Summary
Background
Vincristine plus dexamethasone pulses are generally used throughout continuation treatment for childhood acute lymphoblastic leukemia (ALL). We sought to determine if this therapy can be safely omitted beyond 1 year of treatment.
Methods
We conducted a randomized, open-label, non-inferiority study in children between 0 and 18 years old with newly diagnosed ALL. Patients in continuous remission for 1 year were stratified and randomized to receive or not receive seven pulses of vincristine (1.5 mg/m2) plus dexamethasone (6 mg/m2 per day for 7 days) during the second year of treatment. Randomization and analyses were performed in low-risk and intermediate-/high-risk cohorts separately. Stratification factors included participating center, sex, and age at diagnosis; the low-risk group was additionally stratified for ETV6–RUNX1 status, and the intermediate-/high-risk cohort for cell lineage. Randomizations were performed centrally at the leading institution with assignment sequences generated by the protocol biostatistician. The primary endpoint was 5-year event-free survival, with the non-inferiority margin preset at 0.05 (5%). The analysis was done by intention to treat. We herein report the findings after completion of enrollment and completion of protocol-specified follow-up. The trial is registered with the Chinese Clinical Trial Registry (ChiCTR-IPR-14005706).
Findings
From January 1, 2015 to February 20, 2019, 6108 evaluable patients were enrolled. The median follow-up time for the randomized patients who were alive at the time of analysis was 3.7 years (IQR, 2.8–4.7). Among patients with low-risk ALL, there was no difference in 5-year event-free survival between the 1442 patients treated with and the 1481 treated without additional pulse therapy (90.3% [95% CI, 88.4%−92.2%] vs. 90.2% [95% CI, 88.2%−92.2%], P=0.90). The one-sided 95% upper confidence bound for the difference in 5-year event-free survival probability was 0.024, establishing non-inferiority based on the preset criterion of 0.05. Among patients with intermediate-/high-risk ALL, the 5-year event-free survival was 82.8% [95% CI, 80.0%−85.6%] for the 1071 patients treated with and 80.8% [95% CI, 77.7%−83.90%] for the 1060 patients treated without additional pulse therapy (P=0.90). The one-sided 95% upper confidence bound for the difference in probability of 5-year event-free survival was 0.055, a borderline inferior result for those treated without additional pulse therapy. Patients with intermediate-/high-risk ALL receiving additional pulse therapy were significantly more likely to develop grade 3/4 pneumonia and peripheral neuropathy. Fatal infection occurred in 7 patients with low-risk ALL and in 11 with intermediate-/high-risk ALL with no significant difference in the rate between randomized groups.
Interpretation
Vincristine plus dexamethasone pulses can be omitted beyond 1 year of treatment for children with low-risk ALL. Additional studies are needed for intermediate-/high-risk ALL.
Funding
VIVA China Children’s Cancer Foundation, the National Natural Science Foundation of China, the China fourth round of Three-Year Public Health Action Plan (2015–2017), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, U.S. National Cancer Institute, St. Baldrick’s Foundation, and the American Lebanese Syrian Associated Charities.
Introduction
Adding vincristine plus prednisone (or prednisolone) pulses during continuation treatment of childhood acute lymphoblastic leukemia (ALL) improved 5-year event-free survival from approximately 60% to 70% in multiple clinical trials conducted between the 1970s and mid-1980s.1 Two clinical trials conducted in the 1990s randomized patients to receive dexamethasone or prednisone (or prednisolone) during remission induction and consolidation and throughout continuation treatment, as monthly pulse therapy with vincristine.2,3 In the Children’s Cancer Group study of patients with standard-risk ALL, dexamethasone-treated patients had significantly better 6-year event-free survival (85% vs. 77%) and a lower CNS relapse rate (3.7% vs. 7.1%) than prednisone-treated patients.2 In the UK MRC 97 study of patients in all risk groups, dexamethasone-treated patients also had superior 5-year event-free survival (84.2% vs. 75.6%) and lower CNS relapse rate (2.5% vs. 5%) than did prednisolone-treated patients.3
Several study groups conducted randomized studies between 1995 and 2002 to determine the efficacy of six additional pulses of vincristine plus dexamethasone during continuation treatment in patients with intermediate-risk ALL.1,4,5 Meta-analyses of eight randomized trials based mainly on the Berlin-Frankfurt-Münster regimen yielded similar 5-year event-free survival of approximately 80% between patients treated with or without the six additional pulses of vincristine plus dexamethasone during the first 60 weeks of continuation treatment.1 The improved overall event-free survivals seen in these clinical trials, compared to those of historical studies, led the investigators to attribute the lack of benefit of the six additional pulses of vincristine plus dexamethasone to their use of more intensive backbone therapy, including early intensification of treatment.1 However, the European Organisation for Research and Treatment of Cancer trial 58951 conducted between 1999 and 2002 showed that six additional pulses with vincristine plus prednisolone or dexamethasone given every 10 weeks throughout continuation treatment yielded significantly better 6-year disease-free survival for patients with intermediate-risk ALL (90.6% vs. 82.8%).6 The authors of this trial explained the discrepancies of the results to their longer follow-up as compared to the other eight randomized trials (median, 6 years vs. 3.3 years). Despite these inconclusive results and the lack of randomized studies in patients with low- or high-risk disease, virtually all major study groups have incorporated pulses of vincristine plus steroid (generally dexamethasone) during continuation treatment of various risk groups and for various durations since the 2000s.7–17 Therefore, we conducted this large open-label, randomized non-inferiority study to determine if removing the additional vincristine plus dexamethasone pulses would not lead to an inferior outcome in any subgroup of childhood ALL.
Methods
Study design and participants
Chinese Children’s Cancer Group study ALL-2015 (CCCG-ALL-2015)18 is a prospective, multi-institutional clinical trial conducted by 20 major medical centers across China. Eligible patients were children aged 0–18 years with newly diagnosed ALL. Patients with secondary malignancy or primary immunodeficiency were not eligible for the study. Only prior steroid treatment for less than 1 week was allowed. All patients received minimal residual disease (MRD)-directed, risk-stratified treatment modified from the St. Jude Children’s Research Hospital Total Therapy 15 and 16 studies8,16 and the Shanghai Children’s Medical Center ALL-2005 trial.19
CCCG-ALL-2015 consists of two open-label, randomized studies: one compared the efficacy and toxicity of dasatinib vs. imatinib in patients with Philadelphia chromosome–positive ALL; the results of this study have been published recently.20 The other randomized trial, the subject of this report, determined whether vincristine plus dexamethasone pulses can be omitted from the last half of continuation therapy without compromising the event-free survival of all other patients. The 260 patients with Philadelphia chromosome–positive ALL were not eligible for this study. Patients, guardians, clinicians, and the research staff were not aware of the results at any phase of the trial. The study was approved by the institutional review board of each participating center, and informed consent was obtained from the parents, guardians, or patients, as appropriate, in accordance with the Declaration of Helsinki.
The diagnosis of ALL was based on morphology and immunophenotypic and genetic features of leukemic cells. Patients with B-cell ALL (B-ALL) aged between 1 year and <10 years, and leukocyte count <50 × 109/L, hyperdiploidy >50 chromosomes, or ETV6–RUNX1 oncogene fusion and without CNS3 status, testicular leukemia, MRD <1% on Day 19 of induction, and MRD <0.01% on Day 46 of induction were classified as having low-risk disease. Patients with MRD ≥1% (or ≥5% blasts morphologically without suitable markers for MRD) in bone marrow on Day 46 of induction and infants younger than 6 months with KMT2A rearrangement and leukocyte count ≥300 × 109/L were considered to have high-risk ALL. The remaining cases were classified as intermediate-risk ALL.
Randomization and masking
Approximately 1 year after diagnosis, following remission induction, consolidation, and early continuation therapy (figure 1 and appendix p 8), patients remaining in continuous complete remission were randomized to receive or not receive seven additional vincristine plus dexamethasone pulses, given every 8 weeks, over the following 56 weeks. Randomization was performed in low- and intermediate-/high-risk patient cohorts separately. Stratified block randomization21 was done centrally using an interactive randomization system. The block size was set to six, and the randomization ratio was 1:1. Stratification factors for both risk cohorts included participating center, sex, and age at diagnosis (<1 year, 1 to <10 years, or ≥10 years); the low-risk group was additionally stratified for ETV6–RUNX1 status, and the intermediate-/high-risk cohort for cell lineage (B- vs T-ALL). All therapy was administered as open label without masking. Each participating hospital enrolled patients locally and reported the enrollment, along with the required stratification factors, to the central randomization site at Shanghai Children’s Medical Center. Randomization allocations were generated by the study biostatistician.
Procedures
All patients received dexamethasone for 4–5 days as upfront window therapy, followed by remission induction with prednisone, vincristine, daunorubicin, and pegaspargase from Day 5 to Day 28, and cyclophosphamide, mercaptopurine, and cytarabine from Day 29 to Day 35 (appendix p 2). Patients with B-ALL who had MRD ≥1% on Day 19 of remission induction and those with T-cell ALL (T-ALL) received additional early intensification therapy with cyclophosphamide, cytarabine, mercaptopurine, vincristine, and pegaspargase between Days 50 and 57 (appendix p 3).
Upon completion of induction between Days 46 and 49, consolidation treatment was begun with high-dose methotrexate and triple intrathecal therapy every other week and daily mercaptopurine for four courses (appendix, pp 3–4). Continuation treatment in the first 39 weeks for patients with low-risk ALL consisted of daily mercaptopurine and weekly methotrexate interrupted by pulses of vincristine plus dexamethasone every 3 to 4 weeks and two reinduction treatments (appendix pp 4–6). During the same period, patients with intermediate-/high-risk ALL received intensive multiagent chemotherapy, which was also interrupted by pulses of vincristine plus dexamethasone every 3 to 4 weeks and a reinduction (appendix pp 4–6). The low-risk group received 11 pulses, and the intermediate-/high-risk group received 12 pulses during this period. Complete blood counts were checked twice weekly initially and then every 1 to 2 weeks after the treatment doses were titrated; liver and renal function blood tests were checked monthly. Dosages of mercaptopurine and methotrexate were titrated to keep the white blood cell count between 1.8 and 3.0 × 109/L, absolute neutrophil count between 0.5 and 1.2 × 109/L, and platelet count ≥50 × 109/L. Additionally, dosages of mercaptopurine and methotrexate were decreased if white blood cell count and neutrophil count did not increase by at least 2 fold a week after the start date of vincristine and dexamethasone pulse therapy.
Subsequent continuation therapy (approximately 54 weeks from diagnosis) began with randomized treatment for both cohorts consisting of seven cycles of 8-week treatment. The patients with low-risk ALL received weekly methotrexate and daily mercaptopurine with (Group A, control arm) or without (Group B, experiment arm) pulse therapy with vincristine (1.5 mg/m2) plus dexamethasone (6 mg/m2 per day for 7 days) on Week 8 (appendix p 7). The patients with intermediate-/high-risk ALL received 6 weeks of mercaptopurine and methotrexate, followed by 1 week of treatment with cyclophosphamide and cytarabine with (Group A) or without (Group B) the pulse therapy (same as low-risk ALL, appendix p 7). The entire treatment concluded with daily mercaptopurine and weekly methotrexate until Week 125 from the initial induction treatment. None of the patients were given prophylactic cranial irradiation. Allogeneic hematopoietic cell transplantation or chimeric antigen receptor T-cell therapy was a treatment option for patients with high-risk ALL who had MRD ≥1% at the end of remission induction. The conduct of the protocol included a central review of MRD, major adverse events and toxicities every 6 months; periodic internal and on-site monitoring; and external auditing to ensure protocol compliance and appropriate data management.
Outcomes
The primary endpoint of the trial was event-free survival. The secondary endpoints were any relapse and overall survival. Event-free survival was calculated from the date of diagnosis to the first major adverse event, including induction failure, relapse, death from any cause, development of a second malignant neoplasm, and off-protocol by the decision of the treating physician because of severe toxicity. Overall survival was calculated from the time of diagnosis to death from any cause. Time was censored at the date of last patient contact if no event occurred.
Statistical analysis
The goal of the study was to establish the non-inferiority (≤0.05 absolute difference) in the 5-year event-free survival probability of the patients in the experimental arm (Group B, without the additional pulses), as compared to that of patients in the control arm (Group A, with the additional pulses). The randomization and analyses were conducted separately for the low-risk and intermediate-/high-risk groups.
According to the a priori statistical design, the one-sided 95% upper confidence bound of the difference in the probability of 5-year event-free survival (control minus experiment) was computed; the experimental treatment would be considered non-inferior if the upper confidence bound of difference was less than 0.05 (5%). This non-inferiority criterion was so constructed that by randomizing 1500 patients there will be an 80% probability of declaring non-inferiority if the probabilities of 5-year event-free survival in the control and experimental groups are, in fact, equal. A range of possible 5-year event-free survival probabilities (0.6, 0.7, 0.8, 0.9) was considered in assessing sample size and power. The final sample size was determined to assure 80% power for all the postulated 5-year event-free survival probabilities. The non-inferiority margin was determined by clinical relevance; that is, we regard omitting the late vincristine plus dexamethasone pulses as clinically non-inferior if the resulting 5-year event-free probability decreases by no more than 0.05.
Event-free survival and overall survival functions were estimated using the Kaplan-Meier method and compared using the log-rank test. The cumulative incidence functions of any relapse or toxic death were estimated by the method of Kalbfleisch and Prentice22 and compared using Gray’s test to account for competing events.23 Competing events included any relapse, death during remission, second malignancy, and off-protocol treatment by decision of the treating physician. Treatment abandonment and parental refusal of protocol treatment were censored. In all the analyses, confidence intervals (CIs) were calculated using a large-sample normal approximation.
All eligible children with newly diagnosed ALL enrolled on the study, randomized, and evaluable for the primary objective were included in the analyses of the primary, secondary, and safety outcomes. Patients with incorrectly determined risk category or unrandomized were considered as unevaluable and excluded from analyses (figure 1). By protocol design, the results for the primary analysis were performed, according to intention to treat, on all evaluable patients. Secondary post hoc analysis was performed also according to actual vincristine–dexamethasone pulses received (as-treated) on all evaluable patients. Per protocol design, randomization, analysis, and results were obtained and reported in low-risk and intermediate/high-risk patients separately.
Fig 1.
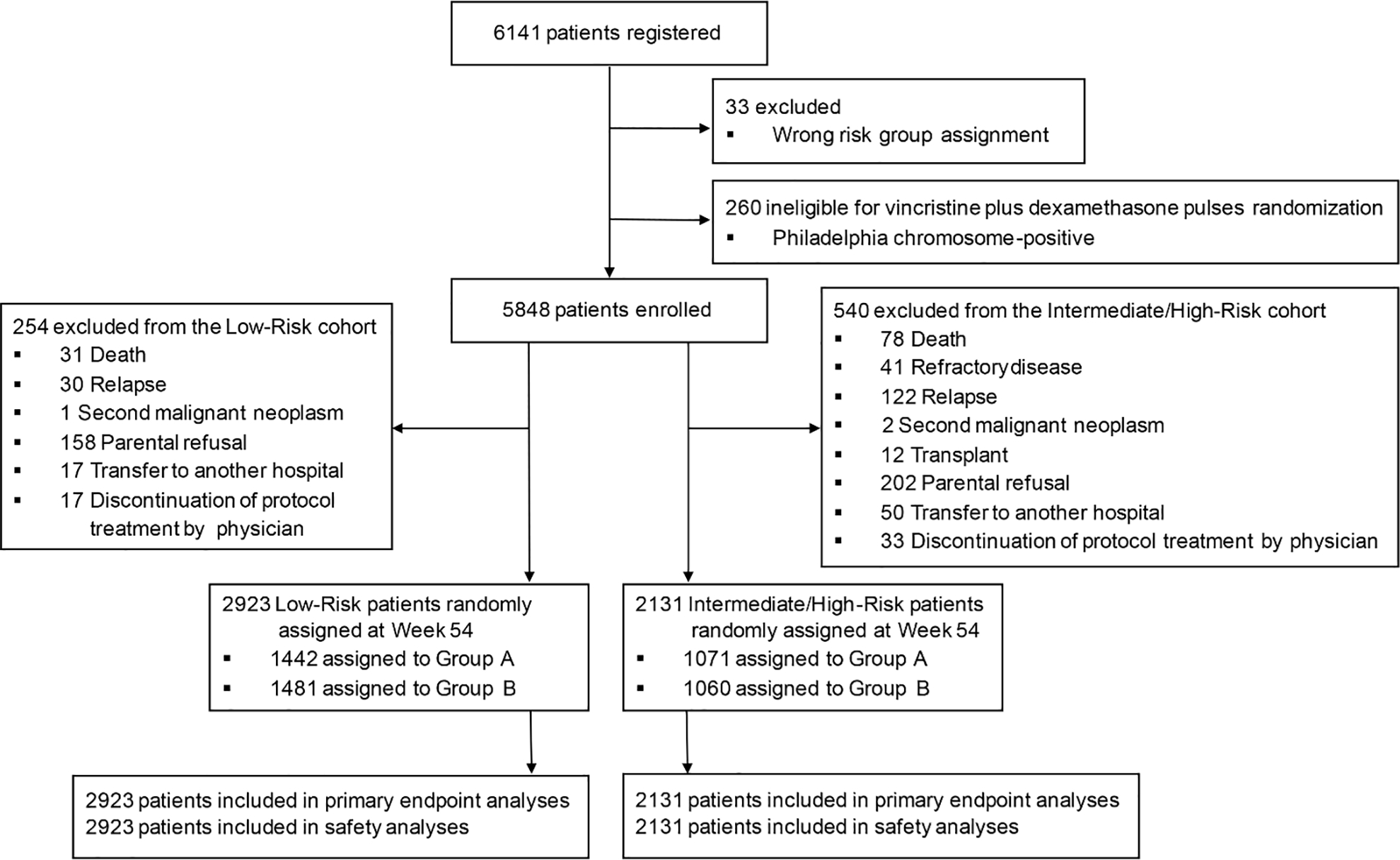
CONSORT diagram.
A post hoc subgroup analysis in intermediate-risk patients was also performed because most reported studies were conducted in this risk group. Additional post hoc subgroup analyses included assessing the relative effects of treatment, with or without vincristine plus dexamethasone pulses, on event-free survival for the entire cohort and various patient subgroups by Peto’s hazard ratio (HR) and 95% CI. Heterogeneity between subgroups was analyzed by the Cochrane Q test.24 Fisher’s exact test was used to post hoc analyze safety data by determining if patients treated in Group B without the additional pulse therapy had less grade 3–5 toxicities than those treated in Group A.
An interim monitoring analysis after 3.5 years of overall enrollment was planned to monitor possible early strong indication of inferiority in event-free survival in the experimental arm, using the log-rank test at the 0.005 significance level. No significant difference was detected by this interim analysis performed on February 17, 2020 (appendix p 1). This paper reports the results after the enrollment and the required follow-up were completed, according to the study design. All statistical analyses were conducted with R statistical software version 3.4.4 (The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/). This trial is registered with Chinese Clinical Trial Registry (ChiCTR-IPR-14005706).
Role of the funding source
The funders of the study had no role in the study design; data collection, analysis, or interpretation; patient recruitment; or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. All authors had full access to the full data in the study and accept responsibility to submit for publication.
Results
Between January 1, 2015 and February 20, 2020, we enrolled 6141 consecutive pediatric patients with newly diagnosed ALL in this randomized study. The median number of patient enrollment per institution was 228 (range, 66 to 717). Because of the lack of difference between the experimental and control arms at the interim analyses and the COVID-19 pandemic, enrollment was terminated on February 20, 2020, so that patients who had not yet been randomized would not have to make clinic visits to receive vincristine beyond 1 year of treatment.
Thirty-three registered patients were not evaluable because of an incorrect risk group assignment. Of the 6108 evaluable patients, 5993 (98.12%) experienced complete morphologic remission. Based on their MRD levels on Days 19 and 46 of remission induction, 3177 patients were classified as having low-risk ALL, 2805 had intermediate-risk ALL, and 126 had high-risk ALL (figure 1). Among the 126 patients with high-risk ALL, hematopoietic cell transplantation was performed in 26 patients, of whom 18 were alive in remission for 0.4 to 4.6 years (median, 2.3 years), and four each died of relapse or transplant-related toxicities. Chimeric antigen receptor T-cell therapy was administered to two patients; one was alive in remission for 1.6 years and the other died of relapse. The 5-year event-free survival was 79.9% (95% CI, 78.7%−81.2%), and the 5-year overall survival was 90.3% (95% CI, 89.4%−91.2%) for all patients.
Approximately 1 year after diagnosis, 2923 patients with low-risk ALL and 2131 with intermediate-/high-risk ALL remaining in continuous remission were randomized for the study; 1442 and 1481 low-risk patients and 1071 and 1060 intermediate-/high-risk patients were randomized to Group A (control arm) and Group B (experimental arm), respectively (figure 1). There were no differences in the demographic and disease characteristics or the levels of MRD at Day 19 or Day 46 of remission induction between patients treated in the control arm (Group A) and those in the experimental arm (Group B) for both risk cohorts (table 1). The only exception was a higher percentage of patients whose leukemic cells were KMT2A rearranged in Group B than in Group A (7.08% vs. 4.48%, P=0.01) among the intermediate-/high-risk cohort (table 1).
Table 1.
Baseline demographic and disease characteristics of patients
| Variable | Low-Risk ALL |
Intermediate-/High-Risk ALL |
||
|---|---|---|---|---|
| Group A n (%) |
Group B n (%) |
Group A n (%) |
Group B n (%) |
|
|
| ||||
| Age, years | ||||
| <1 | 0 (0) | 0 (0) | 31 (2.90) | 37 (3.49) |
| 1–10 | 1421 (98.54) | 1458 (98.45) | 777 (72.55) | 758 (71.51) |
| >10 | 21 (1.46) | 23 (1.55) | 263 (24.56) | 265 (25.00) |
|
| ||||
| Sex | ||||
| Male | 817 (56.66) | 848 (57.26) | 643 (60.04) | 652 (61.51) |
| Female | 625 (43.34) | 633 (42.74) | 428 (39.96) | 408 (38.49) |
|
| ||||
| WBC, ×109/L | ||||
| <100 | 1427 (98.96) | 1462 (98.72) | 863 (80.58) | 857 (80.85) |
| ≥100 | 15 (1.04) | 19 (1.28) | 208 (19.42) | 203 (19.15) |
|
| ||||
| CNS status | ||||
| CNS1 | 1360 (94.31) | 1382 (93.32) | 967 (90.29) | 974 (91.89) |
| CNS2 | 17 (1.18) | 15 (1.01) | 25 (2.33) | 17 (1.60) |
| Traumatic LP | 65 (4.51) | 84 (5.67) | 61 (5.70) | 46 (4.34) |
| CNS3 | 0 (0) | 0 (0) | 18 (1.68) | 23 (2.17) |
|
| ||||
| Immunophenotype | ||||
| B | / | / | 857 (80.02) | 852 (80.38) |
| T | / | / | 214 (19.98) | 208 (19.62) |
|
| ||||
| Ploidy | ||||
| Hyperdiploidy >50 | 285 (19.76) | 276 (18.64) | 104 (9.71) | 111 (10.47) |
| Others | 1157 (80.24) | 1205 (81.36) | 967 (90.29) | 949 (89.53) |
|
| ||||
|
KMT2A
rearrangement | ||||
| Present | / | / | 48 (4.48) | 75 (7.08) |
| Absent | / | / | 1023 (95.52) | 985 (92.92) |
|
| ||||
| TCF3-PBX1 | ||||
| Present | / | / | 133 (12.42) | 130 (12.26) |
| Absent | / | / | 938 (87.58) | 930 (87.74) |
|
| ||||
| ETV6-RUNX1 | ||||
| Present | 479 (33.22) | 491 (33.15) | 60 (5.60) | 61 (5.75) |
| Absent | 963 (66.78) | 990 (66.85) | 1011 (94.40) | 999 (94.25) |
|
| ||||
| MRD D19 | ||||
| <0.01% | 847 (59.77) | 879 (60.33) | 359 (34.75) | 365 (35.33) |
| 0.01–0.09% | 287 (20.25) | 277 (19.01) | 99 (9.58) | 89 (8.75) |
| 0.1–0.99% | 283 (19.97) | 301 (20.66) | 170 (16.46) | 180 (17.70) |
| ≥1% | 0 (0) | 0 (0) | 405 (39.21) | 383 (37.66) |
|
| ||||
| MRD D46 | ||||
| <0.01% | 1325 (96.79) | 1389 (97.13) | 785 (77.19) | 782 (78.20) |
| 0.01–0.09% | 32 (2.34) | 31 (2.17) | 139 (13.67) | 144 (14.40) |
| 0.1–0.99% | 12 (0.88) | 10 (0.70) | 78 (7.67) | 65 (6.50) |
| ≥1% | 0 (0) | 0 (0) | 15 (1.47) | 9 (0.90) |
Abbreviations: CNS, central nervous system; D, day of induction therapy; MRD, minimal residual disease; WBC, white blood cell; LP, lumbar puncture.
Outcome data reported herein were based on intention to treat and were updated on January 31, 2021. The median follow-up time for the 4890 randomized patients who were alive at the time of analysis was 3.7 years (IQR, 2.8–4.7; range, 1.0–6.1 years); 833 patients had been followed for 5 years or more. The follow-up time of the 1500th randomized patient was 3.9 years in the low-risk group and 3.0 years in the intermediate-/high-risk group. Among patients with low-risk ALL, two patients each in Group A and Group B were taken off protocol by the treating physicians. Among patients with intermediate-/high-risk ALL, six patients in Group A and one in Group B were taken off protocol by the treating physicians.
Among patients with low-risk ALL, we have established non-inferiority. The one-sided 95% upper-confidence bound for the difference in the probability of 5-year event-free survival was 0.024, and that for 5-year overall survival was 0.018, both below the preset non-inferiority criterion of 0.05. Secondarily, we found no significant difference between Groups A and B in the 5-year event-free survival (90.3% [95% CI, 88.4%−92.2%] vs. 90.2% [95% CI, 88.2%−92.2%], P=0.90; figure 2A) or overall survival (97.8% [95% CI, 96.9%−98.8%] vs. 97.3% [95% CI, 96.1%−98.5%], P=0.70; figure 2B). There were also no significant differences between Group A and Group B in the 5-year cumulative risk of isolated CNS relapse (1.1% [95% CI, 0.5%−1.7%] vs. 1.0% [95% CI, 0.4%−1.6%], P=0.65), any CNS relapse (1.7% [95% CI, 1.0%−2.4%] vs. 1.6% [95% CI, 0.9%−2.3%], P=0.81), any relapse (9.2% [95% CI, 7.4%−11.0%] vs. 9.1% [95% CI, 7.2%−11.0%], P=0.92), and death during remission (0.2% [95% CI, 0%−0.4%] vs. 0.3% [95% CI, 0%−0.6%], P=0.27) (appendix p 9).
Fig 2.
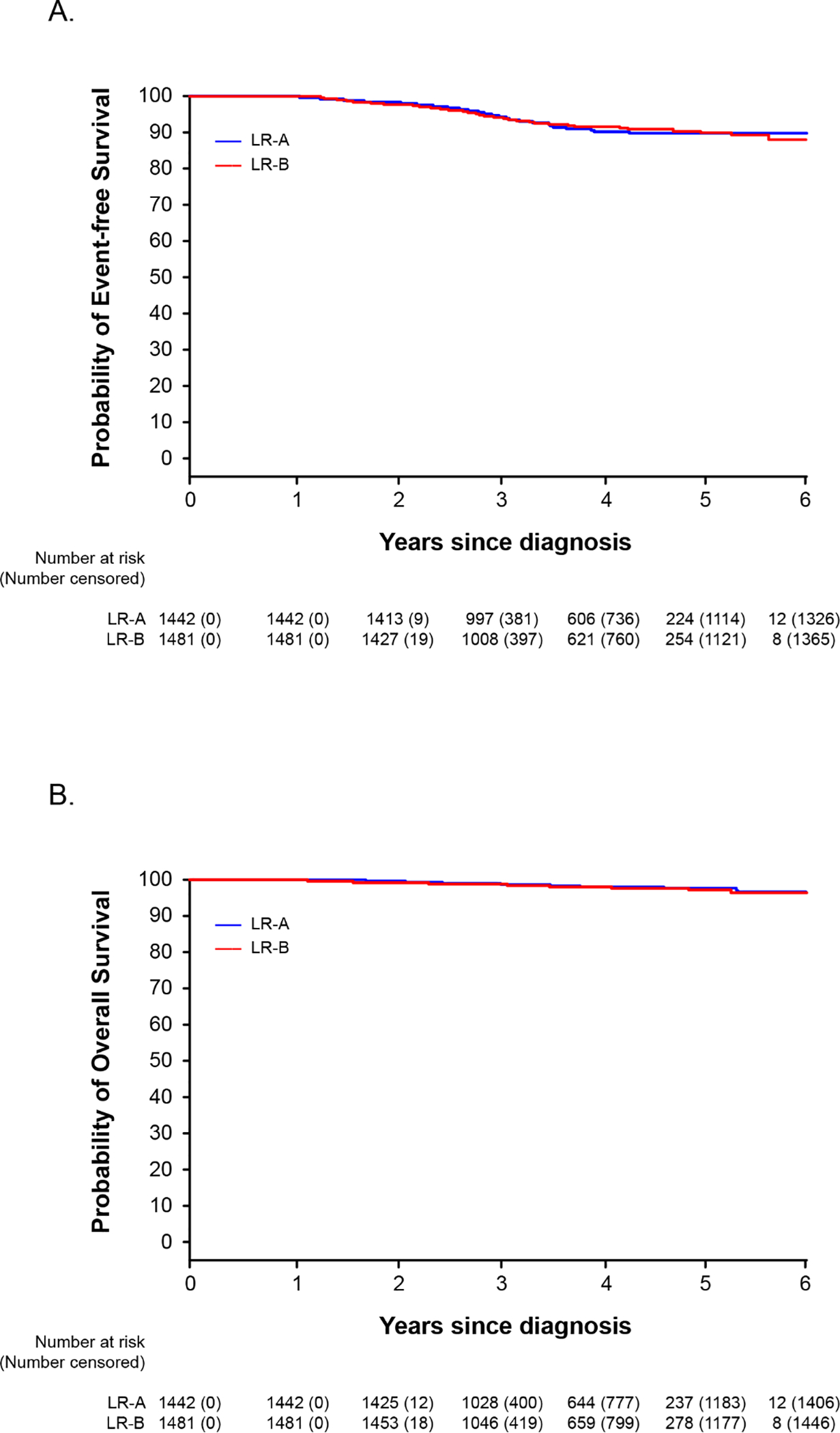
Kaplan-Meier analyses of event-free survival (A) and overall survival (B) of patients with low-risk ALL who were randomized to receive (Group A) or not receive (Group B) seven pulses of vincristine plus dexamethasone after Week 54 of continuation treatment.
Among the patients with intermediate-/high-risk ALL, the one-sided 95% upper confidence bound for the difference in the probability of 5-year event-free survival was 0.055, which slightly exceeded the pre-set non-inferiority margin by 0.005, a borderline inferior result for Group B. The non-inferiority analysis for 5-year overall survival showed a non-inferior result of 0.013. Secondary analysis showed no significant differences between Group A and Group B in the probability of 5-year event-free survival (82.8% [(95% CI, 80.0%−85.7%] vs. 80.8% [95% CI, 77.7%−84.0%], P=0.90; figure 3A) or overall survival (92.3% [95% CI, 90.3%−94.4%] and 93.4% [95% CI, 91.4%−95.4%], P=0.40; figure 3B). There were no significant differences between Group A and Group B in the 5-year cumulative risk of isolated CNS relapse (1.7% [95% CI, 0.9%−2.5%] vs. 1.2% [95% CI, 0.5%−1.9%], P=0.36), any CNS relapse (2.4% [1.2%−3.6%] vs. 2.4% [95% CI, 1.3%−3.5%], P=0.89), any relapse (15.6% [95% CI, 12.9%−18.3%] vs. 17.8% [95% CI, 14.8%−20.8%], P=0.68), and death during remission (0.6% [95% CI, 0.1%−1.1%] vs. 0.5% [95% CI, 0.1%−0.9%], P=0.78; appendix p 10).
Fig 3.
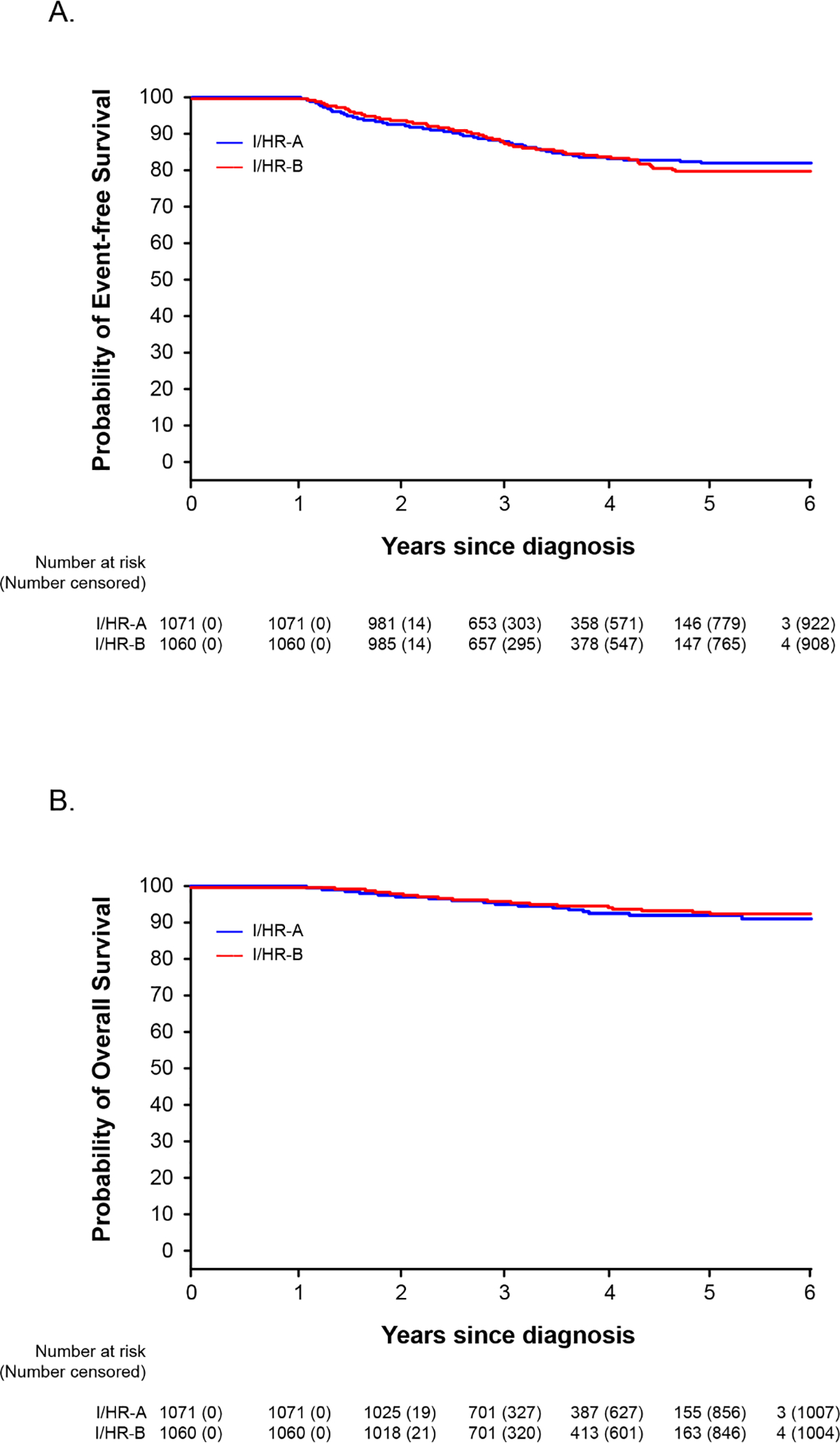
Kaplan-Meier analyses of event-free survival (A) and overall survival (B) of patients with intermediate-/high-risk ALL who were randomized to receive (Group A) or not receive (Group B) seven pulses of vincristine plus dexamethasone after Week 54 of continuation treatment.
Similarly, when the analyses were limited to the patients with intermediate-risk ALL, the 95% upper confidence bound for the difference in 5-year event-free survival probability was 0.056, which slightly exceeded the pre-set non-inferiority margin, a borderline inferior result for Group B; and that for overall survival probability was 0.016, a non-inferior result. There were also no significant differences between Group A and Group B in the 5-year event-free survival (82.7% [(95% CI, 79.9%−85.7%] vs. 80.7% [95% CI, 77.6%−83.9%], P=0.90) or 5-year overall survival (92.5% [95% CI, 90.6%−94.6%] vs. 93.3% [95% CI, 91.3%−95.3%], P=0.50 (appendix p 11).
Among the patients with low-risk ALL, adverse events occurred in 104 of 1442 (7.2%) Group A patients and in 108 of 1481 (7.3%) Group B patients (P=0.45). There was no significant heterogeneity of treatment effect on the event-free survival among subgroups by age, sex, CNS status, presenting leukocyte count, leukemia subtypes, or MRD levels during induction in the low-risk group (appendix p 12). Among the patients with intermediate-/high-risk ALL, adverse events occurred in 146 of 1071 (13.6%) Group A patients and in 148 of 1060 (14%) Group B patients (P=0.80). There was no significant heterogeneity of treatment effect on the event-free survival among various subgroups (appendix p 13). Among patients with intermediate-risk ALL, adverse events occurred in 144 of 1056 (13.6%) Group A patients and in 148 of 1051 (14.1%) Group B patients (P=0.62). There was also no significant heterogeneity of treatment effect on the event-free survival among various subgroups (appendix p 14).
Analyses were also performed by classifying patients based on the actual treatment that they received (i.e., with or without the additional pulse therapy). Among patients with low-risk ALL, there were no significant differences in the 5-year event-free survival (P=0.20) or the 5-year overall survival (P=0.40) between the 1660 patients treated in Group A and the 1263 in Group B; the one-sided 95% upper confidence bound of the difference showed non-inferior results for both (0.043 and 0.023, respectively) (appendix p 15). Among patients with intermediate-/high-risk ALL, there were also no differences in the 5-year event-free survival (P=0.50) or the 5-year overall survival (P=0.90) between the 1242 patients treated in Group A and the 889 in Group B; the one-sided 95% upper confidence bound of the difference in the 5-year event-free probability was 0.051, a very borderline result (exceeding the preset margin by 0.001), and that for overall survival was 0.023, a non-inferior result. Similar results were obtained when the analyses were limited to patients with intermediate-risk ALL alone (appendix p 15).
In the low-risk group, we found no significant differences in the rates of infections, symptomatic osteonecrosis, or other complications during the second year of continuation treatment between the patients treated with or without the additional pulses (table 2). In the intermediate-/high-risk group, we found that grade 3/4 pneumonia (P=0.01) and grade 3/4 vincristine-related peripheral neuropathy (P=0.03) were higher in Group A than in Group B. Similarly, in the intermediate-risk group alone, grade 3/4 severe pneumonia and vincristine-related peripheral neuropathy were higher in Group A than in Group B. Grade 5 fatal infection occurred in seven patients with low-risk ALL and in 11 with intermediate-/high-risk ALL, but the rate did not differ significantly between the randomized groups.
Table 2.
Comparison of toxicity between patients treated with (Group A) or without (Group B) vincristine plus dexamethasone pulses during the second year of continuation treatment.
| Toxicity* | Low-Risk ALL | Intermediate-/High-Risk ALL | Intermediate-Risk ALL | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Group A n=1442(%) |
Group B n=1481 (%) |
P-value | Group A n=1071 (%) |
Group B n=1060 (%) |
P-value | Group A n=1056(%) |
Group B n=1051 (%) |
P-value | |
|
| |||||||||
| Grade 5 (fatal) infection | 2 (0.14) | 5 (0.34) | 0.45 | 6 (0.56) | 5 (0.47) | 0.99 | 6 (0.57) | 5 (0.48) | 0.99 |
| Grade 3/4 Infection | 62 (4.30) | 50 (3.38) | 0.21 | 75 (7.00) | 64 (6.04) | 0.38 | 71 (6.72) | 63 (5.99) | 0.53 |
| Grade 3/4 Sepsis | 15 (1.04) | 10 (0.68) | 0.32 | 19 (1.77) | 21 (1.98) | 0.75 | 18 (1.70) | 21 (2.00) | 0.63 |
| Grade 3/4 Pneumonia | 17 (1.18) | 18 (1.22) | 0.99 | 26 (2.43) | 10 (0.94) | 0.01 | 23 (2.18) | 10 (0.95) | 0.03 |
| Grade 3/4 Osteonecrosis | 1 (0.07) | 2 (0.14) | 0.99 | 6 (0.56) | 8 (0.75) | 0.60 | 6 (0.57) | 8 (0.76) | 0.61 |
| Grade 3/4 Hyperglycemia | 3 (0.21) | 1 (0.07) | 0.37 | 17 (1.59) | 10 (0.94) | 0.24 | 17 (1.61) | 10 (0.95) | 0.24 |
| Grade 3/4 Hypertension | 2 (0.14) | 0 | 0.24 | 1 (0.09) | 3 (0.28) | 0.37 | 0 | 3 (0.29) | 0.12 |
| Grade 3/4 Vincristine-related peripheral neuropathy | 14 (0.97) | 11 (0.74) | 0.55 | 17 (1.59) | 6 (0.57) | 0.03 | 17 (1.61) | 6(0.57) | 0.03 |
Grade 1/2 toxicities are not included.
Discussion
The omission of seven pulses of vincristine plus dexamethasone therapy during the second year of continuation therapy did not adversely affect treatment outcome of childhood ALL, as measured by event-free survival, overall survival, and the cumulative risk of any relapse, in the largest cohort of patients studied to date. All clinical or biological subgroups for both low-risk and intermediate-/high-risk disease, except for Philadelphia chromosome–positive ALL, were included.
With contemporary treatment regimens, the rate of relapse after completion of treatment is less than 10%; 60% of relapsed ALL cases occur within the first 2 years,25 and the rate is even lower among those with low-risk disease.26 Hence, the lack of inferiority of eliminating pulse therapy in the second year for our low-risk group should not change with longer follow-up. The finding for patients in this group is not surprising. In the Tokyo Children’s Cancer Study Group’s L92–13 study, in which continuation therapy was terminated after 1 year of treatment, patients with TCF3–PBX1 or ETV6–RUNX1 fusion oncogenes had excellent 10-year disease-free survival of 90.9% or 93.8%, respectively.27 For the vast majority of patients with these favorable genetic subtypes of ALL, especially those who experience negative MRD after remission induction,26 additional therapy, including the pulse therapy beyond 1 year of treatment, is most likely superfluous. To this end, an additional course of delayed intensification, including 3 weeks of treatment with vincristine plus dexamethasone during the first year of treatment, also failed to improve the outcome of patients with B- or T-ALL with good early treatment response in the Children’s Cancer Group 1961 protocol,7 and for those with low-risk ALL, as defined by MRD at the end of induction in the UKALL 2003 study.9
For patients with intermediate-/high-risk ALL, there were also no significant differences in the event-free survival and overall survival between patients treated with or without additional pulse therapy. However, in the intent-to-treat and as-treated analyses, we did not obtain sufficient statistical evidence to support non-inferiority for patients who did not receive the additional pulse therapy beyond I year of treatment, by the preset statistical design, though the criterion statistic exceeded the preset non-inferiority margin (0.05) only by 0.005 and 0.001, respectively. Although more patients with a KMT2A rearrangement were treated in Group B without additional pulse therapy, patients with this genotype had comparable event-free survival in both treatment groups (appendix p13). The non-inferiority result for the analysis of overall survival may reflect more salvageable relapses in patients who did not receive pulse therapy than in those who did, an observation also made by Eden et al.1 The recent Children’s Oncology Group AALL0932 study showed that decreased frequency of vincristine plus dexamethasone pulses from every 4 weeks to every 12 weeks during continuation treatment yielded the same outstanding event-free survival and overall survival results for their patients with average-risk B-ALL.28 However, the AALL0932 study was not designed as a non-inferiority trial. Therefore, additional studies are needed for patients with average-risk or intermediate-/high-risk ALL.
As expected, infectious complications were observed more often among patients with intermediate-/high-risk disease who received the pulses during the second year of continuation treatment, when they also received cyclophosphamide and cytarabine. The lack of difference in the incidence of osteonecrosis is not surprising because this complication generally occurs early in therapy (i.e., before the time of randomization in this protocol).29 The very low incidence in both groups is consistent with the result of a recent study in Japan, suggesting that East Asians may have a lower risk of this complication.30 It also could be due to the fact that surveillance diagnostic imaging was not performed in this study, leading to the underestimate of this complication because some cases were asymptomatic, and other mild cases of osteonecrosis in children younger than 10 years old might have healed spontaneously.29
There are several limitations in this study. First, because of the limited resources, there was an inadequate number of clinical research associates to capture data on grade 1–2 toxicities. Second, for the same reason, we were not able to collect data on actual treatment doses received by the patients to analyze the effect of dose intensity on outcome. Third, the COVID-19 pandemic further compromised our ability to collect data and enroll additional patients to increase the statistical confidence of our data.
Although we may not have captured all treatment-induced toxicity data, patients who were randomized to not receive additional pulse therapy would most likely have a better quality of life during treatment due to less steroid-induced neuropsychological side effects or vincristine-induced peripheral neuropathy. Future studies of long-term survivors of childhood ALL are needed to determine if the omission of the seven pulses of vincristine plus dexamethasone would significantly decrease the long-term sequelae associated with vincristine and glucocorticoid treatment, such as peripheral neuropathy, metabolic syndrome, muscle weakness, growth retardation, and decreased energy balance.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for reports published in English up to January 2020, using the terms “vincristine,” “dexamethasone,” and “pediatric acute lymphoblastic leukemia.” Of the 385 articles identified, 10 were randomized trials that included vincristine plus dexamethasone pulses, and all involved children with newly diagnosed intermediate- or average-risk ALL. Except for one trial that enrolled patients between 2010 and 2018, the other trials treated patients between 1995 and 2002. Only one trial conducted between 1999 and 2002 showed improved disease-free survival; the other nine showed a lack of benefit of six or more additional pulses, a finding attributed to the use of intensive backbone therapy or early intensification treatment with other drugs. Despite the lack of randomized trials in patients with low- or high-risk ALL and the inconclusive results in the studies of patients with intermediate-risk disease, most contemporary clinical trials incorporated pulses of vincristine plus dexamethasone throughout continuation therapy. We, therefore, conducted a large randomized trial to determine if removing the pulses of vincristine plus dexamethasone in late continuation treatment for any risk groups of childhood ALL would not lead to inferior outcome.
Added value of this study
To our knowledge, this is the first study evaluating vincristine plus dexamethasone pulses in all consecutive pediatric patients with newly diagnosed ALL treated in a contemporary risk-directed protocol. Our study showed that the omission of seven pulses during the second year of continuation therapy did not adversely affect treatment outcome, as measured by the event-free survival, the overall survival, and the cumulative risk of any relapse in all patients. However, the data about intermediate-/high-risk population need to be confirmed by additional studies.
Implications of all the available evidence
Omission of vincristine and dexamethasone pulses beyond 1 year of treatment does not compromise leukemia control and should decrease the acute toxicities and late sequelae in patients, as well as the burden on their families.
Acknowledgements
This study was supported by the VIVA China Children’s Cancer Foundation, the National Natural Science Foundation of China (grant 81670136, J.Y. Tang, J.Y. Cai; grant 81870131, X.F. Zhu; grant 81770175, Y.C. Zhang), the China fourth round of Three-Year Public Health Action Plan (2015–2017) (GWIV-25, S.H. Shen), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant 2017-I2M-1-015, X.F. Zhu), U.S. National Cancer Institute (grant CA21765, C. Cheng, C.-H. Pui), St. Baldrick’s Foundation (581580, H. Zhang), and the American Lebanese Syrian Associated Charities (ALSAC; C. Cheng, J.J. Yang, C.-H. Pui). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health.
Footnotes
Declaration of interests
CHP is on the scientific advisory board of Adaptive Biotechnology, Inc, the Data Monitoring Committee of Novartis, and has received honorariums from Amgen and Erytech. The other authors declared no conflicts of interest.
Data sharing
Individual participant data and other data and documents will not be shared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wenyu Yang, Department of Pediatrics, State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China.
Jiaoyang Cai, Department of Hematology/Oncology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, National Health Committee Key Laboratory of Pediatric Hematology & Oncology, Shanghai, China.
Shuhong Shen, Department of Hematology/Oncology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, National Health Committee Key Laboratory of Pediatric Hematology & Oncology, Shanghai, China.
Ju Gao, Department of Pediatrics, West China Second University Hospital, Sichuan University, Key Laboratory of Birth Defects and Related Disease of Women and Children, Ministry of Education, Chengdu, China.
Jie Yu, Department of Hematology/Oncology, Chongqing Medical University Affiliated Children’s Hospital, Chongqing, China.
Shaoyan Hu, Department of Hematology/Oncology, Children’s Hospital of Soochow University, Suzhou, China.
Hua Jiang, Department of Hematology/Oncology, Guangzhou Women and Children’s Medical Center, Guangzhou, China.
Yongjun Fang, Department of Hematology/Oncology, Children’s Hospital of Nanjing Medical University, Nanjing, China.
Changda Liang, Department of Hematology/Oncology, Jiangxi Provincial Children’s Hospital, Nanchang, China.
Xiuli Ju, Department of Pediatrics, Qilu Hospital of Shandong University, Jinan, China.
Xuedong Wu, Department of Pediatrics, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Xiaowen Zhai, Department of Hematology/Oncology, Children’s Hospital of Fudan University, Shanghai, China.
Xin Tian, Department of Hematology/Oncology, KunMing Children’s Hospital, Kunming, China.
Ningling Wang, Department of Pediatrics, Anhui Medical University Second Affiliated Hospital, Anhui, China.
Aiguo Liu, Department of Pediatrics, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Hui Jiang, Department of Hematology/Oncology, Children’s Hospital Affiliated to Shanghai Jiao Tong University, Shanghai, China.
Runming Jin, Department of Pediatrics, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Lirong Sun, Department of Pediatrics, Affiliated Hospital of Qingdao University, Qingdao, China.
Minghua Yang, Department of Pediatrics, Xiangya Hospital Central South University, Changsha, China.
Alex WK Leung, Department of Pediatrics, Hong Kong Children’s Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China.
Kaili Pan, Department of Hematology/Oncology, Xi ‘an Northwest Women’s and Children’s Hospital, Xi ‘an, China.
Yingchi Zhang, Department of Pediatrics, State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China.
Jing Chen, Department of Hematology/Oncology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, National Health Committee Key Laboratory of Pediatric Hematology & Oncology, Shanghai, China.
Yiping Zhu, Department of Pediatrics, West China Second University Hospital, Sichuan University, Key Laboratory of Birth Defects and Related Disease of Women and Children, Ministry of Education, Chengdu, China.
Hui Zhang, Department of Hematology/Oncology, Guangzhou Women and Children’s Medical Center, Guangzhou, China.
Chunfu Li, Department of Pediatrics, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Jun J Yang, Departments of Oncology, Global Pediatric Medicine, Biostatistics and Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Cheng Cheng, Departments of Oncology, Global Pediatric Medicine, Biostatistics and Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Chi-kong Li, Department of Pediatrics, Hong Kong Children’s Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China.
Jingyan Tang, Department of Hematology/Oncology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, National Health Committee Key Laboratory of Pediatric Hematology & Oncology, Shanghai, China.
Xiaofan Zhu, Department of Pediatrics, State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China.
Ching-Hon Pui, Departments of Oncology, Global Pediatric Medicine, Biostatistics and Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
References
- 1.Eden T, Pieters R, Richards S; Childhood Acute Lymphoblastic Leukaemia Collaborative Group (CALLCG). Systematic review of the addition of vincristine plus steroid pulses in maintenance treatment for childhood acute lymphoblastic leukaemia - an individual patient data meta-analysis involving 5,659 children. Br J Haematol. 2010June;149(5):722–33. [DOI] [PubMed] [Google Scholar]
- 2.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone Versus Prednisone and Daily Oral Versus Weekly Intravenous Mercaptopurine for Patients With Standard-Risk Acute Lymphoblastic Leukemia: A Report From the Children’s Cancer Group. Blood. 2003May15;101(10):3809–17. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of Dexamethasone Compared With Prednisolone for Childhood Acute Lymphoblastic Leukaemia: Results of the UK Medical Research Council ALL97 Randomized Trial Br J Haematol. 2005June;129(6):734–45. [DOI] [PubMed] [Google Scholar]
- 4.Conter V, Valsecchi MG, Silvestri D, et al. Pulses of vincristine and dexamethasone in addition to intensive chemotherapy for children with intermediate-risk acute lymphoblastic leukaemia: a multicentre randomised trial. Lancet. 2007;369(9556), 123–131. [DOI] [PubMed] [Google Scholar]
- 5.Aricò M, Valsecchi MG, Rizzari C, et al. Long-term results of the AIEOP-ALL-95 Trial for Childhood Acute Lymphoblastic Leukemia: insight on the prognostic value of DNA index in the framework of Berlin-Frankfurt-Muenster based chemotherapy. J Clin Oncol. 2008;26(2), 283–289. [DOI] [PubMed] [Google Scholar]
- 6.De Moerloose B, Suciu S, Bertrand Y, et al. Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): report of the EORTC randomized phase 3 trial 58951. Blood. 2010July8;116(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008March1;111(5):2548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013March;14(3):199–209. [DOI] [PubMed] [Google Scholar]
- 10.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014July;15(8):809–18. [DOI] [PubMed] [Google Scholar]
- 11.Liu HC, Yeh TC, Hou JY, et al. Triple intrathecal therapy alone with omission of cranial radiation in children with acute lymphoblastic leukemia. J Clin Oncol. 2014June10;32(17):1825–9. [DOI] [PubMed] [Google Scholar]
- 12.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015December;16(16):1677–90. [DOI] [PubMed] [Google Scholar]
- 13.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. J Clin Oncol. 2016August1;34(22):2591–601. [DOI] [PubMed] [Google Scholar]
- 14.Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018March;32(3):606–615. [DOI] [PubMed] [Google Scholar]
- 15.Winter SS, Dunsmore KP, Devidas M, et al. Improved Survival for Children and Young Adults With T-Lineage Acute Lymphoblastic Leukemia: Results From the Children’s Oncology Group AALL0434 Methotrexate Randomization. J Clin Oncol. 2018October10;36(29):2926–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeha S, Pei D, Choi J, et al. Improved CNS Control of Childhood Acute Lymphoblastic Leukemia Without Cranial Irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019December10;37(35):3377–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salzer WL, Burke MJ, Devidas M, et al. Impact of Intrathecal Triple Therapy Versus Intrathecal Methotrexate on Disease-Free Survival for High-Risk B-Lymphoblastic Leukemia: Children’s Oncology Group Study AALL1131. J Clin Oncol. 2020June4:JCO1902892. : 10.1200/JCO.19.02892.Epub ahead of print. PMID: 32496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang J, Yu J, Cai J, et al. Prognostic factors for CNS control in children with acute lymphoblastic leukemia treated without cranial irradiation. Blood (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Chen J, Tang J, et al. Cost of childhood acute lymphoblastic leukemia care in Shanghai, China. Pediatr Blood Cancer. 2009;53(4):557–562. [DOI] [PubMed] [Google Scholar]
- 20.Shen S, Chen X, Cai J, et al. Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia Chromosome-positive acute lymphoblastic leukemia: a randomized clinical trial. JAMA Oncol. 2020January16;6(3):358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelen M The randomization and stratification of patients to clinical trials. J Chron Dis. 1974; 27:365–375. [DOI] [PubMed] [Google Scholar]
- 22.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure time Data, Second Edition. John Wiley and Sons, Inc. 2002. pp.254–255. [Google Scholar]
- 23.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics, 16:1141–1154, 1988. [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pui CH, Pei D, Campana D, et al. A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia. 2014December;28(12):2336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pui CH, Pei D, Raimondi SC, et al. Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with Response-Adapted therapy. Leukemia. 2017February;31(2):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato M, Ishimaru S, Seki M, et al. Long-term outcome of 6-month maintenance chemotherapy for acute lymphoblastic leukemia in children. Leukemia. 2017March;31(3):580–584. [DOI] [PubMed] [Google Scholar]
- 28.Angiolillo AL, Schore RJ, Kairalla JA, et al. Excellent Outcomes With Reduced Frequency of Vincristine and Dexamethasone Pulses in Standard-Risk B-Lymphoblastic Leukemia: Results From Children’s Oncology Group AALL0932 [published online ahead of print, 2021 Jan 7]. J Clin Oncol. 2021;JCO2000494. doi: 10.1200/JCO.20.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaste SC, Pei D, Cheng C, et al. Utility of early screening magnetic resonance imaging for extensive hip osteonecrosis in pediatric patients treated with glucocorticoids. J Clin Oncol. 2015;33(6):610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakamoto K, Imamura T, Kihira K, et al. Low Incidence of Osteonecrosis in Childhood Acute Lymphoblastic Leukemia Treated With ALL-97 and ALL-02 Study of Japan Association of Childhood Leukemia Study Group. J Clin Oncol. 2018;36(9):900–907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


