Abstract
Introduction
After a vaccine administration, many people have localized symptoms such as pain, redness, warmth, swelling, itching and/or bruising, which usually improve in a few days. If the clinical symptoms do not improve in this period, a shoulder injury related to vaccine administration (SIRVA) should be ruled out. The most common cause of a SIRVA is an improper injection technique. Herein, we reported the first case of combined subacromial-subdeltoid bursitis and supraspinatus tendon tear which was apparently caused by an improper COVID-19 vaccination technique.
Case presentation
A 51-year-old Thai female began to experience severe right shoulder pain about 3 hours after receiving a COVID-19 vaccination. Ultrasonography showed combined subacromial-subdeltoid bursitis and supraspinatus tendon tear. Her clinical symptoms gradually improved after treatment with an oral non-steroidal anti-inflammatory drug. Our investigation found that an improper injection technique had been used, namely inserting the needle too deeply, and using an incorrect landmark.
Conclusion
We report a case of combined subacromial-subdeltoid bursitis and supraspinatus tendon tear following a second dose of the Oxford-AstraZeneca COVID-19 vaccine. This is a rare condition which is usually related to an incorrect injection technique. To reduce the chance of SIRVA, the healthcare worker giving the injection should pay careful attention to find the appropriate landmark, and ensuring the correct needle length and direction of the injection.
Keywords: COVID-19, Injection, Pain, Shoulder, Vaccine
Highlights
-
•
If the clinical symptoms after vaccination do not improve in 2-3 days, a shoulder injury related to vaccine administration should be considered.
-
•
There is a risk of SIRVA if the anatomical landmark of the needle was too high.
-
•
Before diagnosis SIRVA, the serious condition such as septic arthritis of shoulder should be ruled out.
1. Introduction
After any vaccination, the vaccinated person may experience local (pain, redness, warmth, swelling, itching and/or bruising) or systemic (nausea, diarrhea, fever, headache, fatigue, chills, muscle or joint pain) symptoms [1]. These symptoms normally improve in a few days. If the clinical symptoms persist for more than 2–3 days, a shoulder injury related to vaccine administration (SIRVA) should be considered [[2], [3], [4], [5], [6]]. A SIRVA usually occurs due to an incorrect injection technique such as inappropriate needle direction or depth of penetration. This condition usually is the result of an immune response to an intrabursal inoculation which is a good response to oral non-steroidal anti-inflammatory drugs (NSAIDs), oral steroid, an intrabursal steroid injection and a physical therapy [7]. The end of last year, the COVID-19 vaccines have been widely administered due to the COVID-19 pandemic. There have to date been two case reports in the literature of a SIRVA after a vaccination. The first case of subdeltoid bursitis was reported 8 weeks after an Oxford-AstraZeneca COVID-19 injection (Serum Institute of India, India) [8]. The second case was reported by Chuaychoosakoon et al. in which a subdeltoid-subacromial-subcoracoid bursitis occurred 3 days following a Sinovac vaccination (Sinovac Biotech, China) [9]. In this study, we report a case of combined subacromial-subdeltoid bursitis and supraspinatus tendon tear after a second dose of the Oxford-AstraZeneca COVID-19 vaccine. This case is reported according to the SCARE criteria [10], with Ethical Committee approval from the Faculty of Medicine of Prince of Songkla University.
2. Case presentation
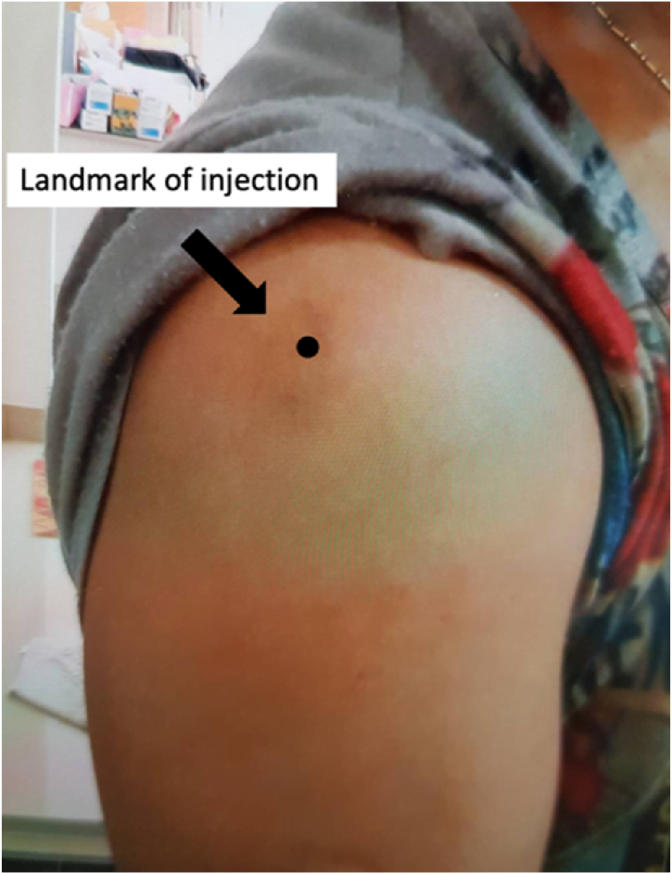
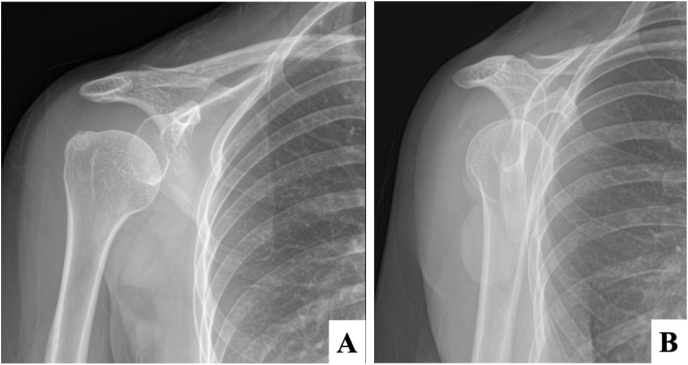
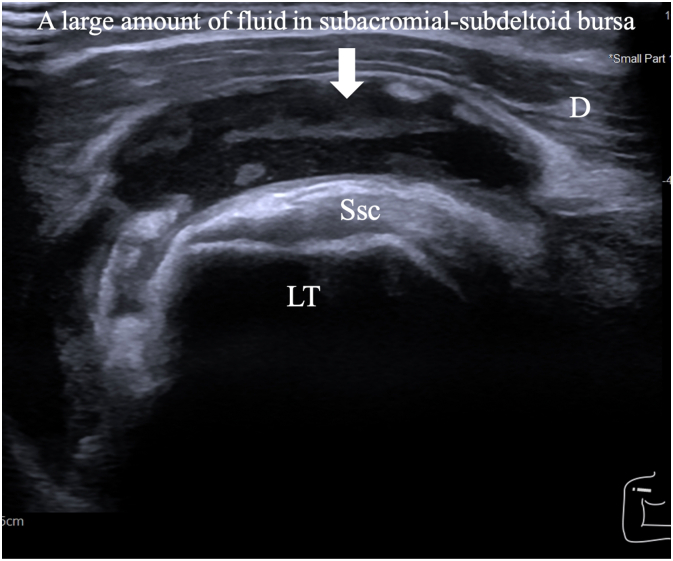
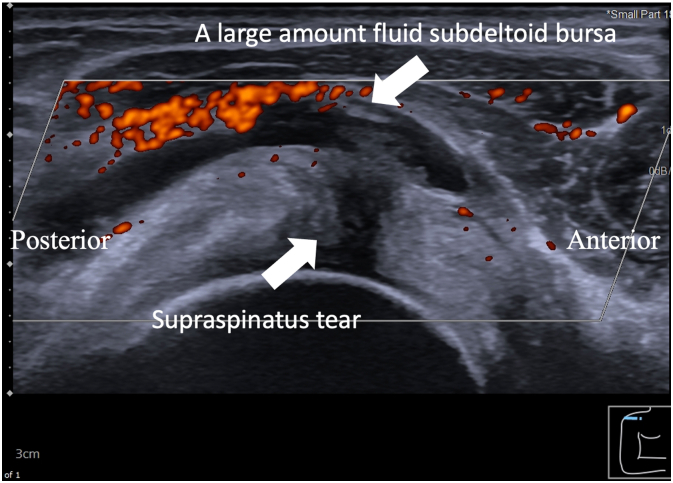
A 51-year-old Thai female with no pre-existing shoulder pain received an Oxford-AstraZeneca COVID-19 vaccine using a 25-gauge needle, 1.5 inches in length, in an injection site based on landmark of 1 finger breadth below the midlateral border of the acromial process (Fig. 1). The needle direction was perpendicular to the skin at the injection site. After returning home, the patient had developed right shoulder pain within 3 hours following the vaccination, and then 4 days later decided to see a doctor when the pain had not improved after taking over-the-counter drugs. A physical examination showed tenderness over the deltoid area, with limited range motion of her right shoulder in all directions. Radiographs of the anteroposterior and lateral transcapular views of the right shoulder showed soft tissue swelling without fracture (Fig. 2A and B). The humeral head was displaced inferiorly with a widened acromiohumeral distance which could have been from joint effusion or distended subacromial-subdeltoid bursa. The greater tuberosity showed cortical irregularities and subcortical sclerosis. Ultrasonography of the right shoulder showed subacromial-subdeltoid bursitis with synovial wall thickening, and internal septa (Fig. 3), tenodesis with small full-thickness tear at the posterior fiber of supraspinatus (Fig. 4). She was treated with oral NSAIDs, and her clinical symptoms gradually improved over the next few days (Fig. 5A–C).
Fig. 1.
The landmark used for the COVID-19 vaccination in this patient.
Fig. 2.
Initial radiographic images of the right shoulder in (A) anteroposterior, and (B) lateral transcapular views.
Fig. 3.
With the right shoulder in the external rotation position, an ultrasonographic image over the lesser tuberosity and subscapularis in a long axis view showed a large amount of fluid in the subacromial-subdeltoid bursa in the anterior right shoulder. Hypoechoic subscapularis tendon was from anisotropy. (D; Deltoid muscle, Ssc; Subscapularis tendon and LT; Lessor tuberosity).
Fig. 4.
With the right shoulder in the modified Crass position, an ultrasonographic image in a short axis view of supraspinatus tendon showed a large amount of fluid in the subdeltoid bursa with subjacent small full-thickness tear at the posterior fiber of supraspinatus.
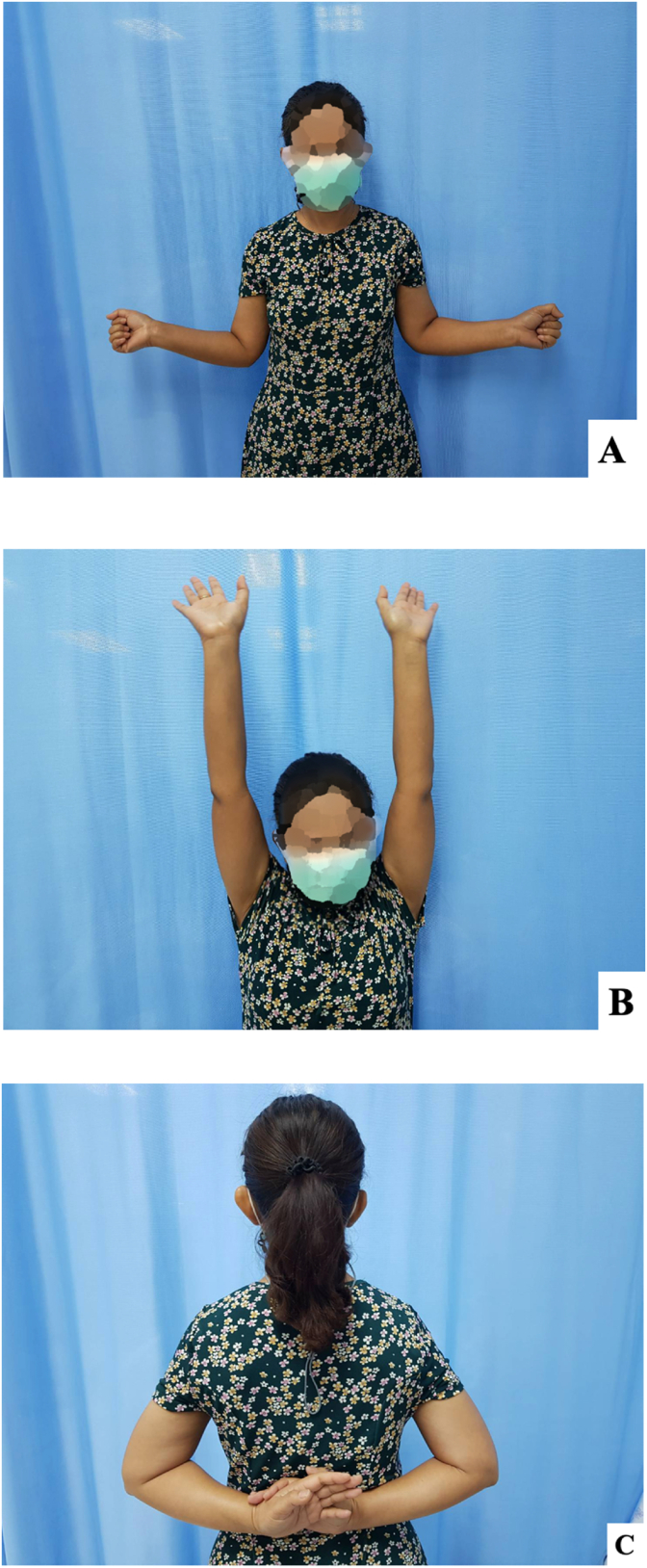
Fig. 5.
Comparing range of motion between right and left shoulders after conservative treatment (A) external rotation in arm at side, (B) forward flexion and (C) internal rotation.
3. Discussion
A SIRVA should be concerned if shoulder pain has not improved within a few days following a vaccination. In this patient, she had developed right shoulder pain following a second dose of COVID-19 Oxford-AstraZeneca vaccine which had not improved in 4 days. The risk factors in her case were the landmark and needle-penetration depth of the injection.
There have to date been two reported cases of a SIRVA following a COVID-19 vaccination. The first case was a 61-year-old male who presented with right shoulder pain 8 weeks after of an Oxford-AstraZeneca COVID-19 injection [8]. His clinical symptoms were improved after received a short course of an oral steroid, vitamin D supplementation and a physical therapy. The second case was a 52-year-old Thai female who developed a right shoulder pain 3 days after receiving the Sinovac COVID-19 vaccine [9]. Ultrasonography showed subacromial-subcoracoid-subdeltoid bursitis. She was treated with antibiotics for 10 days, during which time her clinical symptoms were rapidly improved, and a post-treatment ultrasonography showed decreased bursal fluid. The risk factors of these two cases were the needle direction and penetration depth during their vaccinations, while in our case the risk factors were the landmark and length of needle. In our case, the patient complained of right shoulder pain within 3 hours after her COVID-19 vaccine injection, which persisted for 4 days without improving. When she was examined at the orthopedics clinic, an ultrasonography which showed combined subacromial-subdeltoid bursitis and full-thickness supraspinatus tendon tear. Her pain gradually improved after she was prescribed as oral NSAIDs.
Most SIRVAs involve rotator cuff tendinopathy without tearing and/or bursitis, and are usually diagnosed by ultrasonography and/or MRI [7]. A few SIRVAs have involved rotator cuff tears which occurred following influenza, tetanus or human papillomavirus vaccinations. Our case is the first case to report a supraspinatus tendon tear following a COVID-19 vaccination. There are two hypotheses which could explain this type of rotator cuff tear. The first possibility was round in a case in 2006 in which the patient had a preexisting rotator cuff tear without clinical symptom, until shoulder pain developed after a vaccination which was associated with synovial inflammation [11]. The second possibility is localized rotator cuff tendon inflammation because the rotator cuff tendons are located close to the bursa leading to the possibility of a rotator cuff tear.
To reduce the risk of a SIRVA, all healthcare workers who will be giving injections should be carefully trained regarding the safe way to give vaccine injections. The common pitfalls of intramuscular vaccine injections are the needle direction, needle-penetration depth and an entry point of injection. In this case, the patient was injected using a 1.5-inch 25-gauge needle with the entry point 1 finger breadth below the midlateral border of the acromial process with the direction of the needle perpendicular to the skin. This distance was too small, and left a chance of the needle tip penetrating into a bursa or a rotator cuff, a risk which unfortunately happened, causing the SIRVA in this patient. The recommended injection site is 3 finger breadths below the midlateral border of the acromial process, because the mean distances between the middle part of the acromial process and the axillary nerve are 52.20 ± 4.21 mm with the arm at the side position and 49.66 ± 4.54 mm in the 30° of arm abduction position [12]. The depth of needle-penetration should be at least 5 mm from the skin in a perpendicular direction [13].
4. Conclusions
We report a case of combined subacromial-subdeltoid bursitis and supraspinatus tear following a second dose of the Oxford-AstraZeneca COVID-19 vaccine. This is a rare condition which is usually related to an incorrect injection technique. To reduce the chance of SIRVA, the appropriate landmark, needle length and direction should be confirmed.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
No funding was involved regarding this case report.
Ethical approval
The present study was approved by the Prince of Songkla University Institutional Review Board, Faculty of Medicine, Songklanagarind Hospital, Prince of Songkla University (IRB number REC 64-411-11-1).
Consent
Written informed consent was obtained from the patient for publication.
Author contribution
Chaiwat Chuaychoosakoon —Preparation of case report, Literature review, Writing the paper. Pattira Boonsri —Preparation of case report. Writing the paper.
Research registration (for case reports detailing a new surgical technique or new equipment/technology)
N/A.
Guarantor
Chaiwat Chuaychoosakoon, M.D.
Declaration of competing interest
No conflicts of interest.
Acknowledgements
The authors sincerely thank the patient for allowing us to report this case and David Patterson of the International Affairs Office of the Faculty of Medicine, Prince of Songkla University for English proofreading.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102819.
Contributor Information
Pattira Boonsri, Email: bpattira@medicine.psu.ac.th.
Chaiwat Chuaychoosakoon, Email: psu.chaiwat@gmail.com, chaiwat.c@psu.ac.th.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., Hu C., Selvachandran S., Antonelli M., Murray B., Canas L.S., Molteni E., Graham M.S., Modat M., Joshi A.D., Mangino M., Hammers A., Goodman A.L., Chan A.T., Wolf J., Steves C.J., Valdes A.M., Ourselin S., Spector T.D. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/s1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins M., Rupp D., Goebel L.J. Post-influenza vaccine subdeltoid bursitis. Cureus. 2020;12:10–13. doi: 10.7759/cureus.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook I.F. Subdeltoid/subacromial bursitis associated with influenza vaccination. Hum. Vaccines Immunother. 2014;10:605–606. doi: 10.4161/hv.27232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright A., Patel R., Motamedi D. Influenza vaccine-related subacromial/subdeltoid bursitis: a case report. J. Radiol. Case Rep. 2019;13:24–31. doi: 10.3941/jrcr.v13i6.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Littrell L.A., Leslie D.F., Bierle D.M., Wenger D.E. Progressive monoarticular inflammatory arthritis following influenza vaccination. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021;5:204–209. doi: 10.1016/j.mayocpiqo.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szari S., Belgard A., Adams K., Freiler J. Shoulder injury related to vaccine administration: a rare reaction. Fed. Pract. 2019;36:380–384. [PMC free article] [PubMed] [Google Scholar]
- 7.Atanasoff S., Ryan T., Lightfoot R., Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA) Vaccine. 2010;28:8049–8052. doi: 10.1016/j.vaccine.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Cantarelli Rodrigues T., Hidalgo P.F., Skaf A.Y., Serfaty A. Subacromial-subdeltoid bursitis following COVID-19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA) Skeletal Radiol. 2021 doi: 10.1007/s00256-021-03803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuaychoosakoon C., Parinyakhup W., Tanutit P., Maliwankul K., Klabklay P. Shoulder injury related to Sinovac COVID-19 vaccine: a case report. Ann. Med. Surg. 2021;68:102622. doi: 10.1016/j.amsu.2021.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Reilly P., Macleod I., Macfarlane R., Windley J., Emery R.J.H. Dead men and radiologists don't lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann. R. Coll. Surg. Engl. 2006;88:116–121. doi: 10.1308/003588406X94968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuaychoosakoon C., Suwannaphisit S. The relationship between arm abduction position and the risk of iatrogenic anterior branch of the axillary nerve injuries: a cadaveric study. Orthop. J. Sport. Med. 2021;9 doi: 10.1177/23259671211008834. 232596712110088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima Y., Mukai K., Takaoka K., Hirose T., Morishita K., Yamamoto T., Yoshida Y., Urai T., Nakatani T. Establishing a new appropriate intramuscular injection site in the deltoid muscle. Hum. Vaccines Immunother. 2017;13:2123–2129. doi: 10.1080/21645515.2017.1334747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.