Abstract
The year 2020 marked the thirtieth anniversary of the Nobel Prize in Medicine awarded to E. Donnall Thomas for the development of allogeneic hematopoietic stem cell transplantation (allo-HSCT) to treat hematologic malignancies and other blood disorders. Dr. Thomas, “father of bone marrow transplantation”, first developed and reported this technique in 1957, and in the ensuing decades, this seminal study has impacted fundamental work in hematology and cancer research, including advances in hematopoiesis, stem cell biology, tumor immunology, and T cell biology. As the first example of cancer immunotherapy, understanding the mechanisms of anti-tumor biology associated with allo-HSCT has given rise to many of the principles used today in the development and implementation of novel transformative immunotherapies. Here we review the historical basis underpinning the development of allo-HSCT as well as advances in knowledge obtained by defining mechanisms of allo-HSCT activity. We review how these principles have been translated to novel immunotherapies currently utilized in clinical practice and describe potential future applications for allo-HSCT in cancer research and development of novel therapeutic strategies.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has paved the way for three of the most exciting areas of cancer research: stem cell therapies, immune-modulating techniques and the individualization of cancer therapeutics. After more than 60 years from the first attempted HSCT and with more than one million performed worldwide since (1), allo-HSCT remains the only form of stem cell therapy that is widely clinically available, and the most common form of cellular immunotherapy.
The era of allo-HSCT began in the wake of the first atomic bomb, with the landmark observations that mice could be protected from the lethal effects of radiation by shielding their spleens or femurs with lead (2), or rescued by intravenous infusions of bone marrow (3). While many scientists initially postulated that protection was granted by some “humoral” factor in the spleen or bone marrow able to stimulate recovery, different groups independently demonstrated that radiation protection was due to the transplanted stem cells (4–6). The discovery that protection from radiation could be conferred by stem cell transplant had exciting implications for cell biology and for the therapy of patients with life-threatening hematologic malignancies: it was now possible to treat patients with high doses of chemotherapy or radiotherapy that would be otherwise limited by toxicity to the hematopoietic system. The first suggestion of a donor immune response against leukemia contributing to the overall effect of allogeneic transplantation came in 1956: in murine HSCT attempts, leukemia relapses appeared to be reduced following infusion of allogeneic bone marrow as compared with the syngeneic marrow (7). The following year, the first human allogeneic bone marrow transplantations were reported by E. Donnall Thomas (8). Six patients were treated with myeloablative chemotherapy and radiotherapy, followed by an infusion of bone marrow from a healthy donor. Only two of six patients showed evidence of engraftment and, unfortunately, all died within 100 days of transplantation. Donors and patients were not matched for histocompatibility, as little was known about human histocompatibility antigens at that time. This disappointing result contributed to the general view that the barriers to allogeneic transplantation would never be overcome, causing many researchers, but not Thomas, to abandon the idea that allo-HSCT could be used to treat cancer.
A decade later the discovery of the human leukocyte antigen (HLA) system by Dausset and Van Rood (9) allowed a more careful matching of donor and recipient HLAs, reigniting interest of the scientific community in stem cell transplantation and setting the stage for renewed, and ultimately successful, efforts to treat patients with hematological diseases. In 1965, Mathé reported histocompatibility testing of bone marrow donors for a patient with acute lymphoblastic leukemia (10). The patient was transplanted with a graft derived from six family members (mother, father, 3 brothers and 1 sister) and developed a “secondary syndrome”, later to be recognized as graft versus host disease (GvHD), involving the skin, gastrointestinal tract, and liver. Six months after the transplant, to consolidate an antileukemic effect, an additional bone marrow infusion was performed. To select the donor least likely to exacerbate the secondary syndrome, an elemental histocompatibility test was performed by giving the patient skin grafts from each donor. The donor whose graft survived was selected, but still the allogeneic boost led to a recrudescence of the “secondary syndrome”, which was steroid-responsive.
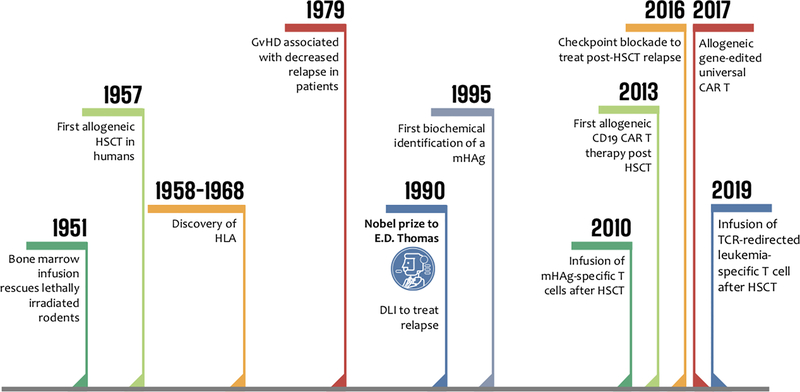
The development of HLA typing techniques for donor-recipient pairs in the mid/late 1960s enabled E. Donnall Thomas to open an allogeneic bone marrow transplantation program in Seattle using HLA-matched donors for patients with acute leukemia, thus configuring the first patient-individualized cancer therapy program. In 1977, they reported 100 transplants from HLA-identical related donors for relapsed and refractory acute leukemia (11). Although only 13 patients were leukemia-free after 1–4.5 years follow-up in this case series, administering transplantation earlier in the course of disease resulted in a cure rate of 50% in patients transplanted in first remission (12). Crucially, Thomas appreciated that the donor immune system played a key role in eliminating residual leukemic cells: although survival was reduced in patients with severe GvHD, most patients did not die of relapse. In 1990, E. Donnall Thomas won a Nobel Prize for his discoveries concerning cell transplantation in the treatment of human disease, laying the foundations of modern cancer immunotherapy (Figure 1). The history of allo-HSCT is one of trials and tribulations, of triumphs, and of perseverance. Here we celebrate E.D. Thomas’s legacy, through five fundamental lessons allo-HSCT has taught us about cancer immunotherapy (Figure 2).
Figure 1: 60 years young: the history of allogeneic HSCT.
Timeline summarizing major milestones in the history of allo-HSCT. HLA: human leukocyte antigen; DLI: donor lymphocyte infusion; mHAg: minor histocompatibility antigen; CAR: chimeric antigen receptor; TCR: T cell receptor.
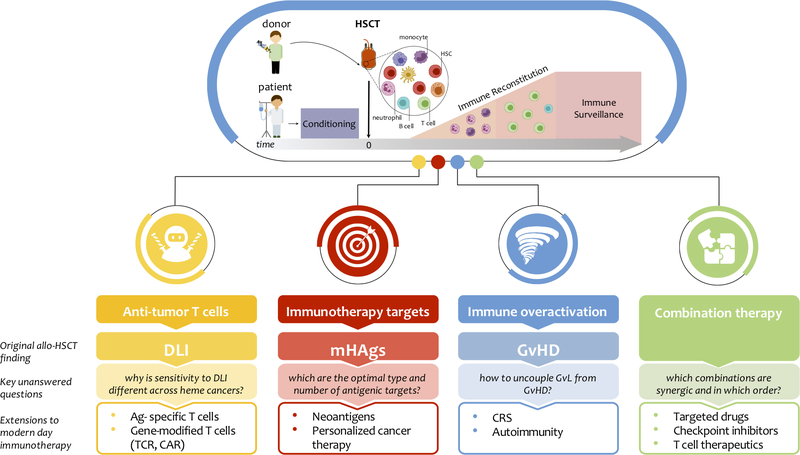
Figure 2: Fundamental lessons and fruits from allo-HSCT.
Top panel: allogeneic HSCT has been the first form of cancer immunotherapy to enter the clinical arena. Hematopoietic stem cells (HSCs) are collected from a donor, while the recipient undergoes conditioning therapy. Upon infusion into the patient, HSCs begin to proliferate and differentiate to repopulate the hematopoietic compartment in a process termed immune reconstitution. The donor-derived immune system is then responsible for long-term immune surveillance against disease recurrence. Bottom panel: allo-HSCT has profoundly influenced cancer immunotherapy in several aspects: for each of them, from top to bottom we report the original allo-HSCT finding, the key unanswered questions in the field and the subsequent extensions to modern day immunotherapy (bullet points). DLI: donor lymphocyte infusion; Ag: antigen; mHAg: minor histocompatibility antigen; GvL: graft vs. leukemia; GvHD: graft vs. host disease; CRS: cytokine release syndrome.
The ABCs of allo-HSCT
The basic principle underpinning allo-HSCT is that of infusing the hematopoietic stem cells (HSCs) and the full immunologic repertoire from a normal donor into a patient to establish donor-derived hematopoiesis and immunity. Originally designed as a stem cell rescue technique, allo-HSCT in its infancy relied on intensive myeloablative conditioning regimens, which limited its applicability to young patients without comorbidities. In the early days of this field, only 25% of patients younger than 35 years of age could be feasibly transplanted with the requirement of using exclusively HLA-identical siblings. In the present day, almost all adult patients in need of a transplant up to 70 years of age may be offered an allo-HSCT, due to several refinements in the transplant procedure. First, while early transplants relied on bone marrow grafts (requiring hospitalization and sedation of the donor), the use of G-CSF to mobilize HSCs to peripheral blood has markedly simplified graft collection (13), such that it is now a minimally-invasive outpatient procedure and provides similar overall survival compared to bone marrow grafts, albeit with a slightly higher incidence of chronic GvHD (14, 15). Second, the increasing appreciation of the graft versus leukemia properties of allo-HSCT allowed the development of reduced-intensity conditioning (RIC) transplants, using lower doses of chemotherapy, which has extended this potentially life-saving treatment to older and frailer patients (16).
Third, we witnessed the expansion of alternative sources of allogeneic HSCs, including unrelated volunteer donors, umbilical cord blood (UCB), and most recently T-replete haploidentical donors. Since the first unrelated donor allo-HSCT in 1979 (17), national donor registries have been successfully established to facilitate registration and identification of potential unrelated donors, as well as collection and transportation of stem cell products across national and international lines. Despite over 10 million HLA-typed registered volunteers worldwide, the chance of finding a suitable donor still poses challenges, particularly for underrepresented populations and for patients in need of a more immediate source of HSCs (18). UCBs can be easily collected after birth, without risks for mothers or donors, banked in dedicated institutions, and available for immediate use. Drawbacks of UCB remain the limited cell dose and slower kinetics of immune reconstitution. Alternatively, for nearly any patient, a first degree haploidentical relative (e.g. parent, child, or partially matched sibling) can be identified. Historically, haploidentical transplants were hampered by high rates of graft failure and severe GvHD, but the introduction of post-transplant cyclophosphamide as an in vivo T cell depletion strategy for GvHD prevention has dramatically improved outcomes of this mode of allo-HSCT (19). It remains unclear whether any of these alternate donor approaches – cord blood, haploidentical, mismatched unrelated – is better than another, and results from ongoing randomized trials are eagerly awaited.
Immunologic impairment is universal after allo-HSCT and commonly persists for several months after transplant, with faster recovery of innate immunity (monocytes, granulocytes, NK cells), followed by a slower recovery of B and T cells, which can take up to 2 years to reconstitute (20). The extent of immunodeficiency and subsequent kinetics of immune reconstitution depend upon many variables, including patient and donor characteristics, graft source, HLA disparity, graft manipulation, and development of GvHD.
Although not the focus of the present review, allo-HSCT represents a curative treatment option also for non-malignant conditions, such as autoimmune diseases and genetic disorders, including hemoglobinopathies and immunodeficiency syndromes, and has set the foundations for the development and clinical application of gene therapy approaches, as comprehensively reviewed elsewhere (21).
Fundamental Lessons and Fruits From HSCT
HSCT as the first consistent human model of effective antitumor immunotherapy
Within a decade of the first successful bone marrow transplants in humans, HLA was identified and its role in typing and histocompatibility began to emerge (10, 22). With this advance in technology, it became possible to HLA type potential bone marrow donors to ensure compatibility. In a study of 100 patients transplanted for acute leukemia, patients who developed GvHD but survived were less likely to die of relapse, and if they did relapse, this tended to occur later in the disease course (11). This provided the first evidence that donor immune responses driving GvHD may also be driving an antileukemia, or graft versus leukemia (GvL) effect (23, 24) and was the first demonstration that the immune system engages in anti-tumor responses, making allo-HSCT the first cancer immunotherapy applied to patients. Additional evidence for an immune-mediated GvL effect included the observation that disease relapse rates increased if donor bone marrow grafts were depleted of T cells in an effort to reduce GvHD (25), that withdrawal of immunosuppressive agents could lead to remission in patients who relapsed after transplant (26), and that some degree of genetic difference between donor and recipient appeared to be protective against relapse (27).
Perhaps the most convincing demonstration of the GvL effect was made in 1990 with the implementation of donor leukocyte infusions (DLI) for treatment of relapsed leukemia following transplant (28). In a landmark study for the field of adoptive immune therapy, three patients with relapsed chronic myeloid leukemia (CML) were infused with interferon-α along with buffy coat preparations from the original bone marrow stem cell donor, resulting in remission of their disease without the use of additional chemotherapy or radiation. Since that time, the use of DLI has become widespread and established for the treatment of relapse after transplant across several hematologic diseases. The GvL effect of DLI is more pronounced in some hematologic malignancies than others, but the underlying mechanisms for these differences remain elusive. CML appears to be the most sensitive to DLI with response rates of up to 90–100% for cytogenetic relapse (29, 30). Unfortunately, however, not all hematological malignancies display the same sensitivity to DLI as CML. However, acute myeloid and lymphoblastic leukemias (AML and ALL, respectively), demonstrate lower response rates to DLI (remission rates of 15–20% and 10–20%, respectively) (31, 32), while aggressive lymphomas and myelomas respond in approximately 30% of cases (33–36).
While DLI is by now an established post-HSCT therapy, the precise mechanisms of its anti-tumor activity remain elusive. Studies in CML have provided the most informative platform thus far for studying how DLI mediates its effects. Analyses of immune reconstitution following DLI have revealed its ability to stimulate both cellular and humoral immunity by leading to increased T and B cell neogenesis, enhanced TCR repertoire diversity, and production of tumor-directed antibodies (37, 38). Furthermore, there is some evidence that DLI stimulates the expansion of pre-existing donor-derived tumor-specific CD8+ T cell responses (39). More recently, we and others, using approaches such as bulk and single cell transcriptome analysis, have demonstrated that a T cell exhaustion phenotype is associated with relapse after transplant, and that DLI is capable of reversing this exhausted phenotype, an effect associated to response to treatment (40–43). Innate immune responses also appear to be critical to an effective anti-tumor response and are stimulated by DLI. Post-DLI plasma from patients with relapsed CML have been shown to have highly upregulated inflammatory cytokines, stimulated by TLR 8/9 activation mediated through tumor-specific immunoglobulin-nucleic acid complexes (44). These data support a critical role for DLI-mediated responses in the arsenal of stem cell therapy tools. However, there remains substantial work to be done in more precisely delineating its mechanisms of activity in harnessing anti-tumor immune responses and defining the molecular determinants of the different susceptibility to DLI of distinct hematologic malignancies to enhance the anti-tumor effect of these therapies.
Other controversial cellular players in the transplant GvL effect are NK cells, innate immune cells capable of distinguishing “self” from “non-self” through killer immunoglobulin-like receptors (KIRs) interacting with KIR ligands on leukemic cells (45). To date, NK cell alloreactivity has proven protective against AML relapse in T cell depleted haploidentical HSCT (45), but definitive proof of NK alloreactivity effectiveness in the context of T cell replete transplants is lacking, although encouraging results from adoptive transfer of donor-derived cytokine-activated NK cells in AML patients relapsing after allo-HSCT have been recently reported (46).
How to tame immune overactivation – the Graft vs Host phenomenon
In the early days of experimentation with transfer of immune cells from one host to another, it was observed that transfer of bone marrow to a lethally irradiated murine host of a different strain led to a clinical syndrome of diarrhea, skin lesions, and wasting within a few weeks after irradiation, and that this process was dependent on genetic differences between donor and host (47, 48). This phenomenon was termed “runt disease” when it was observed that injection of adult lymphoid cells into newborn mice of a different strain led to defects in growth as well as changes in pathology of the spleen, liver, and other organs. The process was determined to result from an immune reaction against the recipient cells by adult donor cells given that: 1) transfer of donor cells did not replicate the disorder, 2) transfer of isologous (genetically identical) cells failed to replicate the phenotype, and 3) the degree of severity of the phenotype was related to the degree of genetic difference between the donor and the host strains (49). Later termed the “secondary syndrome” and now known as graft versus host disease (GvHD), this phenomenon was observed repeatedly in animal models of bone marrow transfer after lethal irradiation (50–54), as well as early attempts at human bone marrow transfer after lethal irradiation gained some early success (55, 56). The study of GvHD, its pathogenesis, and mechanisms of prevention was of utmost interest in the infancy of bone marrow transplantation, as the syndrome frequently led to death of the recipient (10, 57), and continues to be a major area of clinical and basic research.
GvHD is now recognized to encompass two distinct pathologic forms: an acute form that typically occurs early after transplantation and a chronic form that tends to appear later, and the two phenomena are driven by distinct mechanisms (58). Acute GvHD is inflammatory in nature and presents clinically as skin rash, diarrhea, and/or liver inflammation and dysfunction, likely exacerbated by tissue damage and inflammatory changes induced by conditioning chemotherapy regimens peri-transplant. This inflammatory milieu leads to translocation of gut flora and expression of pathogen associated molecular products (PAMPs), resulting in activation of host antigen presenting cells (APCs), expression of inflammatory cytokines, and activation of donor-derived cytotoxic T cells which mediate downstream end-organ damage in the recipient (59–66). This process is thought to be driven primarily by T helper 1 (TH1) and TH17 type immune reactions (67, 68). In contrast, chronic GvHD is more fibrotic in nature, has clinical features more suggestive of autoimmune disorders, and is thought to be primarily mediated by TH2-type cytokine responses. Activation of these cells leads to production of fibrogenic cytokines and is associated with dysregulation of B cell function and skewing toward autoreactive B cell activation (69–72).
The role of immune cells, and of T cells in particular, in mediating pathogenesis of GvHD was recognized early on, as evidenced by two critical observations: first, that matching histocompatibility between donor and recipient resulted in long-term survival of transplanted animals and second, that treating recipient animals with immunosuppressive agents ameliorated GvHD and improved survival (73–77). Methotrexate and cyclosporine were two agents frequently used in early studies of prevention of GvHD in animals. These same techniques were soon applied to transplantation in humans and found to have important clinical benefit in the prevention of GvHD (78–80), with many of these same regimens continuing to be the standard of care. Nevertheless, GvHD continues to be a major cause of post-transplant morbidity and mortality, resulting in death in nearly 15% of transplant patients (81), indicating that there is substantial room for improvement in our current understanding of and strategies for prevention and treatment of GvHD. While treatments for GvHD have made some advances in recent years, the mainstay of therapy continues to be corticosteroids for their anti-inflammatory and immunosuppressive properties (82, 83). There is much interest in developing novel therapeutics and repurposing of agents approved for other inflammatory diseases for treatment of GvHD (84–86). Not surprisingly, the most common complication of DLI is exacerbation of GvHD (87). Methods of manipulating the DLI product to preserve the GvL effect while mitigating the GvHD effect have included depletion of CD8+ T cells in the DLI product, selective depletion of alloreactive T cells (defined by expression of activation markers), and dose escalation of serial DLI infusion, with each strategy achieving modest clinical efficacy (88–91). Administration of immunosuppressive agents after DLI has been shown to significantly reduce GvHD without an appreciable impact on the GvL effect of DLI (92).
Mechanistic insights gleaned from the study of GvHD pathogenesis have made major contributions to basic understanding of the immune system. In particular, studies of T cell mechanism of activity in both acute and chronic GvHD have given rise to an appreciation of the dynamic interplay between cytotoxic T cell responses, tolerogenic versus immunostimulatory roles for both T cells and APCs, T and B cell priming, and remodeling of the immune microenvironment after transplantation leading to autoimmune-like pathophysiology. Several of the inflammatory symptoms associated with GvHD, particularly the acute form, are echoed by toxicities to newer cancer immunotherapies. Common side effects from immune checkpoint blockade (ICB) therapy include colitis, autoimmune hepatitis, and skin rashes, thought to be due to hyperactivated T cells leading to robust cytokine production and cytotoxic T cell activity, similar to what is known about the mechanism of acute GvHD (93, 94). Corticosteroids continue to be the mainstay of treatment for these ICB-associated toxicities, and mechanistic studies in both efficacy and toxicity of ICB therapy reinforce the close relationship between autoimmunity, T cell hyperactivation, and GvHD (95).
Minor Histocompatibility Antigens: the first genomically defined immune targets for personalized cancer immunotherapy
The early observation that GvL and GvHD still occur in patients whose stem cell donors are fully HLA-matched led to the hypothesis that an additional antigen system besides MHC shaped post-transplant immunological reactions. These antigens were originally designated as minor Histocompatibility Antigens (mHAgs) (96), but their nature remained elusive until the mid-nineties, when, fueled by the advances in the understanding of mechanics of T cell recognition (97), pioneering studies demonstrated that mHAgs were polymorphic peptides presented in the context of HLA (98). Today, we know that any non-synonymous variation in the coding region of the genome can potentially result in an immunogenic mHAg after allo-HSCT. Single nucleotide polymorphisms (SNPs) generating amino acid substitutions are the commonest source of known mHAgs (98–101), however base-pair insertions and deletions (indels) (102), as well as frameshifts (103) or copy number variations (104) have been shown to contribute to the mHAg portfolio. There is, however, an upper limit to the number of possible mHAgs for any given donor-recipient pair: first, of the more than least 660,000,000 SNPs and indels in the human genome (105), less than 1% are non-synonymous; second, only non-synonymous SNPs that give rise to mismatches with the correct directionality (recipient homozygous positive or heterozygous, donor homozygous negative for the immunogenic allelic variant) and able to be presented on the available HLA alleles, contribute to the GvL effect. As such, the resulting mHAgs hold the record as the first genomically-identified as well as the first patient-individualized targets for immunotherapy.
Over the decades, the study of mHAgs, initially using laborious and time-consuming T cell expression cloning approaches or biochemical strategies, has been instrumental to our current understanding of the mechanistic basis of the curative potential of allo-HSCT and of the potential source of its toxicities. Indeed, the GvL effect, at least in the HLA-matched transplants, can be conceptualized as the result of the donor-mediated immune responses against mHAgs expressed, though not necessarily limited to hematopoietic cells, while detrimental GvHD depends on the recognition of mHAgs expressed on GvHD-targeted tissues (106).
The discovery of mHAgs encoded by genes preferentially expressed by hematopoietic lineages has long been recognized as a foundation for generating mHAg-based immunotherapy, with the long-sought aim of separating GvL from GvHD effects. Indeed, mHAgs expressed only by hematopoietic cells would allow the selective targeting of the residual or recurrent hematologic malignancies, while the newly reconstituting donor-derived hematopoietic system (i.e. mHAg negative) would remain unharmed. For broad therapeutic application, however, it has been proposed that hematopoietic-specific mHAgs presented by common HLA alleles and with a balanced population prevalence should be prioritized in order to maximize the chances of finding targetable disparities between donor and patient pairs (107). Disappointingly though, the quest for such a panel of ideal mHAgs to date has resulted only in the identification of a handful of targets, some of which have been tested in clinical trials. These have resulted in diverse mHAg-directed immunotherapeutic approaches to either prevent or treat post-HSCT relapse, and have included the infusion of ex vivo expanded (108, 109) or TCR-redirected (110, 111) mHAg-specific T cells, as well as vaccination using dendritic cells loaded with mHAg peptides (112, 113) or mRNAs (NCT02528682). In general, until now, the exploitation of mHAgs in the clinical arena has revealed itself to be more challenging than anticipated due to the inherent difficulty in finding donor-recipient pairs not only suitably mHAg-mismatched but also carrying the appropriate HLA restrictions; thus, studies to target mHAgs have generally resulted in slow enrollment or even premature termination due to poor accrual (NCT00943293).
The discovery of targetable mHAgs is currently undergoing reinvigoration due to the expansion of innovative genomic capabilities available to dissect human samples. The understanding that genetic alterations could result in immunogenic epitopes, illustrated already more than 2 decades ago by mHAgs, was one of the early contributing sources of evidence that encouraged the development of genomics-based approaches for the discovery of tumor neoantigens which are now increasingly the subject of immunotherapeutic targeting for cancers (114). Indeed, the innovations in genomic technology to predict and identify neoantigens are now circling back and fueling new interest in more systematically identifying mHAgs from donor-recipient pairs. While neoantigens are the immunological byproduct of tumor-specific mutations (115), mHAgs are the immunological end result of germline genetic polymorphisms. Hence, the only substantial difference between them is that, at the genetic level, mHAgs are inherited while neoantigens are somatic events; both otherwise conform to the same rules for gene expression, antigen processing and HLA presentation. From the perspective of donor T cells, mHAgs and neoantigens are both sensed as foreign, and therefore there is no thymic negative selection for high-affinity T cell clones (116), and hence mHAg-encoded epitopes, like neoantigens, would be expected to be highly immunogenic. Among the in silico tools available for analysis of genomic data to discover mHAg are included tools to systematically identify germline differences between DNA sequences from donor and recipient, tools for tissue expression profiling (single cell expression atlas (117), GTEx (118, 119), the human Proteome Atlas (120)), algorithms for peptide processing and binding, such as IEDB (121), netMHC (122) and HLAthena (123), and tools for the multidimensional detection of antigen-specific T cells (124). Altogether, these capabilities have, in recent years, all contributed to the establishment of robust pipelines for not only the prediction and selection of neoantigen-derived HLA class I epitopes, which have formed the basis for personal neoantigen-targeting vaccines (125–127), but also for mHAg discovery, with growing attempts at their large-scale prediction (128, 129). Of the predicted mHAgs, only a few have been validated thus far, demonstrating that these approaches require more development in order to fully realize the potency of these candidate targets (130). Assuredly, however, such methods will allow the future identification of novel mHAgs, and as in an analogous fashion as for tumor neoantigens, will form the basis for systematic personalized immunotherapy following allo-HSCT.
From GvL to novel cellular and immunomodulatory therapies to unleash the full power of T cells
Inspired by the GvL effect first recognized in the setting of stem cell transplant, innovative adoptive T cell therapies have made major strides in recent years, with many novel therapeutics demonstrating clear clinical benefit in a variety of malignancies (131–133). Strikingly, most of these advances have been in diseases where transplantation is not the mainstay of treatment.
These new cellular therapies have included the bi-specific T cell engagers (BiTEs) and the chimeric antigen receptor (CAR) T cells. BiTEs directly link CD3 on T cells with a tumor-specific antigen in order to overcome an immunosuppressive tumor microenvironment. This results in direct coupling of tumor cells with T-cells, leading to T-cell activation, proliferation, and anti-tumor cytotoxicity (134). Blinatumomab is a BiTE recognizing CD3 and CD19, a marker specific for B-lineage cells and commonly found on B cell malignancies. Clinical studies have shown considerable efficacy blinatumomab in maintaining remission in B cell acute lymphoblastic leukemia after relapse or in the presence of minimal residual disease (135, 136). Many novel therapeutics extending and enhancing features of BiTEs are currently under development, including bifunctional checkpoint-inhibitory T cell engagers (CiTEs), trispecific killer engagers (TriKEs), and bispecific constructs utilizing T cell receptor based moieties (137).
CAR-T cells employ ex vivo manipulation of autologous or allogeneic T cells engineered with T cell receptor (TCR) specificity to tumor antigens. This specificity allows CAR-T cells to bypass the MHC restriction required for endogenous anti-tumor T cell activity, and to recognize a specific tumor antigen, resulting in CAR-T cell expansion, direct cytotoxicity, and the potential for long-lasting memory responses (133, 138, 139). While first generation CAR-T cell therapy was limited in efficacy, the subsequent addition of co-stimulatory domains in second- and third-generation CAR-T constructs has resulted in impressive clinical responses in a variety of B-cell malignancies (140). CD19 targeting CAR-T cells have been the first targeted cellular therapy to demonstrate significant clinical impact, first in CLL, then B-ALL (138, 141), and ultimately gaining approval for clinical use for refractory large B-cell lymphoma and B-ALL in children and young adults (142–144). There is certainly intense interest in adapting similar therapies to be used for myeloid malignancies, but for now, the optimal design of such therapeutics remains elusive.
The study of T cell anti-tumor biology in HSCT has further contributed to the investigations into the many regulatory molecules expressed on T cells. Immune checkpoint blockade has long been recognized to play a role in GvHD and GvL effects in animal models of bone marrow transplant. Studies in mice have shown an important role for cytotoxic T-lymphocyte associated protein-4 (CTLA-4) and its homologous T cell co-stimulatory protein CD28 in mediating both GvL and GvHD effects after bone marrow transplantation (145, 146). Inhibition of CD28 was shown to effectively reduce GvHD mortality and augment anti-tumor T cell responses, and selective blockade of CTLA-4 enhanced the GvL effect in a model of delayed donor lymphocyte infusions while accelerating mortality due to GvHD. (146). Further, blockade of programmed death-1 (PD-1) was also shown to augment lethality due to GvHD (147). Despite the potential for checkpoint blockade to exacerbate GvHD suggested by these preclinical studies, several lines of evidence have also pointed to the potential beneficial role of checkpoint inhibition in hematologic malignancies. Murine leukemia cell lines demonstrate upregulated expression of PD-L1, the cognate ligand for PD-1, in vivo, and mice treated with anti-PD-L1 have enhanced anti-tumor T cell responses (148). Studies in a murine model of CML showed that disease-specific cytotoxic T cells with an exhausted phenotype upregulate PD-L1, leading to disease progression (149). Inhibition of PD-1/PD-L1 interaction reversed this phenotype and restored function of anti-tumor cytotoxic T cells.
Such promising preclinical work combined with the established role for PD-1 and CTLA-4 blockade across human cancer types has led to recent clinical investigations exploring the role of checkpoint inhibition in the treatment of relapsed hematologic malignancies after HSCT. In this setting, blockade of CTLA-4 with ipilimumab has been shown to result in objective disease response without development of significant GvHD or other treatment related toxicity (150). A subsequent phase I/Ib trial found that ipilimumab led to some durable responses of relapsed hematologic disease after allo-HSCT although with some immune-mediated toxicity and GvHD (151). Responses to ipilimumab treatment were associated with infiltration of cytotoxic CD8+ T cells into the tumor microenvironment, reduced activity of regulatory T cells, and expansion of effector T cells in peripheral blood samples of patients. Optimal use of checkpoint blockade in hematologic malignancies before, during, and after stem cell transplant remains an area of active investigation, aided significantly by a basic understanding of the role of these pathways in mediating anti-tumor immune responses.
The immunological pressure exerted by the donor-derived immune system could also trigger immune evasion mechanisms ultimately leading to disease relapse. Several mechanisms of post-transplant immune escape have been described, including genomic loss of mismatched HLA after mismatched HSCT. In this setting, recurrent leukemic cells lose, through acquired uniparental disomy of chromosome 6p, the mismatched HLA alleles, therefore abrogating recognition by alloreactive donor T cells (152). Initially described in the haploidentical setting, where it accounts for a third of relapses (153), this mechanism has been documented also for unrelated donor transplants (154–156). Non genomic mechanisms of immune evasion include, among others, transcriptional silencing of HLA class II expression and deregulation of costimulatory molecules (157, 158) or production of lactic acid (159). Intriguingly, metabolic reprogramming through the administration of sodium bicarbonate restored GvL activity, suggesting that metabolic fine-tuning of donor T cells may provide antileukemia benefit. Certainly, much remains to be explored in defining and honing the GvL effect of donor immune cells to prevent and treat relapsed disease.
HSCT as a springboard for the rational design of combinatorial immunotherapies
Allogeneic HSCT has extended the lives of innumerable hematologic malignancy patients worldwide, yet relapse of the original malignancy remains the most frequent cause of treatment failure and mortality (160). In efforts to prevent or treat recurrent disease, numerous therapeutic avenues have been explored with the aim of either directly modifying the tumor or indirectly altering the microenvironment to sensitize resistant disease to allogeneic immune elements. In many respects, allo-HSCT can be considered one of the first examples of effective combination immunotherapy – which by now is also a central tenet of cancer immunotherapy in the non-transplant setting – bringing together cytoreduction with coordinated humoral and cellular immunity. At the same time, this complex therapy also serves as an inviting launching point for additional interventions. Indeed, the post-transplant setting represents a versatile platform for immune intervention, with the allogeneic background offering several advantages for reinvigorating the anti-tumor immune response. A crucial aspect of this setting is the fact that the reconstituted donor T cells have not been exposed to the immune suppressive tumor microenvironment, nor to chemotherapy, thereby resulting in the possibility that they are more amenable to in vivo manipulation or ex vivo gene modification (161, 162). In addition, the T cell homeostatic cytokine milieu immediately after stem cell transplantation has been shown to provide favorable conditions for T cell expansion (163, 164).
With the remarkable expansion of the arsenal of mechanistically driven therapeutic options for hematologic malignancies, it has become evident that maintenance therapies might be crucial to sustain and boost the immunotherapeutic effects of allo-HSCT (165, 166). These can include the sequential administration of antigen-specific or whole tumor cell cancer vaccines (167–170), monoclonal antibodies (such as Inotuzumab (NCT03104491), Blinatumumab (171), or gemtuzumab ozogamycin (172, 173), cell-based therapeutics including but not limited to DLI (162, 174, 175) or additional use of targeted drugs. In the last two decades, drug development has taken huge steps forward, with many new molecules developed and marketed for the blood malignancies (176). Some of these have broader activity, such as hypomethylating agents (175, 177, 178) or venetoclax (179), while others possess a narrower scope, such as FLT3 inhibitors for AML (which notably displayed a potent synergistic effect with DLI in the treatment of post-HSCT relapse) (180–183), JAK2 inhibitors for myeloproliferative disorders (184), Tyrosine Kinase inhibitors (TKIs) for Philadelphia positive ALL (185) or Bruton Tyrosine Kinase inhibitors for CLL (186, 187), as a few examples. With the notable exception of TKIs for CML, none of the new drugs have been shown to fully eliminate the need for allo-HSCT and its immunotherapeutic effects, but rather improve bridging to transplantation for high-risk patients, and the optimal timing of allo-HSCT in the setting on these novel agents remains the focus of ongoing clinical investigation.
A major challenge to the success of targeted therapies is development of resistance, as documented for most small molecules that have entered the clinical arena (188). Indeed, clonal heterogeneity and evolution render the tumor a moving target which might be difficult to combat with just one “weapon”, no matter how precise. In this respect, allo-HSCT could complement the selectivity of targeted drugs with the broader, though less precise, effects of polyclonal T cell alloreactivity, potentially improving disease control, as shown by the promising results of dual targeting of FLT3 mutated AML with sorafenib and DLI (180). This could represent a dual approach model for precision cancer medicine, laying the foundation for delineating which therapeutic agents might have the best synergistic effects when used in combination.
Conclusions
As the success of more sophisticated cellular therapies such as CAR-T cells continues to expand, one may wonder whether the genetically engineered precision of CAR-T therapy will one day obviate the need for the nonspecific alloreactivity of traditional allo-HSCT. Despite the unprecedented remission rate and controllable side effects, disease relapse after CAR-T cell therapy remains a considerable hurdle (189, 190). Early loss or exhaustion of CAR-T cells, selection of antigen-negative clones or downregulation of target expression, lineage switching of leukemia, and tumor microenvironment are all important factors now identified as contributing to relapse after CAR-T cell therapy (191). With this respect, CAR-T cell therapy could be envisioned as an effective and safe method to induce complete responses – especially in the challenging context of refractory disease – which could then be consolidated with allo-HSCT. Notably, several studies have affirmed that ALL patients receiving consolidative allo-HSCT have longer leukemia-free survival than those receiving CAR-T cell therapy alone (192–194). Whether the current surge of CAR-T therapy in other disease settings will herald the decline of the more than 60 years of allo-HSCT practice remains to be seen. Indeed, allo-HSCT and CAR-T are tightly intertwined, with lessons being constantly translated from one platform to the other, such as the management of cytokine release syndrome, the benefits of lymphodepletion, and the potential for “off-the-shelf” allogeneic universal CAR-T cells (195). Perhaps, the best testament to the potential for synergic use of CAR-T and HSCT comes from the AML setting, where researchers are trying to overcome the lack of suitable CAR targets by genetically engineering the allograft to remove a candidate surface antigen, such as CD33, from the normal hematopoietic system and transplanting this allograft in sequence with donor-derived CAR-T cells against CD33 (196). Although the coupling of CAR-T with next generation engineered allo-HSCT is still in the preclinical phase of development, it is incredibly exciting that we have reached a point where we can even envision such innovative combination strategies, and we like to think that E.D. Thomas would share our enthusiasm for the unrelenting progress of the HSCT field.
Acknowledgments
This work was supported by NIH grant P01CA229092 (C.J. Wu). N. Cieri is supported by an AACR Immuno-Oncology fellowship (20-40-46-CIER). K. Maurer is supported by NIH/NHLBI (T32HL116324).
Conflicts of Interest: C.J.W. holds equity in BioNTech, Inc, and receives research funding from Pharmacyclics. The authors declare no other relevant financial disclosures or conflicts of interest.
References
- 1.Niederwieser D, Baldomero H, Atsuta Y, Aljurf M, Seber A, Greinix HT, et al. One and Half Million Hematopoietic Stem Cell Transplants (HSCT). Dissemination, Trends and Potential to Improve Activity By Telemedicine from the Worldwide Network for Blood and Marrow Transplantation (WBMT). Blood. 2019;134(Supplement_1):2035-. [Google Scholar]
- 2.Jacobson LO, Marks EK, et al. The role of the spleen in radiation injury. Proc Soc Exp Biol Med. 1949;70(4):740–2. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz E, Uphoff D, Reid TR, Shelton E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J Natl Cancer Inst. 1951;12(1):197–201. [PubMed] [Google Scholar]
- 4.Ford CE, Hamerton JL, Barnes DW, Loutit JF. Cytological identification of radiation-chimaeras. Nature. 1956;177(4506):452–4. [DOI] [PubMed] [Google Scholar]
- 5.Main JM, Prehn RT. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst. 1955;15(4):1023–9. [PubMed] [Google Scholar]
- 6.Trentin JJ. Mortality and skin transplantability in x-irradiated mice receiving isologous, homologous or heterologous bone marrow. Proc Soc Exp Biol Med. 1956;92(4):688–93. [DOI] [PubMed] [Google Scholar]
- 7.Barnes DW, Corp MJ, Loutit JF, Neal FE. Treatment of murine leukaemia with X rays and homologous bone marrow; preliminary communication. Br Med J. 1956;2(4993):626–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas ED, Lochte HL Jr., Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–6. [DOI] [PubMed] [Google Scholar]
- 9.Thorsby E A short history of HLA. Tissue Antigens. 2009;74(2):101–16. [DOI] [PubMed] [Google Scholar]
- 10.Mathe G, Amiel JL, Schwarzenberg L, Cattan A, Schneider M, Devries MJ, et al. Successful Allogenic Bone Marrow Transplantation in Man: Chimerism, Induced Specific Tolerance and Possible Anti-Leukemic Effects. Blood. 1965;25:179–96. [PubMed] [Google Scholar]
- 11.Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49(4):511–33. [PubMed] [Google Scholar]
- 12.Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL, Neiman PE, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N Engl J Med. 1979;301(11):597–9. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz N, Bacigalupo A, Labopin M, Majolino I, Laporte JP, Brinch L, et al. Transplantation of peripheral blood progenitor cells from HLA-identical sibling donors. European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 1996;95(4):715–23. [DOI] [PubMed] [Google Scholar]
- 14.Pulsipher MA, Chitphakdithai P, Logan BR, Navarro WH, Levine JE, Miller JP, et al. Lower risk for serious adverse events and no increased risk for cancer after PBSC vs BM donation. Blood. 2014;123(23):3655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–400. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JA, Clift RA, Thomas ED, Buckner CD, Storb R, Giblett ER. Transplantation of marrow from an unrelated donor to a patient with acute leukemia. N Engl J Med. 1980;303(10):565–7. [DOI] [PubMed] [Google Scholar]
- 18.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30(4):425–37. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari G, Thrasher AJ, Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nat Rev Genet. 2021;22(4):216–34. [DOI] [PubMed] [Google Scholar]
- 22.Dausset J [Iso-leuko-antibodies]. Acta Haematol. 1958;20(1–4):156–66. [DOI] [PubMed] [Google Scholar]
- 23.Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300(19):1068–73. [DOI] [PubMed] [Google Scholar]
- 24.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED, Seattle Marrow Transplant T. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–33. [DOI] [PubMed] [Google Scholar]
- 25.Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78(8):2120–30. [PubMed] [Google Scholar]
- 26.Higano CS, Brixey M, Bryant EM, Durnam DM, Doney K, Sullivan KM, et al. Durable complete remission of acute nonlymphocytic leukemia associated with discontinuation of immunosuppression following relapse after allogeneic bone marrow transplantation. A case report of a probable graft-versus-leukemia effect. Transplantation. 1990;50(1):175–7. [PubMed] [Google Scholar]
- 27.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–62. [PubMed] [Google Scholar]
- 28.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–5. [PubMed] [Google Scholar]
- 29.Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11(4):473–87. [DOI] [PubMed] [Google Scholar]
- 30.Raiola AM, Van Lint MT, Valbonesi M, Lamparelli T, Gualandi F, Occhini D, et al. Factors predicting response and graft-versus-host disease after donor lymphocyte infusions: a study on 593 infusions. Bone Marrow Transplant. 2003;31(8):687–93. [DOI] [PubMed] [Google Scholar]
- 31.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25(31):4938–45. [DOI] [PubMed] [Google Scholar]
- 32.Porter DL, Collins RH Jr., Hardy C, Kernan NA, Drobyski WR, Giralt S, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000;95(4):1214–21. [PubMed] [Google Scholar]
- 33.Peggs KS, Kayani I, Edwards N, Kottaridis P, Goldstone AH, Linch DC, et al. Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2011;29(8):971–8. [DOI] [PubMed] [Google Scholar]
- 34.Bishop MR, Dean RM, Steinberg SM, Odom J, Pavletic SZ, Chow C, et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol. 2008;19(11):1935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell NH, Byrne JL, Faulkner RD, Gilyead M, Das-Gupta EP, Haynes AP. Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36(5):437–41. [DOI] [PubMed] [Google Scholar]
- 36.Lokhorst HM, Wu K, Verdonck LF, Laterveer LL, van de Donk NW, van Oers MH, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103(11):4362–4. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Choi J, Zeng W, Rogers SA, Alyea EP, Rheinwald JG, et al. Graft-versus-leukemia antigen CML66 elicits coordinated B-cell and T-cell immunity after donor lymphocyte infusion. Clin Cancer Res. 2010;16(10):2729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orsini E, Alyea EP, Schlossman R, Canning C, Soiffer RJ, Chillemi A, et al. Changes in T cell receptor repertoire associated with graft-versus-tumor effect and graft-versus-host disease in patients with relapsed multiple myeloma after donor lymphocyte infusion. Bone Marrow Transplant. 2000;25(6):623–32. [DOI] [PubMed] [Google Scholar]
- 39.Orsini E, Bellucci R, Alyea EP, Schlossman R, Canning C, McLaughlin S, et al. Expansion of tumor-specific CD8+ T cell clones in patients with relapsed myeloma after donor lymphocyte infusion. Cancer Res. 2003;63(10):2561–8. [PubMed] [Google Scholar]
- 40.Bachireddy P, Hainz U, Rooney M, Pozdnyakova O, Aldridge J, Zhang W, et al. Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123(9):1412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15(4):201–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Chang YJ, Xu LP, Zhang XH, Wang Y, Liu KY, et al. Reversal of T Cell Exhaustion by the First Donor Lymphocyte Infusion Is Associated with the Persistently Effective Antileukemic Responses in Patients with Relapsed AML after Allo-HSCT. Biol Blood Marrow Transplant. 2018;24(7):1350–9. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Chang YJ, Xu LP, Zhang XH, Wang Y, Liu KY, et al. T cell exhaustion characterized by compromised MHC class I and II restricted cytotoxic activity associates with acute B lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell transplantation. Clin Immunol. 2018;190:32–40. [DOI] [PubMed] [Google Scholar]
- 44.Lin Y, Zhang L, Cai AX, Lee M, Zhang W, Neuberg D, et al. Effective posttransplant antitumor immunity is associated with TLR-stimulating nucleic acid-immunoglobulin complexes in humans. J Clin Invest. 2011;121(4):1574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro RM, Nikiforow S, Rambaldi B, Vergara J, Daley H, Kim HT, et al. Cytokine-Induced Memory-like NK Cells Exhibit Massive Expansion and Long-Term Persistence after Infusion Post-Haploidentical Stem Cell Transplantation: A Report of the First Three Cases in a Phase I Trial. Blood. 2020;136(Supplement 1):8–9.32614959 [Google Scholar]
- 47.Barnes DW, Loutit JF, Micklem HS. “Secondary disease” of radiation chimeras: a syndrome due to lymphoid aplasia. Ann N Y Acad Sci. 1962;99:374–85. [DOI] [PubMed] [Google Scholar]
- 48.Uphoff DE. Genetic factors influencing irradiation protection by bone marrow. I. The F1 hybrid effect. J Natl Cancer Inst. 1957;19(1):123–30. [PubMed] [Google Scholar]
- 49.Billingham RE, Brent L. Quantitative studies on tissue transplantation immunity IV. Induction of tolerance in newborn mice and studies on the phenomenon of runt disease. Phil Trans R Soc Lon. 1959;242(477). [Google Scholar]
- 50.de VM, Crouch BG, van PL, van BD. Pathologic changes in irradiated monkeys treated with bone marrow. J Natl Cancer Inst. 1961;27:67–97. [PubMed] [Google Scholar]
- 51.Loutit JF, Micklem HS. “Secondary disease” among lethally irradiated mice restored with haematopoietic tissues from normal or iso-immunized foreign mice. Br J Exp Pathol. 1962;43:77–87. [PMC free article] [PubMed] [Google Scholar]
- 52.Simonsen M The impact on the developing embryo and newborn animal of adult homologous cells. Acta Pathol Microbiol Scand. 1957;40(6):480–500. [PubMed] [Google Scholar]
- 53.Woodruff JM, Eltringham JR, Casey HW. Early secondary disease in the Rhesus monkey. I. A comparative histopathologic study. Lab Invest. 1969;20(6):499–511. [PubMed] [Google Scholar]
- 54.Balner H, De Vries MJ, Van B. Secondary Disease in Rat Radiation Chimeras. J Natl Cancer Inst. 1964;32:419–59. [PubMed] [Google Scholar]
- 55.Mathe G, Bernard J, de VM, Schwarzenberg L, Larrieu MJ, Lalanne CM, et al. [New trials with homologous bone marrow grafts after total irradiation in children with acute leukemia in remission. The problem of the secondary syndrome in man]. Rev Hematol. 1960;15:115–61. [PubMed] [Google Scholar]
- 56.Kersey JH, Meuwissen HJ, Good RA. Graft versus host reactions following transplantation of allogeneic hematopoietic cells. Hum Pathol. 1971;2(3):389–402. [DOI] [PubMed] [Google Scholar]
- 57.Storb R, Epstein RB, Bryant J, Ragde H, Thomas ED. Marrow grafts by combined marrow and leukocyte infusions in unrelated dogs selected by histocompatibility typing. Transplantation. 1968;6(4):587–93. [DOI] [PubMed] [Google Scholar]
- 58.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. [PubMed] [Google Scholar]
- 60.Broady R, Yu J, Chow V, Tantiworawit A, Kang C, Berg K, et al. Cutaneous GVHD is associated with the expansion of tissue-localized Th1 and not Th17 cells. Blood. 2010;116(25):5748–51. [DOI] [PubMed] [Google Scholar]
- 61.Murphy WJ, Welniak LA, Taub DD, Wiltrout RH, Taylor PA, Vallera DA, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102(9):1742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105(9):1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–9. [PubMed] [Google Scholar]
- 64.Calcaterra C, Sfondrini L, Rossini A, Sommariva M, Rumio C, Menard S, et al. Critical role of TLR9 in acute graft-versus-host disease. J Immunol. 2008;181(9):6132–9. [DOI] [PubMed] [Google Scholar]
- 65.Penack O, Holler E, van den Brink MR. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115(10):1865–72. [DOI] [PubMed] [Google Scholar]
- 66.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59(8):1079–87. [DOI] [PubMed] [Google Scholar]
- 67.Ratajczak P, Janin A, Peffault de Latour R, Leboeuf C, Desveaux A, Keyvanfar K, et al. Th17/Treg ratio in human graft-versus-host disease. Blood. 2010;116(7):1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70. [DOI] [PubMed] [Google Scholar]
- 69.Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16(2):289–95. [PubMed] [Google Scholar]
- 71.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105(5):2227–34. [DOI] [PubMed] [Google Scholar]
- 72.Shlomchik WD. Antigen presentation in graft-vs-host disease. Exp Hematol. 2003;31(12):1187–97. [DOI] [PubMed] [Google Scholar]
- 73.Thomas ED, Kasakura S, Cavins JA, Swisher SN, Ferrebee JW. Significance of Blood Groups in Homotransplantation of Marrow in the Dog. Ann N Y Acad Sci. 1964;120:362–6. [DOI] [PubMed] [Google Scholar]
- 74.Thomas ED, Collins JA, Herman EC Jr., Ferrebee JW. Marrow transplants in lethally irradiated dogs given methotrexate. Blood. 1962;19:217–28. [PubMed] [Google Scholar]
- 75.Epstein RB, Storb R, Clift RA, Thomas ED. Transplantation of stored allogeneic bone marrow in dogs selected by histocompatibility typing. Transplantation. 1969;8(4):496–501. [DOI] [PubMed] [Google Scholar]
- 76.Epstein RB, Storb R, Clift RA, Thomas ED. Autologous bone marrow grafts in dogs treated with lethal doses of cyclophosphamide. Cancer Res. 1969;29(5):1072–5. [PubMed] [Google Scholar]
- 77.Epstein RB, Storb R, Ragde H, Thomas ED. Cytotoxic typing antisera for marrow grafting in littermate dogs. Transplantation. 1968;6(1):45–58. [DOI] [PubMed] [Google Scholar]
- 78.Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, et al. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68(1):119–25. [PubMed] [Google Scholar]
- 79.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729–35. [DOI] [PubMed] [Google Scholar]
- 80.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–8. [PubMed] [Google Scholar]
- 81.Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl. 2010:87–105. [PubMed] [Google Scholar]
- 82.Cutler CS, Koreth J, Ritz J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood. 2017;129(1):22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus Conference on Clinical Practice in Chronic GVHD: Second-Line Treatment of Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2011;17(1):1–17. [DOI] [PubMed] [Google Scholar]
- 84.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med. 2020;382(19):1800–10. [DOI] [PubMed] [Google Scholar]
- 85.Floisand Y, Lazarevic VL, Maertens J, Mattsson J, Shah NN, Zachee P, et al. Safety and Effectiveness of Vedolizumab in Patients with Steroid-Refractory Gastrointestinal Acute Graft-versus-Host Disease: A Retrospective Record Review. Biol Blood Marrow Transplant. 2019;25(4):720–7. [DOI] [PubMed] [Google Scholar]
- 86.Ganetsky A, Frey NV, Hexner EO, Loren AW, Gill SI, Luger SM, et al. Tocilizumab for the treatment of severe steroid-refractory acute graft-versus-host disease of the lower gastrointestinal tract. Bone Marrow Transplant. 2019;54(2):212–7. [DOI] [PubMed] [Google Scholar]
- 87.Marks DI, Lush R, Cavenagh J, Milligan DW, Schey S, Parker A, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002;100(9):3108–14. [DOI] [PubMed] [Google Scholar]
- 88.Soiffer RJ, Alyea EP, Hochberg E, Wu C, Canning C, Parikh B, et al. Randomized trial of CD8+ T-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol Blood Marrow Transplant. 2002;8(11):625–32. [DOI] [PubMed] [Google Scholar]
- 89.Mackinnon S, Papadopoulos EB, Carabasi MH, Reich L, Collins NH, Boulad F, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86(4):1261–8. [PubMed] [Google Scholar]
- 90.Bloor AJ, Thomson K, Chowdhry N, Verfuerth S, Ings SJ, Chakraverty R, et al. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(1):50–8. [DOI] [PubMed] [Google Scholar]
- 91.Hartwig UF, Nonn M, Khan S, Link I, Huber C, Herr W. Depletion of alloreactive donor T lymphocytes by CD95-mediated activation-induced cell death retains antileukemic, antiviral, and immunoregulatory T cell immunity. Biol Blood Marrow Transplant. 2008;14(1):99–109. [DOI] [PubMed] [Google Scholar]
- 92.Huang XJ, Wang Y, Liu DH, Xu LP, Liu KY, Chen H, et al. Administration of short-term immunosuppressive agents after DLI reduces the incidence of DLI-associated acute GVHD without influencing the GVL effect. Bone Marrow Transplant. 2009;44(5):309–16. [DOI] [PubMed] [Google Scholar]
- 93.Oh DY, Cham J, Zhang L, Fong G, Kwek SS, Klinger M, et al. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-cell Repertoire. Cancer Res. 2017;77(6):1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20(9):2424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23(2):211–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goulmy E Minor histocompatibility antigens: allo target molecules for tumor-specific immunotherapy. Cancer J. 2004;10(1):1–7. [DOI] [PubMed] [Google Scholar]
- 97.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987;329(6139):512–8. [DOI] [PubMed] [Google Scholar]
- 98.den Haan JM, Sherman NE, Blokland E, Huczko E, Koning F, Drijfhout JW, et al. Identification of a graft versus host disease-associated human minor histocompatibility antigen. Science. 1995;268(5216):1476–80. [DOI] [PubMed] [Google Scholar]
- 99.den Haan JM, Meadows LM, Wang W, Pool J, Blokland E, Bishop TL, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279(5353):1054–7. [DOI] [PubMed] [Google Scholar]
- 100.Spierings E, Brickner AG, Caldwell JA, Zegveld S, Tatsis N, Blokland E, et al. The minor histocompatibility antigen HA-3 arises from differential proteasome-mediated cleavage of the lymphoid blast crisis (Lbc) oncoprotein. Blood. 2003;102(2):621–9. [DOI] [PubMed] [Google Scholar]
- 101.Griffioen M, van Bergen CA, Falkenburg JH. Autosomal Minor Histocompatibility Antigens: How Genetic Variants Create Diversity in Immune Targets. Front Immunol. 2016;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Rijke B, van Horssen-Zoetbrood A, Beekman JM, Otterud B, Maas F, Woestenenk R, et al. A frameshift polymorphism in P2X5 elicits an allogeneic cytotoxic T lymphocyte response associated with remission of chronic myeloid leukemia. J Clin Invest. 2005;115(12):3506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brickner AG, Evans AM, Mito JK, Xuereb SM, Feng X, Nishida T, et al. The PANE1 gene encodes a novel human minor histocompatibility antigen that is selectively expressed in B-lymphoid cells and B-CLL. Blood. 2006;107(9):3779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murata M, Warren EH, Riddell SR. A human minor histocompatibility antigen resulting from differential expression due to a gene deletion. J Exp Med. 2003;197(10):1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lappalainen T, Scott AJ, Brandt M, Hall IM. Genomic Analysis in the Age of Human Genome Sequencing. Cell. 2019;177(1):70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Bueger M, Bakker A, Van Rood JJ, Van der Woude F, Goulmy E. Tissue distribution of human minor histocompatibility antigens. Ubiquitous versus restricted tissue distribution indicates heterogeneity among human cytotoxic T lymphocyte-defined non-MHC antigens. J Immunol. 1992;149(5):1788–94. [PubMed] [Google Scholar]
- 107.Warren EH. Diversifying the MHC peptide portfolio. Blood. 2012;120(16):3165–7. [DOI] [PubMed] [Google Scholar]
- 108.Warren EH, Fujii N, Akatsuka Y, Chaney CN, Mito JK, Loeb KR, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115(19):3869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meij P, Jedema I, van der Hoorn MA, Bongaerts R, Cox L, Wafelman AR, et al. Generation and administration of HA-1-specific T-cell lines for the treatment of patients with relapsed leukemia after allogeneic stem cell transplantation: a pilot study. Haematologica. 2012;97(8):1205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dossa RG, Cunningham T, Sommermeyer D, Medina-Rodriguez I, Biernacki MA, Foster K, et al. Development of T-cell immunotherapy for hematopoietic stem cell transplantation recipients at risk of leukemia relapse. Blood. 2018;131(1):108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krakow EF SC, Dahlberg A, Bar M, Biernacki MA, Cunningham T, Vartanian N, Hickner M, Chaney C, Habtetsion T, Woodward K, Dossa R, Brault M, Bleadley M. Phase I Study of Adoptive Immunotherapy with HA-1-Specific CD8+ and CD4+ Memory T Cells for Children and Adults with Relapsed Acute Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation (HCT): Trial in Progress [Conference Presentation]. 62nd ASH Annual Meeting and Exposition. 2020. [Google Scholar]
- 112.Oostvogels R, Kneppers E, Minnema MC, Doorn RC, Franssen LE, Aarts T, et al. Efficacy of host-dendritic cell vaccinations with or without minor histocompatibility antigen loading, combined with donor lymphocyte infusion in multiple myeloma patients. Bone Marrow Transplant. 2017;52(2):228–37. [DOI] [PubMed] [Google Scholar]
- 113.Franssen LE, Roeven MWH, Hobo W, Doorn R, Oostvogels R, Falkenburg JHF, et al. A phase I/II minor histocompatibility antigen-loaded dendritic cell vaccination trial to safely improve the efficacy of donor lymphocyte infusions in myeloma. Bone Marrow Transplant. 2017;52(10):1378–83. [DOI] [PubMed] [Google Scholar]
- 114.Chapman M, Warren EH 3rd, Wu CJ. Applications of next-generation sequencing to blood and marrow transplantation. Biol Blood Marrow Transplant. 2012;18(1 Suppl):S151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schumacher TN, Scheper W, Kvistborg P. Cancer Neoantigens. Annu Rev Immunol. 2019;37:173–200. [DOI] [PubMed] [Google Scholar]
- 116.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5(10):772–82. [DOI] [PubMed] [Google Scholar]
- 117.Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, et al. The Human Cell Atlas. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang L, Wang M, Lin S, Jian R, Li X, Chan J, et al. A Quantitative Proteome Map of the Human Body. Cell. 2020;183(1):269–83 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 121.Vita R, Mahajan S, Overton JA, Dhanda SK, Martini S, Cantrell JR, et al. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019;47(D1):D339–D43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48(W1):W449–W54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sarkizova S, Klaeger S, Le PM, Li LW, Oliveira G, Keshishian H, et al. A large peptidome dataset improves HLA class I epitope prediction across most of the human population. Nat Biotechnol. 2020;38(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bentzen AK, Hadrup SR. Evolution of MHC-based technologies used for detection of antigen-responsive T cells. Cancer Immunol Immunother. 2017;66(5):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–6. [DOI] [PubMed] [Google Scholar]
- 127.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Granados DP, Rodenbrock A, Laverdure JP, Cote C, Caron-Lizotte O, Carli C, et al. Proteogenomic-based discovery of minor histocompatibility antigens with suitable features for immunotherapy of hematologic cancers. Leukemia. 2016;30(6):1344–54. [DOI] [PubMed] [Google Scholar]
- 129.Lansford JL, Dharmasiri U, Chai S, Hunsucker SA, Bortone DS, Keating JE, et al. Computational modeling and confirmation of leukemia-associated minor histocompatibility antigens. Blood Adv. 2018;2(16):2052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mutis T, Xagara A, Spaapen RM. The Connection Between Minor H Antigens and Neoantigens and the Missing Link in Their Prediction. Front Immunol. 2020;11:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boni A, Muranski P, Cassard L, Wrzesinski C, Paulos CM, Palmer DC, et al. Adoptive transfer of allogeneic tumor-specific T cells mediates effective regression of large tumors across major histocompatibility barriers. Blood. 2008;112(12):4746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gattinoni L, Powell DJ Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoffman LM, Gore L. Blinatumomab, a Bi-Specific Anti-CD19/CD3 BiTE((R)) Antibody for the Treatment of Acute Lymphoblastic Leukemia: Perspectives and Current Pediatric Applications. Front Oncol. 2014;4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. [DOI] [PubMed] [Google Scholar]
- 136.Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–7. [DOI] [PubMed] [Google Scholar]
- 137.Goebeler ME, Bargou RC. T cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol. 2020;17(7):418–34. [DOI] [PubMed] [Google Scholar]
- 138.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123(17):2625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52. [DOI] [PubMed] [Google Scholar]
- 145.Blazar BR, Taylor PA, Boyer MW, Panoskaltsis-Mortari A, Allison JP, Vallera DA. CD28/B7 interactions are required for sustaining the graft-versus-leukemia effect of delayed post-bone marrow transplantation splenocyte infusion in murine recipients of myeloid or lymphoid leukemia cells. J Immunol. 1997;159(7):3460–73. [PubMed] [Google Scholar]
- 146.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. 1999;162(11):6368–77. [PubMed] [Google Scholar]
- 147.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171(3):1272–7. [DOI] [PubMed] [Google Scholar]
- 148.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114(8):1528–36. [DOI] [PubMed] [Google Scholar]
- 150.Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med. 2016;375(2):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–88. [DOI] [PubMed] [Google Scholar]
- 153.Crucitti L, Crocchiolo R, Toffalori C, Mazzi B, Greco R, Signori A, et al. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia. 2015;29(5):1143–52. [DOI] [PubMed] [Google Scholar]
- 154.Toffalori C, Cavattoni I, Deola S, Mastaglio S, Giglio F, Mazzi B, et al. Genomic loss of patient-specific HLA in acute myeloid leukemia relapse after well-matched unrelated donor HSCT. Blood. 2012;119(20):4813–5. [DOI] [PubMed] [Google Scholar]
- 155.Dubois V, Sloan-Bena F, Cesbron A, Hepkema BG, Gagne K, Gimelli S, et al. Pretransplant HLA mistyping in diagnostic samples of acute myeloid leukemia patients due to acquired uniparental disomy. Leukemia. 2012;26(9):2079–85. [DOI] [PubMed] [Google Scholar]
- 156.Jan M, Leventhal MJ, Morgan EA, Wengrod JC, Nag A, Drinan SD, et al. Recurrent genetic HLA loss in AML relapsed after matched unrelated allogeneic hematopoietic cell transplantation. Blood Adv. 2019;3(14):2199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Toffalori C, Zito L, Gambacorta V, Riba M, Oliveira G, Bucci G, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25(4):603–11. [DOI] [PubMed] [Google Scholar]
- 158.Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. N Engl J Med. 2018;379(24):2330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Uhl FM, Chen S, O’Sullivan D, Edwards-Hicks J, Richter G, Haring E, et al. Metabolic reprogramming of donor T cells enhances graft-versus-leukemia effects in mice and humans. Sci Transl Med. 2020;12(567). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.“Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT)”, Summary Slides 2019, CIBMTR, accessed February 1, 2021, https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx.
- 161.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol. 2016;34(10):1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nature Medicine. 2019;25(7):1064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol. 2005;17(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Soiffer RJ. Maintenance therapy for high-risk acute leukemia after allogeneic hematopoietic cell transplantation: wait a minute. Blood advances. 2020;4(13):3205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Scott BL. Allogeneic stem cell transplantation for high-risk acute leukemia and maintenance therapy: no time to waste. Blood advances. 2020;4(13):3200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ho VT, Vanneman M, Kim H, Sasada T, Kang YJ, Pasek M, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci USA. 2009;106(37):15825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ho VT, Kim HT, Bavli N, Mihm M, Pozdnyakova O, Piesche M, et al. Vaccination with autologous myeloblasts admixed with GM-K562 cells in patients with advanced MDS or AML after allogeneic HSCT. Blood advances. 2017;1(24):2269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burkhardt UE, Hainz U, Stevenson K, Goldstein NR, Pasek M, Naito M, et al. Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J Clin Invest. 2013;123(9):3756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liegel J, Weinstock M, Rosenblatt J, Avigan D. Vaccination as Immunotherapy in Hematologic Malignancies. J Clin Oncol. 2021:JCO2001706. [DOI] [PubMed] [Google Scholar]
- 171.Kebriaei P, Banerjee PP, Ganesh C, Kaplan M, Nandivada V, Cortes AKN, et al. Blinatumomab Is Well Tolerated Maintenance Therapy Following Allogeneic Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia. Blood. 2019;134(Supplement_1):1298-.31416800 [Google Scholar]
- 172.Oshikawa G, Kakihana K, Saito M, Aoki J, Najima Y, Kobayashi T, et al. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. British Journal of Haematology. 2015;169(5):756–9. [DOI] [PubMed] [Google Scholar]
- 173.Zahler S, Bhatia M, Ricci A, Roy S, Morris E, Harrison L, et al. A Phase I Study of Reduced-Intensity Conditioning and Allogeneic Stem Cell Transplantation Followed by Dose Escalation of Targeted Consolidation Immunotherapy with Gemtuzumab Ozogamicin in Children and Adolescents with CD33+ Acute Myeloid Leukemia. Biol Blood Marrow Transplant. 2016;22(4):698–704. [DOI] [PubMed] [Google Scholar]
- 174.Schmid C, Labopin M, Schaap N, Veelken H, Schleuning M, Stadler M, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia - a matched pair analysis by the Acute Leukaemia Working Party of EBMT. British Journal of Haematology. 2019;184(5):782–7. [DOI] [PubMed] [Google Scholar]
- 175.Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplantation. 2019;54(11):1815–26. [DOI] [PubMed] [Google Scholar]
- 176.Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Impact of drug development on the use of stem cell transplantation: a report by the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplantation. 2017;52(2):191–6. [DOI] [PubMed] [Google Scholar]
- 177.Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. The Lancet Oncology. 2018;19(12):1668–79. [DOI] [PubMed] [Google Scholar]
- 178.de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL, et al. CC-486 Maintenance after Stem Cell Transplantation in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol Blood Marrow Transplant. 2018;24(10):2017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kent A, Pollyea DA, Winters A, Jordan CT, Smith C, Gutman JA. Venetoclax Is Safe and Tolerable As Post-Transplant Maintenance Therapy for AML Patients at High Risk for Relapse. Blood. 2020;136(Supplement 1):11–2. [DOI] [PubMed] [Google Scholar]
- 180.Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993–3002. [DOI] [PubMed] [Google Scholar]
- 181.Levis MJ, Hamadani M, Logan BR, Rosales M, Delgado D, Bahceci E, et al. BMT CTN Protocol 1506: A Phase 3 Trial of Gilteritinib As Maintenance Therapy after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with FLT3-ITD+ AML. Blood. 2019;134(Supplement_1):4602-. [Google Scholar]
- 182.Sandmaier BM, Khaled S, Oran B, Gammon G, Trone D, Frankfurt O. Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am J Hematol. 2018;93(2):222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24(3):282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pu JJ, Poulose J, Malysz J, Zhu J, Fanburg Smith JC, Claxton DF, et al. Impact of ruxolitinib on myelofibrosis patients post allogeneic stem cell transplant-a pilot study. British Journal of Haematology. 2019;186(5):e130–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Warraich Z, Tenneti P, Thai T, Hubben A, Amin H, McBride A, et al. Relapse Prevention with Tyrosine Kinase Inhibitors after Allogeneic Transplantation for Philadelphia Chromosome-Positive Acute Lymphoblast Leukemia: A Systematic Review. Biol Blood Marrow Transplant. 2020;26(3):e55–e64. [DOI] [PubMed] [Google Scholar]
- 186.Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ryan CE, Sahaf B, Logan AC, O’Brien S, Byrd JC, Hillmen P, et al. Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood. 2016;128(25):2899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wood KC. Mapping the Pathways of Resistance to Targeted Therapies. Cancer Res. 2015;75(20):4247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-Term Follow-Up of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. J Clin Oncol. 2020;38(32):3805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D’Aveni-Piney M, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood advances. 2020;4(22):5607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Majzner RG, Mackall CL. Tumor Antigen Escape from CAR T-cell Therapy. Cancer discovery. 2018;8(10):1219–26. [DOI] [PubMed] [Google Scholar]
- 192.Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X, Lin YH, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia. 2017;31(12):2587–93. [DOI] [PubMed] [Google Scholar]
- 193.Jiang H, Li C, Yin P, Guo T, Liu L, Xia L, et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am J Hematol. 2019;94(10):1113–22. [DOI] [PubMed] [Google Scholar]
- 194.Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–99. [DOI] [PubMed] [Google Scholar]
- 196.Kim MY, Yu K-R, Kenderian SS, Ruella M, Chen S, Shin T-H, et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell. 2018;173(6):1439–53.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]