Abstract
The adaptive immune checkpoints such as PD-1(programmed death-1)/PD-L1 (programmed death-ligand 1) play an important role in cancer immunotherapy, whereas increasing evidence suggests that cancer cell evades immune surveillance by innate immune checkpoints such as SIRPα (signal-regulatory protein α)/CD47 (cluster of differentiation 47). In multiple types of cancer cells and solid tumor tissues, highly expressed CD47 protein level has been observed, which is triggered by some transcription factors including NFκB, Myc, and HIF. As a transmembrane protein, the binding of CD47 to SIRPα ligand on phagocytes results in phagocytosis resistance and cancer cell immune escape. In contrast, CD47-SIRPα interaction blockade enhances cancer cell clearance by phagocytes such as macrophages and dendritic cells (DCs) to activate an innate immune response, whereas this process could promote antigen cross-presentation by antigen present cells (APCs) leading to T cell priming, consequently, activates an adaptive antitumor immune response. In this review, we discussed the current SIRPα-CD47 axis-mediated cancer cell immune escape and immunotherapy, which could provide an effective antitumor strategy by the innate and adaptive immune response.
Keywords: CD47, SIRPα, immune escape, innate immune response, adaptive immune response, cancer immunotherapy
Introduction
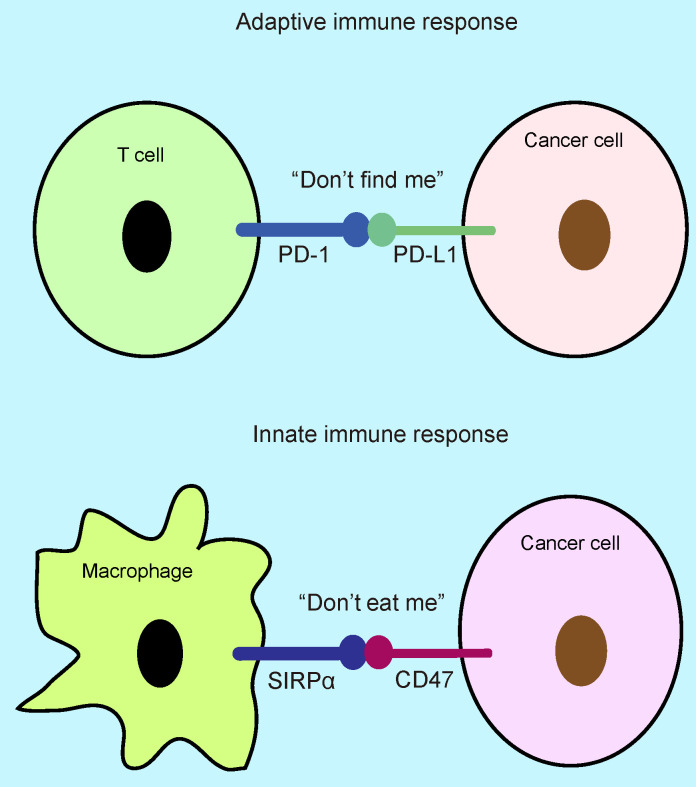
The innate and adaptive immune systems of host play an important role in killing cancer cells and inhibiting tumor progression 1-3, while cancer cell exhibits immune escape by expression of some immune checkpoint proteins such as PD-L1 (programmed death-ligand 1) and CD47 (cluster of differentiation 47) 3, 4. PD-1(programmed death-1)/PD-L1 checkpoint functions as “don't find me” signal to the adaptive immune response 5-7, whereas SIRPα (signal-regulatory protein α)-CD47 axis serves as “don't eat me” signal to the innate immune response 7, 8 (Figure 1). The interaction of PD-L1 with surface PD-1 receptor on T cells leads to inhibition of cancer cell killing 9, 10, whereas the binding of CD47 to surface SIRPα receptor on phagocytes inhibits cancer cell clearance 7, 8, 11. CD47 is a widely expressed glycoprotein in normal and cancer cells with five transmembrane domains 12, 13, which binds to the extracellular domain of SIRPα on phagocytes leading to inhibition of phagocytosis 7, 12. The SIRPα/CD47 checkpoint was first identified in 1999 14, 15, which suppresses phagocytosis of phagocytes and promotes cancer immune escape 8, 16, 17. The clinical analysis shows that CD47 is highly expressed on multiple types of cancer patients including glioblastoma, ovarian, breast, bladder, colon, and hepatocellular carcinoma, which correlates with low survival 18. CD47 expression is trigged by multiple transcriptional factors including NFκB, Myc, and HIF, etc 3, 11, 19, 20, while the SIRPα-CD47 axis blockade enhances phagocytosis by macrophages and DCs to activate innate immune response resulting in tumor regression 3, 7, 11, 12, 18, whereas the phagocytosis by DCs activates DNA-sensing cGAS-STING-INF-γ-mediated adaptive immune response leading to T cell priming 21-23, suggesting that inhibition of SIRPα-CD47 axis could enhance innate and adaptive antitumor immune response. In this review, we discussed the regulatory mechanism of CD47-mediated cancer immune escape and immunotherapy.
Figure 1.
“Don't eat me” and “don't find me” signal. The binding of CD47 to SIRPα on phagocytes inhibits phagocytosis, which functions as “don't eat me” signal, whereas the binding of PD-L1 to PD-1 serves as “don't find me” signal that inhibits T cell killing. APCs: antigen present cells.
SIRPα-CD47 axis protects cancer cell from phagocytosis
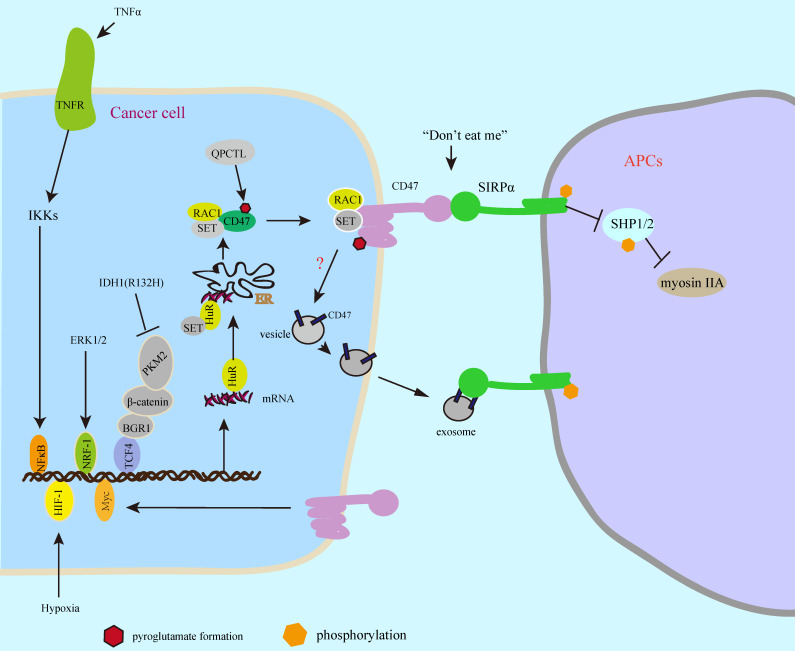
SIRPα is one of the SIRP family members, which was first identified in 1997 24. Thibaudeau et al 25 reports that SIRPα is highly expressed on macrophages. After that, CD47 was identified as the first ligand of SIRPα 14, 15. The binding of SIRPα to CD47 triggers SIRPα phosphorylation of ITIMs (immunoreceptor tyrosine-based inhibitory motifs) resulting in deactivation of myosin IIA, which is a critical step to block phagocytosis 16. CD47 glycoprotein is highly expressed in multiple types of cancer cells and human tumor tissues 7, 18, 26, 27, which is regulated by Myc oncogene 11. In this study, Myc directly binds to CD47 promoter and triggers its gene expression. In T cell acute lymphoblastic leukemia (T-ALL) xenograft tumor model, activation of Myc leads to tumor growth and inhibition of phagocytosis, which is alleviated by Myc inactivation 11. Interestingly, another report shows that silenced CD47 reduces Myc expression in oral squamous cell carcinoma 27, which suggests that CD47 could increase Myc expression. However, it is unclear whether CD47-Myc-CD47 feedback signal could regulate CD47 expression. In addition to direct regulation of CD47 promoter by Myc binding 11, extracellular stimuli also trigger CD47 gene expression 20, 28. In response to TNFα, activated NFκB (nuclear factor- κB) directly binds to a specific constituent enhancer of CD47 and increases its gene expression in MCF-7 breast cancer cells resulting in tumor growth by inhibiting phagocytosis 28. Moreover, under hypoxia condition, HIF-1 (hypoxia-inducible factor 1) binds to CD47 promoter and increases its expression resulting in inhibition of phagocytosis in breast cancer cells, which is a strong correlation between CD47 and HIF-1 by clinical analysis from thousands of breast cancer patients 20. The ChIP-Seq-based analysis shows that nuclear respiratory factor 1 (NRF-1) targets CD47 promoter 29. Consistent with this, oncogenic activation of ERK signal induces CD47 expression by NRF-1-mediated CD47 gene transcription in melanoma cells leading to inhibition of phagocytosis 30. In contrast, IDH1 (R132H) mutation in gliomas, negatively regulates CD47 gene transcription, which disrupts the binding of PKM2/β-catenin/BRG1 complex to TCF4 transcription factor resulting in inhibition of TCF4-mediated CD47 expression 31. In addition, Berkovits and Mayr 32 have described the mechanism of how does the new synthesized CD47 to be delivered to the cell surface. This study suggests that CD47 protein is present on the cell surface and intracellular, whereas the long 3'UTR of CD47 is critical for its surface localization. Mechanistically, the binding of HuR to long 3'UTR recruits SET to develop CD47 mRNA/HuR/SET complex and targets the endoplasmic reticulum (ER) surface, subsequently, the binding of SET to the new synthesized cytoplasmic domains of CD47 recruits RAC1 and forms a CD47/SET/RAC1 complex leading to the plasma membrane translocation of CD47 protein 32. Moreover, the expression of CD47 protein undergoes transcriptional modification by glutaminyl-peptide cyclotransferase-like (QPCTL), which induces CD47 pyroglutamate formation shortly after biosynthesis 17. In this study, it shows that the formation of pyroglutamate on CD47 enhances the binding of SIRPα to CD47, consequently, inhibits cancer cell clearance by phagocytes. In addition to present on cell surface, CD47 protein is observed on exosomes 33-35. Exosomes are extracellular vesicles (30-150 nm) with double-layer membrane, which is secreted from cells and effectively enter into other cells 36. High CD47 levels on the exosomes of breast cancer patients may be unfavourable 33, 34, and CD47 on the exosomes inhibits cancer cell clearance by phagocytes in pancreatic cancer 35, while it still unclear the secreted mechanism of CD47 on the exosomes. Taken together, CD47 gene expressions are regulated by multiple transcription factors, which could be transcriptional modification by pyroglutamate formation that enhances the binding of CD47 to SIRPα, consequently, inhibits phagocytosis by phagocytes and promotes cancer cell immune escape. CD47 on the exosomes also decreases antitumor activity by inhibition of phagocytosis. So, the surface CD47 on cancer cells and exosomes should be blocked for cancer immunotherapy (Figure 2).
Figure 2.
SIRPα-CD47 axis protects cancer cell from phagocytosis. Multiple transcription factors regulate CD47 expression in response to intracellular oncogenic activation pathways or extracellular stimuli. The new synthesized CD47 protein is delivered to the cellular surface by binding to SET/RAC complex proteins, which undergoes pyroglutamate formation by cyclotransferase-like (QPCTL) shortly after biosynthesis leading to increased phagocytosis resistance. In addition, the CD47 protein on the surface of the exosomes inhibits phagocytosis.
CD47 blockade enhances the innate and adaptive antitumor immune response
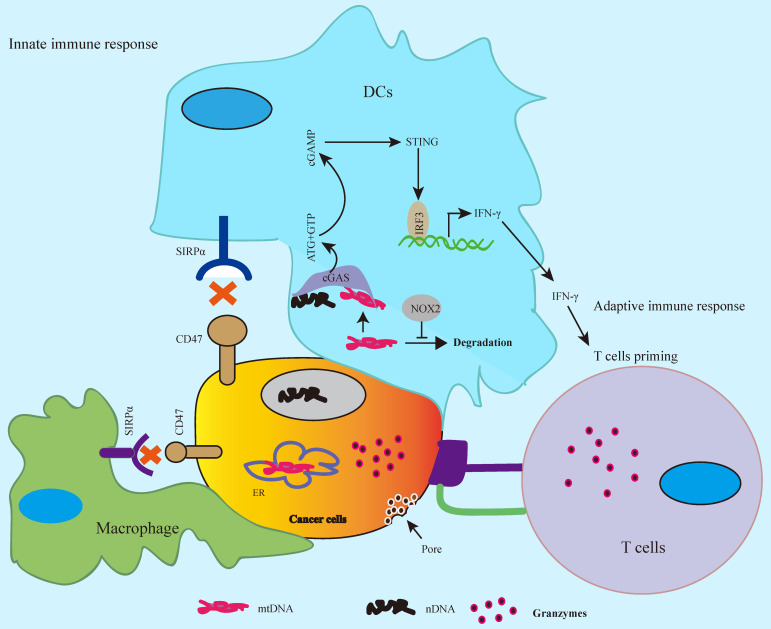
CD47-SIRPα axis serves as “don't eat me” signal to the innate immune response 7, 8, whereas SIRPα-CD47 checkpoint blockade promotes phagocytosis by phagocytes such as macrophages and DCs leading to tumor regression by activation of innate immune response 23, 37, 38. The antitumor activity by CD47 blockade enhances cancer cell clearance by both of phagocytes and T cells 26, 37, and anti-CD47 antibody enhances CD8+ T cells killing but not CD4+ T cell in colon cancer cells 37. Silenced CD47 in T cells leads to enhanced T cell killing in irradiated melanoma cells 26. Vaccination with CD47 knockout tumor cells induces CD11c+SIRPα+ DCs activation and enhances T cell response in B16F0 melanoma mouse tumor model 39. As APCs, DCs engulf cancer cells and tumor-derived DNA in DCs activates cGAS (cGAMP), a cytosolic DNA sensor, subsequently, activates the downstream cGAS-cGAMP-STING innate immune response that exhibits antitumor activity 23, 40, whereas highly expressed CD47 inhibits this signaling pathway in cancer cells leading to tumor immune escape 7, 8. CD47-SIRPα blockade by anti-CD47 antibody enhances antigen cross-presentation by DCs and promotes T cell priming, consequently, CD8+ T cells, but not CD4+ T cells, mediate killing on colon cancer cells. In this process, cytosolic DNA sensor STING (Stimulator of interferon genes) is required for anti-CD47 antibody-mediated tumor regression 23. In addition, bispecific anti-PD-L1-SIRPα, both of SIRPα/CD47 and PD-1/PD-L1 checkpoints blockade, significantly enhances CD8+ T cell killing on colon cancer cells compared to SIRPα/CD47 or PD-1/PD-L1 blockade alone, which is involved in activation of STING-IFN-γ pathway in DCs 41. Combined CD47/SIRPα blockade with temozolomide in glioblastoma enhances phagocytosis and promotes T cell priming by activation of STING-IFN-γ pathway in DCs 38. In addition to tumor-derived DNA, the tumor mitochondrial DNA (mtDNA) can also trigger the cGAS-cGAMP-STING innate immune response 42. In this study, it reports that CD47 blockade leads to inhibition of degradation of tumor mtDNA by activation of NADPH oxidase NOX2 in DCs, consequently, activates the mtDNA-cGAS-STING-IFN-γ pathway in DCs. These findings suggest that CD47 blockade activates cGAS-cGAMP-STING-mediated innate immune response as well as adaptive immune response by cGAS-STING-IFN-γ signal-mediated T cell priming (Figure 3).
Figure 3.
CD47 blockade activates innate and adaptive antitumor immune response. CD47-SIRPα axis blockade activates cGAS-cGAMP-STING-mediated innate immune response by tumor-derived nuclear DNA (nDNA) or mitochondrial DNA (mtDNA) in DCs, whereas the release of IFN-γ via cGAS-cGAMP-STING-INFR signal results in cytotoxic T cell priming and activates adaptive antitumor immune response.
SIRPα-CD47 checkpoint blockade in cancer immunotherapy
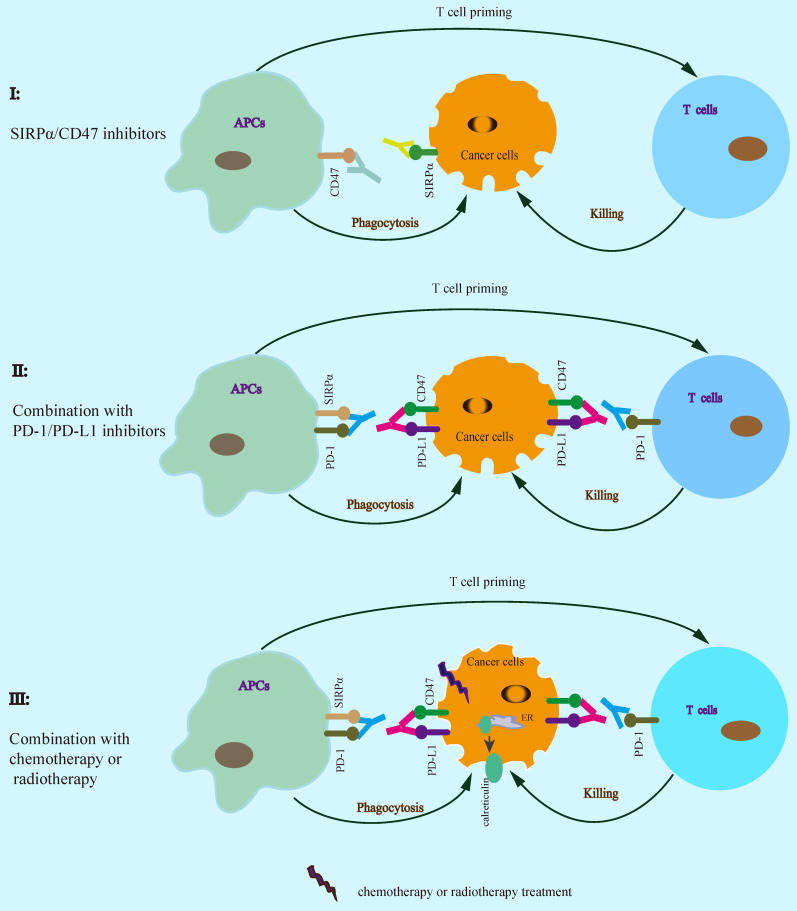
Increasing evidence suggests that SIRPα-CD47 checkpoint blockade enhances the efficacy of cancer immunotherapy (Table 1). SIRPα-CD47 axis blockade by using anti-CD47 antibody significantly enhances phagocytosis by macrophages and inhibits tumor growth 8, 18, 23, 26, 37, 43. Furthermore, SIRPα specific monoclonal antibody KWAR23 disrupts SIRPα-CD47 interaction resulting in inhibition of tumor growth by increasing phagocytosis 44. TTI-621 (SIRPαFc), a recombinant protein for CD47 binding, activates macrophage phagocytosis and inhibits tumor growth 45, 46. In addition to block CD47/SIRPα checkpoint alone, combined SIRPα/CD47 with PD-1/PD-L1 blockade enhances the efficiency of antitumor immunotherapy 41, 47, 48, suggesting that SIRPα-CD47 axis blockade could enhance PD-1/PD-L1 blockade therapy for cancer. The binding of the bispecific anti-PD-L1-SIRPα fusion protein to both of PD-L1 and CD47 on cancer cells significantly enhances antitumor activity in MC-38 colon cell xenograft tumor model 41. Similarly, anti-CD47 antibody synergizes with PD-L1 blockade for cancer immunotherapy in B16F10 melanoma tumor model 47. Moreover, combined with chemotherapy or radiotherapy also enhances the efficacy of cancer immunotherapy, which could increase T cell priming via the release of tumor-derived antigens consequent activation of APCs 21, 22. Similarly, cotrimoxazole synergizes with anti-CD47 antibody treatment leading to enhanced antitumor activity by both of phagocytosis and cGAS-STING DNA sensing signal 23. In response to mitoxantrone, anti-CD47 antibody significantly enhances antitumor activity in breast cancer cells 49. SIRPα-CD47 axis blockade enhances cancer cell clearance by phagocytes, which in turn promotes antigen cross-presentation by APCs resulting in enhanced T cell priming. Therefore, a rational combination of SIRPα-CD47 axis blockade contributes to cancer immunotherapy (Figure 4, Table 1).
Table 1.
SIRPα/CD47 blockade and antitumor immunotherapy
| Targets | Tumor model | Reference |
|---|---|---|
| Anti-CD47+BRAF/MEK inhibitors | Melanoma | 30 |
| Anti-CD47 | Acute myeloid leukemia (AML) stem cells | 8 |
| Anti-CD47 | Breast cancer | 49 |
| KWAR23 (Anti-SIRPα) | Burkitt's lymphoma | 44 |
| TTI-621 (SIRPαFc) | Lymphoma. AML | 46 |
| Anti-PD-L1-SIRPα | Colon cancer | 41 |
| Anti-CD47+ anti-PD-L1 | Melanoma | 47 |
| Cotrimoxazole+anti-CD47 | Colon, B cell lymphoma | 23 |
| Mitoxantrone+anti-CD47 | Breast cancer | 49 |
| 1H9(anti-SIRPα)+anti-PD-L1 | Melanoma | 48 |
| SRF23(anti-CD47) | Burkitt's lymphoma | 43 |
Figure 4.
SIRPα-CD47 checkpoint blockade enhances antitumor immunotherapy. A rational combination of SIRPα-CD47 axis blockade contributes to enhancing the efficacy of cancer immunotherapy.
Conclusion
Highly expressed CD47 levels are present in multiple types of cancers including solid tumors and hematologic malignancies, which is major regulated by some transcription factors such as NFκB, Myc, HIF-1 and NRF-1. Although CD47 protein on exosomes has been observed, the mechanism of secreted pathway is still unclear. Given that normal and blood red cells are widely expressed CD47 that will limit the efficiency of anti-CD47 antibody therapy, therefore, a specific anti-CD47 antibody for CD47/SIRPα blockade is necessary. Especially, combined CD47/SIRPα with PD-1/PD-L1 checkpoints blockade will be preferred to inhibit cancer cell immune evasion. Since DNA damage stimuli could trigger an adaptive antitumor immune response by DNA-sensing cGAS-STING-INF-γ pathway or release of tumor-derived antigens, a rational combined anti-CD47 antibody with chemotherapy or radiotherapy could enhance the efficiency of antitumor immunotherapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81972618, 81872275); Jiangsu Commission of Health Natural Science Foundation (M2020002), Changzhou Sci & Tech Program. Grant No. CJ20200004.
Abbreviations
- PD-1
programmed death-1
- PD-L1
programmed death-ligand 1
- SIRPα
signal-regulatory protein α
- CD47
cluster of differentiation 47
- DCs
dendritic cells
- APCs
antigen presenting cells
- ITIMs
immunoreceptor tyrosine-based inhibitory motifs
- T-ALL
T cell acute lymphoblastic leukemia
- NFκB
nuclear factor- Κb
- HIF-1
hypoxia-inducible factor 1
- QPCTL
glutaminyl-peptide cyclotransferase-like
- STING
Stimulator of interferon genes
References
- 1.Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nature reviews Immunology. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanker A, Verdeil G, Buferne M, Inderberg-Suso EM, Puthier D, Joly F. et al. CD8 T cell help for innate antitumor immunity. Journal of immunology. 2007;179:6651–6662. doi: 10.4049/jimmunol.179.10.6651. [DOI] [PubMed] [Google Scholar]
- 3.Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nature reviews Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature medicine. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr. et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24:26. doi: 10.1186/s12929-017-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN. et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends in cell biology. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 13.Yanagita T, Murata Y, Tanaka D, Motegi SI, Arai E, Daniwijaya EW. et al. Anti-SIRPalpha antibodies as a potential new tool for cancer immunotherapy. JCI Insight. 2017;2:e89140. doi: 10.1172/jci.insight.89140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seiffert M, Cant C, Chen Z, Rappold I, Brugger W, Kanz L. et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94:3633–3643. [PubMed] [Google Scholar]
- 15.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. The Journal of biological chemistry. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 16.Tsai RK, Discher DE. Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logtenberg MEW, Jansen JHM, Raaben M, Toebes M, Franke K, Brandsma AM. et al. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPalpha axis and a target for cancer immunotherapy. Nature medicine. 2019;25:612–619. doi: 10.1038/s41591-019-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS. et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang WT, Huang AM. Alpha-Pal/NRF-1 regulates the promoter of the human integrin-associated protein/CD47 gene. The Journal of biological chemistry. 2004;279:14542–14550. doi: 10.1074/jbc.M309825200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D. et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E6215–6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA. et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nature medicine. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 25.Veillette A, Thibaudeau E, Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. The Journal of biological chemistry. 1998;273:22719–22728. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz AL, Nath PR, Allgauer M, Lessey-Morillon EC, Sipes JM, Ridnour LA. et al. Antisense targeting of CD47 enhances human cytotoxic T-cell activity and increases survival of mice bearing B16 melanoma when combined with anti-CTLA4 and tumor irradiation. Cancer Immunol Immunother. 2019;68:1805–1817. doi: 10.1007/s00262-019-02397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai S, Bamodu OA, Lin YK, Lin CS, Chu PY, Chien MH, CD47-SIRPalpha Signaling Induces Epithelial-Mesenchymal Transition and Cancer Stemness and Links to a Poor Prognosis in Patients with Oral Squamous Cell Carcinoma. Cells. 2019. 8. [DOI] [PMC free article] [PubMed]
- 28.Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M. et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun. 2017;8:14802. doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh J, Kawana N, Yamamoto Y. Pathway Analysis of ChIP-Seq-Based NRF1 Target Genes Suggests a Logical Hypothesis of their Involvement in the Pathogenesis of Neurodegenerative Diseases. Gene Regul Syst Bio. 2013;7:139–152. doi: 10.4137/GRSB.S13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu F, Jiang CC, Yan XG, Tseng HY, Wang CY, Zhang YY. et al. BRAF/MEK inhibitors promote CD47 expression that is reversible by ERK inhibition in melanoma. Oncotarget. 2017;8:69477–69492. doi: 10.18632/oncotarget.17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gowda P, Patrick S, Singh A, Sheikh T, Sen E. Mutant Isocitrate Dehydrogenase 1 Disrupts PKM2-beta-Catenin-BRG1 Transcriptional Network-Driven CD47 Expression. Molecular and cellular biology. 2018;38:e00001–18. doi: 10.1128/MCB.00001-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkovits BD, Mayr C. Alternative 3' UTRs act as scaffolds to regulate membrane protein localization. Nature. 2015;522:363–367. doi: 10.1038/nature14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kibria G, Ramos EK, Lee KE, Bedoyan S, Huang S, Samaeekia R. et al. A rapid, automated surface protein profiling of single circulating exosomes in human blood. Scientific reports. 2016;6:36502. doi: 10.1038/srep36502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Ji S, Shao G, Zhang J, Zhao K, Wang Z. et al. Effect of exosome biomarkers for diagnosis and prognosis of breast cancer patients. Clin Transl Oncol. 2018;20:906–911. doi: 10.1007/s12094-017-1805-0. [DOI] [PubMed] [Google Scholar]
- 35.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 37.Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB. et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Roemeling CA, Wang Y, Qie Y, Yuan H, Zhao H, Liu X. et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat Commun. 2020;11:1508. doi: 10.1038/s41467-020-15129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Zhang M, Wang X, Liu W, Wang H, Yang YG. Vaccination with CD47 deficient tumor cells elicits an antitumor immune response in mice. Nat Commun. 2020;11:581. doi: 10.1038/s41467-019-14102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Liu L, Ren Z, Yang K, Xu H, Luan Y. et al. Dual Targeting of Innate and Adaptive Checkpoints on Tumor Cells Limits Immune Evasion. Cell Rep. 2018;24:2101–2111. doi: 10.1016/j.celrep.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 42.Xu MM, Pu Y, Han D, Shi Y, Cao X, Liang H. et al. Dendritic Cells but Not Macrophages Sense Tumor Mitochondrial DNA for Cross-priming through Signal Regulatory Protein alpha Signaling. Immunity. 2017;47:363–373. doi: 10.1016/j.immuni.2017.07.016. e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peluso MO, Adam A, Armet CM, Zhang L, O'Connor RW, Lee BH. et al. The Fully human anti-CD47 antibody SRF231 exerts dual-mechanism antitumor activity via engagement of the activating receptor CD32a. J Immunother Cancer. 2020;8:e000413. doi: 10.1136/jitc-2019-000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM. et al. Anti-SIRPalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017;114:E10578–E10585. doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrova PS, Viller NN, Wong M, Pang X, Lin GH, Dodge K. et al. TTI-621 (SIRPalphaFc): A CD47-Blocking Innate Immune Checkpoint Inhibitor with Broad Antitumor Activity and Minimal Erythrocyte Binding. Clin Cancer Res. 2017;23:1068–1079. doi: 10.1158/1078-0432.CCR-16-1700. [DOI] [PubMed] [Google Scholar]
- 46.Lin GHY, Chai V, Lee V, Dodge K, Truong T, Wong M. et al. TTI-621 (SIRPalphaFc), a CD47-blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PLoS One. 2017;12:e0187262. doi: 10.1371/journal.pone.0187262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC. et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E2646–2654. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Xavy S, Mihardja S, Chen S, Sompalli K, Feng D. et al. Targeting macrophage checkpoint inhibitor SIRPalpha for anticancer therapy. JCI Insight. 2020;5:e134728. doi: 10.1172/jci.insight.134728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iribarren K, Buque A, Mondragon L, Xie W, Levesque S, Pol J. et al. Anticancer effects of anti-CD47 immunotherapy in vivo. Oncoimmunology. 2019;8:1550619. doi: 10.1080/2162402X.2018.1550619. [DOI] [PMC free article] [PubMed] [Google Scholar]