Abstract
Background:
Patients with Tetralogy of Fallot with pulmonary stenosis (ToF/PS), the most common form of cyanotic congenital heart disease (CHD), develop adverse right ventricular (RV) remodeling, leading to late heart failure and arrhythmia. We recently demonstrated that overactive p-adrenergic receptor signaling inhibits cardiomyocyte division in ToF/PS infants, providing a conceptual basis for the hypothesis that treatment with the P-adrenergic receptor blocker, propranolol, early in life would increase cardiomyocyte division. No data are available in ToF/PS infants on the efficacy of propranolol as a possible novel therapeutic option to increase cardiomyocyte division and potentially reduce adverse RV remodeling.
Methods:
Using a randomized, double-blind, placebo-controlled trial, we will evaluate the effect of propranolol administration on reactivating cardiomyocyte proliferation to prevent adverse RV remodeling in 40 infants with ToF/PS. Propranolol administration (1 mg/kg po QID) will begin at 1 month of age and last until surgical repair. The primary endpoint is cardiomyocyte division, quantified after 15N-thymidine administration with Multi-isotope Imaging Mass Spectrometry (MIMS) analysis of resected myocardial specimens. The secondaty endpoints are changes in RV myocardial and cardiomyocyte hypertrophy.
Conclusion:
This trial will be the first study in humans to assess whether cardiomyocyte proliferation can be pharmacologically increased. If successful, the results could introduce a paradigm shift in the management of patients with ToF/PS from a purely surgical approach, to synergistic medical and surgical management. It will provide the basis for future multi-center randomized controlled trials of propranolol administration in infants with ToF/PS and other types of CHD with RV hypertension.
Clinical trial registration:
The trial protocol was registered at clinicaltrials.gov (NCT04713657).
Keywords: Congenital heart disease, Tetralogy of Fallot, Cardiomyocyte division, Beta-blocker, Ventricular hypertrophy
1. Introduction
Congenital heart disease (CHD) is the most common birth defect, affecting almost 40,000 infants each year in the US [1,2]. Improved diagnosis and surgical care have led to 1 million patients living with CHD in the US [3] in whom the right ventricle (RV) is often negatively impacted. Patients with Tetralogy of Fallot with pulmonary stenosis (ToF/PS), the most common form of cyanotic CHD, have prolonged RV hypertension as infants, and subsequently develop RV hypertrophy. These patients undergo cardiac surgery early in life to physiologically correct the defects. Although patients with ToF/PS undergo a complete repair, they are at risk for the development of heart failure [4] and arrythmia [5] later in life. This is likely multifactorial due to both residual lesions, i.e., pulmonary valve insufficiency and stenosis, as well as intrinsic differences in cardiomyocyte proliferation. The current understanding of the underlying pathobiology and therapies in this population are very limited [3].
Cardiomyocyte proliferation is a biological basis of heart muscle growth during development and regeneration [6]. Whether and how CHD could alter cardiomyocyte proliferation is a relatively new research direction. It has been thought that the RV remodeling in ToF patients is a consequence of surgical repair and the resulting anatomic lesions [7]. However, we have recently shown that ToF/PS patients already have decreased cardiomyocyte cycle activity [8] and division at the time of surgery [9], suggesting an independent role for decreased cardiomyocyte proliferation in the development of CHD-associated RV heart failure that had not been previously acknowledged.
Although the need for cardiomyocyte regeneration in adult patients is high, cardiomyocyte division is very rare in adults [10,11]. However, studies in mammalian model organisms and humans demonstrate cardiomyocyte proliferation in the young [10,12]. In humans, the percentages of cardiomyocytes in mitosis and cytokinesis are the highest in infants [10]. As such, the barrier for stimulating cardiomyocyte proliferation in infants seems to be lower than in adults, making infants the ideal patient population for strategies to promote cardiomyocyte proliferation. Additionally, patients with ToF/PS require surgical repair during infancy, which includes myocardial resection, providing a source of myocardium to study at no additional risk to the patient.
Excessive cardiac hypertrophy, as well as decreased cardiomyocyte cell cycle activity [8] and division [9] are characteristics of ToF/PS. Studies in animals and humans show that a high prevalence of bi- and multi-nucleated cardiomyocytes and cardiomyocytes with polyploid nuclei indicates decreased ability to proliferate [10,13–15]. The percentage of bi- and multi-nucleated cardiomyocytes indicates the degree to which failure of cardiomyocyte division occurred. Infants with ToF/PS exhibit a 30% increase in cytokinesis failure relative to infants without heart disease [9]. We have demonstrated that increased β-adrenergic receptor signaling (β-AR), triggered by increased wall stress in the hypertensive RV in ToF/PS, decreases cardiomyocyte division [9]. β-AR activation is also a strong stimulus for cardiac hypertrophy [16,17]. Although both cardiomyocyte proliferation and hypertrophy are the basic mechanisms of heart growth during development, excessive hypertrophy is pathological and leads to adverse remodeling and heart failure. As such, β-blockers have the potential to be beneficial by increasing cardiomyocyte division and decreasing cardiomyocyte and myocardial hypertrophy in ToF/PS (Fig. 1)
Fig. 1.
The proposed study will quantify the basic mechanisms of heart growth, i.e., cardiomyocyte division and hypertrophy. The measurement for the primary outcome is cardiomyocyte division, and for the secondary outcome, cardiomyocyte and myocardial hypertrophy. β-Adrenergic receptor (β-AR) signaling inhibits cardiomyocyte division and stimulates cardiac hypertrophy. Cardiomyocyte division is the mechanism of proliferation, which counterbalances cardiac hypertrophy. If the results of the primary outcome show that cardiomyocyte division is increased, this may counteract cardiomyocyte hypertrophy and, thereby, reduce adverse cardiac hypertrophy.
This paper describes the rationale and design of the first clinical trial to determine if cardiomyocyte division can be altered therapeutically in humans. Quantification of cardiomyocyte cell cycle activity in humans is challenging since existing methods require administration of radiolabeled substances [18], antibodies to detect cell cycle phase-specific proteins [10,19], or carbon dating [11]. Each of these methods has specific limitations [20]. The presented trial overcomes these challenges by utilizing an innovative approach [20] of administering thymidine labeled with a stable isotope tag (15N-thymidine) that can be quantitatively imaged in individual cardiomyocyte nuclei with Multi-isotope Imaging Mass Spectrometry (MIMS) analyses of RV myocardial sections. This novel approach enables unequivocal visualization and quantification of the generation of mono- and binucleated cardiomyocytes and of polyploid cardiomyocyte nuclei in ToF/PS [9,18], which was previously not possible.
The goal of the current trial is to test if β-blocker administration, utilizing oral propranolol, can shift heart muscle growth from hypertrophic to proliferative mechanisms (Fig. 1). As such, the primary objective of this trial is to assess the effectiveness of propranolol administration for increasing cardiomyocyte division in infants with ToF/PS. The secondary objective is to evaluate the effect of oral propranolol on RV cardiomyocyte and myocardial hypertrophy.
2. Methods
2.1. Overview of the clinical trial design and relevant rationale
This will be a single-center, randomized (1:1), double-blind, placebo-controlled clinical trial design of 40 infants with ToF/PS. Permuted block randomization (block size of 4) will be used and implemented via a web-based data management system. Infants will receive the study drug starting at 1 month after birth, which will continue until surgical repair (commonly between 2 and 9 months of age). 15N-thymidine will be administered one week after the initiation of the study drug to label newly generated cardiomyocytes. RV size, mass, and function will be determined at baseline (prior to administration of the study drug) and at the time of surgical repair (Fig. 2). Pieces of RV myocardium will be resected during surgery and analyzed with MIMS to quantify cardiomyocyte division and with histology to quantify their size. The clinical trial protocol has been approved by the Institutional Review Board at the University of Pittsburgh (STUDY20020132) and the Federal Drug Administration (IND 150748), and was registered at clinicaltrials.gov (NCT04713657).
Fig. 2.
Diagram of the nial design depicts temporal relationship of assessments to study drug administration. The administration of 15N-thymidine enables direct quantification of cardiomyocyte division to compare propranolol and placebo at the end of the study period. Echocardiograms and cardiac MRIs will be performed at baseline (before begin of propranolol administration) and at the end of the study period (24 h after the last dose of propranolol) to avoid biasing the researchers who might recognize a lower heart rate in propranolol-ueated study patients.
2.2. Study population
All potentially eligible patients who receive care at the University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh and UPMC Magee Women’s Hospital will be considered. Inclusion and exclusion criteria are listed in Table 1. All participating subject families will provide written, informed consent before any study-specific evaluations or procedures are performed.
Table 1.
Inclusion and exclusion criteria.
| Inclusion | • Diagnosis of ToF/PS by echocardiogram • <1 month of age • >2 kg at the time of consent • Tolerating enteral feeds • Parental consent • English speaking |
| Exclusion | • Infants of diabetic mothers, asthma, or underlying respiratory disease • Double outlet right ventricle (DORV) variant of ToF/PS • Gestation age < 35 weeks • Known genetic disorders such as trisomy 21 or 22q deletion syndromes, Alagille syndrome • Major aortopulmonary collateral arteries • Congenital atrio-ventricular block on EKG (PR interval >120 ms) • Severe ventricular dysfunction assessed qualitatively • Current β-blocker use • Current intravenous inotropic drugs • Concomitant medication administration that interacts with propranolol • Inability to complete Cardiac MRI • Ongoing or planned participation in another research protocol that would either prevent successful completion of planned study testing or invalidate its results • Noncardiac medical, psychiatric, and/or social condition that would prevent successful completion of planned study • Cardiac care, ongoing or planned, at a nonstudy center that would impede study completion • Refusal of parents to provide written informed consent/assent • In the opinion of the primary cardiologist, the patient family is likely to be noncompliant with the study protocol |
2.3. Sample size justification
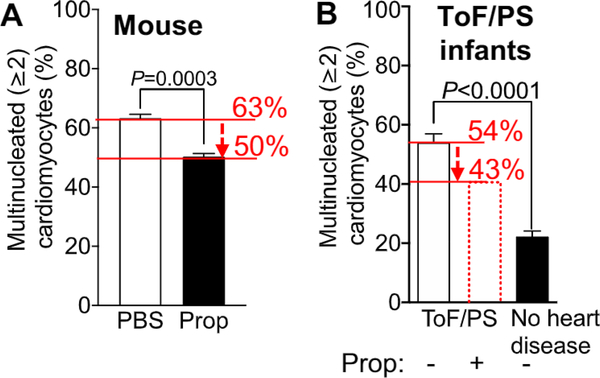
Our previous work established the expected average percentage level of bi- and multinucleated cardiomyocytes (primary outcome) in ToF/PS patients in the placebo group at 54% (SD = 8.8%, n = 8) [9]. To estimate the anticipated difference due to intervention, we utilized our mouse results [9], as no data exist for humans. We have shown that propranolol-treated mice have 50% multi-nucleated cardiomyocytes compared with 63% in control mice, indicating a difference of 13% (Fig. 3A). Because infants with ToF/PS have 54% multinucleated cardiomyocytes, i.e., 9% lower than mice (Fig. 3B), we anticipate that the effect size will be proportionately smaller than that in mice, i.e., 13% x 0.91 = 11%, a conservative effect size. To detect a propranolol-induced reduction of multinucleated cardiomyocytes from 54% to 43% with a 90% power, a sample size of 30 ToF/PS patients (n = 15 per group) is estimated, assuming the published SD = 8.8% [9] of bi- and multinucleated cardiomyocytes in humans in both groups, and using a Two-Sample T-Test with equal variance, α = 0.05. With an anticipated 75% retention rate in this trial, the calculated sample size has been inflated to 40 patients (20 per group).
Fig. 3.
The effect size of propranolol-increased cardiomyocyte division in mice and available results defining the similar proportion of binucleated caidiomyocytes in infants with ToF/PS provide an estimate of the anticipated effect size. (A) Mice naturally have increased cardiomyocyte cytokinesis failure, leading to the formation of 63% bi- and multi-nucleated cardiomyocytes by eight days after birth. Propranolol administration decreases the cytokinesis failure, resulting in 50% bi- and multi-nucleated cardiomyocytes, a 13% decrease. (B) Infants with ToF/PS have increased cardiomyocyte cytokinesis failure, leading to the development of 54% multinucleated cardiomyocytes by 6 months after birth. Because the underlying molecular mechanisms are the same in humans and in mice, the propranolol-induced decrease of cytokinesis failure is anticipated to be proportional to the effect in mice, reducing the percentage of bi- and multinucleated cardiomyocytes from 54 to 43% (indicated in the red dotted bar). Humans with no heart disease have 20% multinucleated cardiomyocytes, shown in the black bar, indicating that the anticipated effect size is a conseivative estimate. Results are from Liu et al., Sci Transi Med 2019.
2.4. Recruitment plan and feasibility
The trial protocol is designed to identify infants as early as possible on the basis of pre- and post-natal echocardiography diagnosis. In the past 5 years, an average of 15–18 infants each year undergo ToF/PS repair at our Heart Institute. Given an anticipated consent rate of 80%, it is feasible to enroll 10 to 13 patients per year for a total of 40 patients over 4 years.
2.5. Study intervention: drug administration, rationale for dose selection, and blinding
2.5.1. Study drug dosing, dosing interval, and safety
Patients will be randomized to either oral propranolol (1 mg/kg PO QID) or placebo. Safe and effective prophylaxis of supraventricular tachycardia in infants uses four doses per day of 1 mg/kg p.o. of propranolol [21]. Previous work in infantile hemangioma reported administration of 1.5 mg/kg po per dose given twice per day [21–24]. There is a long standing history of using propranolol in ToF/PS infants in dosing ranges of 2–6 mg/kg/day with no adverse effects on surgical morbidity [25–27]. With regard to the dosing interval, oral dose administration every 6 h is required to prevent hypercyanotic spells in ToF/PS patients [28]. Thus, the available data support the administration of 1 mg/kg propranolol p.o. every 6 h, corresponding to 4 mg/kg/day, in this trial. Potential side effects associated with propranolol use are rare and include trouble sleeping, bronchial irritation, hypoglycemia, hypotension, and bradycardia [29]. Patients will be observed during the initiation of the study drug. Study drug doses will be adjusted by weight every month and patients will be screened for side effects every two weeks. Protocols for screening, monitoring, and dosing of propranolol were developed from the American Academy of Pediatrics consensus recommendations for the initiation and treatment of infantile hemangioma [30].
2.5.2. Timing of study drug administration
In terms of the normal hemodynamic workload on the RV, the first month after birth is similar to the fetal circulation, i.e., the RV pressure is still high [31]. However, due to the pulmonary stenosis in ToF/PS, the RV does not experience the decline of pressure, which normally occurs in the first month after birth [32]. This leads to increased β-adrenergic receptor (β-AR) signaling in the RV myocardium [32]. Consequently, study drug administration will begin at 1 month after birth and continue until 24 h before ToF/PS surgery, when RV pressure returns to normal or near-normal (Fig. 2). This treatment regimen should cover the period of pathological β-AR signaling increase due to RV hypertension. Additionally, preoperative treatment with propranolol does not negatively impact post-operative varriables in infants with ToF/PS [27].
2.5.3. Study drug adherence
To determine adherence to the study drug, families will be provided with a written log to document administration, and bottles will be returned to the Research Pharmacy. Adherence will be assessed by measuring the remaining volume in the bottles and comparing it to the predicted remaining volume based on the prescribed administration, as well as by reviewing the administration log documents returned by families. We define an adherer as a study participant who received 85% of the study drug from both validation methods.
2.5.4. Blinding of investigators
To mask the study drug (double-blinding), propranolol and the placebo will be prepared by the UPMC Children’s Hospital of Pittsburgh Research Pharmacy using an identical oral sweetener and will be dispensed in identical bottles. The researchers will remain blinded as to the contents of the study drug containers. Assessments (echocardiography, MRI) will be performed before (baseline assessments) and after study drug administration (prior to surgical repair), so as not to bias the researchers with the possible heart rate reduction of propranolol. Physicians interpreting both the echocardiogram and cardiac MRI will also be blinded to treatment assignment.
2.6. Trial outcomes
2.6.1. The primary study end point: cardiomyocyte division
Multi-isotope Imaging Mass Spectrometry (MIMS) will be used to quantify cardiomyocyte division. Using our innovative approach [20] of administering 15N-thymidine followed by MIMS analysis of myocardial samples, individual cardiomyocyte nuclei will be imaged so that newly generated mono- and binucleated cardiomyocytes and cardiomyocytes with polyploid nuclei can be quantified. The use of stable isotopes as chemical labels is innocuous in vivo [33], in contrast to potential toxicities of labeling with radioactive isotopes, fluorescent proteins, and halogens. In addition, 15N-thymidine is stable over time compared with immunofluorescence microscopy detection of cell cycle proteins, which are subject to degradation.
MIMS measures stable isotope tracers with a new-generation secondary ion mass spectrometer (NanoSIMS) that incorporates crucial advances in ion optics. MIMS synergistically merges imaging resolution, approaching that of transmission electron microscopy with the power of quantitative analysis by mass spectrometry. MIMS has recently been applied to a range of fundamental biological questions [34,35] and to examine the division of blood [36] and fat cells in humans [37].
Right ventricular myocardial samples will be processed for MIMS. Ten areas (400 × 400 μm) per heart, selected by random-systematic sampling to reduce bias, will be examined. A researcher blinded to sample identity will identify cardiomyocytes by their association with sarcomeres, as previously described [20]. 15N-positive mononucleated cardiomyocytes indicate cell division, 15N-positive bi–/multinucleated cardiomyocytes indicate failure of cell division, and 15N-positive cardiomyocyte nuclei indicate failure of nuclear division [38]. To determine mean percentages of mono- and bi/multi-nucleated cardiomyocytes, we will quantify the number of 15N-thymidine-positives in ≥1000 mononucleated cardiomyocytes and in ≥1000 bi–/multinucleated cardiomyocytes per heart using digital analysis as described [9,20]. The percentages of unlabeled, as well as labeled, mono- and bi–/multinucleated cardiomyocytes will be calculated and compared between the trial groups. Although we anticipate that the percentage of cardiomyocytes with polyploid nuclei does not change in ToF/PS infants [9], we will quantify the nuclear ploidy of 15N-positive and -negative cardiomyocyte nuclei in the mono- and binucleated cardiomyocyte populations.
2.6.2. The secondary study end points: cardiomyocyte and RV myocardial hypertrophy
To quantify RV cardiomyocyte hypertrophy, we will examine RV myocardial pieces obtained on the day of surgery and quantify mean cardiomyocyte volume as described [9,10,39].
Cardiac MRI and transthoracic echocardiography [40] will be used to measure RV size, function, and mass. Both imaging modalities will be performed at one month of age, i.e., immediately before study drug administration, and on the day of surgery, i.e., after the end of study drug administration (see Fig. 2). The baseline MRI will be performed using a feed and bundle technique. The second MRI will be performed under general anesthesia in conjunction with surgical repair. Measurements will be made by a single physician and verified by a second reader, and both will be blinded to treatment assignment. All quantitative measurements will be indexed to body surface area (BSA). Both MRIs will follow the same protocol and will be read by a single physician. Echocardiograms will also follow the same protocols and will be read by two physicians, any difference between subjective assessment of ventricular size and function will be averaged between both reviewers.
Cardiac MRIs will provide measures for RV end diastolic and systolic volumes, and RV mass, all calculated by standard segmentation of the myocardial, endocardial, and epicardial borders in a short-axis cine steady-state free precession (SSFP) stack using Circle cvi42 software (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada) [41]. Anterior RV wall thickness will also be measured to compare to the 2-dimensional échocardiographie measurements. RV mass estimates are not standard given the typically thin RV myocardium; however, the RV hypertrophy seen in ToF/PS is amenable to quantification by MRI, and reproducibility of RV volumes and mass have been previously demonstrated [42]. Limited phase contrast imaging in the main pulmonary artery and ascending aorta will assist in verifying ventricular volumes and, thus, myocardial mass.
Standard 2-dimensional, M-mode, color, and spectral Doppler échocardiographie interrogation will be performed on both the RV and LV according to guidelines by the American Society of Echocardiography [40]. RV wall thickness as a surrogate for myocardial mass will be measured in the parasternal short axis and subcostal sagittal views, as previously validated [43]. Following 2-dimensional échocardiographie acquisition, a 3-dimensional full volume whole-heart acquisition will be performed from both apical and subcostal coronal windows. Subsequent analysis with the “4D RV-FUNCTION” tool using TOMTEC-ARENA (TOMTEC Imaging Systems GmbH, Germany) will provide both enddiastolic and end-systolic volume measurements, ejection fraction, stroke volume, and RV global longitudinal strain values, i.e., complete evaluation of the RV. Additional analysis of RV epicardial volumes in short-axis reconstructions of the volumetric data will allow for the calculation of RV mass, as previously validated [44]. Diastolic function will be assessed by tricuspid and mitral inflow valve spectral Doppler patterns, tissue Doppler assessment of the valve annuli, and 2-dimensional assessment of atrial size.Table 2 provides measurements to be obtained via MRI and transthoracic echocardiogram.
Table 2.
RV myocardial hypertrophy assessments at one-month age and at time of surgery.
| Cardiac MRI | RV end-diastolic volume RV end-systolic volume RV myocardial mass RV wall thickness (anterior free wall) |
| Transthoracic echocardiogram | RV end-diastolic volume RV end-systolic volumes RV myocardial mass RV wall thickness (anterior free wall) Tricuspid inflow spectral Doppler patterns Tissue Doppler assessment of the valve annuli Atrial size RV global longitudinal strain values |
2.7. Statistical analysis
An “intention to treat” approach will be used. The primary outcome (percentage of 15N-thymidine positive bi—/multinucleated cardiomyocytes) will be compared between the two groups using a Mann-Whitney test with an α-level = 0.05. Both within and between groups, changes in RV hypertrophy measured by MRI (myocardial volume), echocardiography (wall thickness), and cardiomyocyte volume will be assessed. The Wilcoxon signed-rank test will be used to test changes within each group, while the Mann-Whitney test will be used to test whether changes in measurements of RV hypertrophy differ between groups.
2.8. Ethical considerations and study oversight
The study will be conducted in compliance with the Good Clinical Practice protocols and Declaration of Helsinki principles. The trial will be controlled and monitored by a Steering Committee and a Data Safety and Monitoring Board (DSMB), which will be provided by the University of Pittsburgh Clinical and Translational Sciences Institute. The trial will maintain timely, accurate, and complete safety information, in order to ensure the protection of study participants. All adverse events will be reported to the DSMB every 6 months and serious adverse events will be reported to the Medical Safety Officer within 24 h of each event occurrence.
3. Discussion
β-blockers have been used for decades in patients with ToF to prevent and treat cyanotic attacks and prevent dyspneic spells [25,28]. A previous randomized clinical trial of propranolol-administration in adults who had undergone surgical correction of ToF did not indicate any beneficial effect on right ventricular dysfunction [45]. However, in ToF/PS, most cytokinesis failure occurs 1–3 months after birth [9]; thus, early administration of β-blockers is likely critical for maximizing cardiomyocyte division [9]. No previous study has assessed a potential beneficial effect of β-blockers on cardiomyocyte cytokinesis in infants with ToF/PS early in life. Prior research by our group identified a critical decrease of cardiomyocyte proliferation in infants with ToF/PS [8,9], indicating stimulation of heart regeneration as a potential novel therapeutic strategy.
This mechanistic trial will test if propranolol can stimulate endogenous cardiomyocyte division in infants. Our previous work provides the basic mechanistic pathway through which β-blockers might increase cardiomyocytes division [9]. We showed that Ect2, a critical protein triggering the constriction of the cleavage furrow during cell division [13], is downregulated in cycling cardiomyocytes of infants with ToF/PS. We additionally showed that the Hippo tumor suppressor pathway decreases Ect2 gene expression, thus reducing cardiomyocyte cytokinesis. In cardiomyocytes, β-adrenergic receptor signaling activates the Hippo pathway [46]. Our work in mice showed that β-blockers could increase Ect2 levels, thus promoting cytokinesis and cardiomyocyte proliferation.
Major strengths of our trial includes the use of an unequivocal assay to quantify cardiomyocyte proliferation. The MIMS approach, using a stable isotope label (15N), avoids limitations of traditional approaches, such as the possible degradation of cell cycle markers whose detection is used to calculate cardiomyocyte proliferation. The comprehensive assessment of the secondary outcome is another strength of our study. Cardiomyocyte hypertrophy will be assessed at both the clinical level, using echocardiography and cardiac MRI, as well as at the cellular level, with microscopy.
In summary, the anticipated results of this trial will validate β-blocker therapy to increase cardiomyocyte proliferation in CHD. If successful, this will be the first definitive demonstration in humans that cardiomyocyte generation can be pharmacologically modified. The anticipated results will advance a paradigm in which increased β-adrenergic receptor signaling reduces cardiomyocyte proliferation and validate a strategy to interrupt this pathology with β-blocker administration. This initial clinical trial will provide the basis for future multi-center randomized controlled trials of propranolol administration in infants with ToF/PS and other types of CHD with prolonged infantile and childhood exposure to RV hypertension, such as hypoplastic left heart syndrome and pulmonary hypertension. Conceptually, the anticipated results could introduce a new paradigm of combined medical and surgical interventions to manage CHD.
Acknowledgements
The authors thank the patients and their families who have contributed to the studies leading to the development of this protocol. We would also like to recognize the IRB (University of Pittsburgh) and the Federal Drug Administration for their efforts related to protocol development and comments to increase research patient safety. We would like to recognize the faculty and staff of the Heart Institute for their referrals of research subjects (UPMC Children’s Hospital of Pittsburgh). We thank Jennifer S. Li (Duke University) and Bryan H. Goldstein and Brian D. Feingold (University of Pittsburgh) for critical reading and commenting on this manuscript.
Sources of funding
This research was supported by the National Institutes of Health (NIH R01HL155597, R01HL151415, and R01HL106302), the Department of Pediatrics, the Richard King Mellon Institute for Pediatric Research at UPMC Children’s Hospital of Pittsburgh, Leducq Foundation (15CVD03), HeartFest (to BK), and DP2CA216362 (to MLS). JWY was supported, in part, by the NIH (T32HD071834) and by the Sang Park Endowed Fellowship Fund for Cardiology Fellows research. Research leading to this publication was supported by the NIH, National Center for Advancing Translational Sciences (NCATS) through Grant Number (s) UL1 TR001857, KL2 TR001856, and/or TL1 TR001858. The funding agencies had no influence on the contents of this manuscript.
Footnotes
Disclosures
BK and HL are inventors on a patent application (PCT/US20/41808; 62/873,483) filed by the University Pittsburgh that covers the use of β-blockers for preventing increased cardiomyocyte cytokinesis failure in pediatric patients. The authors declare that they have no further competing financial or other interests.
References
- [1].Hoffman JI, Kaplan S, The incidence of congenital heart disease, J. Am. Coll. Cardiol. 39 (12) (2002) 1890–1900. [DOI] [PubMed] [Google Scholar]
- [2].Wames CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, et al. , Task force 1: the changing profile of congenital heart disease in adult life, J. Am. Coll. Cardiol. 37 (5) (2001) 1170–1175. [DOI] [PubMed] [Google Scholar]
- [3].Bums KM, Byrne BJ, Gelb BD, Kuhn B, Leinwand LA, Mitai S, et al. , New mechanistic and therapeutic targets for pediatric heart failure: report from a National Heart, Lung, and Blood Institute working group, Circulation 130 (1) (2014) 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bradley E, Parker J, Novak E, Ludbrook P, Billadello J, Cedars A, Cardiovascular disease in late survivors of tetralogy of fallot: a tertiary care center experience, Tex. Heart Inst. J. 40 (4) (2013) 418–423. [PMC free article] [PubMed] [Google Scholar]
- [5].Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, et al. , Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study, Lancet 356 (9234) (2000) 975–981. [DOI] [PubMed] [Google Scholar]
- [6].Tzahor E, Poss KD, Cardiac regeneration strategies: staying young at heart, Science 356 (6342) (2017) 1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Geva T, Indications for pulmonary valve replacement in repaired tetralogy of fallot: the quest continues, Circulation 128 (17) (2013) 1855–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Polizzotti BD, Ganapathy B, Walsh S, Choudhury S, Ammanamanchi N, Bennett DG, et al. , Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window, Sci. Transi. Med. 7 (281) (2015) 281ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu H, Zhang CH, Ammanamanchi N, Suresh S, Lewarchik C, Rao K, et al. , Control of cytokinesis by beta-adrenergic receptors indicates an approach for regulating cardiomyocyte endowment, Sci. Transi. Med. 11 (2019) 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mollova M, Bersell KR, Walsh S, Savia J, Das LT, Park SY, et al. , Cardiomyocyte proliferation contributes to heart growth in young humans, Proc. Natl. Acad. Sci. U. S. A. 110 (4) (2013) 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. , Evidence for cardiomyocyte renewal in humans, Science 324 (5923) (2009) 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. , Dynamics of cell generation and turnover in the human heart, Cell 161 (7) (2015) 1566–1575. [DOI] [PubMed] [Google Scholar]
- [13].Gonzalez-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, et al. , Myocardial polyploidization creates a barrier to heart regeneration in Zebrafish, Dev. Cell 44 (4) (2018) 433–446 (e7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, et al. , Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration, Nat. Genet. 49 (9) (2017) 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bersell KR, Arab S, Haring B, Kuhn B, Neuregulinl/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury, Cell 138 (2) (2009) 257–270. [DOI] [PubMed] [Google Scholar]
- [16].Vidal M, Wieland T, Lohse MJ, Lorenz K, Beta-adrenergic receptor stimulation causes cardiac hypertrophy via a Gbetagamma/Erk-dependent pathway, Cardiovasc. Res. 96 (2) (2012) 255–264. [DOI] [PubMed] [Google Scholar]
- [17].de Lucia C, Eguchi A, Koch WJ, New insights in cardiac beta-adrenergic signaling during heart failure and aging, Front. Pharmacol. 9 (2018) 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Duque A, Rakic P, Different effects of bromodeoxyuridine and [3H] thymidine incorporation into DNA on cell proliferation, position, and fate, J. Neurosci. 31 (42)(2011) 15205–15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC, Reduced Ki67 staining in the postmortem state calls into question past conclusions about the lack of turnover of adult human beta-cells, Diabetes 64 (5) (2015) 1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yester JW, Liu H, Gyngard F, Ammanamanchi N, Little KC, Thomas D, Sullivan MLG, Lal S, Steinhauser ML, Kuhn B, Use of stable isotope-tagged thymidine and multi-isotope imaging mass spectrometry (MIMS) for prospective quantification of human cardiomyocyte division, Nat. Protoc. 16 (2021) 1995–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barton AL, Moffett BS, Valdes SO, Miyake C, Kim JJ, Efficacy and safety of high-dose propranolol for the management of infant supraventricular tachyarrhythmias, J. Pediatr. 166 (1) (2015) 115–118. [DOI] [PubMed] [Google Scholar]
- [22].Leaute-Labreze C, Boccara O, Degrugillier-Chopinet C, Mazereeuw-Hautier J, Prey S, Lebbe G, et al. , Safety of Oral Propranolol for the Treatment of Infantile Hemangioma: A Systematic Review, Pediatrics 138 (2016) 4. [DOI] [PubMed] [Google Scholar]
- [23].Krowchuk DP, Frieden IJ, Mancini AJ, Darrow DH, Blei F, Greene AK, et al. , Clinical practice guideline for the management of infantile hemangiomas, Pediatrics 143 (2019) 1. [DOI] [PubMed] [Google Scholar]
- [24].Leaute-Labreze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. , A randomized, controlled trial of oral propranolol in infantile hemangioma, N. Engl. J. Med. 372 (8) (2015) 735–746. [DOI] [PubMed] [Google Scholar]
- [25].Garson A Jr., Gillette PC, McNamara DG, Propranolol: the preferred palliation for tetralogy of Fallot, Am. J. Cardiol. 47 (5) (1981) 1098–1104. [DOI] [PubMed] [Google Scholar]
- [26].Fanous E, Mogyorosy G, Does the prophylactic and therapeutic use of beta-blockers in preoperative patients with tetralogy of Fallot significantly prevent and treat the occurrence of cyanotic spells? Interact. Cardiovasc. Thorac. Surg. 25 (4) (2017) 647–650. [DOI] [PubMed] [Google Scholar]
- [27].Graham EM, Bandisode VM, Bradley SM, Crawford FA Jr., Simsic JM, Atz AM, Effect of preoperative use of propranolol on postoperative outcome in patients with tetralogy of Fallot, Am. J. Cardiol 101 (5) (2008) 693–695. [DOI] [PubMed] [Google Scholar]
- [28].Cumming GR, Propranolol in tetralogy of Fallot, Circulation 41 (1) (1970) 13–15. [DOI] [PubMed] [Google Scholar]
- [29].Droitcourt C, Kerbrat S, Rault C, Botrel MA, Happe A, Garlantezec R, et al. , Safety of oral propranolol for infantile hemangioma, Pediatrics 141 (2018) 6. [DOI] [PubMed] [Google Scholar]
- [30].Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. , Initiation and use of propranolol for infantile hemangioma: report of a consensus conference, Pediatrics 131 (1) (2013) 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rudolph AM, Congenital Diseases of the Heart: Clinical-Physiological Considerations, Futura Publishing Company, Inc., 2001. [Google Scholar]
- [32].Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. , Transient regenerative potential of the neonatal mouse heart, Science. 331 (6020)(2011) 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bodamer OA, Halliday D, Uses of stable isotopes in clinical diagnosis and research in the paediatric population, Arch. Dis. Child. 84 (5) (2001) 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Steinhauser ML, Lechene CP, Quantitative imaging of subcellular metabolism with stable isotopes and multi-isotope imaging mass spectrometry, Semin. Cell Dev. Biol. 34 (8–9) (2013) 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Steinhauser ML, Guillermier C, Wang M, Lechene CP, Approaches to increasing analytical throughput of human samples with multi-isotope imaging mass spectrometry, Surf. Interface Anal. 46 (Suppl. 1) (2014) 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Steinhauser ML, Bailey AP, Senyo SE, Guillermier C, Perlstein TS, Gould AP, et al. , Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism, Nature 481 (7382) (2012) 516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guillermier C, Fazeli PK, Kim S, Lun M, Zuflacht JP, Milian J, et al. , Imaging mass spectrometry demonstrates age-related decline in human adipose plasticity, JCI Insight. 2 (5) (2017), e90349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Han L, Choudhury S, Mich-Basso JD, Ammanamanchi N, Ganapathy B, Suresh S, et al. , Lamín B2 levels regulate polyploidization of cardiomyocyte nuclei and myocardial regeneration, Dev. Cell 53 (1) (2020) 42–59, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu H, Bersell KR, Kuhn B, Isolation and characterization of intact cardiomyocytes from frozen and fresh human myocardium and mouse hearts, Methods Mol. Biol. 2158 (2021) 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, et al. , Guidelines and standards for performance of a pediatric echocardiogram: a report from the task force of the Pediatric Council of the American Society of Echocardiography, J. Am. Soc. Echocardiogr. 19 (12) (2006) 1413–1430. [DOI] [PubMed] [Google Scholar]
- [41].Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, et al. , Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease, J. Cardiovasc. Magn. Reson. 15 (2013) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ, Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance, Am. Heart J. 147 (2) (2004) 218–223. [DOI] [PubMed] [Google Scholar]
- [43].Seliem MA, Wu YT, Glenwright K, Relation between age at surgery and regression of right ventricular hypertrophy in tetralogy of Fallot, Pediatr. Cardiol. 16 (2) (1995) 53–55. [DOI] [PubMed] [Google Scholar]
- [44].Grapsa J, O’Regan DP, Pavlopoulos H, Durighel G, Dawson D, Nihoyannopoulos P, Right ventricular remodelling in pulmonary arterial hypertension with three-dimensional echocardiography: comparison with cardiac magnetic resonance imaging, Eur. J. Echocardiogr. 11 (1) (2010) 64–73. [DOI] [PubMed] [Google Scholar]
- [45].Norozi K, Bahlmann J, Raab B, Alpers V, Arnhold JO, Kuehne T, et al. , A prospective, randomized, double-blind, placebo controlled trial of beta-blockade in patients who have undergone surgical correction of tetralogy of Fallot, Cardiol. Young 17 (4) (2007) 372–379. [DOI] [PubMed] [Google Scholar]
- [46].Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. , Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling, Cell. 150 (4) (2012) 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]