that have disease progression after at least 2 lines of therapy (7,8).
Checkpoint inhibition with pembrolizumab (1), nivolumab (5), or the combination nivolumab and ipilimumab (5, 6) in patients with previously-treated MSI-H/dMMR mCRC led to durable overall response rates (ORR) in over a third of patients. Previously, patients with MSI-H/dMMR CRC in the second-line setting were generally treated with chemotherapy and monoclonal antibodies targeting either the vascular endothelial growth factor pathway or the epidermal growth factor receptor (if RAS wild type), the same standard of care (SOC) treatments that are used to treat patients with CRC that are non-MSI-H.
This article summarizes the FDA’s review of data and regulatory considerations regarding the approval of pembrolizumab for the treatment of patients with unresectable or metastatic MSI-H or dMMR CRC. Results have been previously published (9).
Clinical trial design
Keynote-177 (NCT02563002) was an open-label, randomized (1:1) trial in patients with MSI-H/dMMR mCRC, as determined by a local lab using polymerase chain reaction (PCR) or immunohistochemistry (IHC). Eligible patients had to have met the following inclusion criteria to be enrolled: presence of metastatic disease, untreated with systemic therapy for metastatic disease, Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and presence of measurable disease. Patients who received prior adjuvant therapy for CRC were permitted if treatment was completed at least 6 months before randomization. Patients were excluded if they had active autoimmune disease requiring systemic treatment (except hormone replacement) within the prior two years, immunodeficiency, or known active CNS metastasis. There were no stratification factors used in this study. Patients received either pembrolizumab 200 mg intravenously on Day 1 of every 21-day cycle or treatment with mFOLFOX6 or FOLFIRI (standard of care [SOC] arm) with or without bevacizumab or cetuximab (9). Treatment was administered until intolerable toxicity or disease progression. Crossover to receive pembrolizumab was permitted for patients randomized to chemotherapy at the time of disease progression. Disease response and progression were measured at baseline and every 9 weeks. The primary endpoints were progression-free survival (PFS) as assessed by a blinded, independent review committee (BIRC), and overall survival (OS). The key secondary endpoint was BIRC-assessed ORR using RECIST 1.1 (10).
The planned sample size was 300 patients and with 209 PFS events, the study had 98% power to detect a hazard ratio (HR) of 0.55 for PFS using a log-rank test at a one-sided significance level of 0.0125 assuming a median PFS of 10 months in the SOC arm. For OS, the study would have up to 85% power, with 190 OS events and depending on cross-over effects, to detect a HR of 0.62 using a log-rank test at a one-sided significance level of 0.0125 assuming a median OS of 24 months in the SOC arm. Two interim analyses (IA) were planned in this study: IA1, an interim analysis of PFS and OS when 162 PFS events had occurred and 6 months of minimum follow-up, and IA2, an interim analysis of OS at the time of PFS final analysis.
The efficacy analyses were conducted in the intention-to-treat population (ITT), defined as all patients who were randomly assigned to treatment. The log-rank test was used to compare OS and PFS between arms and the Cox proportional hazard model was used to estimate the HRs. The Kaplan–Meier (KM) method was applied to summarize OS, PFS, and duration of response. The Miettinen and Nurminen method was used to compare ORR. Safety was analyzed in patients who received at least one dose of study treatment.
Results
Efficacy
A total of 307 patients were randomized to receive pembrolizumab (n=153) or SOC (n=154). All patients randomized to the pembrolizumab arm were treated per protocol. Of the 154 patients randomized to the SOC arm, 143 received chemotherapy per the protocol (of these 143 patients, 56% received FOLFOX and 44% FOLFIRI; 70% received SOC plus bevacizumab and 11% received SOC plus cetuximab). Baseline demographics and disease characteristics for patients in the ITT population were mostly balanced; there were more women and patients with lung or liver metastases in the pembrolizumab arm (Table 1). The study enrolled 14 patients (4.6%) across both arms who identified as Black and 21 (6.8%) who identified as Hispanic or Latino.
Table 1:
Keynote-177: Demographic Characteristics
| Pembrolizumab (n: 153) |
SOC (n: 154) |
|
|---|---|---|
| Median age, years (range) | 63 (24, 93) | 62.5 (26, 90) |
| Male sex, n (%) | 71 (46%) | 82 (53%) |
| Western Europe/North America | 109 (71%) | 113 (73%) |
| ECOG PS 0/1, n (%) | 75 (49%)/ 78 (51%) | 84 (55%)/ 70 (45%) |
| Site of primary tumor | ||
| Right | 102 (67%) | 107 (70%) |
| Left | 46 (30%) | 42 (27%) |
| Site of metastases | ||
| Liver or lung | 86 (56%) | 73 (49%) |
| Other sites | 67 (44%) | 81 (53%) |
| Newly diagnosed stage | 73 (48%) | 80 (52%) |
| No prior neo/adjuvant therapy | 115 (75%) | 109 (71%) |
| Mutation status | ||
| Wild type BRAF*/RAS | 34 (22%) | 35 (23%) |
| Mutant RAS/Wild type BRAF | 33 (22%) | 38 (25%) |
| Mutant BRAF/Wild type RAS | 34 (22%) | 40 (26%) |
| Mutant RAS and BRAF | 0 | 3 (2%) |
ECOG PS: Eastern Cooperative Group Performance Status
BRAF: BRAF V600E
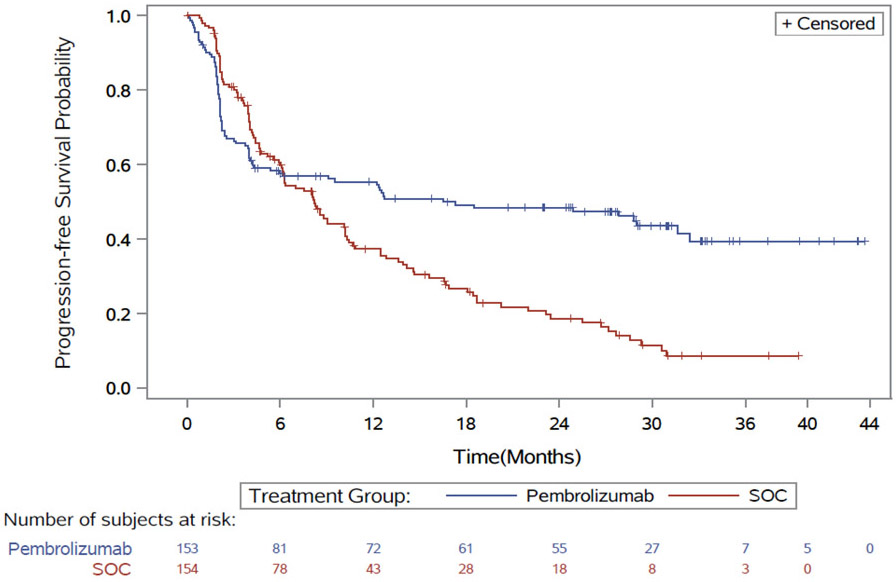
At the final PFS analysis and second pre-specified interim analysis of survival (data cutoff February 19, 2020), 195 PFS events and 125 deaths had occurred. With a median follow-up duration of 28.4 months vs 27.2 months in the pembrolizumab and SOC respectively, Keynote-177 demonstrated a statistically significant improvement in BICR-assessed PFS, with a HR of 0.60 (95% CI: 0.45, 0.80; two-sided p-value= 0.0004); the estimated median PFS was 16.5 months (95% CI: 5.4, 32.4) vs. 8.2 months (95% CI: 6.1, 10.2) in the pembrolizumab and SOC arms, respectively. As shown in the Kaplan Meier curves (Figure 1), the hazards for PFS are not proportional, and curves cross around 6 months. Additional exploratory treatment effect estimates using Kaplan-Meier based restricted mean survival time supported the primary analysis. Sensitivity analyses show that the overall treatment effect was generally consistent across most exploratory subgroups (age, sex, ECOG PS, site of primary tumor, BRAF status, etc.).
Figure 1:
Keynote-177: K-M Curves of PFS
FDA assessed unblinded OS data during the review of the application and identified no safety concerns that would preclude approval of this supplement.
The independently assessed ORR per RECIST 1.1 was 43.8% (95% CI: 35.8, 52.0) in the pembrolizumab arm vs. 33.1% (95% CI: 25.8, 41.1) in the SOC arm. Complete response rate was 11% and 4% in the pembrolizumab and SOC arms, respectively. The median response duration could not be estimated in the pembrolizumab arm (range: 2.3+ - 41.4+ months) and was estimated at 10.6 months (range: 2.8 - 37.5+) in the SOC arm. Efficacy analyses are summarized in Table 2.
Table 2:
Keynote-177: Efficacy results
| Pembrolizumab (n: 153) |
SOC (n: 154) |
|
|---|---|---|
| Progression-Free Survival | ||
| Number of events (%) | 82 (54%) | 113 (73%) |
| Median in months (95% CI) | 16.5 (5.4, 32.4) | 8.2 (6.1, 10.2) |
| HR1 (95% CI), p-value2 | 0.60 (0.45, 0.80), 0.0004 | |
| Overall Response Rate 3 (ORR), RECIST 1.1 | ||
| ORR (%) (95% CI) | 43.8% (35.8, 52.2) | 33.1% (25.8, 41.1) |
| Complete responses , n (%) | 11% | 4% |
| Partial responses, n (%) | 33% | 29% |
| Median duration of response3, months (Range) | NR (2.3+, 41.4+) | 10.6 (2.8, 37.5+) |
| % duration4 ≥ 12 months | 75% | 37% |
| % duration4 ≥ 24 months | 43% | 18% |
Based on a Cox-proportional regression model
Two-sided p-value based on log-rank test (compared to a significance level of 0.0234 for PFS); Based on confirmed response by independent review
Based on n=67 patients with a response in the pembrolizumab arm and n=51 patients with a response in the SOC arm
Based on observed duration of response; NR=Not Reached; RECIST 1.1= Response Evaluation Criteria in Solid Tumors v1.1
Safety
The safety analysis was based on 296 patients who received at least one dose of pembrolizumab (n= 153) or SOC (n=143). The most common (≥ 20%) treatment emergent adverse events (TEAEs) in the pembrolizumab arm were fatigue/asthenia (49%), diarrhea (44%), nausea (31%), abdominal pain (27%), decreased appetite (24%), vomiting (22%), and cough (20%); in the SOC arm, TEAEs >20% were diarrhea (63%), nausea (59%), fatigue/asthenia (51%), neutropenia/neutrophils decreased (43%), decreased appetite (42%), peripheral neuropathy/peripheral sensory neuropathy/polyneuropathy (40%), vomiting (38%), abdominal pain (31%), constipation (32%), stomatitis (30%), anemia (24%), and alopecia (20%).
The most common Grade 3-4 TEAEs occurring in ≥ 5% of patients in the pembrolizumab arm were hypertension (7%), hyponatremia (7%), anemia (5%), diarrhea (5%), and abdominal pain (5%); in the SOC arm, Grade 3-4 TEAs in ≥ 5% were ANC decreased/neutropenia (31%), anemia (11%), diarrhea (11%), fatigue (11%), abdominal pain (6%), hypokalemia (6%), decreased appetite (6%), hypertension (5%), febrile neutropenia (5%), vomiting (5%), and embolism (5%). Table 3 summarizes Grade 3-4 TEAEs.
Table 3:
Keynote-177: Grade 3-4 adverse events (incidence ≥ 3 %)
| AEs | Pembrolizumab N: 153; n(%) |
SOC N: 143; n(%) |
|---|---|---|
| ANC decreased/neutropenia1 | 0 | 45 (31) |
| Anemia | 8 (5) | 16 (11) |
| Diarrhea | 8 (5) | 16 (11) |
| Fatigue | 6 (4) | 15 (10) |
| Hypertension | 11 (7) | 7 (5) |
| Hyponatremia | 10 (7) | 6 (4) |
| Abdominal pain | 8 (5) | 8 (6) |
| Decreased appetite | 1 (1) | 9 (6) |
| Hypokalemia | 2 (1) | 9 (6) |
| GGT2 increased | 7 (5) | 1 (1) |
| Febrile neutropenia | 1 (1) | 7 (5) |
| Vomiting | 2 (1) | 7 (5) |
| Embolism | 0 | 7 (5) |
| Nausea | 4 (3) | 6 (4) |
| Asthenia | 3 (2) | 6 (4) |
| Stomatitis | 0 | 6 (4) |
| WBC3 decreased | 0 | 6 (4) |
| AST4 increased | 4 (3) | 4 (3) |
| Dehydration | 3 (2) | 5 (3) |
| Pulmonary embolism | 3 (2) | 4 (3) |
| Small intestinal obstruction | 2 (1) | 5 (3) |
| Urinary tract infection | 1 (1) | 4 (3) |
| Peripheral neuropathy5 | 1 (1) | 4 (3) |
| ALT6 increased | 4 (3) | 3 (2) |
| Pneumonia | 5 (3) | 3 (2) |
| ALK7 increased | 4 (3) | 2 (1) |
| Hyperglycemia | 4 (3) | 1 (1) |
composite term, absolute neutrophil count decreased and neutropenia
gamma glutamyl transpeptidase
white blood cells count
aspartate aminotransferase
composite term, peripheral neuropathy, peripheral sensory neuropathy, and polyneuropathy
alanine aminotransferase
blood alkaline phosphatase.
Grade 3-4 TEAEs associated with an immune etiology were reported in 14 (9%) and 3 (2%) patients in the pembrolizumab and SOC arms, respectively. The most common immune-related TEAEs (≥ 2% incidence) in the pembrolizumab group were hypothyroidism, hyperthyroidism, colitis, pneumonitis, adrenal insufficiency, hepatitis, and infusion reactions; 5 (3%) patients experienced Grade 3-4 colitis events and 4 patients discontinued treatment because of the event. Immune-related TEAEs leading to the discontinuation of pembrolizumab were reported in 7% patients; most events were managed with systemic corticosteroids, supportive care, and dose interruption.
The most common Grade 3-4 lab abnormalities occurring in ≥ 5% of patients in the pembrolizumab arm were hyponatremia (12%), hyperglycemia (10%), low hemoglobin (7%), hyperkalemia (6%), and hypokalemia (5%); in the SOC arm, Grade 3-4 lab abnormalities occurring in ≥ 5% of patients were decreased neutrophil count (34%), decreased hemoglobin (14%), hypokalemia (11%), hyponatremia (10%), decreased white blood cells count (8%), and hyperglycemia (5%).
Fatal TEAEs were reported in 6 (4%) and 7 (5%) patients in the pembrolizumab and SOC arms, respectively. There was no leading cause or toxicity pattern associated with fatal events, and it was difficult to attribute most specific events (e.g., intestinal perforation) to treatment versus underlying disease.
The median duration of exposure to pembrolizumab was 11.1 months and to SOC was 5.7 months; 48% patients in the pembrolizumab arm and 22% patients in the SOC arm remained on treatment for longer than 12 months. Treatment was discontinued because of toxicity in 14% and 12% patients in the pembrolizumab and SOC arms, respectively. The most common AEs leading to treatment discontinuation in the pembrolizumab arm were immune-related events (7%). The most common AEs leading to SOC treatment (4%) were cardiovascular/cerebrovascular events.
Regulatory considerations
This is the first FDA approval for first-line immunotherapy in patients with MSI-H/dMMR unresectable or metastatic CRC. Although pembrolizumab, nivolumab, and the combination of nivolumab and ipilimumab have been approved in patients with previously-treated disease, these approvals were based on durable responses in single-arm studies. Keynote-177 showed significantly better PFS in patients receiving pembrolizumab compared to patients receiving SOC multi-agent chemotherapy. The study results are robust and consistent across most subgroups of patients. Due to the magnitude of effect on PFS observed in the pembrolizumab arm when compared with an active treatment, FDA considered that clinical benefit was demonstrated and FDA assessment of unblinded OS data identified no safety concerns.
The PFS KM curves crossed early (approximately 6 months for PFS). This crossed pattern has been observed for PFS and OS in other trials (in different disease settings like melanoma, non-small cell lung cancer, etc.) (11) comparing immune checkpoint inhibition therapy versus chemotherapy. As a result, the estimated medians and hazard ratio based on Cox-proportional model may not provide a comprehensive summary measure of treatment benefit. FDA conducted additional supportive analyses, such as the restricted mean survival time estimates at multiple follow-up times, to account for the non-proportional hazards effect associated with immunotherapies, which supported the results of the primary analysis. Merck agreed to submit the results of the final OS analysis as a post-marketing commitment. Note that the trial was not designed to assess whether patients might benefit from chemoimmunotherapy or from chemotherapy first, followed by immunotherapy.
Supporting the effect on PFS, there were more responders and complete responses in patients treated with pembrolizumab. Although the number of patients with observed sustained responses at 6 months was similar in both arms (91% vs. 84% in the pembrolizumab and SOC arms, respectively), in parallel with the PFS results, a higher proportion of responders in the pembrolizumab arm had a sustained response at 12 months (75% and 37% continuous responders at 12 months in the pembrolizumab and SOC arms, respectively).
The median length of treatment in Keynote-177 was almost double in the pembrolizumab arm compared to the SOC arm (11.1 months vs. 5.7 months). The safety profile of adverse events observed with pembrolizumab was consistent with its known safety profile. Despite longer exposure, patients in the pembrolizumab arm experienced less toxicity overall, less Grade 3-4 adverse events and a similar incidence of fatal adverse events than patients treated with chemotherapy.
The results of Study Keynote-177 demonstrated superior efficacy of single agent pembrolizumab compared with multi-agent chemotherapy, likely leading to a change in the treatment paradigm for patients with advanced or metastatic MSI-H/dMMR CRC. Prior to the approval of pembrolizumab in the first-line setting, pembrolizumab, nivolumab, and nivolumab in combination with ipilimumab, received accelerated approval in patients treated with prior fluoropyrimidine-, oxaliplatin-, and irinotecan-based therapy. Clinical trials are needed to address important remaining questions regarding the optimal sequencing of therapies in patients who progress on or following treatment with pembrolizumab and to identify those patients who may benefit from upfront chemotherapy in combination with pembrolizumab.
Accruing a diverse population more representative of U.S. patients remains a challenge in contemporary cancer trials. A limitation of the Keynote-177 trial is the inclusion of only 14 of 307 patients (4.6%) identified as Black and 21 (6.8%) identified as Hispanic or Latino. Increased efforts are needed to enroll a more representative patient population in future clinical trials.
Conclusions:
In summary, pembrolizumab for the treatment of patients with unresectable or metastatic MSI-H/dMMR mCRC has a favorable benefit-risk profile, with a clinically meaningful and statistically significant improvement in PFS demonstrated in Study Keynote-177. FDA assessed unblinded OS data during the review of the application and identified no safety concerns that would preclude approval of this supplement. No new safety signals were identified for pembrolizumab in the MSI-H/dMMR population, and although the treatment duration was markedly longer with pembrolizumab, there was no increased incidence of adverse events. Overall, based on the safety profile and duration of treatment, pembrolizumab appears to be reasonably tolerated with a favorable risk:benefit assessment given the demonstrated benefits in patients with MSI-H/dMMR advanced CRC.
Pembrolizumab for the first-line treatment of MSI-H/dMMR advanced or metastatic CRC was approved on June 29, 2020, less than a month after submission. The application was reviewed under various programs designed to expedite the review of applications for patients with cancer including the Real-Time Oncology (RTOR) program (12) entailing early receipt of datasets prior to application submission; Assessment Aid (13) (a voluntary review template submission from the applicant to facilitate FDA’s assessment); and Project Orbis (14), under which FDA reviewed the pembrolizumab application in collaboration with Health Canada, Swissmedic, and Australia’s Therapeutic Goods Administration. Although collaboration under Project Orbis fostered discussion of issues pertinent to the review of the pembrolizumab application, the application review was ongoing in Canada, Switzerland, and Australia at the time that FDA approved the application.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors report no financial interests or relationships with the commercial sponsors of any products discussed in this report.
References
- 1.Drugs@FDA [database on the Internet]. Silver Spring (MD): U.S. Food and Drug Administration. [cited 2020 November 2]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s084lbl.pdf [Google Scholar]
- 2.Flaherty KT, Le DT, Lemery S., Tissue-agnostic drug development. Am Soc Clin Oncol Educ Book 2017;37:222–30. [DOI] [PubMed] [Google Scholar]
- 3.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus L, Lemery S, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. CCR 2019; July1;25(13):3753–3758 [DOI] [PubMed] [Google Scholar]
- 5.Cancer Stat Facts, https://seer.cancer.gov/statfacts/html/colorect.html
- 6.Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007March1;25(7):767–72. [DOI] [PubMed] [Google Scholar]
- 7.Opdivo USPI, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125554s083lbl.pdf
- 8.Yervoy USPI, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s111lbl.pdf
- 9.André T, Shiu KK, Kim TW, Jensen B, et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N Engl J 2020; 383:2207–2218. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009January;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 11.Keytruda USPI. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s088lbl.pdf.
- 12.Real-Time Oncology Review Pilot Program [cited 2020 November 2]. Available from: https://www.fda.gov/about-fda/oncology-center-excellence/real-time-oncology-review-pilot-program.
- 13.Assessment Aid [cited 2020 November 2]. Available from: https://www.fda.gov/about-fda/oncology-center-excellence/assessment-aid
- 14.Project orbis. [cited 2020 November 2]. Available from: https://www.fda.gov/about-fda/oncology-center-excellence/project-orbis