Abstract
Children with sickle cell disease (SCD) are at high-risk of progressive, chronic pulmonary and cardiac dysfunction. In this prospective multicenter Phase II trial of myeloimmunoablative conditioning followed by haploidentical stem cell transplantation in children with high-risk SCD, 19 patients, 2.0–21.0 years of age, were enrolled with one or more of the following: history of 1) overt stroke; 2) silent stroke; 3) elevated transcranial Doppler velocity; 4) multiple vaso-occlusive crises; and/or 5) two or more acute chest syndromes and received haploidentical transplants from 18 parental donors. Cardiac and pulmonary centralized cores were established. Pulmonary function results were expressed as percent of the median of healthy reference cohorts, matched for age, sex, height and race At 2 years, pulmonary functions including forced expiratory volume (FEV), FEV1/ forced vital capacity (FVC), total lung capacity (TLC), diffusing capacity of lung for carbon monoxide (DLCO) were stable to improved compared to baseline values. Importantly, specific airway conductance was significantly improved at 2 years (p < 0.004). Left ventricular systolic function (fractional shortening) and tricuspid regurgitant velocity were stable at 2 years. These results demonstrate that haploidentical stem cell transplantation can stabilize or improve cardiopulmonary function in patients with SCD.
Introduction
Sickle cell disease (SCD) is an autosomal recessive genetic disorder, characterized by repetitive vaso-occlusive crises, resulting in chronic organ dysfunction including progressive pulmonary hypertension, chronic lung disease and cardiac failure that predispose patients with severe disease to a shortened lifespan (median age 45 years) [1–11]. Thus, pulmonary and cardiac complications currently account for over 45% of all the causes of mortality in adults with SCD who have not undergone allogeneic stem cell transplantation (AlloSCT) [2]. Each year, over 300,000 babies worldwide are born with SCD. SCD is now recognized by the United Nations and World Health Organization as a global health problem [12–14].
Patients with SCD often have abnormal pulmonary function [15–17]. While studies have confirmed reduced lung function compared to healthy age-matched controls, most are either within the normal range or low normal in the pediatric age group [18]. In children, an obstructive pattern is most often seen [7, 19, 20]. In adults a restrictive pattern is more likely to emerge [21], often with a reduced diffusing capacity for carbon monoxide (DLCO) [22]. However, restrictive defects can be overestimated and obstructive defects underestimated if based on spirometry alone [23, 24].
Reduced pulmonary function in some subjects following AlloSCT is well established, presumably due to cryptogenic organizing pneumonia or bronchiolitis obliterans syndrome (BOS) [25, 26]. In one study, the incidence of BOS was 5.5% and its prevalence was 15% among patients with chronic graft-versus-host disease (CGVHD) [27, 28]. One study demonstrated a rapid decline in pulmonary function forced expiratory volume (FEV1) during the six months before the diagnosis of BOS, with a lower FEV1 (% predicted) at diagnosis associated with poor survival [29].
The cardiovascular pathophysiology of SCD involves intravascular hemolysis, vascular occlusion with resultant ischemia, and chronic anemia, which contribute to the development of pulmonary hypertension, left ventricular (LV) diastolic dysfunction, dysrhythmias, and an increased risk of death [3]. SCD is associated with a 26% prevalence of pulmonary hypertension even in the pediatric age group [30]. While human leukocyte antigen (HLA) matched sibling donor AlloSCT is currently the best chance of cure for these patients, it itself has been associated with the development of congestive heart failure as well as subclinical systolic cardiac dysfunction [31]. Although cardiac biomarkers such as troponin and B-type natriuretic peptide are elevated following AlloSCT, they are not associated with long-term systolic function changes [32, 33].
The current long-term cure for patients with high-risk SCD with the best outcomes is a HLA matched sibling donor AlloSCT [34, 35]. We and others have demonstrated ≥90% event-free survival following both myeloablative and reduced toxicity conditioning and HLA sibling matched AlloSCT [36–44]. Unfortunately, it is estimated that there is only a one in six (16%) chance of finding a HLA matched sibling donor for a child with high-risk SCD who doesn’t also have homozygous SCD within any given family in the United States [45]. Despite this low probability, there has been a dramatic increase in AlloSCT over the past decade in part due to the use of alternative donor sources including HLA matched unrelated adult donors (URD), unrelated cord blood donors (UCB) and familial haploidentical donors [46]. Unfortunately, utilizing URD and UCB donors have been limited by a paucity of unrelated matched donors in the unrelated donor registries and an unacceptable rate of graft failure following UCB transplantation and CGVHD following URD AlloSCT as reported by our group and others [47–51]. We previously reported the success of CD34+ enrichment and mononuclear cell addback (2 × 105 CD34/kg) following URD transplantation in children and adolescents [52]. We therefore investigated in a prospective multicenter Phase II study of the safety and efficacy of a novel method of myeloimmunoablative conditioning (MIAC) and haploidentical stem cell transplantation (HISCT) in children and adolescents with high-risk SCD. We previously reported the main outcome results of this study and now focus on and report the long-term pulmonary and cardiac effects following this innovative therapeutic approach [53].
Patients and methods
Patients aged ≥2.0 and ≤20.99 years of age that were homozygous for hemoglobin S with one or more high-risk features, adequate organ function and without an HLA matched unaffected sibling donor were eligible and enrolled from September 2012 to September 2017, as we have previously reported [53]. The protocol was approved at each institutional review board, informed consent was obtained and assent was obtained if clinically applicable and the study was registered at ClinicalTrials.gov NCT01461837. HLA typing at intermediate resolution for Class I (HLA A & B) and high resolution for Class II (DRB1) was performed on the patient and parental donor as we have previously described [38, 50, 52]. Stem cells from familial (parental) donors were mobilized with filgrastim (15 mcg/kg/day) divided twice a day (bid) x eight doses for four days prior to peripheral blood stem cell collection, and a CD34+ enrichment was performed utilizing the CliniMACS CD34+ reagent system generously supplied by Miltenyi Biotec, Bergisch Gladbach, Germany, and cryopreserved prior to conditioning as we have previously described [53, 54]. MIAC consisted of hydroxyurea and azathioprine from day −59 to day −11 (as an outpatient) and fludarabine day −17 -to −13, busulfan day −12 to −9; thiotepa day −8, cyclophosphamide on days −7 to −4, rabbit anti-thymocyte globulin day −5 to −2, and total lymphocyte radiation on day −2 (as an inpatient), as we have previously described (Supplementary Fig. 1) [53]. Acute graft-versus-host disease (AGVHD) prophylaxis only consisted of tacrolimus and supportive care and infection prophylaxis were as we have previously described [38, 50, 52, 53].
Pulmonary function
Pulmonary function tests (PFTs) were performed at baseline prior to transplant, and approximately every year thereafter. All studies were performed in the clinical pulmonary function laboratories at each site, using their own equipment with their own chosen reference values. All pulmonary function laboratories adhered to American Thoracic Society Guidelines in performing their own PFTs [55–57]. Results were expressed as a percent of the median of healthy reference cohorts, matched for age, sex, height, and race [58, 59]. All subjects underwent spirometry, measurement of lung volumes and specific airway conductance, estimated with body plethysmography [57], and DLCO, corrected for hemoglobin [60]. Spirometry was repeated after an inhaled bronchodilator in all subjects. At one center (New York Medical College), additional studies were performed, including post-bronchodilator plethmosgraphy, estimation of respiratory system mechanics obtained with forced oscillation [61] and Lung Clearance Index (LCI) obtained with inert nitrogen washout [62]. An LCI greater than 7.2, >2 standard deviations above the normal mean, was considered abnormal [63]. A 12% increase in FEV1 and a 55% increase in a specific airway conductance (sGaw) after an inhaled bronchodilator were considered significant [64, 65]. The pulmonary function core was based at the Maria Fareri Children’s Hospital and was directed by Allen J. Dozor, MD.
Cardiac function and tricuspid regurgitant jet (TRJ) velocity
Patients underwent noninvasive cardiac evaluations prior to transplant, Day +30, +100, 1 year, and 2 years after AlloSCT. Transthoracic echocardiograms were performed using Acuson Sequoia Ultrasound Systems (Siemens Medical Solutions, Malvern, PA) or the Philips IE33 ultrasound system (Philips Medical Systems, Bothell, WA) or equivalent with appropriate transducer selection based on body size and image quality. Complete standard views and measurements were obtained based on American Society of Echocardiography standards. Pulmonary artery pressure was assessed based on methods of Pashankar et al [66]. Tricuspid regurgitation velocity (TRV) measurements were attempted from multiple views (apical four chamber, parasternal short axis, parasternal long axis) to obtain the optimal flow signals by pulsed wave or continuous Doppler, averaging 5 successive cardiac cycles. The right ventricular to right atrial systolic pressure gradient was calculated using the modified Bernoulli equation: 4 x the square of the maximum velocity. The mean right atrial pressure was assumed to be 5 mm Hg, and this value was added to the above calculated right ventricular systolic peak pressure to derive the estimated pulmonary artery systolic pressure. Pulmonary diastolic pressure was also estimated from the Bernoulli equation by measuring the end diastolic velocity of the pulmonary insufficiency jet and adding that again to the estimated 5 mm Hg right ventricular end diastolic pressure. The threshold for diagnosing pulmonary hypertension was defined as a peak TRV of at least 2.5 m/sec, which equals an estimated pulmonary artery systolic pressure of at least 30 mm Hg. By convention, mild pulmonary hypertension was defined as a TRV of 2.5 to 2.9 m/sec (which is a pulmonary artery systolic pressure of 30 to 39 mm Hg), and moderate pulmonary hypertension was assumed when the peak TRV was equal to or greater than 3 m/sec (pulmonary artery systolic pressure of at least 40 mm Hg). If the TRV was less than 2.5 m/sec or not measurable, the patient was assumed to have normal pulmonary artery pressures. The Pulmonary Hypertension Core was based at Maria Fareri Children’s Hospital and was directed by Deborah Friedman, MD. Participant flow and testing diagram is noted in Supplementary Fig. 2.
Statistical analysis
Descriptive statistics were calculated for patient measured parameters at each time point (Baseline, 1 year post, 2 years post, 4 years post). Repeated measures analysis of variance analysis was used to assess parameter differences between time points followed by Bonferroni post hoc calculation when the p values were significant (<0.05). The Prism 8 statistical program (GraphPad v. 8.4.3, San Diego, CA) was used for all statistical determinations. Data were analyzed from start of study to October 2020.
Results
There were 19 patients from 18 parental donors that proceeded to HISCT. There were no major protocol deviations. Age, gender, HLA match, primary risk factor, CD34 × 106/kg infused, days to ANC and platelet reconstitution, busulfan steady state concentration, maximal Grade II-IV AGVHD and extensive CGVHD, 1 year whole blood donor and red blood cell chimerism and viral studies are summarized in Table 1. The mean±standard error of mean (SEM) age of the parental donors was 41.3±1.8 (30–55) years of age with a gender ratio female/male (F/M) (15/3) with a CD34 recovery of 66.0±3.4% (39.7–96), T cell depletion (mean±SEM CD3/kg) 4.8±0.1 (4.0–6.0) logs, mean±SEM viability of 96.3±0.7% (86.3–99.8) and the mean±SEM enriched PBSC infusion was 10.94±0.4×106 CD34/kg with mononuclear cell addback (2×105 CD3/kg) as we have previously described [53, 54]. The median time to ANC and platelet engraftment was 9 and 19 days, respectively, cumulative incidence of Grade II-IV AGVHD and extensive CGVHD was 6.2% and 6.7%, respectively and probability of 1-year event free survival/overall survival was 90% (95% CI, 64.1–97.3%) as we have previously described [53]. The median follow-up is now 2064 days (59–2985) and the average inpatient stay was 8 weeks.
Table 1.
Clinical characteristics and clinical outcome results of HISCT recipients (n = 19)
| Pt Recipient # | Age yr | Gender (M/F) | HLA Match Out of 6 | Primary Risk Factor | CD34 × 106/kg infused | Neutrophil engraft-ment Day + | Platelet engraft-ment Day + | Bu Css ng/ml,a | Max Grade II-IV AGVHD | Max moderate/extensive CGVHD | 1y WB donor chimerism % | 1y RBC donor chimerism % | Status Day + |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 526–001 | 10 | M | 3/6 | TCD | 12.0 | 13 | 33 | 596 | None | None | 99 | 99 | A/2985 |
| 526–002 | 13 | F | 3/6 | Stroke | 11.99 | 9 | 16 | 590,a | None | None | 98 | 98 | A/2872 |
| 526–003 | 20 | F | 4/6 | Stroke | 11.57 | 9 | 19 | 722 | None | None | 100 | 100 | A/2578 |
| 526–004 | 18 | F | 3/6 | ACS | 9.78 | 10 | NE | 779 | None | None | N/A | N/A | D/59 |
| 526–005 | 20 | M | 3/6 | VOC | 9.4 | 9 | 12 | 839 | None | None | 100 | 99 | A/2368 |
| 526–006 | 12 | M | 3/6 | VOC | 9.97 | 9 | 44 | 479,a | None | None | 95 | 98 | A/2348 |
| 526–007 | 8 | M | 3/6 | Silent infarct | 12.76 | 11 | 21 | 521a | None | None | 99 | 99 | A/2299 |
| 526–008 | 9 | F | 3/6 | Stroke | 11.2 | 9 | 17 | 622 | None | None | 100 | 100 | A/2194 |
| 526–009 | 15 | M | 3/6 | Stroke | 8.52 | 6 | 8 | 652 | None | None | 99 | 99 | A/2012 |
| 526–010 | 4 | M | 3/6 | ACS | 12.9 | 10 | 15 | 548a | None | None | 96 | 91 | A/2064 |
| 526–011 | 17 | F | 3/6 | Silent infarct | 11.83 | 9 | NE | 809 | III | None | N/A | N/A | D/141 |
| 526–012 | 14 | M | 3/6 | TCD | 7.37 | 9 | 90 | 606,a | None | None | 95 | 98 | A/1966 |
| 526–013 | 12 | F | 3/6 | Stroke | 10.9 | 9 | 14 | 746 | None | Severe | 99 | 99 | D/390 |
| 526–014 | 20 | F | 3/6 | Stroke | 10.64 | 9 | 18 | 698 | None | None | 100 | 100 | A/1893 |
| 526–015 | 10 | M | 3/6 | ACS | 9.88 | 10 | 33 | 383,a | None | None | 99 | 97 | A/1711 |
| 526–016 | 20 | M | 3/6 | Stroke | 9.38 | 10 | 19 | 715 | None | None | 100 | 100 | A/1529 |
| 526–018 | 3 | M | 3/6 | ACS | 15.2 | 10 | 16 | 544,a | None | None | 73,b | 70,b | A/1532 |
| 526–019 | 11 | M | 3/6 | Silent infarct | 10.6 | 10 | 33 | 969,a | None | None | 98,c | 98,c | A/1263 |
| 526–020 | 11 | M | 3/6 | Silent infarct | 11.88 | 9 | 8 | 494,a | None | None | 98,d | 94,d | A/1256 |
| Summary mean ± SEM | 13 ± 1.2 | 12/7 (M/F) | 3/6 N=18 4/6 N=1 |
N/A | 10.9 ± 0.4 | 9.5 ± 0.3 | 24.5 ± 4.7 | 648 ± 33 ng/ml | 1/19 | 1/19 | 97.0 ± 1.6 | 96.4 ± 1.7 | med f/u 2064days (59–2985) |
Abbreviations: n number, HISCT haploidentical stem cell transplantation, Pt patient, M male, F female, TCD transcranial Doppler, AGVHD acute graft versus host disease, CGVHD chronic graft versus host disease, WB whole blood, RBC red blood cell, TCD transcranial Doppler, A alive, ACS acute chest syndrome, NE not engrafted, D death (004, VOC, 011, AGVHD, 013, CGVHD), VOC venoocclusive disease, N/A not applicable, med f/u median follow up.
Bu- dose adjusted.
Bu- dose adjusted Day +438.
Bu- dose adjusted Day +109.
Bu- dose adjusted Day +176.
Pulmonary function
PFTs are reported in 17 subjects at baseline, in 13 subjects 1 year after HISCT, in 11 after 2 years. Standard PFTs are outlined in Table 2. At baseline, spirometry and lung volumes were in the normal range. Seven subjects (41.2%) had an FEV1 <80% of predicted value, 3 (17.6%) had an FEV1/FVC <0.80 and 2 (11.8%) had a TLC <80% of predicted. Mean DLCO, corrected for hemoglobin was 76.8±15/4% of predicted and 11 (64.7) subjects had a baseline DLCO <80 of predicted. Means sGaw was 69.4±20.9% of predicted and 10 (71.74%) subjects had a sGaw <80% of predicted. Mean % changes in FEV1 and sGaw after albuterol were 3.9±5.4 and 67.6±27.7, respectively. One subject demonstrated ≥12% change in FEV1 and 12 of 13 (92%) demonstrated >55% change in sGaw with albuterol.
Table 2.
Pulmonary function tests
| FVC (% Pred) |
FEV1 (% Pred) |
FEV1/FVC (Absolute) | FEF25–75 (% Pred) |
TLC (% Pred) |
DLCO, Adj (% Pred) |
|
|---|---|---|---|---|---|---|
|
Baseline,
n = 17 |
90.0±12.5 | 84.5±11.5 | .84±.04 | 76.2±20.5 | 87.2±7.7 | 76.8±15.4 |
|
1 yr Post-AlloSCT,
n = 13 |
81.2±14.1 | 80.3±13.9 | .87±.06 | 78.7±16.7 | 80.9±12.1 | 68.4±17.7 |
|
2 yr Post-AlloSCT,
n = 11 |
84.9±15.8 | 82.4±14.5 | .86±.03 | 81.3±14.6 | 81.7±9.9 | 65.4±18.9 |
|
4 yr Post-AlloSCT,
n = 4 |
86.5±9.9 | 85.5±7.0 | .86±.09 | 87.0±27.3 | 89.1±10.2 | 79.3±11.8 |
| P value, 1 year post transplant compared to baseline | 0.075 | 0.365 | 0.050 | 0.721 | 0.091 | 0.163 |
| P value, 2 years post-transplant compared to baseline | 0.351 | 0.671 | 0.136 | 0.484 | 0.115 | 0.087 |
| P value, 4 years post-transplant compared to baseline | 0.609 | 0.867 | 0.451 | 0.379 | 0.686 | 0.767 |
Abbreviations: FVC forced vital capacity, Pred predicted, FEV forced expiratory volume, FEF25–75, forced expiratory flow, TLC total lung capacity, DLCO diffusing capacity of lung for carbon monoxide adjusted for Hgb, n number, yr, year, AlloSCT allogeneic stem cell transplantation.
All values reported as mean±SD.
There were significant improvements in sGaw at 2 years post HISCT (p < 0.004) (Fig. 1). There were no significant changes over time in the proportion of subjects with an FEV1 < 80% predicted, FEV1/FVC <0.80, or DLCO <80% of predicted. The proportion with a TLC <80% predicted was 11.8% at baseline, 53.8% at 1 year (p = 0.02), and 45.5% at 2 years post-HISCT (p = 0.004). The proportion of subjects with sGaw <80% predicted was 71.4% at baseline, 50% at 1 year, (p = 0.422), and 20% at 2 years, (p = 0.036). Forced oscillatory mechanics and LCI, performed in a subset of patients at one center (New York Medical College) are shown in Table 3. All results remained within the normal range and stable at all-time points with no significant changes from baseline. LCI was greater than 7.2 in 41.7% of subjects at baseline, 30% at 1 year, and 25% at 2 years post HISCT.
Fig. 1. Specific conductance (% predicted) before and after myeloimmunoablative conditioning and haploidentical stem cell transplantation (HISCT).
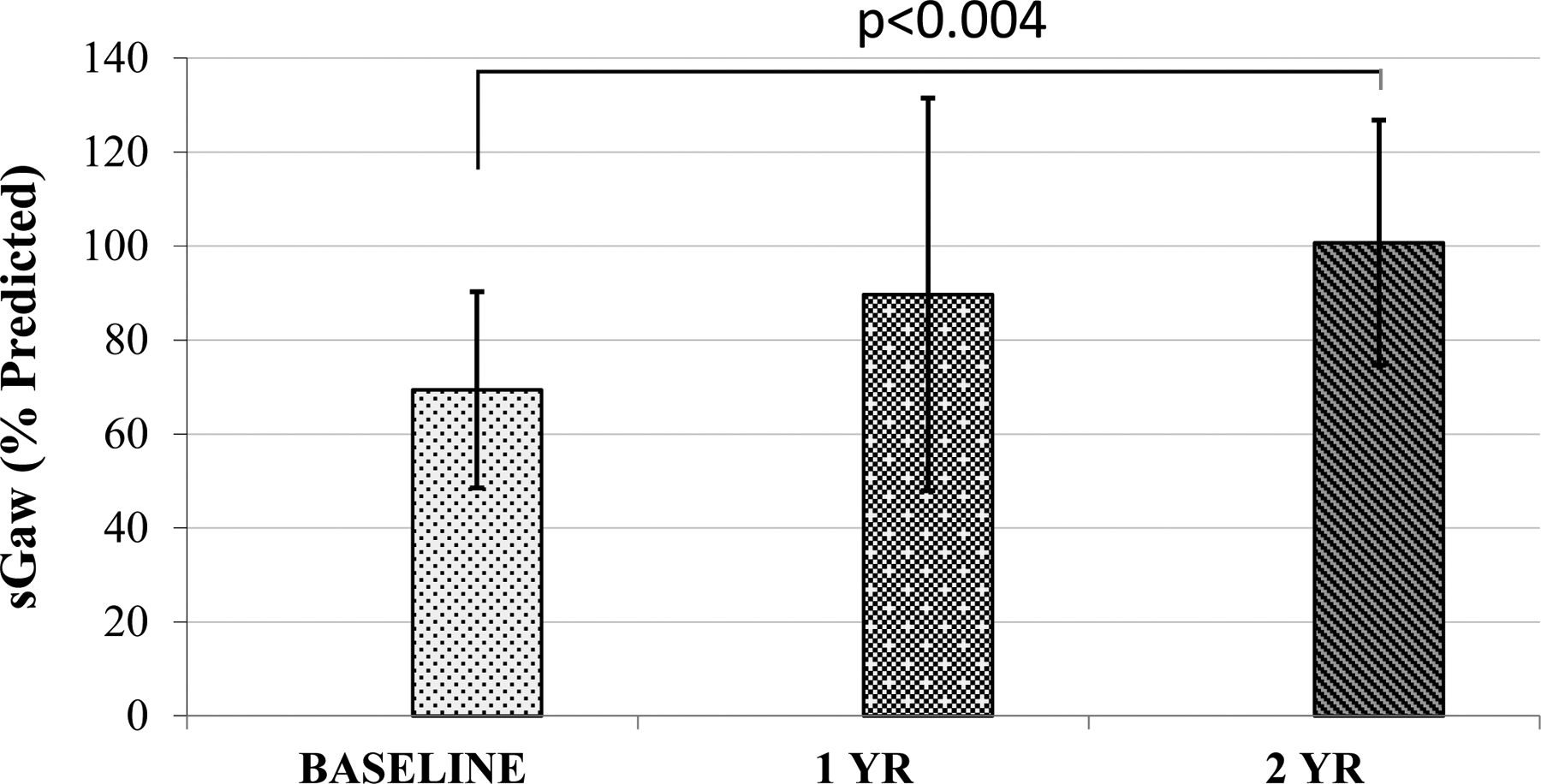
All means expressed as ± standard deviation (SD).
Table 3.
Forced oscillatory mechanics and lung clearance index
| R5 (cm H20/L/sec) |
R20 (cm H20/L/sec) |
R5-R20 (cm H20/L/sec) |
AX (cm H20/L) |
X5 (cm H20/L/sec) |
Fres (Hz) |
LCI | |
|---|---|---|---|---|---|---|---|
|
Baseline,
n = 17 |
6.39±1.96 | 4.42±1.16 | 1.88±.89 | 18.81±11.9 | −2.71±1.36 | 19.51±2.94 | 7.07±1.56 |
|
1 yr Post-HSCT,
n = 13 |
5.53±1.75 | 3.93±1.08 | 1.60±.98 | 13.18±10.52 | −2.27±1.28 | 17.40±1.96 | 6.64±1.30 |
|
2 yr Post-HSCT,
n = 11 |
5.51±1.75 | 3.68±1.09 | 0.99±1.52 | 14.77±9.26 | −2.08±1.03 | 18.74±2.86 | 6.81±1.14 |
|
4 yr Post-HSCT,
n = 4 |
4.81±1.16 | 3.75±.83 | 1.07±.35 | 9.57±3.81 | −1.87±0.50 | 17.57±2.77 | 6.00±.95 |
| P value, 1 year post transplant compared to baseline | 0.288 | 0.315 | 0.473 | 0.251 | 0.438 | 0.101 | 0.417 |
| P value, 2 years post-transplant compared to baseline | 0.262 | 0.126 | 0.968 | 0.368 | 0.217 | 0.664 | 0.549 |
| P value, 4 years post-transplant compared to baseline | 0.152 | 0.306 | 0.097 | 0.154 | 0.253 | 0.325 | 0.197 |
Abbreviations: R5 Respiratory system resistance at 5 hz, R20 Respiratory system resistance at 20 hz, AX area under the reactance curve, X5
Respiratory system reactance 5 hz, Fres Resonant frequency, LCI Lung Clearance Index, n number, AlloSCT allogeneic stem cell transplantation.
All values reported as mean±SD.
Cardiac and TRJ
Echocardiographic data were centrally available for review in 17 patients at baseline, 16 patients at 1-year post HISCT and 15 patients 2 years after HISCT. We analyzed echocardiographic data looking specifically at cardiac systolic function, volumes, and measures of pulmonary hypertension (Table 4). None of the patients had pathologic pulmonary hypertension at baseline, and none developed post HISCT as determined by TRJ velocity (Fig. 2). The LV systolic function as measured by fractional shortening was normal at baseline and values remained stable and normal post HISCT vs baseline. The hematocrit was low as expected at baseline and increased to normal after the HISCT. LV dimensions did correlate with body surface area (BSA) throughout the study as expected.
Table 4.
Cardiac fractional shortening and left ventricular end-diastolic volume
| Day 0 | Day 30 | Day 100 | Day 365 | Day 730 | |
|---|---|---|---|---|---|
| FS% | 35.2±2.9 % | 36.4±5.4% | 37.6±3.8% | 36.6±3.7% | 35.4±3.6% |
| LVEDV | 4.74±0.47 cm | 4.59±0.41 cm | 4.39±0.6 cm | 4.30±0.45 cm | 4.35±0.46 cm |
Abbreviations: FS fractional shortening, LVEDV left ventricular end-diastolic volume.
Mean values ± standard deviation given for each time point (p = NS).
Fig. 2. Tricuspid regurgitation gradient before and after myeloimmunoablative conditioning and haploidentical stem cell transplantation (HISCT).
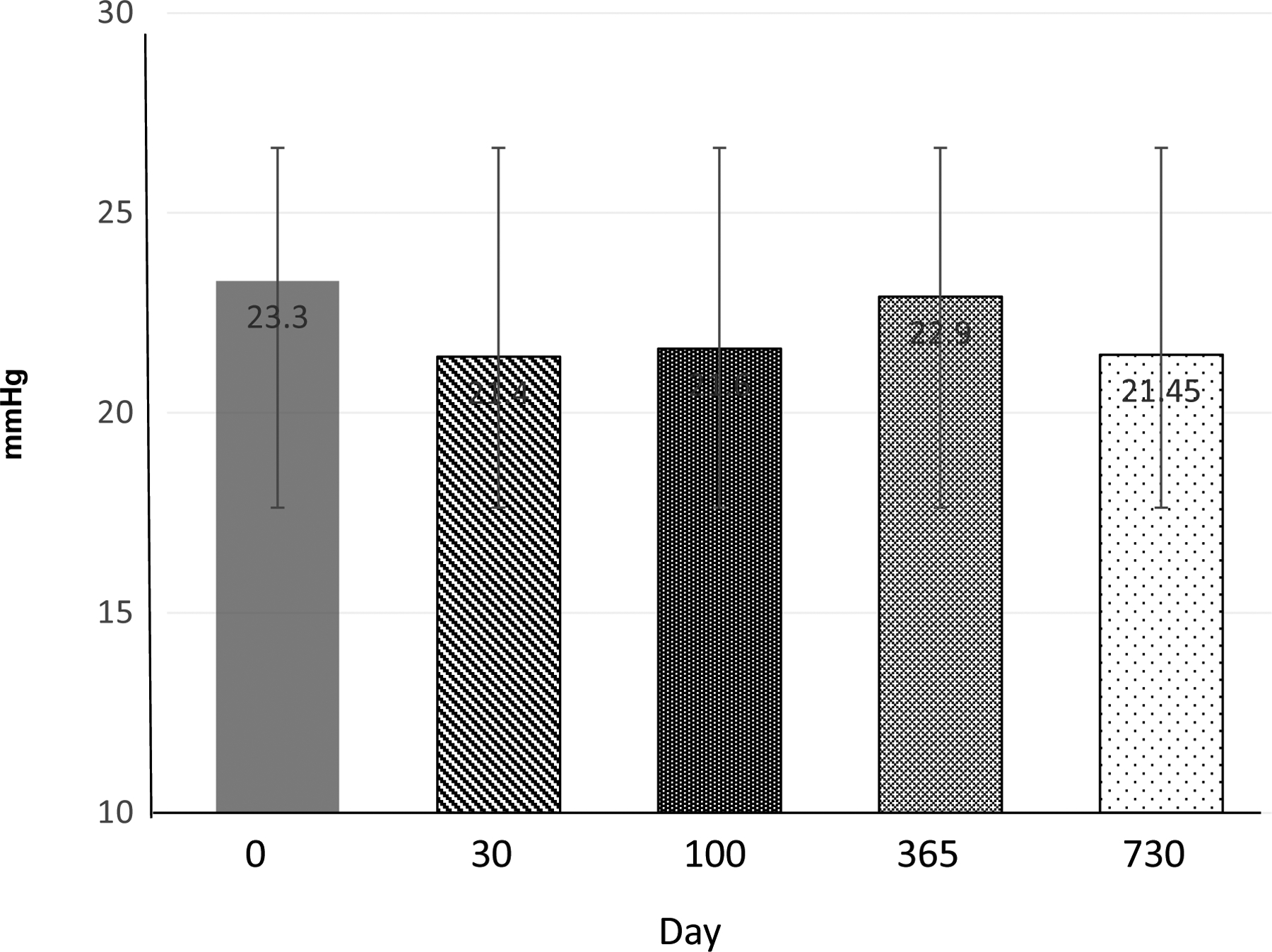
All means expressed as ± standard deviation (SD).
Discussion
Despite the utilization of myeloimmunoablative therapy prior to HISCT in children and adolescents with high-risk SCD, we report stable to improved pulmonary function, cardiac function and cardiac TRJ velocity. Importantly, we also demonstrated for the first time a significant improvement in specific pulmonary conductance and return into a normal range post HISCT (sGaw). Furthermore, no patient developed signs of pulmonary hypertension as measured by TRJ velocity post HISCT.
Though patients had pulmonary function within the normal range prior to HISCT, there was evidence for a mild obstructive defect and/or reduced diffusing capacity in a significant proportion. Only 2 subjects (11.8%) had a restrictive defect at baseline. These results are consistent that younger patients with SCD are less likely to be restricted and more likely to be obstructed [7, 15–18, 20]. There was essentially no change in FEV1 with albuterol, though almost all subjects studied with post-bronchodilator plethysmography had a significant increase in sGaw [64, 65]. This is consistent with a number of studies suggesting that changes in sGaw may be a more sensitive marker of bronchodilator responsiveness than change in FEV1 in patients with SCD [67, 68]. The proportion with obstructive defects as assessed with spirometry did not change, though the proportion with abnormal sGaw decreased. The proportion with a reduced diffusing capacity did not change, but the proportion with restriction (TLC, % predicted), appeared to have increased, likely related to myeloablative conditioning therapy.
At one center, additional pulmonary function tests were obtained, including post-bronchodilator plethysmography, impulse oscillometry, and LCI. Impulse oscillometry appears to be more sensitive than spirometry in detecting small airways disease [61]. LCI appears to be sensitive to unevenness of gas mixing in the lungs [69, 70], and 41.7% of our subjects had an elevated LCI. There were no significant changes in these measures over time in this study, but interpretation of the results is hampered by limited reference values in healthy age and race-matched populations.
Pulmonary hypertension occurs in 10% of patients with SCD [66]. There are multiple mechanisms by which this may occur such as hemolysis, hypoxia, thromboembolism, as well as abnormal nitric oxide signaling or indirectly from LV hypertrophy and dysfunction. Those patients with evidence of pulmonary hypertension have a markedly higher risk of death [71]. Therefore it is prudent to routinely screen patients for progressive elevations in pulmonary artery pressure. TRV has been shown to be predictive of pulmonary hypertension and mortality and is elevated in 21% of children and 30% of adults [72]. In this study of pediatric patients, there were no patients who had evidence of pulmonary hypertension at the start of the study. In the natural history of the disease we would expect that some might develop it, however, we did not observe this.
SCD can lead to a high cumulative incidence of cardiac abnormalities in children that progresses with age [73]. In a study of sickle cell patients, these abnormalities were greatest for LV size and mass, but were lowest for gross systolic function measures such as LV fractional shortening, and for TRV. The initial abnormalities may portend the more relevant changes in function parameters and measures of pulmonary hypertension that occur later. LV diastolic dysfunction is also common in patients with SCD and may be related to the ventricular dilation, chronic anemia, or the concentric hypertrophy as a response to relative systemic hypertension [71]. In addition, while iron overload is a significant concern in transfusion-dependent anemias and has been shown to cause worsening cardiac dysfunction especially those with thalassemia. SCD patients receiving chronic transfusions may not demonstrate significant cardiac iron loading irrespective of ferritin trends, liver iron content, or erythropoiesis suppression [74].
AlloSCT has been shown to be successful in SCD and eliminating chronic vaso-occlusive endothelial induced end organ damage and thereby the progression of cardiopulmonary dysfunction. However, AlloSCT can be associated with progressive cardiovascular complications in the long term [33]. In fact, cardiovascular disease is a leading cause of non-relapse morbidity and mortality in AlloSCT survivors of malignancies [75]. These patients have a two to four-fold greater risk of cardiovascular related mortality [75]. It has been demonstrated that long term survivors of pediatric AlloSCT have systolic dysfunction at rates up to 26% [76]. We were able to demonstrate overall stable cardiac function in SCD patients through the course of AlloSCT up to two years post-transplant. However, one of the challenges is the crudeness of routine echocardiographic measurements for systolic function such as ejection fraction and shortening fraction. However, speckle tracking and global longitudinal strain have demonstrated an ability to detect subclinical changes in ventricular dysfunction. Through this method, one study demonstrated abnormal baseline longitudinal and radial strain that returned to normal by 1 year after AlloSCT and remained stable at 2 years after AlloSCT. This may demonstrate an ability to detect subclinical changes in systolic function prior to routine echocardiographic measures. In the same study, LV mass index decreased from baseline to 2 years post-transplant, implying improved cardiac hypertrophy [76].
Conclusion
In summary, children with high-risk SCD following MIAC and HISCT had stable or improved pulmonary and cardiac function at 2 years versus baseline. Longer follow-up of this cohort is ongoing to determine the durability of these findings. Although this study did not detect any significant changes in overall normal systolic function or pulmonary artery pressure estimated with routine echocardiograms, future studies may benefit from more sensitive techniques such as diastolic function measurements, LV longitudinal strain, or alternative imaging such as MRI.
Supplementary Material
Acknowledgements
This study was supported in large part by FDA R01FD004090 held by Dr. Cairo and in small part by the Pediatric Cancer Research Foundation, held by Dr. Cairo. We would like to thank Virginia Davenport, RN for her superb editorial assistance. We would also like to thank the patients and families who participated in this clinical trial and all the members of the external data safety monitoring committee and external advisory committee (Supplementary Fig. 3)
Competing Interest:
MSC has received funding from the FDA (R01FD004090), the Pediatric Cancer Research Foundation (PCRF), Otsuka and Miltenyi. Although not related to this study, MCW is a consultant for Bluebird Bio, Inc., Bioverativ, Sangamo, Veevo, Editas Medicine and is medical director for AllCells, Inc. Biosciences. SKP is a consultant to Seattle Genetics, also unrelated to this study. EV is a consultant for ApoPharma, on the advisory committee for Global Blood Therapeutics and receives royalties from UptoDate. All other authors declare no conflicts of interest.
Footnotes
Ethics The protocol was approved at each institutional review board, informed consent was obtained and assent was obtained if clinically applicable and the study was registered at ClinicalTrials.gov NCT01461837.
This research has been presented in part at the American Society of Hematology (ASH), December 2017, San Diego, CA.
References
- 1.Caughey MC, Poole C, Ataga KI, Hinderliter AL. Estimated pulmonary artery systolic pressure and sickle cell disease: a meta-analysis and systematic review. Br J Haematol. 2015;170:416–24. [DOI] [PubMed] [Google Scholar]
- 2.Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X, Wright EC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387:2565–74. [DOI] [PubMed] [Google Scholar]
- 4.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359:2254–65. [DOI] [PubMed] [Google Scholar]
- 6.Lanzkron S, Carroll CP, Haywood C Jr. Mortality rates and age at death from sickle cell disease: U.S., 1979–2005. Public Health Rep. 2013;128:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLean JE, Atenafu E, Kirby-Allen M, MacLusky IB, Stephens D, Grasemann H, et al. Longitudinal decline in lung volume in a population of children with sickle cell disease. Am J Respir Crit Care Med. 2008;178:1055–9. [DOI] [PubMed] [Google Scholar]
- 8.Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. N Engl J Med. 2017;376:1561–73. [DOI] [PubMed] [Google Scholar]
- 9.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–44. [DOI] [PubMed] [Google Scholar]
- 10.Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–30. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Regional Office for Africa. Sickle-cell disease: a strategy for the WHO African Region. Report of the Regional Director. Equatorial Guinea. In: WHO, 2010. [Google Scholar]
- 13.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piel FB, Tatem AJ, Huang Z, Gupta S, Williams TN, Weatherall DJ. Global migration and the changing distribution of sickle haemoglobin: a quantitative study of temporal trends between 1960 and 2000. Lancet Glob Health. 2014;2:e80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field JJ, Glassberg J, Gilmore A, Howard J, Patankar S, Yan Y, et al. Longitudinal analysis of pulmonary function in adults with sickle cell disease. Am J Hematol. 2008;83:574–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klings ES, Wyszynski DF, Nolan VG, Steinberg MH. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173:1264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koumbourlis AC. Lung function in sickle cell disease. Paediatr Respir Rev. 2014;15:33–7. [DOI] [PubMed] [Google Scholar]
- 18.Field JJ, DeBaun MR, Yan Y, Strunk RC. Growth of lung function in children with sickle cell anemia. Pediatr Pulmonol. 2008;43:1061–6. [DOI] [PubMed] [Google Scholar]
- 19.Koumbourlis AC, Zar HJ, Hurlet-Jensen A, Goldberg MR. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. J Pediatr. 2001;138:188–92. [DOI] [PubMed] [Google Scholar]
- 20.Lunt A, McGhee E, Sylvester K, Rafferty G, Dick M, Rees D, et al. Longitudinal assessment of lung function in children with sickle cell disease. Pediatr Pulmonol. 2016;51:717–23. [DOI] [PubMed] [Google Scholar]
- 21.Lunt A, Desai SR, Wells AU, Hansell DM, Mushemi S, Melikian N, et al. Pulmonary function, CT and echocardiographic abnormalities in sickle cell disease. Thorax. 2014;69:746–51. [DOI] [PubMed] [Google Scholar]
- 22.Arteta M, Campbell A, Nouraie M, Rana S, Onyekwere OC, Ensing G, et al. Abnormal pulmonary function and associated risk factors in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2014;36:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunt A, McGhee E, Robinson P, Rees D, Height S, Greenough A. Lung function, transfusion, pulmonary capillary blood volume and sickle cell disease. Respir Physiol Neurobiol. 2016;222:6–10. [DOI] [PubMed] [Google Scholar]
- 24.Ruhl AP, Sadreameli SC, Allen JL, Bennett DP, Campbell AD, Coates TD, et al. Identifying Clinical and Research Priorities in Sickle Cell Lung Disease. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2019;16:e17–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica. 2017;102:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roca J, Granena A, Rodriguez-Roisin R, Alvarez P, Agusti-Vidal A, Rozman C. Fatal airway disease in an adult with chronic graft-versus-host disease. Thorax. 1982;37:77–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:S106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng GS, Storer B, Chien JW, Jagasia M, Hubbard JJ, Burns L, et al. Lung Function Trajectory in Bronchiolitis Obliterans Syndrome after Allogeneic Hematopoietic Cell Transplant. Ann Am Thorac Soc. 2016;13:1932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambrusko SJ, Gunawardena S, Sakara A, Windsor B, Lanford L, Michelson P, et al. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006;47:907–13. [DOI] [PubMed] [Google Scholar]
- 31.Genberg M, Oberg A, Andren B, Hedenstrom H, Frisk P, Flachskampf FA. Cardiac function after hematopoietic cell transplantation: an echocardiographic cross-sectional study in young adults treated in childhood. Pediatr Blood Cancer. 2015;62:143–7. [DOI] [PubMed] [Google Scholar]
- 32.Rotz SJ, Dandoy CE, Taylor MD, Jodele S, Jefferies JL, Lane A, et al. Long-term systolic function in children and young adults after hematopoietic stem cell transplant. Bone Marrow Transplant. 2017;52:1443–7. [DOI] [PubMed] [Google Scholar]
- 33.Rotz SJ, Ryan TD, Hayek SS. Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation. J Thromb Thrombolysis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talano JA, Cairo MS. Smoothing the crescent curve: sickle cell disease. Hematology Am Soc Hematol Educ Program. 2014;2014:468–74. [DOI] [PubMed] [Google Scholar]
- 35.Talano JA, Cairo MS. Hematopoietic stem cell transplantation for sickle cell disease: state of the science. Eur J Haematol. 2015;94:391–9. [DOI] [PubMed] [Google Scholar]
- 36.Bernaudin F, Dalle JH, Bories D, de Latour RP, Robin M, Bertrand Y, et al. Long-term event-free survival, chimerism and fertility outcomes in 234 patients with sickle-cell anemia younger than 30 years after myeloablative conditioning and matched-sibling transplantation in France. Haematologica. 2020;105:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–56. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia M, Jin Z, Baker C, Geyer MB, Radhakrishnan K, Morris E, et al. Reduced toxicity, myeloablative conditioning with BU, fludarabine, alemtuzumab and SCT from sibling donors in children with sickle cell disease. Bone Marrow Transplant. 2014;49:913–20. [DOI] [PubMed] [Google Scholar]
- 39.Gluckman E, Cappelli B, Bernaudin F, Labopin M, Volt F, Carreras J, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129:1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guilcher GMT, Monagel DA, Nettel-Aguirre A, Truong TH, Desai SJ, Bruce A, et al. Nonmyeloablative Matched Sibling Donor Hematopoietic Cell Transplantation in Children and Adolescents with Sickle Cell Disease. Biol Blood Marrow Transplant. 2019;25:1179–86. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King AA, Kamani N, Bunin N, Sahdev I, Brochstein J, Hayashi RJ, et al. Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. Am J Hematol. 2015;90:1093–8. [DOI] [PubMed] [Google Scholar]
- 43.Locatelli F, Kabbara N, Ruggeri A, Ghavamzadeh A, Roberts I, Li CK, et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122:1072–8. [DOI] [PubMed] [Google Scholar]
- 44.Walters MC, Patience M, Leisenring W, Eckman JR, Scott JP, Mentzer WC, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–76. [DOI] [PubMed] [Google Scholar]
- 45.Mentzer WC, Heller S, Pearle PR, Hackney E, Vichinsky E. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am J Pediatr Hematol Oncol. 1994;16:27–9. [PubMed] [Google Scholar]
- 46.Stenger EO, Shenoy S, Krishnamurti L. How I treat sickle cell disease with hematopoietic cell transplantation. Blood. 2019;134:2249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abraham A, Cluster A, Jacobsohn D, Delgado D, Hulbert ML, Kukadiya D, et al. Unrelated Umbilical Cord Blood Transplantation for Sickle Cell Disease Following Reduced-Intensity Conditioning: Results of a Phase I Trial. Biol Blood Marrow Transplant. 2017;23:1587–92. [DOI] [PubMed] [Google Scholar]
- 48.Kamani NR, Walters MC, Carter S, Aquino V, Brochstein JA, Chaudhury S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: results of one cohort from the phase II study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN). Biol Blood Marrow Transplant. 2012;18:1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ngwube A, Shah N, Godder K, Jacobsohn D, Hulbert ML, Shenoy S. Abatacept is effective as GVHD prophylaxis in unrelated donor stem cell transplantation for children with severe sickle cell disease. Blood Adv. 2020;4:3894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radhakrishnan K, Bhatia M, Geyer MB, Del Toro G, Jin Z, Baker C, et al. Busulfan, fludarabine, and alemtuzumab conditioning and unrelated cord blood transplantation in children with sickle cell disease. Biol Blood Marrow Transplant. 2013;19:676–7. [DOI] [PubMed] [Google Scholar]
- 51.Shenoy S, Eapen M, Panepinto JA, Logan BR, Wu J, Abraham A, et al. A trial of unrelated donor marrow transplantation for children with severe sickle cell disease. Blood. 2016;128:2561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geyer MB, Ricci AM, Jacobson JS, Majzner R, Duffy D, Van de Ven C, et al. T cell depletion utilizing CD34(+) stem cell selection and CD3(+) addback from unrelated adult donors in paediatric allogeneic stem cell transplantation recipients. Br J Haematol. 2012;157:205–19. [DOI] [PubMed] [Google Scholar]
- 53.Cairo MS, Talano JA, Moore TB, Shi Q, Weinberg RS, Grossman B, et al. Familial Haploidentical Stem Cell Transplant in Children and Adolescents With High-Risk Sickle Cell Disease: A Phase 2 Clinical Trial. JAMA Pediatr. 2020;174:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flower A, Long B, Keever-Taylor C, Fabricatore S, Morris E, Mahanti H, et al. Safety and efficacy of high dose granulocyte colony stimulating factor mobilization in familial haploidentical adult donors with sickle cell trait followed by CD34+ enrichment and mononuclear cell add-back prior to haploidentical allogeneic transplantation in high-risk sickle cell disease recipients. Br J Haematol. 2020. [Google Scholar]
- 55.Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49. [DOI] [PubMed] [Google Scholar]
- 56.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. [DOI] [PubMed] [Google Scholar]
- 58.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. [DOI] [PubMed] [Google Scholar]
- 59.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. [DOI] [PubMed] [Google Scholar]
- 61.Mondal P, Yirinec A, Midya V, Sankoorikal BJ, Smink G, Khokhar A, et al. Diagnostic value of spirometry vs impulse oscillometry: A comparative study in children with sickle cell disease. Pediatr Pulmonol. 2019;54:1422–30. [DOI] [PubMed] [Google Scholar]
- 62.Nyilas S, Baumeler L, Tamm M, Halter JP, Savic S, Korten I, et al. Inert Gas Washout in Bronchiolitis Obliterans Following Hematopoietic Cell Transplantation. Chest. 2018;154:157–68. [DOI] [PubMed] [Google Scholar]
- 63.Verbanck S, Thompson BR, Schuermans D, Kalsi H, Biddiscombe M, Stuart-Andrews C, et al. Ventilation heterogeneity in the acinar and conductive zones of the normal ageing lung. Thorax. 2012;67:789–95. [DOI] [PubMed] [Google Scholar]
- 64.Bussamra MH, Cukier A, Stelmach R, Rodrigues JC. Evaluation of the magnitude of the bronchodilator response in children and adolescents with asthma. Chest. 2005;127:530–5. [DOI] [PubMed] [Google Scholar]
- 65.Tavares e Castro A, Matos P, Tavares B, Matos MJ, Segorbe-Luis A. Alternative functional criteria to assess airflow-limitation reversibility in asthma. Rev Port Pneumol (2006). 2015;21:69–75. [DOI] [PubMed] [Google Scholar]
- 66.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121:777–82. [DOI] [PubMed] [Google Scholar]
- 67.Morice AH, Waterhouse JC, Peers EM, Parry-Billings M. Use of whole-body plethysmography to compare bronchodilator inhaler efficacy. Respiration. 1998;65:120–4. [DOI] [PubMed] [Google Scholar]
- 68.Skinner C, Palmer KN. Changes in specific airways conductance and forced expiratory volume in one second after a bronchodilator in normal subjects and patients with airways obstruction. Thorax. 1974;29:574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aurora P, Kozlowska W, Stocks J. Gas mixing efficiency from birth to adulthood measured by multiple-breath washout. Respir Physiol Neurobiol. 2005;148:125–39. [DOI] [PubMed] [Google Scholar]
- 70.Usemann J, Yammine S, Singer F, Latzin P. Inert gas washout: background and application in various lung diseases. Swiss Med Wkly. 2017;147:w14483. [DOI] [PubMed] [Google Scholar]
- 71.Gordeuk VR, Castro OL, Machado RF. Pathophysiology and treatment of pulmonary hypertension in sickle cell disease. Blood. 2016;127:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127:750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrington JK, Krishnan U, Jin Z, Mardy C, Kobsa S, Lee MT. Longitudinal Analysis of Echocardiographic Abnormalities in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 2017;39:500–5. [DOI] [PubMed] [Google Scholar]
- 74.Badawy SM, Liem RI, Rigsby CK, Labotka RJ, DeFreitas RA, Thompson AA. Assessing cardiac and liver iron overload in chronically transfused patients with sickle cell disease. Br J Haematol. 2016;175:705–13. [DOI] [PubMed] [Google Scholar]
- 75.Armenian SH, Ryan TD, Khouri MG. Cardiac Dysfunction and Heart Failure in Hematopoietic Cell Transplantation Survivors: Emerging Paradigms in Pathophysiology, Screening, and Prevention. Heart Fail Clin. 2017;13:337–45. [DOI] [PubMed] [Google Scholar]
- 76.Covi S, Ravindranath Y, Farooqi A, Savasan S, Chu R, Aggarwal S. Changes in Bi-ventricular Function After Hematopoietic Stem Cell Transplant as Assessed by Speckle Tracking Echocardiography. Pediatr Cardiol. 2018;39:365–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


