Abstract
Covalently attaching ubiquitin (Ub) to cellular proteins as a post-translational modification can result in altered function of modified proteins. Enzymes regulating Ub as a post-translational modification, such as ligases and deubiquitinases, are challenging to characterize in part due to the low throughput of in-vitro assays. Single-molecule nanopore based assays have the advantage of detecting proteins with high specificity and resolution, and in a label-free, real-time fashion. Here we demonstrate the use of a MspA nanopore for discriminating and quantifying Ub proteins. We further applied the MspA pore to measure the Ub-chain disassembly activity of UCH37, a proteasome associated deubiquitinase. The implementation of this MspA system into nanopore arrays could enable high throughput characterizations of unknown deubiquitinases as well as drug screening against disease related enzymes.
Keywords: deubiquitinase activity assay, MspA, nanopore, sensing, ubiquitin
Graphical Abstract
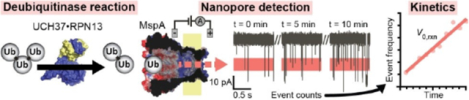
A MspA nanopore is demonstrated as a sensor for discriminating and quantifying various ubiquitin proteins. Real-time measurement of polymeric ubiquitin chain disassembly by a deubiquitinating enzyme was performed and enzyme kinetics were determined.
Ubiquitination, a post-translational modification (PTM), plays a prominent role in cell signaling networks that regulate many important biological processes. Protein ubiquitination is a process in which the C-terminal carboxyl group of the small protein ubiquitin (Ub) is covalently attached to the ε-amino group of lysine side chains. In addition to modifying the vast majority of proteins in a cellular proteome, Ub can also modify itself at eight different sites (M1, K6, K11, K27, K29, K33, K48, K63), resulting in the formation of polymeric Ub chains. In cells, ~10% of Ub proteins are in polymeric chains[1] with various functions depending on the Ub linkage type.[2] For example, K48 linkages function in proteolysis[3] while K6 and K33 chains regulate the DNA damage response.[4] The functional significance of Ub chain types is currently the focus of many efforts aimed at understanding the ‘Ub code’.[2a,5]
The enzymes responsible for sculpting the Ub code include the conjugation machinery-E2s and E3s-along with the deconjugating enzymes-deubiquitinases (DUBs). Considering the coordinated activities of these enzymes regulate the vast majority of cellular processes it is important to understand how specificity is achieved.
Access to robust activity assays is key to these efforts.
There are a variety of methods currently used for assessing DUB activity and specificity.[6] One of the most common approaches is sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). However, SDS-PAGE suffers from poor sensitivity, and assays cannot be performed in real-time. Fluorescence based assays, on the other hand, enable real-time measurements and are high-throughput.[7] The challenge is that fluorophores can disrupt native interactions with DUBs and thus provide inaccurate activity readings or alter specificity altogether. Western blotting and mass spectrometry-based approaches provide valuable insights into DUB activity, but often require considerable optimization and are generally low throughput. An ideal in-vitro DUB assay would use label-free substrates, and enable simultaneous detection of multiple different products in real-time.
Nanopore sensing involves measuring ion flows through individual nano-sized conduits embedded in a membrane, which can be applied to detect a variety of analytes. Nanopore based sensors offer label-free, real-time detection with single-molecule sensitivity, which enable novel methods for biophysical and biochemical characterizations.[8] Nanopores can be integrated into array architectures to yield versatile and convenient sensing devices.[9] Nanopores can be derived from synthetic materials (solid-state)[10] or biological pore forming proteins. Solid-state nanopores are mechanically robust and chemically inert, while biological nanopores have higher reproducibility in achieving atomic structural precision.
Biological nanopore sensing of analytes can be done in several ways: through nonspecific interactions with the nanopore;[15] specific interactions with the integrated ligand,[16] aptamer,[17] and protein binding motifs,[18] or via adaptor binding domains[19] and large molecular machines.[9d,20] Funnel-shaped biological nanopore geometries, such as Fragaceatoxin C (FraC) and Cytolysin A (ClyA) can be used as nanopore tweezers; where an applied voltage can hold a natively-folded protein within the nanopore vestibule, without translocating it, for protein identification and characterization. To date, nanopore tweezers have been used to identify protein PTMs,[21] sense protein-ligand interactions,[22] and measure enzyme kinetics.[23]
Different nanopore funnel sizes and vestibule geometries may be better suited for different molecular tweezer applications. For example, a prior work using ClyA nanopores was effective for sensing the Ub ligation state of target proteins, however, was unable to detect individual Ub monomers.[24] Given that the ClyA funnel geometry terminates at a 3 nm constriction region, it is likely that Ub monomers (~2 nm diameter) may translocate through the ClyA pore undetected. Mycobacterium smegmatus outer membrane protein A (MspA) has been previously developed for genomic DNA sequencing.[12] MspA has a funnel geometry grading from ~5 nm at the entrance to a ~1 nm constriction region. Therefore MspA may be of a suitable geometry for sensing Ub molecules and their polymers, since MspA’s constriction is smaller than Ub diameter (Figure 1A). In this work, we tested the ability of the MspA nanopore to detect Ub species and devised a real-time assay for measuring the activity of the proteasome associated DUB UCH37, which has recently been shown to promote protein degradation by debranching Ub chains (Figure 1B).[25]
Figure 1.
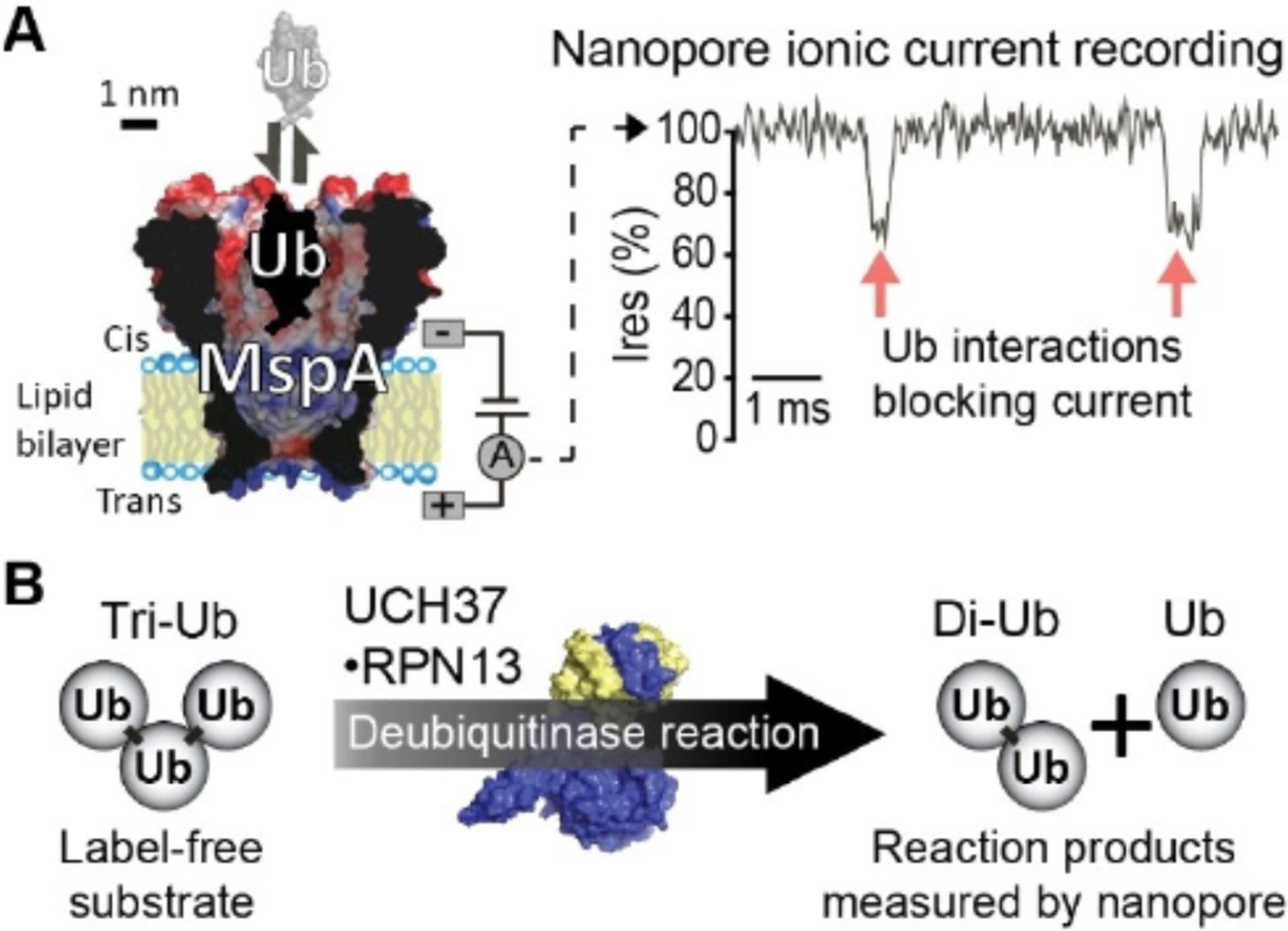
The MspA nanopore assay for DUB activity. A) Schematic showing ubiquitin (Ub, PDB: 1UBQ[11]) interacting with the MspA nanopore (M2-MspA-NNN,[12] adapted from PDB: 1UUN[13]) under a positive trans voltage. Current through MspA is recorded, transient blockages in the current indicate MspA: Ub interactions. B) Ub trimer (tri-Ub) substrate is broken down into a Ub dimer (di-Ub) and a Ub monomer product by the DUB UCH37 ·RPN13 (PDB: 4UEM[14]).
We first characterized the ability of the MspA nanopore to detect components of an in vitro assay with UCH37. Single channel current measurements of MspA were separately performed in the presence of Ub, ubiquitin dimer (di-Ub), ubiquitin trimer (tri-Ub), and UCH37 bound to its partner protein RPN13 (Figure 2A–D). The UCH37·RPN13 complex frequently triggered permanent blockades indicating the enzyme complex was stuck inside the nanopore (Figure 2D). Reversing the applied voltage to a negative potential recovered the open pore current presumably by allowing the DUB to escape from the nanopore (Figure S1D–E). Therefore, when recording the MspA current in the presence of UCH37·RPN13, the voltage polarity was periodically reversed at 3 s intervals using an episodic voltage stimulation protocol (Figure S1A–C).
Figure 2.
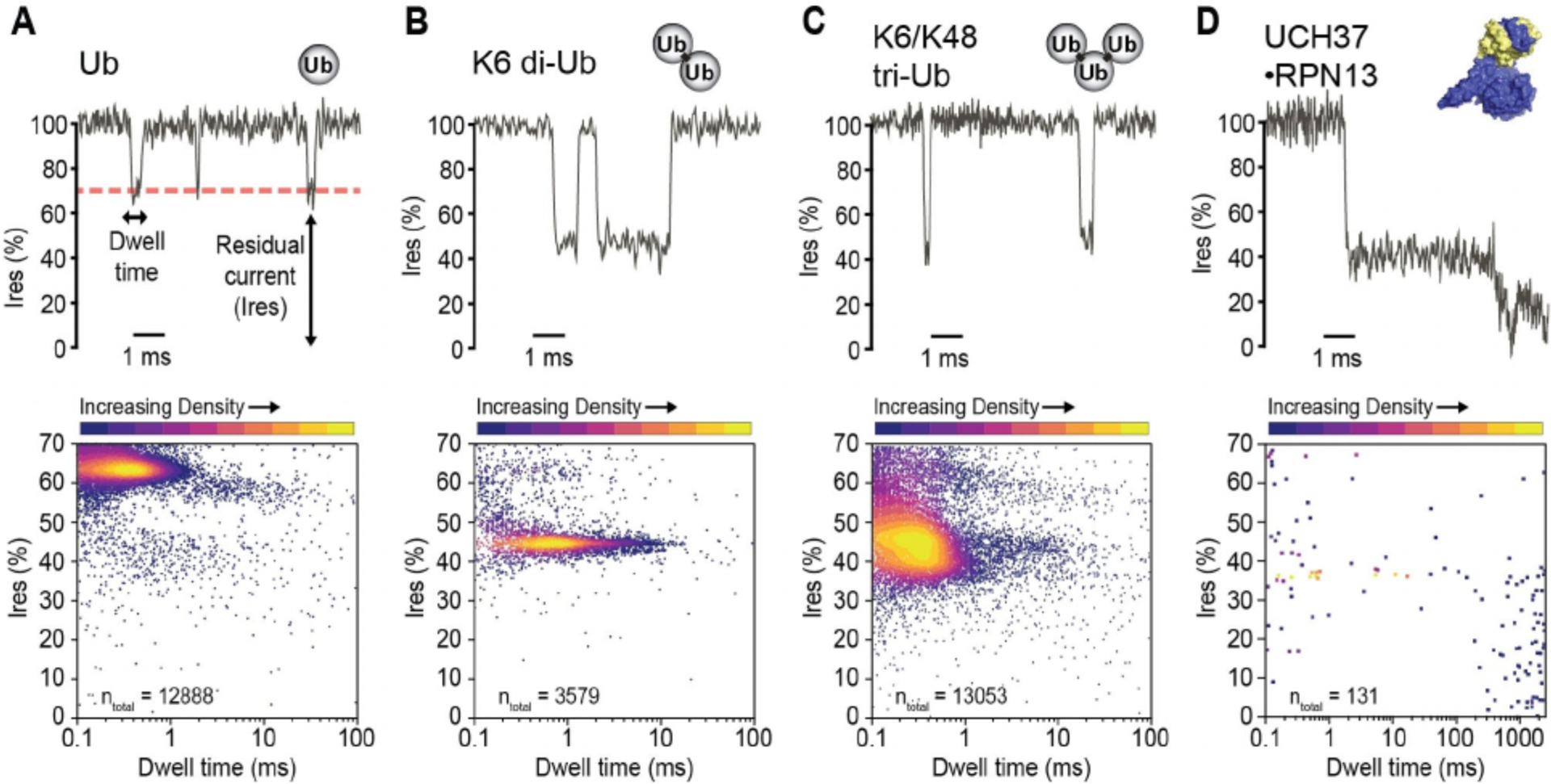
Event characteristics of different analytes. A–D) (Top) Representative current recordings for each analyte. (Bottom) Residual current (Ires %) and dwell times of a sampling of events for each analyte. Single channel current measurements were performed in buffer containing 150 mM NaCl, 1% glycerol (v/v), 20 mM HEPES, pH 7.4 at 23 °C, and under an applied transmembrane potential of +200 mV (applied to the trans electrode). Events were detected by MOSAIC ADEPT 2-state algorithm.[26]
The dwell times for all Ub species were less than 10 ms (Table S1, Figure S2D). Increasing Ub chain length was observed to correlate with relatively lower event blockade residual current (Ires %). Ub monomer events presented at 55–70% Ires, which differed substantially from di-Ub and tri-Ub events. Di-Ub blocked more current (~45% Ires) than Ub monomer (~64% Ires). Tri-Ub, however, showed considerable heterogeneity in event Ires, which may result from its diverse interaction modes with MspA owing to the varying geometry of tri-UB originating from the flexible ubiquitin chain. UCH37·RPN13 exhibited a wider range of event signatures (Figure S3), however these events were not analyzed in greater detail since it was unnecessary to quantify these events for enzyme kinetics analysis.
To establish a method to quantify Ub, we carried out the single channel measurements with MspA in the presence of increasing concentrations of Ub (Figure 3A). Events occurring in the range of 50–70% Ires were partitioned and analyzed separately as Ub events occurred most frequently in this range, henceforth referred to as partition 1 (P1). For each minute of recording in the presence of Ub, the number of events occurring in P1 were counted and expressed as an event frequency (ƒP1, in min−1). At each Ub concentration, three separate pores were measured for 3 minutes each, and each minute of measurement was independently analyzed to yield event frequency values, which were then pooled and averaged. Analysis of P1 event frequency versus Ub concentration showed a linear relationship, and the slope derived from the linear regression was calculated as the concentration-calibrated event frequency (K[Ub],ƒP1, Figure 3B). Similarly, we calibrated the P1 event and the total event frequencies against varying concentrations of other Ub analytes (Figure S2). P1 event frequencies showed a high specificity to Ub monomers (247.9 ± 18.8 min−1·μM−1), as compared to relatively low contributions from the other product di-Ub (13.6 ± 0.9 min−1·μM−1) and the reactant tri-Ub (5.5 ± 0.3 min−1·μM−1) (Figure 3C). This indicated that the UCH37·RPN13 deubiquitination reaction with tri-Ub could be quantified from a nanopore recording by tracking changes in P1 event frequency over time as Ub monomers are produced.
Figure 3.
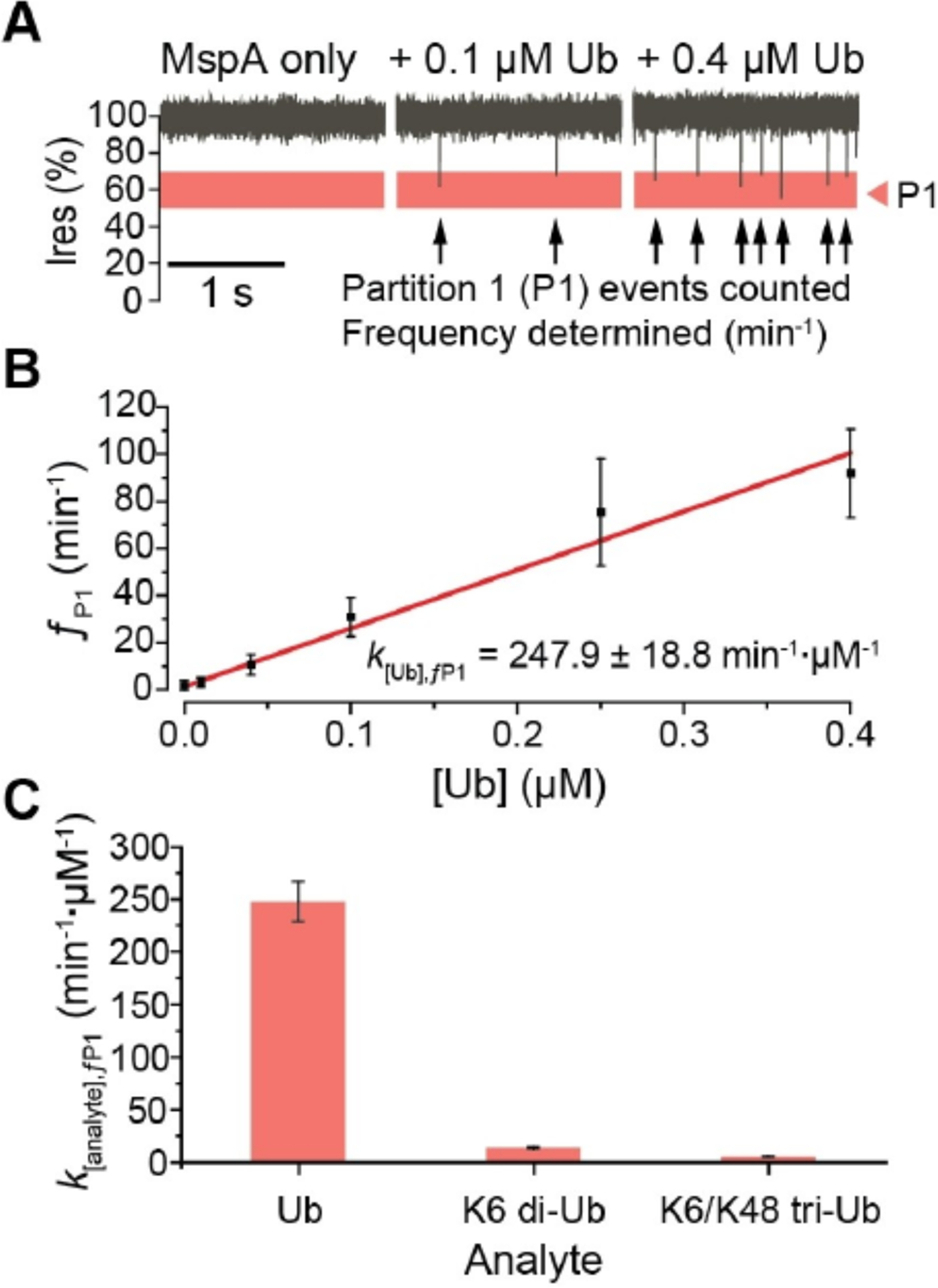
Concentration dependence of Ub analytes. A) Current traces of MspA pore in the presence of increasing concentrations of Ub monomer. Single channel current measurements were performed in buffer containing 150 mM NaCl, 1% glycerol (v/v), 20 mM HEPES, pH 7.4 at 23 °C, and under an applied transmembrane potential of +200 mV (applied to the trans electrode). B) Concentration calibration curve of Ub monomer P1 event frequencies (ƒP1). C) Concentration calibrated event frequencies for each analyte in P1 (k[analyte],ƒP1).
We then performed a DUB assay in situ with MspA pore continuously monitoring the proteolytic reaction (Figure 4A). The frequencies of P1 events were determined for each minute up to 10 minutes, and linearly fit to determine an initial velocity (v0,ƒP1) in terms of the P1 event frequency change over time (min−2) (Figure 4B, Figure S5). UCH37·RPN13 exhibited some events in P1 (Figure 2D), but remain constant throughout the reaction. Therefore, the P1 events originating from UCH37·RPN13 do not influence the initial velocity determinations. Calculation of Ub product formation velocity, i.e. the reaction initial velocity v0,rxn from the P1 event frequency velocity v0,ƒP1, was achieved by factoring in the concentration calibrated P1 event frequencies from the substrate and both products (Equation S1). Initial reaction rates were thus determined, at an enzyme concentration of 250 nM and substrate concentrations ranging from 5 to 50 μM. Michaelis-Menten enzyme kinetic parameters were obtained by plotting the initial reaction rate against the substrate concentration (Figure 4C, Figure S6).
Figure 4.
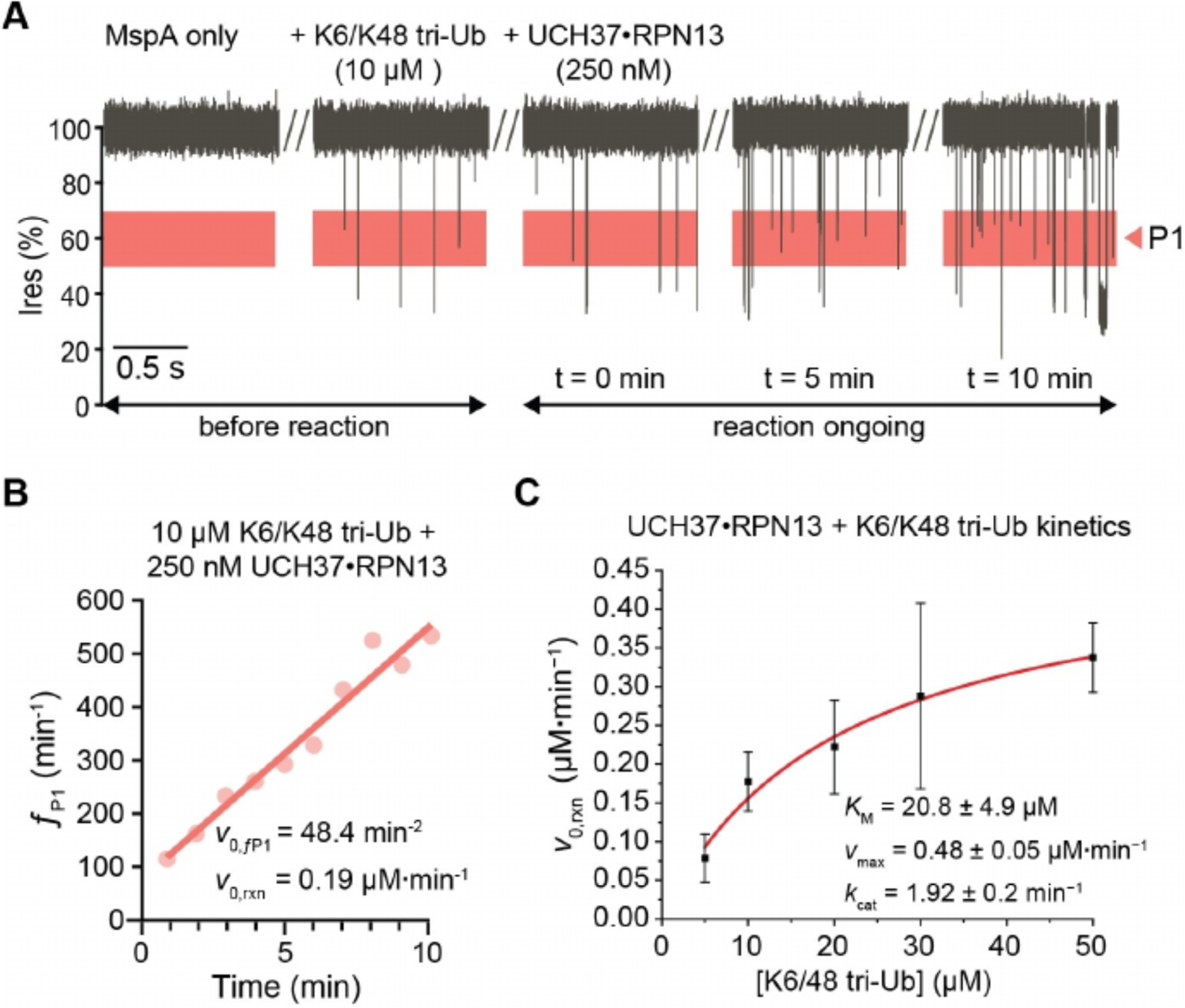
Real-time activity monitoring of UCH37 ·RPN13-catalyzed cleavage reactions with the K6/K48 tri-Ub substrate. A) Representative current recordings of MspA in the presence of K6/K48 tri-Ub (10 μM) before and after adding UCH37·RPN13 (250 nM); Ub product formation in P1 Ires % range highlighted in red. Single channel current measurements were performed in buffer containing 150 mM NaCl, 1% glycerol (v/v), 20 mM HEPES, pH 7.4 at 23 °C, and under an applied transmembrane potential of +200 mV (applied to the trans electrode). B) P1 events from first 10 minutes of recording were binned in minute intervals, and fit for an initial velocity. C) The rate of Ub product formation was calculated and fit to the Michaelis-Menten equation. Each substrate concentration was tested on three separate pores, the initial velocity of P1 events were fit independently for each real-time kinetic.
The kinetic constants derived for the activity of UCH37·RPN13 against the branched K6/K48 tri-Ub are shown in Table S2. The measured KM is 20.8 ± 4.9 μM, which is in agreement with the KM 25±2 μM obtained from a gel-based SDS-PAGE assay previously described by Deol et al. (Table S2).[25] The kcat (1.92 ± 0.2 min−1) is slightly lower than that of the gel-based measurement of 3.0 ± 0.1 min−1. The small difference in kcat is likely attributable to the higher salt concentration used in the nanopore assay (150 mM NaCl vs 50 mM NaCl), which is also known to slow DUB activity.[27] Deol et al. also showed that a catalytically inactive UCH37C88A· RPN13 bound to homotypic K48 tri-Ub and branched K6/K48 tri-Ub with similar affinities (0.23 and 0.49 μM Kd respectively), while the active UCH37·RPN13 enzyme showed over ~20 fold less activity against K48 tri-Ub compared to K6/K48 tri-Ub, thereby showing UCH37·RPN13’s specificity for the K6/K48 tri-Ub substrate.[25] We further tested the activity of UCH37·RPN13 against K48 tri-Ub using the nanopore assay and found a comparable ~15-fold reduction in activity compared to K6/48 tri-Ub (0.013 μM·min−1 vs 0.19 μM·min−1 respectively at 10 μM substrate) (Figure S7). Together, these results show that the activity and substrate specificity of the UCH37·RPN13 were consistently reproduced with the real-time nanopore assay as compared with the gel-based method.
Nanopore sensors are increasingly being developed as novel tools for the study of enzymes.[8,28] Previous studies demonstrated end-point detection of DUB reaction products using a solid-state nanopore,[29] and real-time detection of Ub ligation activity using a ClyA nanopore.[24] In this study, we have shown the use of a MspA nanopore for analysis of DUB activity in real-time. In future work, we will pursue efforts to engineer MspA towards extending MspA’s ability to discriminate a wider variety of Ub chain types. We may integrate these engineered variants with nanopore array technology for measuring the activity and specificity of uncharacterized DUBs as well as screening for DUB inhibitors.
Supplementary Material
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Experimental Section
Details of the experimental procedures are provided in the Supporting Information.
Supporting information and the ORCID identification numbers for the authors of this article can be found under https://dx.doi.org/10.1002/cbic.202100092.
References
- [1].Clague MJ, Trends Cell Biol. 2015, 25, 417–426. [DOI] [PubMed] [Google Scholar]
- [2].a) Yau R, Rape M, Nat. Cell Biol 2016, 18, 579–586; [DOI] [PubMed] [Google Scholar]; b) Swatek KN, Komander D, Cell. Res 2016, 26, 399–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chau V, Tobias J, Bachmair A, Marriott D, Ecker D, Gonda D, Varshavsky A, Science 1989, 243, 1576–1583. [DOI] [PubMed] [Google Scholar]
- [4].Elia AEH, Boardman AP, Wang DC, Huttlin EL, Everley RA, Dephoure N, Zhou C, Koren I, Gygi SP, Elledge SJ, Mol. Cell 2015, 59, 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Haakonsen DL, Rape M, Trends Cell Biol. 2019, 29, 704–716; [DOI] [PubMed] [Google Scholar]; b) Huang Q, Zhang X, Proteomics 2020, 20, 1900100. [DOI] [PubMed] [Google Scholar]
- [6].Kemp M, in Progress in Medicinal Chemistry, Elsevier, 2016, pp. 149–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dang LC, Melandri FD, Stein RL, Biochemistry 1998, 37, 1868–1879. [DOI] [PubMed] [Google Scholar]
- [8].a) Deamer D, Akeson M, Branton D, Nat. Biotechnol 2016, 34, 518–524; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Willems K, Van Meervelt V, Wloka C, Maglia G, Phil. Trans. R. Soc. B 2017, 372, 20160230; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Robertson JWF, Reiner JE, Proteomics 2018, 18, 1800026; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Sheng Y, Zhang S, Liu L, Wu H, ChemBioChem 2020, 21, 2089–2097. [DOI] [PubMed] [Google Scholar]
- [9].a) Wang Y, Yan S, Zhang P, Zeng Z, Zhao D, Wang J, Chen H, Huang S, ACS Appl. Mater. Interfaces 2018, 10, 7788–7797; [DOI] [PubMed] [Google Scholar]; b) Niedzwiecki DJ, Chou Y-C, Xia Z, Thei F, Drndić M, Rev. Sci. Instrum 2020, 91, 031301; [DOI] [PubMed] [Google Scholar]; c) Maggiori C, Stromberg J, Blanco Y, Goordial J, Cloutis E, García-Villadangos M, Parro V, Whyte L, Astrobiology 2020, 20, 375–393; [DOI] [PubMed] [Google Scholar]; d) Palla M, Punthambaker S, Stranges B, Vigneault F, Nivala J, Wiegand D, Ayer A, Craig T, Gremyachinskiy D, Franklin H, Sun S, Pollard J, Trans A, Arnold C, Schwab C, Mcgaw C, Sarvabhowman P, Dalal D, Thai E, Amato E, Lederman I, Taing M, Kelley S, Qwan A, Fuller CW, Roever S, Church GM, ACS Nano 2021, 15, 489–502. [DOI] [PubMed] [Google Scholar]
- [10].a) Lee K, Park K-B, Kim H-J, Yu J-S, Chae H, Kim H-M, Kim K-B, Adv. Mater 2018, 30, 1704680; [DOI] [PubMed] [Google Scholar]; b) Feng J, Liu K, Bulushev RD, Khlybov S, Dumcenco D, Kis A, Radenovic A, Nat. Nanotechnol 2015, 10, 1070–1076; [DOI] [PubMed] [Google Scholar]; c) Yusko EC, Bruhn BR, Eggenberger OM, Houghtaling J, Rollings RC, Walsh NC, Nandivada S, Pindrus M, Hall AR, Sept D, Li J, Kalonia DS, Mayer M, Nat. Nanotechnol 2017, 12, 360–367; [DOI] [PubMed] [Google Scholar]; d) Hu R, Rodrigues JV, Waduge P, Yamazaki H, Cressiot B, Chishti Y, Makowski L, Yu D, Shakhnovich E, Zhao Q, Wanunu M, ACS Nano 2018, 12, 4494–4502; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Cadinu P, Campolo G, Pud S, Yang W, Edel JB, Dekker C, Ivanov AP, Nano Lett. 2018, 18, 2738–2745; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Chien C-C, Shekar S, Niedzwiecki DJ, Shepard KL, Drndić M, ACS Nano 2019, 10545–10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vijay-kumar S, Bugg CE, Cook WJ, J. Mol. Biol 1987, 194, 531–544. [DOI] [PubMed] [Google Scholar]
- [12].Butler TZ, Pavlenok M, Derrington IM, Niederweis M, Gundlach JH, Proc. Natl. Acad. Sci. USA 2008, 105, 20647–20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Faller M, Science 2004, 303, 1189–1192. [DOI] [PubMed] [Google Scholar]
- [14].Sahtoe DD, van Dijk WJ, El Oualid F, Ekkebus R, Ovaa H, Sixma TK, Mol. Cell 2015, 57, 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Tan CS, Fleming AM, Ren H, Burrows CJ, White HS, J. Am. Chem. Soc 2018, 140, 14224–14234; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li S, Cao C, Yang J, Long Y-T, ChemElectroChem 2019, 6, 126–129; [Google Scholar]; c) Ouldali H, Sarthak K, Ensslen T, Piguet F, Manivet P, Pelta J, Behrends JC, Aksimentiev A, Oukhaled A, Nat. Biotechnol 2020, 38, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pham B, Eron SJ, Hill ME, Li X, Fahie MA, Hardy JA, Chen M, Biophys. J 2019, 117, 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rotem D, Jayasinghe L, Salichou M, Bayley H, J. Am. Chem. Soc 2012, 134, 2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Thakur AK, Movileanu L, Nat. Biotechnol 2019, 37, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zernia S, van der Heide NJ, Galenkamp NS, Gouridis G, Maglia G, ACS Nano 2020, 14, 2296–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ho C-W, Van Meervelt V, Tsai K-C, De Temmerman P-J, Mast J, Maglia G, Sci. Adv 2015, 1, e1500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Restrepo-Pérez L, Wong CH, Maglia G, Dekker C, Joo C, Nano Lett. 2019, 19, 7957–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li X, Lee KH, Shorkey S, Chen J, Chen M, ACS Nano 2020, 14, 1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].a) Derrington IM, Craig JM, Stava E, Laszlo AH, Ross BC, Brinkerhoff H, Nova IC, Doering K, Tickman BI, Ronaghi M, Mandell JG, Gunderson KL, Gundlach JH, Nat. Biotechnol 2015, 33, 1073–1075; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kwak D-K, Kim J-S, Lee M-K, Ryu K-S, Chi S-W, Anal. Chem 2020, 92, 14303–14308. [DOI] [PubMed] [Google Scholar]
- [24].Wloka C, Van Meervelt V, van Gelder D, Danda N, Jager N, Williams CP, Maglia G, ACS Nano 2017, 11, 4387–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Deol KK, Crowe SO, Du J, Bisbee HA, Guenette RG, Strieter ER, Mol. Cell 2020, 80, 796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].a) Balijepalli A, Ettedgui J, Cornio AT, Robertson JWF, Cheung KP, Kasianowicz JJ, Vaz C, ACS Nano 2014, 8, 1547–1553; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Forstater JH, Briggs K, Robertson JWF, Ettedgui J, Marie-Rose O, Vaz C, Kasianowicz JJ, Tabard-Cossa V, Balijepalli A, Anal. Chem 2016, 88, 11900–11907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Villamil MA, Chen J, Liang Q, Zhuang Z, Biochemistry 2012, 51, 2829–2839. [DOI] [PubMed] [Google Scholar]
- [28].a) Harrington L, Alexander LT, Knapp S, Bayley H, ACS Nano 2019, 13, 633–641; [DOI] [PubMed] [Google Scholar]; b) Zhou S, Wang L, Chen X, Guan X, ACS Sens. 2016, 1, 607–613; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ying Y-L, Yang J, Meng F-N, Li S, Li M-Y, Long Y-T, Research 2019, 2019, 1–8.. [Google Scholar]
- [29].Nir I, Huttner D, Meller A, Biophys. J 2015, 108, 2340–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


