Abstract
Introduction:
Oral health is closely related to extra-oral disease status, as may be represented by the manifestations of gastrointestinal and liver diseases.
Areas covered:
This review focuses on the roles that the oral–gut or the oral–gut–liver axis play in the pathogenesis of inflammatory bowel disease, colorectal cancer, metabolic fatty liver disease, and nonalcoholic steatohepatitis. The discussion will begin with clinical data, including data from preclinical animal models, to elucidate mechanisms. We will also discuss ways to target oral dysbiosis and oral inflammation to treat gastrointestinal and liver diseases.
Expert opinion:
Several studies have demonstrated that oral pathobionts can translocate to the gastrointestinal tract where they contribute to inflammation and tumorigenesis. Furthermore, oral bacteria that migrate to the gastrointestinal tract can disseminate to the liver and cause hepatic disease. Thus, oral bacteria that ectopically colonize the intestine may serve as biomarkers for gastrointestinal and liver diseases. Also, understanding the characteristics of the oral–gut and oral–gut–liver microbial and immune axes will provide new insights into the pathogenesis of these diseases.
Keywords: Oral microbiota, Gut microbiota, Inflammatory bowel disease, Colorectal cancer, Metabolic fatty liver disease, Nonalcoholic steatohepatitis
1.0. Introduction
The NIH Human Microbiome Project, launched as a part of the NIH Roadmap for Medical Research, recognized the need to accelerate our understanding of how our bodies and microorganisms interact to influence health and disease [1–3]. The complex ecosystem of the human gastrointestinal tract consists of many microorganisms, including bacteria, viruses, and fungi [4]. Advances in next-generation sequencing have made it possible to obtain large amounts of DNA sequencing data from all types of samples [5]. In particular, 16S rRNA analysis has enabled a high level of quantification in estimating the composition and abundance of bacteria in the community. Several studies have elucidated the vital role that the gut microbiota plays in host physiology, such as energy acquisition and the development of the immune system [1]. On the other hand, abnormalities in the gut microbiome, characteristic of various disease conditions, namely dysbiosis, have been reported. It is believed that gut dysbiosis is not simply a consequence of the disease. Rather, the dysbiotic microbiota harbors abnormalities in the context of its metabolic and immunogenic functions, and thereby directly contributes to disease pathogenesis [6].
The oral microbiome is the second-largest microbial community in the human body. As in the gut, resident microbes in the oral cavity play a vital role in homeostasis and disease. Conditions such as decreased salivary gland function, decreased oral clearance, decreased salivary pH, and changes in immunoglobulin A in saliva can affect the composition of the oral microbiome and cause oral dysbiosis [7]. It has been reported that certain oral bacteria, such as Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Prevotella intermedia, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans, are enriched in patients with oral disease, including periodontitis, and contribute to the disease pathogenesis [8,9]. Notably, accumulating evidence indicates that these oral pathobionts affect systemic diseases, such as arteriosclerosis, diabetes, and rheumatoid arthritis [10,11]. Furthermore, research supports a possible pathogenic impact of the oral microbiome and oral disease on gastrointestinal and liver diseases [12–14]. Here, we review the recent findings in studies of the oral–gut and the oral–gut–liver axes in the context of the pathogenesis of gastrointestinal and liver diseases. We also discuss the potential therapeutic options that target the oral microbiome and oral diseases to treat gastrointestinal and liver diseases. A research collection was performed using the PubMed electronic database for relevant full-text original articles or review articles published until April 2021.
2.0. The oral–gut and oral–gut–liver axes: Lessons from clinical and preclinical studies
2.1. Inflammatory bowel disease
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic inflammatory disease of the gastrointestinal tract. Accumulating evidence suggests that aberrant immune responses to the gut microbiota are closely associated with the pathogenesis of IBD. It has been repeatedly demonstrated that atypical, potentially pathogenic members of commensal bacteria (pathobionts) are markedly enriched in the stool and the intestinal mucosa of IBD patients compared to non-IBD individuals [15,16]. The perturbed microbial community in the gut, so-called gut dysbiosis, is a hallmark of IBD [17]. For example, Gevers and colleagues analyzed the microbiome of newly diagnosed treatment-naïve pediatric patients with CD [18]. This study revealed a significant correlation between microbial alterations in the intestinal mucosa and disease status, with an increased abundance of Veillonellaceae, Pasteurellaceae, Enterobacteriaceae, Nisseriaceae, Gemellaceae, and Fusobacteriaceae, and a decreased abundance of Bacteroidales, Erysipelotrichales, and Clostridiales [12,18]). Notably, the enriched bacterial taxa are considered typical oral resident bacteria. Similarly, the accumulation of oral-derived bacteria in the gut is also reported in patients with UC. A cohort of new-onset, treatment-naïve pediatric UC patients with severe inflammation exhibited a striking increase in the abundance of oral bacteria, such as Veillonella dispar, Aggregatibacter segnis, Campylobacter spp, Lachnospiraceae, Veillonella parvula, Haemophilus parainfluenzae, and Megasphaera spp. [19]. Thus, bacteria derived from the oral cavity ectopically colonize the gut in patients with IBD, both CD and UC (Figure 1).
Figure 1. Oral bacteria found in patients with gut or liver pathology.
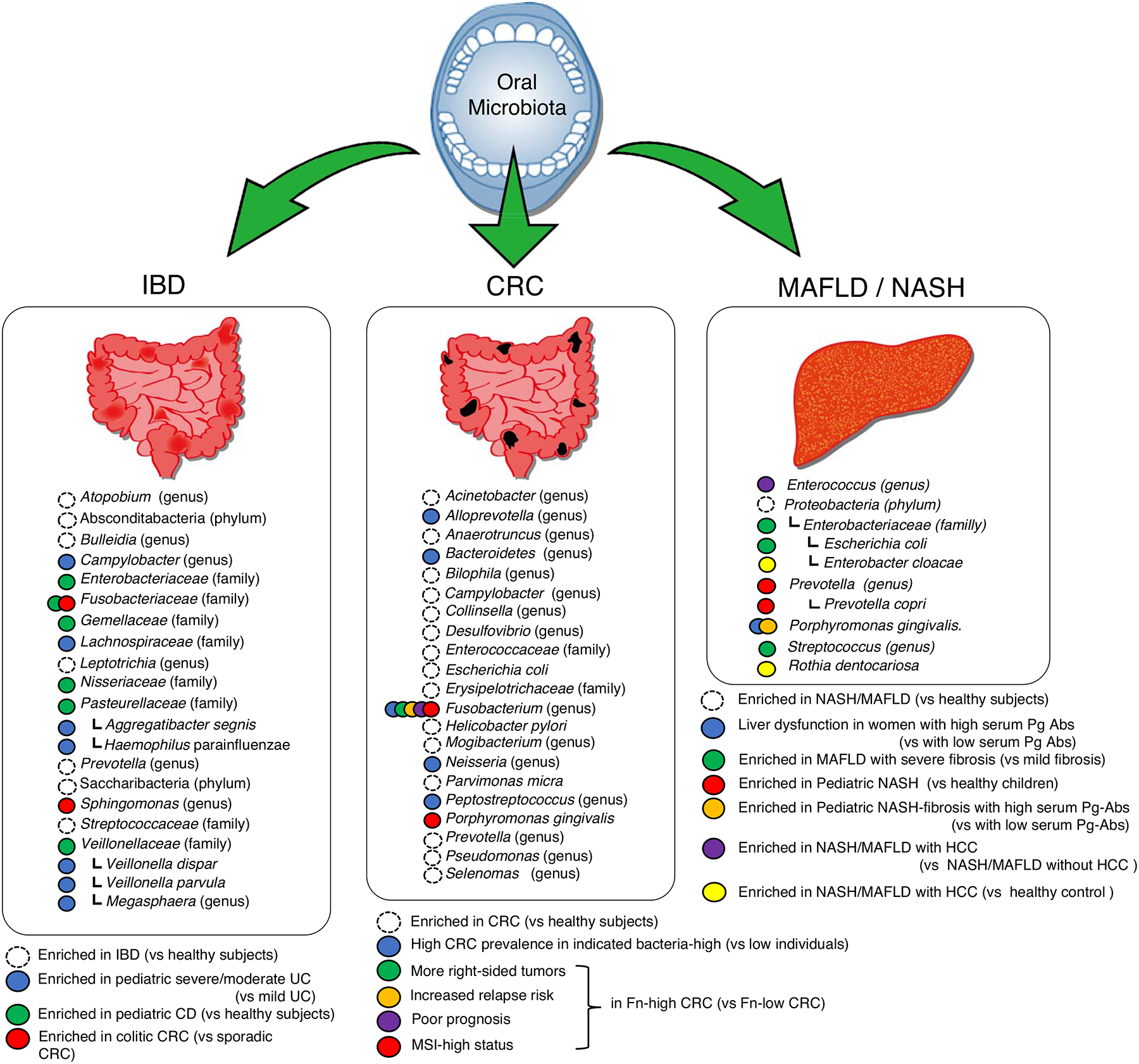
Oral bacterial species found in the gut of patients with inflammatory bowel disease (IBD), colorectal cancer (CRC), metabolic fatty liver disease (MAFLD), and nonalcoholic steatohepatitis (NASH) are listed. The taxonomic rank is shown in parentheses in the case that species information is not defined in references.
The possible impact of gut colonization by oral bacteria has been studied in IBD preclinical animal models. The oral administration of P. gingivalis disrupts the intestinal barrier, thereby increasing the severity of dextran sulfate sodium (DSS)-induced colitis in mice [20]. Likewise, gut colonization by F. nucleatum results in the skewed polarization of macrophages toward the M1 phenotype, disrupting the epithelial barrier, and thereby rendering the host susceptible to DSS-induced colitis [21,22]. Moreover, Atarashi and colleagues reported that transplantation of salivary bacteria isolated from IBD patients leads to colitis in genetically susceptible germ-free (GF) mice through the induction of pathogenic Th1 cell differentiation [23]. In this study, members of the family Enterobacteriaceae, in particular Klebsiella spp. were identified as the key colitogenic pathobionts that reside in the saliva of IBD patients. Likewise, we demonstrated that Klebsiella and Enterobacter spp. isolated from the oral cavity can exacerbate colitis when they ectopically colonize the gut through activation of the inflammasome in intestinal mononuclear cells [24]. Notably, we showed that oral inflammation promotes ectopic gut colonization by oral bacteria. In this study, mice that developed periodontitis displayed oral dysbiosis with the accumulation of Klebsiella and Enterobacter spp. [24]. This expansion of Klebsiella and Enterobacter spp. in the oral cavity resulted in an increased gut colonization by the same bacterial species, thereby increasing the susceptibility to DSS-induced colitis [24]. Besides the translocation of oral bacteria to the gut mucosa, it has become evident that immune cells, educated in the oral mucosa, can transmigrate to the gut and contribute to the pathogenesis of gastrointestinal disease. In this regard, it has been demonstrated that periodontitis leads to the expansion of oral pathobiont-reactive, pathogenic Th17 cells in the murine oral mucosa [24]. The oral pathogenic Th17 cells that arise during periodontitis express gut-homing markers, such as α4β7 integrin and CCR9, which enable the migration of these cells to the gut mucosa [24]. Gut-migrated oral pathogenic Th17 cells are then activated by gut-colonized oral pathobionts, exacerbating colitis [24]. All of these observations suggest that the inflamed oral mucosa, a characteristic feature of periodontitis, essentially serves colitogenic pathobionts and pathogenic immune cells to the gastrointestinal tract.
In this context, oral dysfunction accompanied by oral mucosal inflammation and oral dysbiosis are often manifest in patients with IBD. The prevalence of periodontitis is considerably increased in patients with IBD, particularly CD, compared to individuals who do not have IBD [25]. Moreover, oral salivary dysfunction and other oral health problems are often observed in CD and UC patients compared to healthy individuals [26,27]. For example, CD patients have a significantly higher concentration of mucin 5B (MUC5B) during active intestinal disease [26]. Given that MUC5B serves as a nutrient source for oral bacteria [28], an altered MUC5B level in the saliva may promote oral dysbiosis in patients with CD. Indeed, bacterial dysbiosis in saliva is more evident in IBD patients compared to healthy individuals [29]. Thus, in IBD patients, compromised oral health, such as periodontitis, leads to oral dysbiosis with the expansion of pathobionts in the oral cavity. Expanded oral pathobionts may naturally translocate to the lower digestive tract and may serve as colitogenic pathobionts. Unlike animal models, the immunological connection between the oral and gut mucosa in humans remains unexplored. Further studies are required to explore the potential role of oral immune cells, such as Th17 cells, in the pathogenesis of gastrointestinal inflammation in patients with IBD.
Some resident bacteria in the gut play a protective role of gut inflammation [30]. For example, some bacteria, in particular members of the phylum Firmicutes, generate beneficial metabolites, such as short-chain fatty acids (SCFAs), including butyrate, propionate, and acetate. SCFAs promotes the epithelial barrier integrity [31] and/or induce the differentiation of regulatory T cells [32–34], thereby regulating the homeostasis of the gastrointestinal tract. In addition to the accumulation of pathobionts, the reduction of these beneficial bacteria is also closely associated with the risk of IBD. However, there are no reports showing the relationship between SCFA-producing bacteria in the oral cavity and the potential risk of IBD.
2.2. Colorectal cancer (CRC)
Colorectal cancer (CRC) is one the most common types of cancer in both men and women [35]. Given that most of the known CRC risks such as age, obesity, and inflammation are closely associated with gut dysbiosis, it is conceivable that gut microbes contribute to the pathogenesis of CRC [12]. Indeed, gut microbiome and metabolome analyses have demonstrated the indispensable role of resident microbes and microbial by-products in the pathogenesis of CRC [36,37]. Several animal models of CRC facilitate the study of the causal role of the gut microbiota in carcinogenesis in the colon [38]. The pivotal role of the gut microbiota in the development of chemically induced colorectal tumors in rats was first reported in 1975 [39]. Investigators observed significantly fewer tumors in the colon of GF rats compared to conventional rats [39]. Several later reports confirmed the vital role of the gut microbiota in colonic tumorigenesis and proposed various carcinogenic mechanisms induced by bacteria [40–42]. For example, human colonic commensal bacteria promote colon tumorigenesis through the activation of Th17 responses [43]. Also, adherent–invasive Escherichia coli facilitate carcinogenesis by inducing genotoxic capabilities [44].
Nakatsu and colleagues reported that the enrichment of specific bacterial taxa in humans is an early sign of dysbiosis in adenoma, and coexclusive relationships are subsequently more common in cancer [45]. Another study of patients with adenomas identified multiple taxa that were significantly more abundant, including Bilophila and Desulfovibrio spp., proinflammatory bacteria in the genus Mogibacterium, and multiple species in the phylum Bacteroidetes [46]. Furthermore, increased numbers of Acinetobacter, Helicobacter, and Pseudomonas spp. have been reported in patients with rectal adenomas than in individuals without adenomas [47]. Although most CRC microbiome studies are conducted using fecal samples [48,49], some studies analyze the bacteria enriched in colorectal tissue specimens. Kostic and colleagues confirmed that Fusobacterium was enriched in carcinomas using quantitative PCR and 16S rDNA sequence analysis of 95 carcinoma/normal DNA pairs, whereas Bacteroidetes and Firmicutes were depleted [50]. These findings suggest that CRC may be initiated by modification of the balanced interaction between the host and the microbiome through the adoption of a proinflammatory profile by the intestinal microbiota [51].
Besides gut resident bacteria, mounting evidence suggests the possible involvement of oral bacteria in colonic tumorigenesis [49] (Figure 1). To reiterate, Fusobacterium species, including F. nucleatum, are significantly enriched in patients with CRC [50,52,53]. Colorectal carcinoma harboring F. nucleatum are more abundant in the proximal colon (cecum) than distal colon (rectum) [54]. The abundance of F. nucleatum positively correlate with microsatellite instability (MSI)-high status and shorter survival, indicating the association of the abundance of F. nucleatum and worse clinical outcomes [55,56]. Moreover, the abundance of F. nucleatum is associated with the risk of relapse after neoadjuvant chemoradiotherapy [57]. The monoassociation of F. nucleatum promotes colonic tumors in GF ApcMin/+ mice, suggesting that F. nucleatum is the direct cause of tumorigenesis in the gut [53]. Also, F. nucleatum is capable of activating β-catenin signaling and oncogenic responses through its unique adhesin called FadA, which binds to E-cadherin expressed on colorectal cancer cells [58] Fap2 protein in F. nucleatum directly interacts with TIGIT expressed on natural killer cells, thereby inhibiting their antitumor immunity [59]. Moreover, F. nucleatum enhances the proliferation and invasion of tumor cells through the activation of TLR4 signaling and the upregulation of miR21 expression [60]. The overgrowth of F. nucleatum in CRC tissue samples is associated with KRAS mutation and tumor size and correlated with reduced overall survival [61]. Further, the amount of tissue F. nucleatum in CRC tissue is inversely correlated with the density of CD3+ but not FOXP3+ T cells [62]. These findings suggest that the oral pathobiont F. nucleatum can trigger or promote tumor development in the gut. F. nucleatum usually coexists with other members of the oral microbiota, such as Porphyromonas spp., mainly P. gingivalis, which are some of the most typically increased taxa in individuals with CRC [45,63,64]. One study showed higher amounts of P. gingivalis, as well as F. nucleatum, in the saliva of CRC patients [65]. P. gingivalis, which is a primary causative bacterium of periodontitis, may be a risk factor for various systemic diseases, including Alzheimer disease, cardiovascular disease, diabetes, and rheumatoid arthritis [66–68]. P. gingivalis remodels the oral microbiota into a dysbiotic state by exploiting complement [69]. This pathobiont may also significantly affect the pathogenesis of CRC.
Chronic inflammation in the gut is considered as a potent risk factor for CRC. Richard and colleagues reported that patients with colitis-associated cancer (CAC) display different microbial profiles compared to the sporadic CRC. In patients with CAC, Enterobacteriacae and Sphingomonas are increased, while Fusobacterium and Ruminococcus are decreased compared to sporadic CRC [70].
2.3. MAFLD / NASH
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a phenotype of liver diseases associated with metabolic syndrome [71–73]. It was previously referred to as nonalcoholic fatty liver disease (NAFLD); in 2020, the new name MAFLD was proposed to more accurately reflect the pathogenesis of fatty liver disease and to help stratify patients for better management [71]. It has been reported that nonalcoholic steatohepatitis (NASH) is closely related to the lifestyle diseases obesity and diabetes [74] and leads to the development of hepatocirrhosis and hepatic carcinoma [75]. The global prevalence of NAFLD is 25.24% (95% CI: 22.10–28.65), with the highest prevalence in the Middle East and South America and the lowest in Africa [76]. Although a fatty liver is induced by hepatic fat accumulation arising from lifestyle diseases, Younossi and colleagues revealed the weak predictivity of the prevalence of obesity and daily caloric excess for the prevalence of NAFLD and NASH [76,77]. In this context, the two-hit theory is widely supported as a possible mechanism of the onset and progression of NASH [78,79]. Obesity causes fatty liver development – the first hit. Then, NASH develops due to oxidative stress, dysregulated cytokines, and mitochondrial dysfunction – the second hit [79]. Various factors, such as genetic susceptibility, induce hepatic inflammation and fibrosis in the fatty liver [80,81].
Given the direct connection between the intestine and the liver by way of the portal vein, the gut microbiota is believed to influence the pathophysiology of NAFLD and NASH [81,82]. Bacterial phylum Proteobacteria, family Enterobacteriaceae, and genus Escherichia are enriched in patients with NASH compared to healthy individuals [83]. Children with NAFLD show the increased abundance of Gammaproteobacteria and Prevotella [84] and increased Prevotella copri is associated with the severity of fibrosis in children with NASH [85]. Enterobacteriaceae, Streptococcus, and Gallibacterium are enriched and Akkermansia is decreased in NAFLD-cirrhosis patients [86–89]. Studies in animal models and humans have shown that colonic inflammation increases intestinal permeability in NASH cirrhosis, resulting in the translocation of bacteria and bacterial products to the liver [13,90,91] (Figure 1). In this context, it is possible that bacteria or their by-products in the oral cavity disseminate to the liver – the secondary event, after the oral–gut bacterial translocation – and thus contribute to the pathogenesis of liver diseases [13]. Indeed, it is a routine clinical practice to treat periodontitis prior to liver transplantation to eliminate all potential infectious foci and reduce the risk of postoperative complications [13,92]. Consistent with this notion, P. gingivalis, a well-known causative agent of periodontitis, is associated with liver inflammation. Several reports describe the relationship between NASH and P. gingivalis in clinical settings. Yoneda and colleagues reported that the detection frequency of P. gingivalis in saliva from NASH patients is significantly higher than in saliva from healthy individuals [93]. Nakahata and colleagues reported a significant correlation between serum IgG antibody titers against P. gingivalis fimA and the progression of liver fibrosis in 200 patients with biopsy-proven NASH [94]. Moreover, a cross-sectional analysis of adult outpatients conducted by Takamisawa and colleagues suggested the association between P. gingivalis infection and alanine aminotransferase (ALT) levels in women [95]. The application of the serum IgG antibody titer against P. gingivalis as a screening test for periodontitis has been evaluated [96].
Hepatocellular carcinoma (HCC) is an end-stage manifestation of a variety of chronic liver diseases. MAFLD and NASH have a low relative individual risk of developing HCC but, due to their high incidence, have a high overall population contribution to the development of HCC [86,97]. Enterobacter cloacae, Methylorubrum populi BJ001, and Rothia dentocariosa are enriched in HCC tissues compared to adjacent normal tissues in a subset of patients (HBV negative drinkers) [98]. Ponziani and colleagues reported that the gut microbiota of NAFLD-related cirrhosis with HCC is enriched with Bacteroides, Ruminococcaceae, Enterococcus, Phascolarctobacterium, and Oscillospira compared to patients with cirrhosis but without HCC [89]. Given E. cloacae, R. dentocariosa, and Enterococcus spp. are considered to be components of the oral microbiome [99–101], it is possible that the oral-gut-liver axis may be associated with HCC. Similar to studies of IBD and CRC, several animal experiments have proven that oral bacteria may contribute to the pathogenesis of NASH and MAFLD. Of note, experimental periodontitis caused by P. gingivalis in animals fed a high-fat diet induces the development of NASH [93,102,103]. Moreover, P. gingivalis infection exacerbates the hepatic fibrosis of NASH through the activation of hepatic stellate cells [104]. Likewise, P. gingivalis–derived lipopolysaccharide contributes to the accumulation of intracellular lipids and the elicitation of inflammatory reactions in hepatocytes through the activation of NF-κB and JNK signaling pathways [105]. These findings increase the understanding of the oral–gut–liver axis in the pathogenesis of NASH and advance the design of new treatment strategies for this disease.
3.0. Targeting oral inflammation and dysbiosis to treat gut and liver diseases
3.1. Oral maintenance therapy
Given that oral conditions may closely associate with the pathogenesis of gastrointestinal and liver diseases, it is plausible that the maintenance of oral health may affect disease activity in these distant organs (Figure 2). In this context, Kamata and colleagues devised a prospective, multicenter, randomized comparison trial to evaluate the impact of periodontal treatment on patients with NAFLD [106]. This ongoing clinical trial is expected to show that patients with liver disease who receive periodontal treatment will have improved outcomes. IBD is associated with several extraintestinal manifestations [107,108]. Among them, oral lesions are relatively common, with a prevalence ranging from 5% to 50% [109]. Likewise, IBD patients show a significantly higher risk of periodontitis compared to non-IBD patients [110]. Although the impact of oral inflammation on IBD outcomes remains largely unknown, knowledge from animal studies supports the possible benefits of treating oral conditions for intestinal inflammation in IBD. As well as gastrointestinal and liver diseases, oral maintenance may also affect the outcomes of other diseases. It was reported that periodontal treatment affects serum antibody levels to P. gingivalis and decreases the C-reactive protein (CRP) level and the disease activity score in patients with rheumatoid arthritis [111].
Figure 2. Targeting oral dysbiosis to diagnose and treat gut and liver diseases.
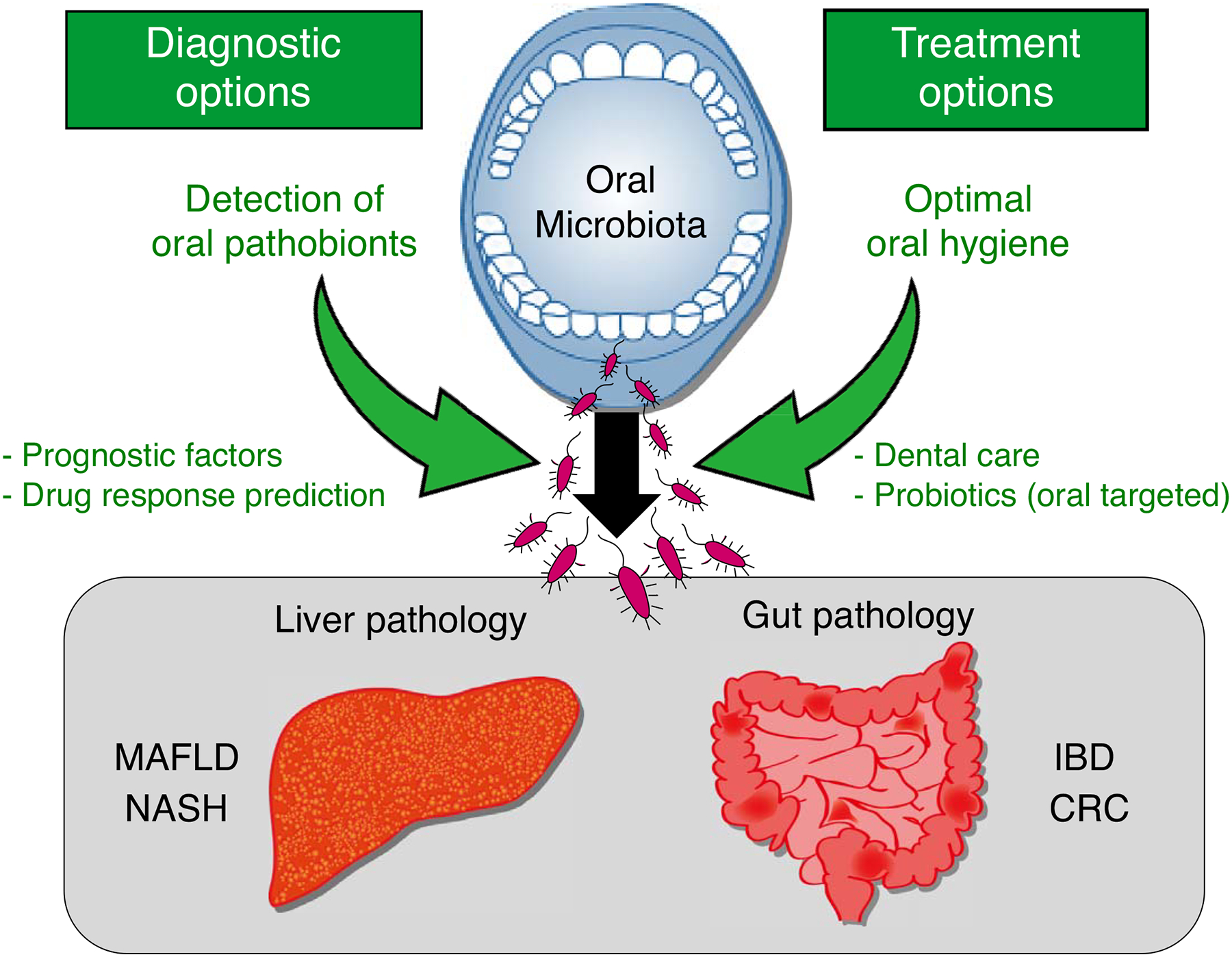
Monitoring of oral health and the oral dysbiosis (e.g., the abundance of oral pathobionts) could be used as a biomarker for inflammatory bowel disease (IBD), colorectal cancer (CRC), metabolic fatty liver disease (MAFLD), and nonalcoholic steatohepatitis (NASH). Also, optimal oral care has the potential to reduce the risk for these diseases.
3.2. Probiotics
Probiotic bacteria have a positive impact on the resident microorganisms and host immunity when administrated in an appropriate proportion [112]. Probiotics exert their immunomodulatory effects on many processes [113], and several studies have demonstrated their efficacy in the treatment of gastrointestinal and liver diseases, including IBD, CRC, and NASH [114–122]. Although the precise mechanisms of action are unclear, probiotics may prevent ectopic gut colonization by oral pathobionts, which in turn, reduces the intestinal or liver inflammation caused by these bacteria (Figure 2).
Also, it is possible that probiotics affect the inflammation in the oral cavity, as they are generally delivered through oral gavage. In this regard, Shimauchi and colleagues indicated that periodontal clinical parameters were improved in volunteers after an 8-week intervention using freeze-dried Lactobacillus salivarius, and especially in the current smokers who had a high risk of periodontal disease [123]. The review of a meta-analysis confirmed that probiotic treatment improves several clinical parameters, such as bleeding on probing, probing depth, and gingival index, but has no significant effect on colony-forming unit (CFU) counts of periodontal pathogens [124]. Randomized controlled trials with homogeneous methodologies and long-term follow-up periods are needed to confirm the contribution of probiotics in the management of periodontal diseases.
3.3. Prognostic marker
As oral dysbiosis is observed in various gut and liver diseases and may correlate with risk or severity, it may serve as a prognostic marker for these diseases (Figure 2). A meta-analysis has indicated that periodontal bacterial infection increases the incidence of cancer and is associated with poor overall survival, disease-free survival, and cancer-specific survival [125]. Further subgroup analysis indicated that the risk of cancer, including CRC, was associated with P. gingivalis and P. intermedia infections [125]. As the abundance of F. nucleatum in CRC tissue correlates with tumor size and KRAS mutation and is significantly associated with shorter overall survival, the measurement of F. nucleatum levels may help predict clinical outcomes in CRC [61]. Wong and colleagues used the abundance of F. nucleatum in feces as a biomarker alongside fecal immunochemical test (FIT) results to demonstrate a complementary role for the bacterial marker to detect lesions missed by FIT alone [126]. Consistently, the frequency of detection of F. nucleatum in colorectal specimens significantly correlates with CRC [127–129]. Moreover, the combination of F. nucleatum, Enterococcus feacalis, Streptococcus bovis, Enterotoxigenic Bacteroides fragilis, and Porphyromonas spp. showed improved diagnostic performance, compared to each bacterium alone [130]. Thus, the measurement of oral pathobionts in the gut is a potential CRC screening tool.
Reports have indicated that NASH severity is associated with gut dysbiosis and a shift in the metabolic function of the gut microbiota. Thus, gut microbiota analysis may provide valuable information to predict the severity of NASH [131]. There is preliminary evidence that nonsurgical periodontal treatment of 10 patients with NAFLD for 3 months ameliorates liver function parameters, such as the serum levels of AST and ALT [106]. These findings suggest that oral dysbiosis could be a prognostic marker for liver diseases.
4.0. Conclusion
Clinical and preclinical studies have shown that inflammation or microbial dysbiosis in the oral cavity affect the pathophysiology of gastrointestinal and liver diseases, including IBD, CRC, NASH, and NAFLD. Optimal oral care has the potential to reduce the risk for these diseases. Moreover, the monitoring of oral health and the oral microbiome could be used as a biomarker for these diseases.
5.0. Expert opinion
It has been suggested that the typical members of the oral bacterial community accumulate in extra-oral organs in certain disease conditions, such as IBD and CRC [18,19,49]. Furthermore, oral bacteria that relocate to the gut can further disseminate from the gut lumen to the liver by way of the portal vein and cause liver disease, such as NAFLD, NASH, and hepatocellular carcinoma [81,82]. Notably, the results of animal studies suggest that the commensal symbionts in a healthy oral cavity are harmless and have no adverse effect on gastrointestinal diseases even if they reach the gastrointestinal tract [24]. On the other hand, oral pathobionts that accumulate during the course of an oral disease, such as periodontitis, have pathogenetic characteristics and can induce intestinal inflammation if they ectopically colonize the gastrointestinal tract [23,24]. As discussed, oral inflammation and dysbiosis are often observed in patients with IBD, indicating that oral pathobionts may be enriched in the disease state compared to the healthy state. Therefore, optimal oral health may reduce the risk of extra-oral diseases, such as IBD, through decreasing the colonization of the gut by opportunistic oral pathobionts.
The expansion of oral pathobionts due to oral inflammation, such as periodontitis, may be the decisive trigger for the ectopic colonization of extra-oral organs such as the gut and the liver by these bacteria. Other factors affecting the integrity of the gut microbiota or the luminal metabolic microenvironment may also determine the susceptibility to opportunistic colonization by oral pathobionts. Patients with gastrointestinal and liver diseases have been reported to have a perturbed composition of the gut microbiota (i.e., gut dysbiosis) [17,83]. Gut dysbiosis may lead to a decrease in metabolic competitors and promote the growth and colonization of exogenous bacteria of oral origin. In addition, intestinal inflammation creates a microenvironment conducive to the colonization and expansion of pathogenic bacteria, including oral pathobionts, by supplying them with inflammation-related nutrients (e.g., iron, by-products of reactive oxygen species) and altering the epithelial metabolism [132]. Thus, the treatment of gut inflammation and gut dysbiosis may prevent the ectopic gut colonization by oral pathobionts, even in the presence of oral disease. Nevertheless, the simultaneous treatment of oral and intestinal diseases may have synergistic effects for patients with IBD, CRC, and liver diseases.
In addition to treating oral inflammation and oral dysbiosis as complementary therapy for intestinal inflammation, the condition of the oral cavity, especially the oral microbiome, can be a valuable biomarker for gastrointestinal and liver diseases. Once the causative oral pathobionts that correlate with the risk of extra-oral diseases are identified, detecting the presence or increased presence of these bacteria in the oral cavity can predict the risk of disease, such as the flare of IBD or the development of CRC. For example, oral dysbiosis with the expansion of Enterobacteriaceae, such as Klebsiella and Enterobacter spp., could be used as an indicator to predict the patients at risk for disease flares [23,24]. The composition of the oral microbiota may also identify subpopulations of patients who will respond to specific therapies, such as biologics or fecal microbiota transplantation. We expect that more studies will correlate specific changes in the oral microbiota with disease outcome and response to treatment. This will increase the potential for the use of the oral microbiome as a biomarker for extra-oral diseases. It is noteworthy that oral pathobionts can be detected in saliva. As the collection of a saliva sample is noninvasive and self-administered, it would be a cost-effective and convenient way to monitor the risk for gastrointestinal and liver diseases and to predict the response to treatment.
Article highlights.
Oral bacteria can translocate to the gastrointestinal tract where they contribute to inflammation and tumorigenesis.
Gut colonized oral bacteria can further disseminate to the liver and cause liver pathologies.
Oral treatment has the potential to improve the gastrointestinal and liver diseases.
Funding
This work was supported by Japan Society for the Promotion of Science 19K17413 (to JI), Takeda Japan Medical Office Funded Research Grant 2019 18J02400 (to JI), the University of Michigan Center for Gastrointestinal Research NIH P30 DK034933 (to SK and NK), the Office of the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense through the Peer Reviewed Cancer Research Program under Award No. W81XWH2010547 (to SK), National Institutes of Health grants DK108901, DK119219, AI142047, and DK125087 (to NK).
Footnotes
Declaration of Competing Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Consortium HMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012June;486(7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Consortium HMP. A framework for human microbiome research. Nature. 2012June;486(7402):215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson J, Garges S, Giovanni M, et al. The NIH Human Microbiome Project. Genome Res. 2009December;19(12):2317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillman ET, Lu H, Yao T, et al. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017December;32(4):300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010January;11(1):31–46. [DOI] [PubMed] [Google Scholar]
- 6.Nagao-Kitamoto H, Shreiner AB, Gillilland MG, et al. Functional Characterization of Inflammatory Bowel Disease-Associated Gut Dysbiosis in Gnotobiotic Mice. Cell Mol Gastroenterol Hepatol. 2016July;2(4):468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynge Pedersen AM, Belstrøm D. The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent. 201901;80Suppl 1:S3–S12. [DOI] [PubMed] [Google Scholar]
- 8.Bodet C, Chandad F, Grenier D. [Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis]. Pathol Biol (Paris). 20072007Apr-May;55(3–4):154–62. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Chaparro PJ, Gonçalves C, Figueiredo LC, et al. Newly identified pathogens associated with periodontitis: a systematic review. J Dent Res. 2014September;93(9):846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graves DT, Corrêa JD, Silva TA. The Oral Microbiota Is Modified by Systemic Diseases. J Dent Res. 201902;98(2):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minty M, Canceil T, Serino M, et al. Oral microbiota-induced periodontitis: a new risk factor of metabolic diseases. Rev Endocr Metab Disord. 201912;20(4):449–459. [DOI] [PubMed] [Google Scholar]
- 12.Kitamoto S, Nagao-Kitamoto H, Hein R, et al. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J Dent Res. 2020August;99(9):1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 201710;2(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponziani FR, Zocco MA, Cerrito L, et al. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol. 2018July;12(7):641–656. [DOI] [PubMed] [Google Scholar]
- 15.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006February;55(2):205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo T, Kamm MA, Colombel JF, et al. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 201807;15(7):440–452. [DOI] [PubMed] [Google Scholar]
- 17.Ni J, Wu GD, Albenberg L, et al. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017October;14(10):573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.*.Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014March;15(3):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large-cohort study clarifying the presence of oral bacteria in the intestine of treatment-naive Crohn’s disease patients
- 19.Schirmer M, Denson L, Vlamakis H, et al. Compositional and Temporal Changes in the Gut Microbiome of Pediatric Ulcerative Colitis Patients Are Linked to Disease Course. Cell Host Microbe. 201810;24(4):600–610.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuzuno T, Takahashi N, Yamada-Hara M, et al. Ingestion of Porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J Periodontal Res. 2021April;56(2):275–288. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Liang L, Liang H, et al. Aggravates the Progression of Colitis by Regulating M1 Macrophage Polarization via AKT2 Pathway. Front Immunol. 2019;10:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su W, Chen Y, Cao P, et al. Promotes the Development of Ulcerative Colitis by Inducing the Autophagic Cell Death of Intestinal Epithelial. Front Cell Infect Microbiol. 2020;10:594806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.**.Atarashi K, Suda W, Luo C, et al. Ectopic colonization of oral bacteria in the intestine drives T. Science. 201710;358(6361):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study showing the Th1-mediated colitogenic capacity of human oral bacteria isolated from saliva of IBD patients
- 24.**.Kitamoto S, Nagao-Kitamoto H, Jiao Y, et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell. 2020July;182(2):447–462.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study demonstrating the causative link between periodontal and intestinal inflammation in vivo from the immunological and microbiological points of views.
- 25.Brandtzaeg P Inflammatory bowel disease: clinics and pathology. Do inflammatory bowel disease and periodontal disease have similar immunopathogeneses? Acta Odontol Scand. 2001August;59(4):235–43. [DOI] [PubMed] [Google Scholar]
- 26.de Vries SAG, Tan CXW, Bouma G, et al. Salivary Function and Oral Health Problems in Crohn’s Disease Patients. Inflamm Bowel Dis. 201805;24(6):1361–1367. [DOI] [PubMed] [Google Scholar]
- 27.Goldinova A, Tan CX, Bouma G, et al. Oral health and salivary function in ulcerative colitis patients. United European Gastroenterol J. 2020November;8(9):1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickström C, Svensäter G. Salivary gel-forming mucin MUC5B--a nutrient for dental plaque bacteria. Oral Microbiol Immunol. 2008June;23(3):177–82. [DOI] [PubMed] [Google Scholar]
- 29.*.Said HS, Suda W, Nakagome S, et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res. 2014February;21(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study showing the oral dysbiosis in saliva of IBD patients
- 30.Axelrad JE, Cadwell KH, Colombel JF, et al. The role of gastrointestinal pathogens in inflammatory bowel disease: a systematic review. Therap Adv Gastroenterol. 2021;14:17562848211004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015May;17(5):662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox MA, Jackson J, Stanton M, et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol. 2009November;15(44):5549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhaskaran N, Quigley C, Paw C, et al. Role of Short Chain Fatty Acids in Controlling T. Front Microbiol. 2018;9:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015March;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 36.Abreu MT, Peek RM. Gastrointestinal malignancy and the microbiome. Gastroenterology. 2014May;146(6):1534–1546.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arthur JC, Jobin C. The struggle within: microbial influences on colorectal cancer. Inflamm Bowel Dis. 2011January;17(1):396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014March;15(3):317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisburger JH, Reddy BS, Narisawa T, et al. Germ-free status and colon tumor induction by N-methyl-N’-nitro-N-nitrosoguanidine. Proc Soc Exp Biol Med. 1975April;148(4):1119–21. [DOI] [PubMed] [Google Scholar]
- 40.Vannucci L, Stepankova R, Kozakova H, et al. Colorectal carcinogenesis in germ-free and conventionally reared rats: different intestinal environments affect the systemic immunity. Int J Oncol. 2008March;32(3):609–17. [PubMed] [Google Scholar]
- 41.Elsalem L, Jum’ah AA, Alfaqih MA, et al. The Bacterial Microbiota of Gastrointestinal Cancers: Role in Cancer Pathogenesis and Therapeutic Perspectives. Clin Exp Gastroenterol. 2020;13:151–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rea D, Coppola G, Palma G, et al. Microbiota effects on cancer: from risks to therapies. Oncotarget. 2018April;9(25):17915–17927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.*.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009September;15(9):1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study defining immunological mechanisms of colonic carcinogenesis elicited by a human commensal bacterium
- 44.Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012October;338(6103):120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.*.Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015October;6:8727. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study identifying bacterial signatures in different stages of colorectal tumorigenesis
- 46.Hale VL, Chen J, Johnson S, et al. Shifts in the Fecal Microbiota Associated with Adenomatous Polyps. Cancer Epidemiol Biomarkers Prev. 201701;26(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanapareddy N, Legge RM, Jovov B, et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012October;6(10):1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu N, Yang X, Zhang R, et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol. 2013August;66(2):462–70. [DOI] [PubMed] [Google Scholar]
- 49.Koliarakis I, Messaritakis I, Nikolouzakis TK, et al. Oral Bacteria and Intestinal Dysbiosis in Colorectal Cancer. Int J Mol Sci. 2019August;20(17):4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012February;22(2):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Candela M, Turroni S, Biagi E, et al. Inflammation and colorectal cancer, when microbiota-host mutualism breaks. World J Gastroenterol. 2014January;20(4):908–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marchesi JR, Dutilh BE, Hall N, et al. Towards the human colorectal cancer microbiome. PLoS One. 2011;6(5):e20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.*.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013August;14(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the milestone studies showing the machanism by which Fusobacterium nucleatum modulates the tumor-immune micrenvironment in the pathogenesis of CRC.
- 54.Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin Transl Gastroenterol. 2016November;7(11):e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016January;22(2):557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 201612;65(12):1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serna G, Ruiz-Pace F, Hernando J, et al. Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Ann Oncol. 202010;31(10):1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.*.Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013August;14(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the milestone studies unveiling a mechanism by which Fn can drive CRC and identifying FadA as a potential diagnostic and therapeutic target for CRC.
- 59.Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015February;42(2):344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 201703;152(4):851–866.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaoka Y, Suehiro Y, Hashimoto S, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol. 2018April;53(4):517–524. [DOI] [PubMed] [Google Scholar]
- 62.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015August;1(5):653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013December;105(24):1907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen W, Liu F, Ling Z, et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7(6):e39743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guven DC, Dizdar O, Alp A, et al. Analysis of. Biomark Med. 201906;13(9):725–735. [DOI] [PubMed] [Google Scholar]
- 66.Ryder MI. Porphyromonas gingivalis and Alzheimer disease: Recent findings and potential therapies. J Periodontol. 202010;91Suppl 1:S45–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pizzo G, Guiglia R, Lo Russo L, et al. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010December;21(6):496–502. [DOI] [PubMed] [Google Scholar]
- 68.Figuero E, Sánchez-Beltrán M, Cuesta-Frechoso S, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011October;82(10):1469–77. [DOI] [PubMed] [Google Scholar]
- 69.Maekawa T, Krauss JL, Abe T, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014June;15(6):768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richard ML, Liguori G, Lamas B, et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes. 201803;9(2):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eslam M, Sanyal AJ, George J, et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 202005;158(7):1999–2014.e1. [DOI] [PubMed] [Google Scholar]
- 72.Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. 202007;17(7):387–388. [DOI] [PubMed] [Google Scholar]
- 73.Shiha G, Alswat K, Al Khatry M, et al. Nomenclature and definition of metabolic-associated fatty liver disease: a consensus from the Middle East and north Africa. Lancet Gastroenterol Hepatol. 202101;6(1):57–64. [DOI] [PubMed] [Google Scholar]
- 74.Sanyal AJ. NASH: A global health problem. Hepatol Res. 2011July;41(7):670–4. [DOI] [PubMed] [Google Scholar]
- 75.Liou I, Kowdley KV. Natural history of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006March;40Suppl 1:S11–6. [DOI] [PubMed] [Google Scholar]
- 76.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 201607;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 77.Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology. 201607;64(1):19–22. [DOI] [PubMed] [Google Scholar]
- 78.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998April;114(4):842–5. [DOI] [PubMed] [Google Scholar]
- 79.Cave M, Deaciuc I, Mendez C, et al. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007March;18(3):184–95. [DOI] [PubMed] [Google Scholar]
- 80.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008December;40(12):1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suk KT, Kim DJ. Gut microbiota: novel therapeutic target for nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019March;13(3):193–204. [DOI] [PubMed] [Google Scholar]
- 82.Leung C, Rivera L, Furness JB, et al. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 201607;13(7):412–25. [DOI] [PubMed] [Google Scholar]
- 83.**.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013February;57(2):601–9. [DOI] [PubMed] [Google Scholar]; A study Characterizing the signature of the gut microbiome in patients with NASH
- 84.Michail S, Lin M, Frey MR, et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015February;91(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwimmer JB, Johnson JS, Angeles JE, et al. Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology. 201910;157(4):1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.*.Caussy C, Tripathi A, Humphrey G, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. 201903;10(1):1406. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study characterizing the signature of the gut microbiome in patients with NAFLD-cirrhosis
- 87.Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014September;513(7516):59–64. [DOI] [PubMed] [Google Scholar]
- 88.Zhou J, Tripathi M, Sinha RA, et al. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. 2021;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.*.Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 201901;69(1):107–120. [DOI] [PubMed] [Google Scholar]; A study characterizing the signature of the gut microbiome in NAFLD-cirrhosis patinets with HCC compared to those without HCC
- 90.Brignardello J, Morales P, Diaz E, et al. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther. 2010December;32(11–12):1307–14. [DOI] [PubMed] [Google Scholar]
- 91.Farhadi A, Gundlapalli S, Shaikh M, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008August;28(7):1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014March;59(3):1144–65. [DOI] [PubMed] [Google Scholar]
- 93.Yoneda M, Naka S, Nakano K, et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012February;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.**.Nakahara T, Hyogo H, Ono A, et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J Gastroenterol. 2018February;53(2):269–280. [DOI] [PubMed] [Google Scholar]; A study clarifying that Porphyromonas gingivalis infection is a risk factor for pathological progression of NAFLD
- 95.Takamisawa K, Sugita N, Komatsu S, et al. Association between serum IgG antibody titers against. Heliyon. 2020November;6(11):e05531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kudo C, Naruishi K, Maeda H, et al. Assessment of the plasma/serum IgG test to screen for periodontitis. J Dent Res. 2012December;91(12):1190–5. [DOI] [PubMed] [Google Scholar]
- 97.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013November;10(11):656–65. [DOI] [PubMed] [Google Scholar]
- 98.Chakladar J, Wong LM, Kuo SZ, et al. The Liver Microbiome Is Implicated in Cancer Prognosis and Modulated by Alcohol and Hepatitis B. Cancers (Basel). 2020June;12(6):1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Komiyama EY, Lepesqueur LS, Yassuda CG, et al. Enterococcus Species in the Oral Cavity: Prevalence, Virulence Factors and Antimicrobial Susceptibility. PLoS One. 2016;11(9):e0163001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davis IJ, Richards H, Mullany P. Isolation of silver- and antibiotic-resistant Enterobacter cloacae from teeth. Oral Microbiol Immunol. 2005June;20(3):191–4. [DOI] [PubMed] [Google Scholar]
- 101.Tsuzukibashi O, Uchibori S, Kobayashi T, et al. Isolation and identification methods of Rothia species in oral cavities. J Microbiol Methods. 201703;134:21–26. [DOI] [PubMed] [Google Scholar]
- 102.Furusho H, Miyauchi M, Hyogo H, et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J Gastroenterol. 2013November;48(11):1259–70. [DOI] [PubMed] [Google Scholar]
- 103.Sasaki N, Katagiri S, Komazaki R, et al. Endotoxemia by. Front Microbiol. 2018;9:2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nekvapil F, Pinzaru SC, Barbu-Tudoran L, et al. Color-specific porosity in double pigmented natural 3d-nanoarchitectures of blue crab shell. Sci Rep. 202002;10(1):3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ding LY, Liang LZ, Zhao YX, et al. Porphyromonas gingivalis-derived lipopolysaccharide causes excessive hepatic lipid accumulation via activating NF-κB and JNK signaling pathways. Oral Dis. 2019October;25(7):1789–1797. [DOI] [PubMed] [Google Scholar]
- 106.**.Kamata Y, Kessoku T, Shimizu T, et al. Efficacy and safety of PERIOdontal treatment versus usual care for Nonalcoholic liver disease: protocol of the PERION multicenter, two-arm, open-label, randomized trial. Trials. 2020March;21(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]; A ranomized clinical trial that evaluates the effect of oral maintenance therapy on fatty liver disease
- 107.Lankarani KB, Sivandzadeh GR, Hassanpour S. Oral manifestation in inflammatory bowel disease: a review. World J Gastroenterol. 2013December;19(46):8571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011April;7(4):235–41. [PMC free article] [PubMed] [Google Scholar]
- 109.Ribaldone DG, Brigo S, Mangia M, et al. Oral Manifestations of Inflammatory Bowel Disease and the Role of Non-Invasive Surrogate Markers of Disease Activity. Medicines (Basel). 2020June;7(6):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Papageorgiou SN, Hagner M, Nogueira AV, et al. Inflammatory bowel disease and oral health: systematic review and a meta-analysis. J Clin Periodontol. 2017April;44(4):382–393. [DOI] [PubMed] [Google Scholar]
- 111.Okada M, Kobayashi T, Ito S, et al. Periodontal treatment decreases levels of antibodies to Porphyromonas gingivalis and citrulline in patients with rheumatoid arthritis and periodontitis. J Periodontol. 2013December;84(12):e74–84. [DOI] [PubMed] [Google Scholar]
- 112.Jakubczyk D, Leszczyńska K, Górska S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)-A Critical Review. Nutrients. 2020July;12(7):1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Azad MAK, Sarker M, Wan D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res Int. 2018;2018:8063647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, et al. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand J Gastroenterol. 2008;43(7):842–8. [DOI] [PubMed] [Google Scholar]
- 115.Kato K, Mizuno S, Umesaki Y, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004November;20(10):1133–41. [DOI] [PubMed] [Google Scholar]
- 116.Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004November;53(11):1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Henker J, Müller S, Laass MW, et al. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: an open-label pilot study. Z Gastroenterol. 2008September;46(9):874–5. [DOI] [PubMed] [Google Scholar]
- 118.Montalban-Arques A, Scharl M. Intestinal microbiota and colorectal carcinoma: Implications for pathogenesis, diagnosis, and therapy. EBioMedicine. 2019October;48:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chitapanarux I, Tungkasamit T, Petsuksiri J, et al. Randomized control trial of benzydamine HCl versus sodium bicarbonate for prophylaxis of concurrent chemoradiation-induced oral mucositis. Support Care Cancer. 2018March;26(3):879–886. [DOI] [PubMed] [Google Scholar]
- 120.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015November;350(6264):1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013October;19(40):6911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kobyliak N, Abenavoli L, Mykhalchyshyn G, et al. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J Gastrointestin Liver Dis. 201803;27(1):41–49. [DOI] [PubMed] [Google Scholar]
- 123.Shimauchi H, Mayanagi G, Nakaya S, et al. Improvement of periodontal condition by probiotics with Lactobacillus salivarius WB21: a randomized, double-blind, placebo-controlled study. J Clin Periodontol. 2008October;35(10):897–905. [DOI] [PubMed] [Google Scholar]
- 124.Seminario-Amez M, López-López J, Estrugo-Devesa A, et al. Probiotics and oral health: A systematic review. Med Oral Patol Oral Cir Bucal. 2017May;22(3):e282–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiao L, Zhang Q, Peng Y, et al. The effect of periodontal bacteria infection on incidence and prognosis of cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2020April;99(15):e19698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wong SH, Kwong TNY, Chow TC, et al. Quantitation of faecal. Gut. 201708;66(8):1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janati AI, Karp I, Laprise C, et al. Detection of Fusobaterium nucleatum in feces and colorectal mucosa as a risk factor for colorectal cancer: a systematic review and meta-analysis. Syst Rev. 202012;9(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gethings-Behncke C, Coleman HG, Jordao HWT, et al. in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev. 202003;29(3):539–548. [DOI] [PubMed] [Google Scholar]
- 129.Zhang X, Zhu X, Cao Y, et al. Fecal Fusobacterium nucleatum for the diagnosis of colorectal tumor: A systematic review and meta-analysis. Cancer Med. 201902;8(2):480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rezasoltani S, Sharafkhah M, Asadzadeh Aghdaei H, et al. Applying simple linear combination, multiple logistic and factor analysis methods for candidate fecal bacteria as novel biomarkers for early detection of adenomatous polyps and colon cancer. J Microbiol Methods. 201812;155:82–88. [DOI] [PubMed] [Google Scholar]
- 131.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016March;63(3):764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo Y, Kitamoto S, Kamada N. Microbial adaptation to the healthy and inflamed gut environments. Gut Microbes. 202011;12(1):1857505. [DOI] [PMC free article] [PubMed] [Google Scholar]


