Abstract
Objectives:
Our study’s primary objective was to compare 1-year survival rates between serum cryptococcal antigen (sCrAg)-positive and sCrAg-negative HIV-positive individuals with CD4 counts <100 cells/μl without symptoms of meningitis in Zimbabwe.
Design:
This was a prospective cohort study.
Methods:
Participants were enrolled as either sCrAg-positive or sCrAg-negative and followed up for ≤52 weeks, with death as the outcome. Lumbar punctures (LPs) were recommended to all sCrAg-positives and inpatient management with intravenous amphotericin B and high-dose fluconazole was recommended to those with disseminated Cryptococcus. Antiretroviral therapy was initiated immediately in sCrAg-negatives and after ≥4 weeks following initiation of antifungals in sCrAg-positives. Multivariable logistic regression models were used to determine risk factors for mortality.
Results:
We enrolled 1320 participants and 130 (9.8%) were sCrAg positive, with a median sCrAg titre of 1:20. Sixty-six (50.8%) sCrAg-positives had LPs and 16.7% (11/66) had central nervous system (CNS) dissemination. Cryptococcal blood cultures were performed in 129 sCrAg-positives, with 10 (7.8%) being positive. One-year (48–52 weeks) survival rates were 83.9% and 76.1 % in sCrAg-negatives and sCrAg-positives, respectively, p=0.011. Factors associated with increased mortality were a positive sCrAg, CD4 count <50 cells/μl and having presumptive tuberculosis (TB) symptoms.
Conclusion:
Our study reports a high prevalence of subclinical cryptococcal antigenemia and reiterates the importance of TB and a positive sCrAg as risk factors for mortality in advanced HIV disease (AHD). Therefore, TB and sCrAg screening remains a crucial component of AHD package, hence it should always be part of the comprehensive clinical evaluation in AHD patients.
Keywords: HIV, Sub-Saharan, Cryptococcal, Antigenemia, Meningitis
Introduction
Cryptococcal meningitis (CM) is a leading cause of meningitis among human immunodeficiency virus (HIV)-infected individuals in Sub-Saharan Africa (SSA) (1–3). Prior to improved access to antiretroviral therapy (ART), CM was the leading cause of death in several HIV-infected cohorts in SSA (4–8). Introduction of ART led to a decline in incidence of CM in the developed world (9–11). However, despite improvements in access to antifungal therapy and ART, mortality from CM remains high in low-income settings (2, 12).
Detection of cryptococcal antigen (CrAg) in serum may precede onset of symptoms(6). Early detection and pre-emptive antifungal treatment of subclinical disease may avert fulminant disease, particularly in the setting of ART initiation or optimization following treatment failure. Rapid CrAg kits with high sensitivity and specificity, like the lateral flow immunoassay (LFA), allow for simple low-cost diagnosis of cryptococcosis (13, 14), and have been strongly recommended by World Health Organisation (WHO) for patients with CD4 count <100 cells/μl (15). Widespread implementation of this screen and treat approach in SSA has been sub-optimal (16). Prospective data on the cost-effectiveness and impact on outcomes of a screen and treat approach for patients with subclinical disease are limited, with data based on modelling or cohorts including symptomatic individuals for whom management is well defined (14, 17–19). Data on detection of disseminated disease in patients with subclinical antigenemia is sparse and evidence-based management guidelines are lacking (20).
In Zimbabwe, data on prevalence of subclinical cryptococcal antigenemia in patients with advanced HIV disease and their survival experience were unknown. Therefore, this study’s aim was to compare 1-year (48 to 52 weeks) survival rates between sCrAg-positive and sCrAg-negative HIV-positive individuals with CD4 counts <100 cells/μl and without symptoms of meningitis, receiving care at public HIV/ART treatment programs. We also determined the prevalence of subclinical cryptococcal antigenemia in this cohort.
Methods
Study design
This study was a prospective cohort study. We implemented sCrAg screening in adult (age ≥18 years) patients living with HIV who were both ART naïve (never taken ART) and ART experienced (either had been on ART but defaulted or currently on ART), with CD4 count <100 cells/μl, receiving care at 20 outpatient facilities in Harare, Zimbabwe between 2015 and 2016. Those who were sCrAg positive were considered exposed and sCrAg-negatives were regarded as unexposed. They were then longitudinally followed up for a maximum of 52 weeks from the day of enrolment.
Exclusion criteria
CD4 count ≥100 cells/ μl
Signs and symptoms suggestive of a meningitis namely headache, confusion, vision change, seizure, altered behaviour, focal weakness and neck stiffness.
Diagnosis of CM in the preceding 2 weeks of enrolment,
Previous drug reaction(s) to amphotericin B and /or fluconazole,
An estimated glomerular filtration rate (eGFR) ≤30 ml/minute,
Alanine transaminase (ALT) ≥ 5 times the upper limit of normal,
Pregnant women (confirmed by point-of-care urine dipsticks). They were excluded from the study because of concerns about using high-dose fluconazole for pre-emptive treatment for those found to be sCrAg positive.
All ineligible patients were referred back into the public healthcare system for further management.
Study Procedures
i. Screening
Patients were enrolled for screening at the study sites based on the most recently documented CD4 count of ≤100 cells/μl. Samples for a repeat CD4 count and reflexive sCrAg testing were collected.
ii. Enrolment
At enrollment, sCrAg testing was done only on samples with CD4 count of <100 cells/μl on repeat CD4 count testing using LFA (Immuno-Mycologics Inc., Norman, OK 73071, USA). Cryptococcal blood cultures and sCrAg titre measurements were performed on sCrAg-positive samples. An LP was offered to all participants who were sCrAg positive to exclude subclinical meningitis. Laboratory tests performed on CSF included CrAg (cCrAg) LFA and titres, India ink staining, and routine bacterial and fungal cultures.
iii. Management of asymptomatic antigenemia
Participants with positive cCrAg LFA, India ink staining, CSF and/or blood cryptococcal culture were deemed to have disseminated cryptococcal disease and hence offered hospitalization for induction with a 2-week course of intravenous amphotericin B deoxycholate (0.7mg – 1mg/kg daily) plus high dose (800mg daily) fluconazole and CSF pressure management in accordance with standard protocols(21–23). Participants who had no evidence of disseminated disease (i.e negative cCrAg LFA, India ink staining, CSF and/or blood cryptococcal culture), those who refused an LP and those with disseminated disease who refused hospitalization were put on a 2-week induction high-dose fluconazole (800mg daily) only as outpatients as per the existing guidelines at the time of the study(23–25) (Figure 1). Following the induction phase, all participants were put on fluconazole 400mg daily for 8 weeks as consolidation therapy. They were then maintained on fluconazole 200mg daily for at least a year.
Figure 1:
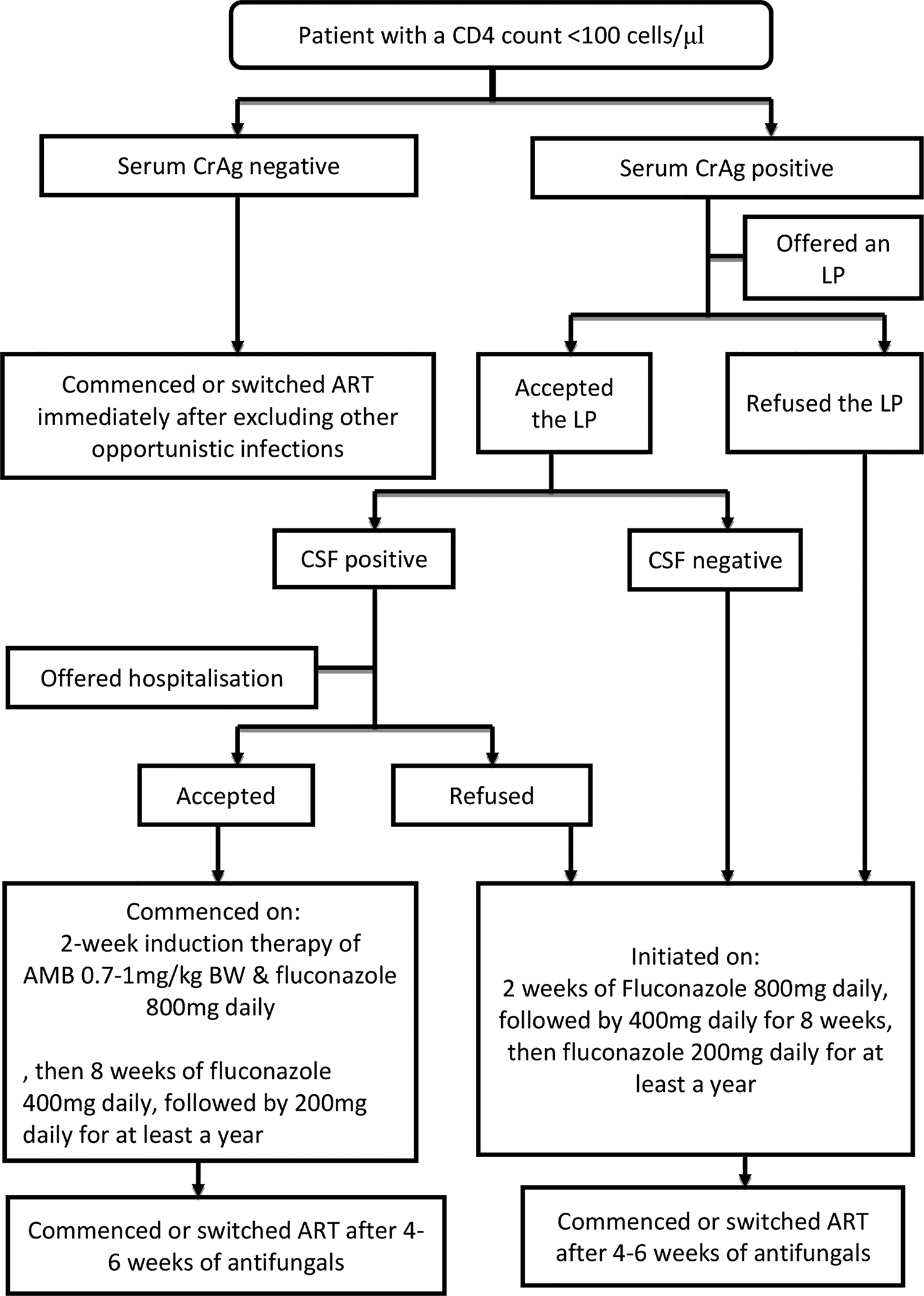
Flowchart of study procedures
CrAg-cryptococcal antigen, LP-lumbar puncture, CSF-cerebrospinal fluid, AMB-Amphotericin B, ART-antiretroviral therapy
iv. Antiretroviral therapy
If no contraindications, first-line ART was initiated or some were switched to second-line ART immediately among sCrAg-negatives and at least after 4 weeks in sCrAg-positives as per the Zimbabwean national ART standard treatment guidelines at time of the study(24).
Study participants follow-up
Participants were followed up at the health facilities where they had been receiving their primary HIV care for a maximum of 52 weeks. At every study visit, they would be reviewed by both their primary HIV care provider(s) and study member(s). HIV management that included ART initiation or switch, choice of the ART regimen and management of other opportunistic conditions like Kaposi Sarcoma were provided by the primary HIV care provider using guidance from the existing guidelines(23, 24). TB symptoms (defined as cough, fever, weight loss, night sweats and chronic fatigue) screening and/or diagnosis, using microscopy and/or GeneXpert, were conducted by both the primary HIV care provider and research nurse at every study visit as per the national guidelines(24).
Outcome measures
The study’s outcome of interest was 1-year (48 to 52 weeks) all-cause mortality in sCrAg-positive and sCrAg-negative participants. To determine the survival, participants were considered to be alive if they had completed 48 to 52 weeks of study follow-up or were known to be alive through use of their primary HIV clinic records at the end of the study. The causes of death for participants were obtained by review of medical records or death certificates or through verbal reports from relatives. No post-mortems were conducted. Loss to follow-up (LTFU) was defined as failure to return to the clinic for care or prescription pick up within 3 months of the last documented visit and/or failure by the study team, during the entire course of the study, to establish contact with the participant either through telephone or home visits. Participants were considered as having transferred care if they had moved out of the initial clinic but were accessing care at other centers outside Harare.
Statistical analysis
Data were analyzed using Stata version 15.1 (StataCorp, College Station, Texas, USA). Baseline demographic and clinical characteristics were summarized in a table using means and standard deviations for normally distributed data or median and interquartile ranges (IQRs) for non-parametric continuous variables and frequencies and proportions for categorical variables. We used Student’s t-test or Wilcoxon rank sum tests to compare sCrAg group means or medians for continuous variables respectively. We compared frequency distribution of categorical characteristics between sCrAg groups using chi-squared tests. We summarized sCrAg titers among sCrAg-positive participants using medians and IQRs.
Participant outcomes were summarized using frequencies and proportions, stratified by sCrAg status. For each participant, we estimated the time (in weeks) from enrolment to either death, LTFU, or to end of study (52 weeks). Participants who did not die were censored at the last date of follow-up which was anytime between 48 and 52 weeks. We estimated survival rates and associated 95% confidence intervals (CIs) for the study period using Kaplan Meier estimation stratified by sCrAg status.
Using univariate binomial regression models, we explored potential factors associated with mortality. Factors associated with mortality at p<0.1 were included in a multivariable binomial regression model to identify independent factors associated with death. Relative risk (RR) and associated 95% CIs were used to quantify the effect size of risk factors on the outcome.
Ethics Approvals
The study was approved by Joint Research Ethics Committee for University of Zimbabwe College of Health Sciences and Parirenyatwa Group of Hospitals, the Medical Research Council of Zimbabwe, the Research Council of Zimbabwe and the Partners Human Research Committee at Massachusetts General Hospital. It was reviewed by the Centers for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research but CDC was not engaged. The study was also registered with the Clinical trials (NCT 02434172). All participants provided written informed consent.
Results
Socio-demographic and clinical characteristics
Between April 2015 and June 2016, 1597 individuals were screened for study eligibility and 1320 participants with CD4 count <100 cells/μl were enrolled into the study (Figure 2). The median age was 37.4 years (IQR: 31.8–43.4) and 745 (56.4%) were males. Median CD4 count was 31 cells/μl (IQR: 14–55) and 923 participants (69.9%) had a CD4 count <50 cells/μl. About a tenth of those enrolled (10.2%, n=135) were ART experienced with median duration on ART of 48 months (IQR: 24–63). At enrollment, a previous diagnosis of TB was documented in 211 (16.0%) participants and 458 (34.7%) had ≥2 presumptive TB symptoms, (Table 1).
Figure 2.
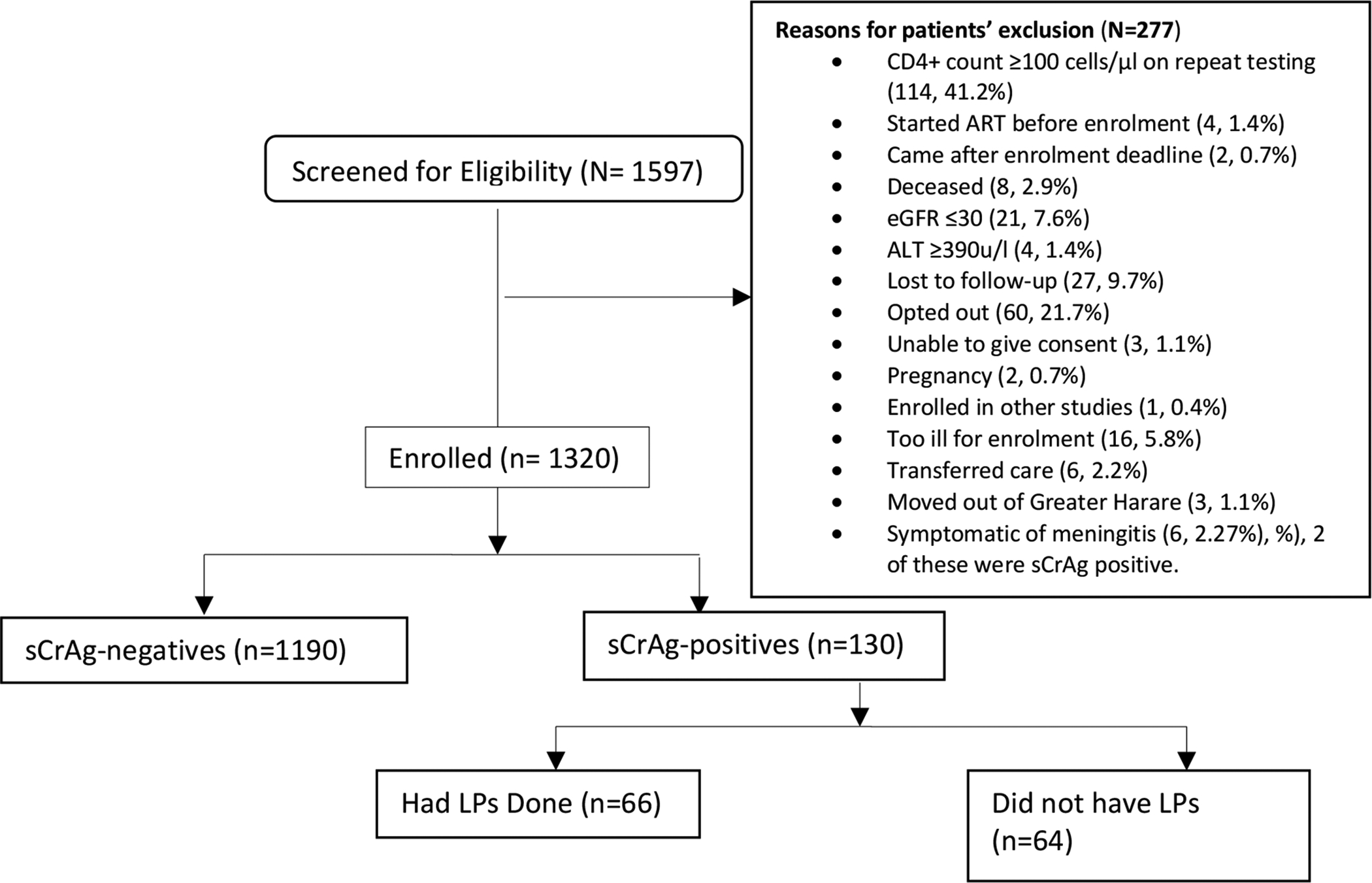
Study participants’ disposition
eGFR- estimated glomerular filtration rate, ALT- alanine aminotransferase, CSF-cerebrospinal fluid, LP – lumbar puncture, sCrAg –serum cryptococcal antigen
Table 1.
Baseline characteristics of 1320 participants by serum cryptococcal antigen status.
| Characteristic | Total N=1320 (100%) |
sCrAg-positive N=130 (100%) |
sCrAg-negative N=1190 (100%) |
p-value+ |
|---|---|---|---|---|
| Median age [years] (IQR) | 37.4 (31.8–43.4) | 38.6 (33.5–42.9) | 37.2 (31.5–43.4) | 0.168 |
| Gender (Male, %) ** | 745 (56.5) | 77 (59.2) | 668 (56.2) | 0.506 |
| CD4 count at enrolment | ||||
| Median (IQR) | 31 (14–55) | 26.5 (11–44) | 32 (14–56) | 0.034 |
| <100 cells/μl (%)∞ | 1320 (100) | 130 (100) | 1190 (100) | |
| 50–100 cells/μl (%)∞ | 397 (30.1) | 29 (22.3) | 368 (30.9) | |
| CD4 <50 cells/μl (%)∞ | 923 (69.9) | 101 (77.7) | 822 (69.1) | 0.042 |
| ART naive (%) | 1185 (89.8) | 117 (90) | 1068 (89.8) | 0.928 |
| Previous History of TB (%) | 211 (16) | 20 (15.4) | 191 (16.1) | 0.841 |
| ≥2 symptoms * associated with TB (%) | 458 (34.7) | 35 (26.9) | 423 (35.6) | 0.05 |
Comparison of distribution of categorical and continuous characteristics between the sCrAg groups was done using chi-squared tests and non-parametric Wilcoxon rank sum tests respectively.
Symptoms were defined as chronic cough, fever, weight loss, drenching night sweats, and chronic fatigue,
Missing gender status for one sCrAg-negative participant, IQR-interquartile range, TB-tuberculosis, sCrAg- serum cryptococcal antigen, ART-antiretroviral therapy,
column percentages.
Cryptococcal antigenemia
Subclinical antigenemia was detected in 130 (9.8%) participants with CD4 count <100cells/μl and in 101 (10.9%) among those with a CD4 count <50 cells/μl. The median (IQR) CD4 count was 26.5 (11–44) cells/μl in sCrAg-positive participants, and 32 (14–56) cells/μl in sCrAg-negative participants, p=0.034 (Table 1). Among the sCrAg-positive participants, the median serum CrAg titre was 1:20 (IQR: 1:5–1:160) (See Supplementary Figure 2).
CNS disease
To rule out subclinical cryptococcal CNS disease, all sCrAg-positive participants were offered a diagnostic LP. Sixty-six (50.8%) participants agreed to have the LP. Of these 66 participants who had LPs, 11 (16.7%) had CNS disease as defined by a positive CSF cryptococcal culture. Of these 11 participants, 10 were cCrAg positive, 9 were India ink positive and 1, who had a sCrAg titre of 1:20, was cCrAg negative but India ink positive. The median (IQR) serum CrAg titre was 1:480 (1:80–1:2560) in the cCrAg-positive compared with 1:10 (1:5–1:40) in cCrAg-negative participants, p<0.001. The median (IQR) CD4 count was 10 (IQR; 6–13) cells/μl in the cCrAg-positives and 32 (IQR; 17–52) cells/μl in the cCrAg-negative participants, p=0.001. In the binomial regression model, every 10 cells/μl increase in CD4 count was associated with a decrease (relative risk [RR] = 0.67, 95% CI: 0.49 – 0.92) in risk of CNS cryptococcal dissemination. Among the 11 CSF culture-positive participants, 10 were hospitalized and received amphotericin B and high dose fluconazole as per national guidelines (24) and one refused to be hospitalized (see Supplementary Table 1).
Fungal cultures
Cryptococcal blood cultures were performed in 129 (99.2%) sCrAg-positive participants and 10 (7.8%) were positive. All the participants with a positive blood culture had sCrAg titres of 1:2560 with an exception of one with a titre of 1:5. In contrast, the median (IQR) sCrAg titre in those with negative blood cultures was 1:20 (IQR: 1:5–1:80) (p <0.001). Among the 11 participants who were CSF culture positive, 7 (63.6%) also had positive cryptococcal blood cultures.
Study follow-up
By the end of the study (which was between 48 and 52 weeks), 756 (57.3%) participants had completed all the study procedures as per the protocol; 84 (6.4%) were LTFU, 73 (5.5%) transferred to facilities outside greater Harare, 175 (13.3%) were determined to be alive either through telephone contact or home visit but could not return to the clinic to complete their study visits, 23 (1.7%) had withdrawn consent, and 209 (15.8%) were deceased (see Supplementary Figure 3). Among the 209 participants who died, 143 (68.4%) died in a healthcare facility. The causes of deaths were known for 85 (40.7%) participants and of these, TB was the leading cause of the death (43.5%, n=37/85). The remaining 124 (59.3%) patients were reported to have died of “unknown” causes of death (see Supplementary Figure 1). A total of 49 participants were hospitalized during the course of the study and TB was the leading cause of the hospitalizations (28.6%, n=14).
Cryptococcal disease was a known cause of death for six (7%, n=6/85) participants. All these six participants were sCrAg-positive; 3 participants had a sCrAg titre ≥1:2560, one had a titre of 1:5 and two had titres of 1:20. All were CSF culture positive. The median (IQR) time from enrolment to death for these participants who died of cryptococcal disease was 11.6 (3.57–16.1) weeks.
Survival
The estimated survival rate at 24 weeks in the sCrAg-negative and sCrAg-positive participants was 89.1% (95% CI: 87.1–90.8) and 78.9 % (95% CI: 70.2– 85.3) respectively. The estimated 48–52 weeks survival rate among sCrAg-negative participants was 83.9% (95% CI: 81.5–86.0) compared to 76.1% (95% CI: 67.1–83.0) among sCrAg-positive participants (p=0.015) (Figure 3). After adjusting for age and sex, mortality was independently associated with low CD4 count of <50 cells/μl (adjusted relative risk (aRR) = 1.71 (95% CI: 1.22–2.40, p=0.002), a positive sCrAg (aRR = 1.48, 95% CI: 1.02–2.16, p=0.041) and presence of ≥2 presumptive TB symptoms (aRR = 1.69 (95% CI: 1.31–2.20, p<0.001) (Table 2). Among sCrAg-positive participants, sCrAg titres ≥1:160 were crudely associated with an increased risk of death (RR=2.03, 95% CI: 1.14–3.62, p=0.017). Counter-intuitively, the mortality rates between sCrAg-positive individuals who had LPs and those who did not, were similar (24.2% vs 26.6%).
Figure 3:
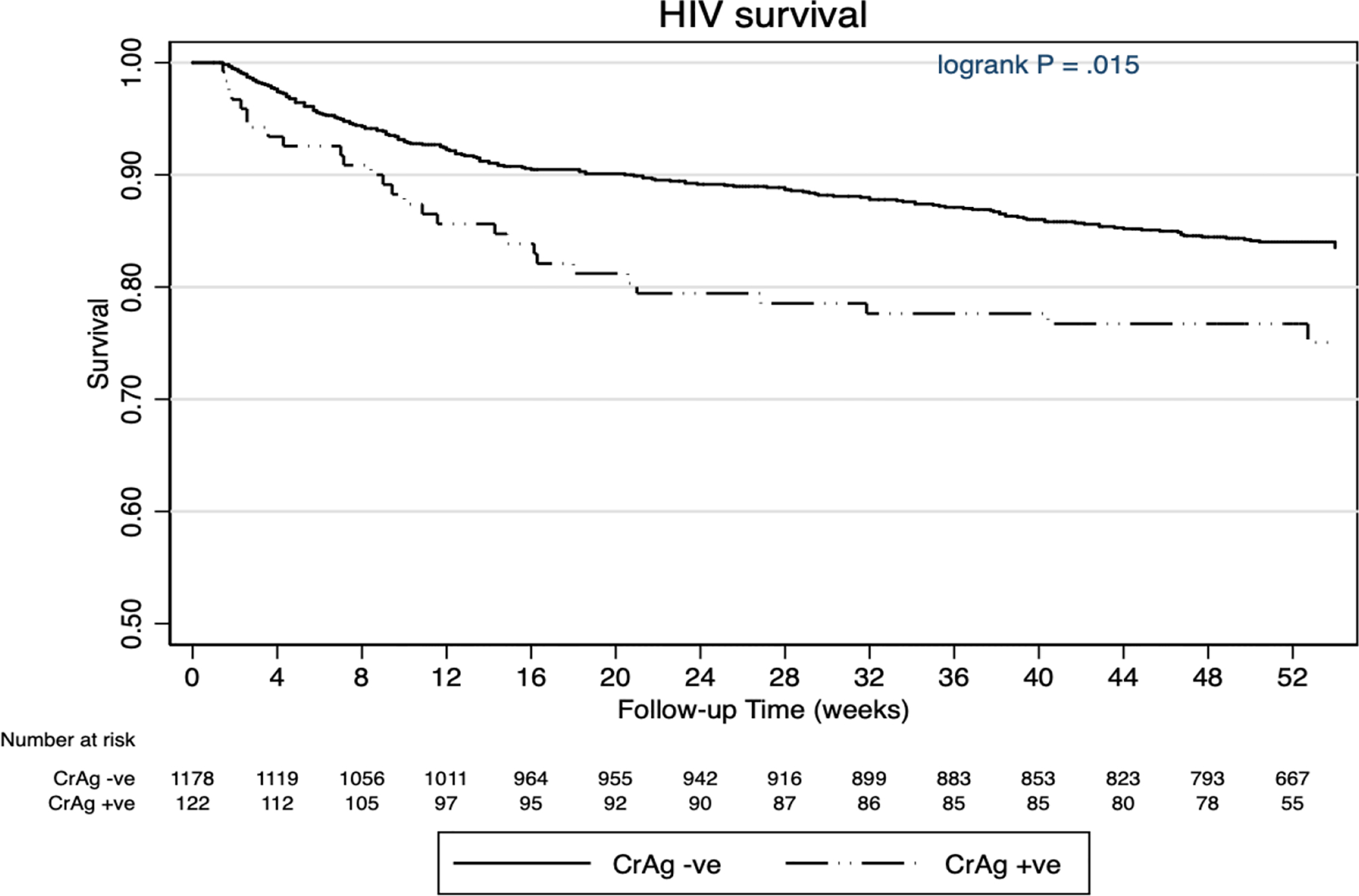
Kaplan Meier estimation curve showing survival probability for serum cryptococcal antigen positive and negative participants.
There were 20 patients whose last study follow-up dates were unknown and these were excluded from the Kaplan Meier survival curves. Hence, the total number of patients at week 0 is 1300 instead of 1320.
CrAg- serum cryptococcal antigen, HIV- Human Immunodeficiency Virus, −ve – negative, +ve – positive.
Table 2.
Multivariable binomial regression showing factors associated with mortality among study participants.
| Characteristics | Died N=209 (%) |
Unadjusted Risk Ratio (95% CI) | Adjusted Risk Ratio (95 % CI) | p-value |
|---|---|---|---|---|
| Age | ||||
| <35 years | 85 (16.4) | reference | reference | |
| >=35 years | 123 (15.4) | 0.94 (0.73 – 1.21) | 0.88 (0.68 – 1.14) | 0.339 |
| Missing | 1 | |||
| Gender | ||||
| Male | 125 (16.8) | reference | reference | |
| Female | 83 (14.5) | 0.86 (0.67 – 1.11) | 0.92 (0.71 – 1.19) | 0.524 |
| Missing | 1 | |||
| CD4 count (cells/μl) | ||||
| >=50 | 40 (10.1) | reference | reference | |
| <50 | 169 (18.3) | 1.82 (1.31 – 2.51) | 1.71 (1.22 – 2.40) | 0.002 |
| CD4 count* | 0.985 (0.98 – 0.99) | 0.988 (0.98 – 0.99 | <0.001 | |
| sCrAg Status | ||||
| Negative | 176 (14.8) | reference | reference | |
| Positive | 33 (24.4) | 1.72 (1.24 – 2.38) | 1.48 (1.02 – 2.16) | 0.041 |
| Education | ||||
| Primary or less | 34 (17.2) | reference | ||
| Secondary | 155 (16.2) | 0.94 (0.67 – 1.32) | ||
| Higher education or | ||||
| job training | 20 (12.0) | 0.70 (0.42 – 1.16) | ||
| Income (United States dollars) | ||||
| <$100 | 122 (17.1) | reference | ||
| $100 – $500 | 77 (14.1) | 0.83 (0.64 – 1.08) | ||
| >$500 | 10 (16.4) | 0.96 (0.53 – 1.73) | ||
| ART status | ||||
| Naïve | 188 (15.9) | reference | ||
| Experienced | 21 (15.6) | 0.98 (0.65 – 1.48) | ||
| Number of presumptive TB symptoms | ||||
| <2 | 105 (12.2) | reference | reference | |
| ≥2 | 104 (22.7) | 1.86 (1.46 – 2.38) | 1.69 (1.31 – 2.20) | <0.001 |
sCrAg- serum cryptococcal antigen, TB- tuberculosis, ART- antiretroviral therapy *(as a continuous variable)
Discussion
This large cohort study assessed the prevalence of subclinical cryptococcal antigenemia and survival in advanced HIV disease (CD4 count <100 cells/μl) following its management. The burden of cryptococcal disease substantially decreased following introduction of ART in resource-rich settings (9, 10), however in this cohort in a low-resource setting, we still observed a high rate of subclinical cryptococcal antigenemia (9.8%), despite improved access to ART and antifungal therapy (i.e amphotericin B deoxycholate and high dose fluconazole). Given that significant numbers of HIV-infected people in SSA continue to present for care with severe immunosuppression, our data emphasizes the ongoing threat posed by cryptococcal disease (18, 26–30).
Serum cryptococcal antigenemia
The sCrAg titres observed in this cohort were low (median 1:20 (IQR: 1:5–1:160). Despite having excluded patients with symptoms consistent with meningitis, we observed disseminated cryptococcal disease in nearly a fifth (16.7%) of participants. Our participants had subclinical CNS disease that needed testing for serum CrAg and thus would not have been detected by screening for symptoms alone. Participants with subclinical CNS dissemination had higher sCrAg titres (median 1:480 [IQR 1:80–1:2560]) and lower CD4 count (median 10 [IQR: 6–13]) compared to those who did not. Moreover, sCrAg titres ≥1:160 were crudely associated with an increased risk of death. These findings should help in risk stratifying patients according to their serum CrAg titres regarding the need and urgency of an LP. They also suggest that it may be reasonable for clinicians to offer intravenous amphotericin B, based on a high sCrAg titres, to those asymptomatic patients who may have refused an LP following serum CrAg positivity.
Systemic dissemination
Systemic dissemination, defined by a positive cryptococcal blood culture, was identified in a minority (7.8%) of sCrAg-positive participants. In our cohort, nearly two thirds (63.6%) of participants who had evidence of subclinical CNS disease i.e. positive CSF CrAg or India ink staining or positive CSF cultures, also had positive cryptococcal blood cultures. Cryptococcal blood cultures have been noted to be positive in 47–70% of individuals with symptomatic HIV-associated CM (31). All patients, except one, with a positive blood culture had very high sCrAg titres (1:2560) suggesting possible utility of quantitative LFA testing prior to giving intravenous amphotericin B where blood cultures or LP are not available.
CNS dissemination
Although we found 16.7% of CNS disease in the sCrAg-positive participants, this was in only half of the participants who agreed to have a lumbar puncture. This challenge was similar to what has been experienced in other settings (32). Thus, we had challenges getting LPs performed in those that needed them. In much of SSA, LPs and blood cultures are not routinely available, making it challenging for most healthcare facilities to identify those with CNS disease, especially in asymptomatic patients like those in our cohort. Therefore, use of high serum CrAg titres obtained using semi-quantitative LFA may contribute to timeous management of cryptococcal CNS disease despite failure to perform a lumbar puncture.
The management of cryptococcal disease in settings of HIV co-infection is well defined (33), however, evidence-based guidelines for the management of subclinical cryptococcal disease are lacking. Invasive procedures, such as LP, CSF pressure management and induction therapy with amphotericin B with flucytosine or fluconazole are recommended for induction therapy for CM in addition to other regimens (15, 22). However, there is limited evidence to support invasive procedures to exclude disseminated disease and the use of amphotericin B and flucytosine or alternative regimens used for induction of CM in those with subclinical disease i.e. asymptomatic patients but sCrAg-positive.
Only a half of our participants agreed to have an LP and the acceptance or refusal of these LPs by participants was most likely non-random. Therefore, our study was most likely not powered to compare the all-cause mortality between serum CrAg-positive patients receiving IV Amphotericin B following a LP and those receiving just oral fluconazole after refusing an LP. However, it was noteworthy, in this cohort study, that there was no difference in all-cause mortality between the sCrAg-positive individuals who agreed to have LPs and/or IV amphotericin plus oral fluconazole and those who did not agree to have a lumbar puncture and hence did not get amphotericin B as part of their treatment.
Survival
We also observed an increased risk of mortality associated with the presence of ≥2 symptoms suggestive of TB (cough, fever, weight loss, night sweats, and fatigue) at enrolment and TB was also the leading known cause of death (43.5%, [37/85]) among those with a well documented cause of death. These data strongly emphasize the importance of coupling interventions to address TB and CM in the management of individuals with advanced HIV infection as recommended by other guidelines(34).
The deaths observed at around one year (48–52 weeks) in this study were many, which may reflect the high mortality associated with advanced immunosuppression (35). However, due to limitations in available pathology and post-mortem services, deaths at home and incomplete public death records, our ability to determine causes of deaths for most participants was limited with only 85 out of 209 having documented causes of deaths. None of these participants underwent post-mortem and hence even in these “known’ causes of deaths may have been inaccurate.
Generalizability
Although additional resources were available for additional diagnostic and monitoring tests, and patient hospitalization and follow-up, these study findings are generalizable for most resource-limited settings. Our study was conducted within the context of routine care, making the data applicable to practitioners in similar settings in SSA. The data also highlight the complexities of addressing issues such as offering lumbar punctures and patient follow-up in a resource-limited setting.
Limitations of this study
The study, though large and meeting its primary objectives, had some limitations. The number of individuals who were sCrAg positive and agreed to an LP was low, but consistent with this clinical setting (36). Although the data suggests that an LP may not impact overall mortality among asymptomatic individuals who were sCrAg positive, there is a need to conduct a randomized controlled trial to further investigate this finding which is counter-intuitive. This study has shown that performing lumbar punctures remains a challenge in our setting and there will be need to address barriers to LPs if we are to be offering LPs to all those with asymptomatic cryptococcal disease.
In this study, the dose of fluconazole (800mg) used for participants with disseminated disease was lower than the currently approved dose in the new WHO CM treatment guidelines (15) for induction and consolidation phase. Higher fluconazole dosing (1200mg) may have attenuated the mortality difference between the sCrAg-positive and sCrAg-negative individuals.
Conclusions
We found quite a high prevalence (9.8%) of subclinical cryptococcal disease in this cohort of patients with CD4 count <100 cells/μl and our study results confirm the importance of TB and a positive sCrAg as risk factors for mortality in this patient population. Thus, CD4 count testing should still be performed even with the ‘test and treat’ approach at ART initiation or optimization, followed by screening for sCrAg in those with advanced HIV disease despite absence of signs and symptoms suggestive of meningitis. In addition, symptomatic screening for TB also needs to be strengthened given the finding of at least a third dying with TB in this study cohort.
Supplementary Material
Acknowledgments:
We thank the participants who participated in this study and their families, and the City of Harare Clinics and Staff. We also thank the CryptoART study staff who made this study possible: Exavior Chivige, Bevlyn Muhwati, G Philemon Chimbganda, Edward Makaha, Taddy Mwarumba, Donnely Nyoni, Ograh Gundani, Charity Musinake, Tarirai Madovi, Fanuel Maveve, Molly Sibanda, Tawanda Manyange, Chiratidzo Gumbo, Tarisiro Matiza, Sidney Sithole, Christine Mandisodza, Zorodzai Tangwena, Anesu Gumbo, and Aaron Zee. The study was sponsored by CDC.
Funding:
This work was supported by the Centers for Disease Control and Prevention [1U01GH000737] and National Institutes of Health [5K08AI104348].
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of Centers for Disease Control and Prevention (CDC) and other funding agencies.
Footnotes
Conflict of Interest:
The funding was awarded to Tariro Azure MAKADZANGE (ATM). ATM is currently an employee of Gilead Sciences, but was not at the time of conception and completion of the study. Christine ROSS, Snighdha VALLABHANENI and Shirish BALACHANDRA are employees of the Centers for Disease Control and Prevention. All other authors declare no competing interests.
References
- 1.Veltman JA, Bristow CC, Klausner JD. Meningitis in HIV-positive patients in sub-Saharan Africa: a review. J Int AIDS Soc. 2014;17:19184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajasingham R, Rhein J, Klammer K, Musubire A, Nabeta H, Akampurira A, et al. Epidemiology of meningitis in an HIV-infected Ugandan cohort. Am J Trop Med Hyg. 2015;92(2):274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. Aids. 2000;14(10):1401–7. [DOI] [PubMed] [Google Scholar]
- 4.Okongo M, Morgan D, Mayanja B, Ross A, Whitworth J. Causes of death in a rural, population-based human immunodeficiency virus type 1 (HIV-1) natural history cohort in Uganda. Int J Epidemiol. 1998;27(4):698–702. [DOI] [PubMed] [Google Scholar]
- 5.Mwaba P, Mwansa J, Chintu C, Pobee J, Scarborough M, Portsmouth S, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77(914):769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. Aids. 2002;16(7):1031–8. [DOI] [PubMed] [Google Scholar]
- 7.Heyderman RS, Gangaidzo IT, Hakim JG, Mielke J, Taziwa A, Musvaire P, et al. Cryptococcal meningitis in human immunodeficiency virus-infected patients in Harare, Zimbabwe. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1998;26(2):284–9. [DOI] [PubMed] [Google Scholar]
- 8.Park BJ, Shetty S, Ahlquist A, Greenbaum A, Miller JL, Motsi A, et al. Long-term follow-up and survival of antiretroviral-naive patients with cryptococcal meningitis in the pre-antiretroviral therapy era, Gauteng Province, South Africa. Int J STD AIDS. 2011;22(4):199–203. [DOI] [PubMed] [Google Scholar]
- 9.Hajjeh RA, Conn LA, Stephens DS, Baughman W, Hamill R, Graviss E, et al. Cryptococcosis: population-based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. Cryptococcal Active Surveillance Group. J Infect Dis. 1999;179(2):449–54. [DOI] [PubMed] [Google Scholar]
- 10.Mirza SA, Phelan M, Rimland D, Graviss E, Hamill R, Brandt ME, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000. Clin Infect Dis. 2003;36(6):789–94. [DOI] [PubMed] [Google Scholar]
- 11.Dromer F, Mathoulin S, Dupont B, Laporte A. Epidemiology of cryptococcosis in France: a 9-year survey (1985–1993). French Cryptococcosis Study Group. Clin Infect Dis. 1996;23(1):82–90. [DOI] [PubMed] [Google Scholar]
- 12.Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol. 2014;6:169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozel TR, Bauman SK. CrAg lateral flow assay for cryptococcosis. Expert Opin Med Diagn. 2012;6(3):245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rick F, Niyibizi AA, Shroufi A, Onami K, Steele SJ, Kuleile M, et al. Cryptococcal antigen screening by lay cadres using a rapid test at the point of care: A feasibility study in rural Lesotho. PLoS One. 2017;12(9):e0183656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organisation. Guidelines for the diagnosis, prevention and management of Cryptococcal disease in HIV-infected adults, adolescents and children. supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva. 2018. pg 1–64. [PubMed] [Google Scholar]
- 16.Coetzee LM, Cassim N, Sriruttan C, Mhlanga M, Govender NP, Glencross DK. Cryptococcal antigen positivity combined with the percentage of HIV-seropositive samples with CD4 counts <100 cells/mul identifies districts in South Africa with advanced burden of disease. PLoS One. 2018;13(6):e0198993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51(4):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longley N, Jarvis JN, Meintjes G, Boulle A, Cross A, Kelly N, et al. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clin Infect Dis. 2016;62(5):581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis JN, Harrison TS, Lawn SD, Meintjes G, Wood R, Cleary S. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS One. 2013;8(7):e69288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, et al. High Cryptococcal Antigen Titers in Blood Are Predictive of Subclinical Cryptococcal Meningitis Among Human Immunodeficiency Virus-Infected Patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(5):686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolfes MA, Hullsiek KH, Rhein J, Nabeta HW, Taseera K, Schutz C, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis. 2014;59(11):1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Rapid Advice. Diagnosis, prevention and management of cryptococcal disease in HIV-Infected adults, adolescents and children. Geneva. 2011. pg 1–44. [PubMed] [Google Scholar]
- 24.Guidelines for Antiretroviral Therapy for the Prevention and Treatment of HIV in Zimbabwe. ART Guideline Review 2016. pg 44–46. [Google Scholar]
- 25.Southern African HIV Clinicians Society. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. 2013. 2013;14(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for Cryptococcal Antigenaemia in Patients Accessing an Antiretroviral Treatment Program in South Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(7):856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osazuwa F, Dirisu JO, Okuonghae PE, Ugbebor O. Screening for Cryptococcal Antigenemia in Anti-Retroviral Naïve AIDS Patients in Benin City, Nigeria. Oman Med J. 272012. p. 228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawadogo S, Makumbi B, Purfield A, Ndjavera C, Mutandi G, Maher A, et al. Estimated Prevalence of Cryptococcus Antigenemia (CrAg) among HIV-Infected Adults with Advanced Immunosuppression in Namibia Justifies Routine Screening and Preemptive Treatment. PLoS One. 112016. p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lara-Peredo O, Cuevas LE, French N, Bailey JW, Smith DH. Cryptococcal infection in an HIV-positive Ugandan population. J Infect. 41. England2000. p. 195. [DOI] [PubMed] [Google Scholar]
- 30.Kisenge PR, Hawkins AT, Maro VP, McHele JP, Swai NS, Mueller A, et al. Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis. 72007. p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antinori S New Insights into HIV/AIDS-Associated Cryptococcosis. ISRN AIDS. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwizera R, Sadiq A, Ndyetukira JF, Nalintya E, Williams D, Rhein J, et al. Impact of community engagement and social support on the outcomes of HIV-related meningitis clinical trials in a resource-limited setting. Research involvement and engagement. 2020;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makadzange AT, McHugh G. New approaches to the diagnosis and treatment of cryptococcal meningitis. Semin Neurol. 2014;34(1):47–60. [DOI] [PubMed] [Google Scholar]
- 34.Meintjes G, Moorhouse MA, Carmona S, Davies N, Dlamini S, van Vuuren C, et al. Adult antiretroviral therapy guidelines 2017. South Afr J HIV Med. 182017. p. 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabillard D, Lewden C, Ndoye I, Moh R, Segeral O, Tonwe-Gold B, et al. Mortality, AIDS-morbidity, and loss to follow-up by current CD4 cell count among HIV-1-infected adults receiving antiretroviral therapy in Africa and Asia: data from the ANRS 12222 collaboration. J Acquir Immune Defic Syndr. 2013;62(5):555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thakur KT, Mateyo K, Hachaambwa L, Kayamba V, Mallewa M, Mallewa J, et al. Lumbar puncture refusal in sub-Saharan Africa: A call for further understanding and intervention. Neurology. 2015;84(19):1988–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


