Abstract
Familial Adenomatous Polyposis (FAP) is a hereditary colorectal cancer (CRC) syndrome that results in the development of hundreds of adenomatous polyps carpeting the gastrointestinal tract. Non-steroidal anti-inflammatory drugs (NSAIDs) have reduced polyp burden in FAP patients and synthetic rexinoids have demonstrated the ability to modulate cytokine-mediated inflammation and WNT signaling. This study examined the use of the combination of an NSAID (sulindac) and a rexinoid (bexarotene) as a durable approach for reducing FAP colonic polyposis to prevent CRC development. Whole transcriptomic analysis of colorectal polyps and matched normal mucosa in a cohort of FAP patients to identify potential targets for prevention in FAP was performed. Drug-dose synergism of sulindac and bexarotene in cell lines and patient-derived organoids was assessed, and the drug combination was tested in two different mouse models. This work explored microRNA as a potential predictive serum biomarker for this combination in FAP. Overall, transcriptomic analysis revealed significant activation of inflammatory and cell proliferation pathways. A synergistic effect of sulindac (300 μM) and bexarotene (40 μM) was observed in FAP colonic organoid systems with primary targeting of polyp tissue compared to normal mucosa. This combination translated into a significant reduction in polyp development in ApcMin/+ and ApcLoxP/+-Cdx2 mice. Lastly, the reported data suggests microRNA-21 could serve as a predictive serum biomarker for polyposis burden in FAP patients. These findings support the clinical development of the combination of sulindac and bexarotene as a treatment modality for FAP patients.
Keywords: Familial Adenomatous Polyposis, Chemoprevention, Hereditary Colorectal Cancer, Next-generation Sequencing
Introduction
Colorectal cancer (CRC) remains the third leading cause of cancer-related deaths affecting both men and women (1). The lifetime risk of developing CRC in the general population nears 5 percent; however, patients with hereditary cancer syndromes have a 10–20 fold higher risk (2). Familial Adenomatous Polyposis (FAP) is an autosomal dominant hereditary cancer syndrome associated with pathogenic germline mutations in the Adenomatous polyposis coli (APC) gene (3), which is commonly mutated in CRC on a somatic basis (4). FAP patients develop hundreds of adenomatous polyps throughout the gastrointestinal tract, including the duodenum, small intestine, colon, and rectum, that develop into tumors by the third and fourth decade of life. The current standard of care for FAP management includes endoscopic polypectomy. When polyp burden cannot be controlled endoscopically, patients typically seek a prophylactic colectomy with the creation of an intestinal pouch or ileo-rectal anastomosis; however, rectal polyps often present more frequently after prophylactic surgery (5).
FAP patients require prophylactic, risk-reducing procedures given the lack of potency and long-term efficacy of most single-agent therapies to control polyposis. For decades, the use of non-steroidal anti-inflammatory drugs (NSAIDs) has provided promising benefits as a CRC chemopreventive agent due to its effects on the inhibition of pro-tumorigenic inflammation. Several studies have demonstrated the clinical benefits of NSAIDs to reduce colorectal polyposis in FAP via the inhibition of the inflammatory cyclooxygenase 2 (COX2) pathway (6, 7). Preclinical trials using NSAIDs and COX2 inhibitors revealed a reduction in polyp burden in ApcMin/+ mice, which is considered the best model for small intestine polyposis (8, 9). Therefore, combinations of different anti-inflammatory drugs present potential opportunities for cancer interception strategies to target an array of mRNAs and miRNAs playing pivotal roles in inflammation and signal transduction pathways in colorectal carcinogenesis (10). The main focus of the work presented in this manuscript is to assess the activity of the combination of sulindac and bexarotene. Sulindac, a nonselective COX inhibitor, was the first NSAID shown to decrease the number and size of polyps in FAP patients (11). Bexarotene, a synthetic rexinoid, has been reported to reduce inflammation via COX2 inhibition within breast cancer and FAP animal models (12, 13). Furthermore, bexarotene reduced tumor progression in non-small cell lung cancer and skin squamous cell carcinoma animal models via the inhibition of the Epidermal Growth Factor Receptor (EGFR) and WNT signaling pathways (14, 15).
Recent studies have shown that microRNA (miRNAs) are key drivers for the initiation and progression of COX, EGFR, and WNT-mediated carcinogenesis, and thus have a prognostic value of disease outcome (16, 17). Among them, miR-20a, miR-21, miR-103a, miR-106, miR-143, and miR-215 are predictive biomarkers for stage II CRC (18). It has been reported that miR-135a/b miRNAs that are overexpressed in CRC inhibit APC expression and activate WNT signaling, whereas miR-21, a highly expressed and the most prominent biomarker for CRC, promotes Prostaglandin E2 (PGE2) levels by inhibiting the expression of the tumor suppressor, PDCD4 (19). Additionally, miRNA modulators such as miR-7 or miR-133b have improved the potency of anti-EGFR therapies in colon cancer cells (20, 21). Yet, the action of these miRNAs as interventions to inhibit oncogenic miRNA in CRC needs further investigation.
Overall, these studies suggest that the combination of sulindac and bexarotene may improve cancer interception at early stages of carcinogenesis through inhibition of COX-2, WNT, and EGFR signaling pathways, and miRNAs. Therefore, the overarching goal of this study was to examine the use of an NSAID and rexinoid combination as a novel chemopreventive strategy for colonic polyps in the context of FAP and to understand the molecular mechanisms underlying the preventive activity of these drugs in colon cancer mouse models.
Materials and Methods
Study Approval.
Written informed consent was obtained from all study participants under The University of Texas MD Anderson Cancer Center (MDACC) Institutional Review Board (IRB) protocol (IRB #PA12–0327) and the U.S. Common Rule regulations. All animal experiments were conducted in compliance with the National Institutes of Health guidelines for animal research and approved by MDACC Institutional Animal Care and Use Committee (IACUC, Protocol #488-RN02).
Transcriptome Analysis and Bioinformatics Analysis.
RNA sequencing was performed on 36 samples (18 matched pairs of colorectal polyps and adjacent normal mucosa) from 18 FAP patients followed in MDACC for their standard of care surveillance (Tables S1–2). Sample preparation, library construction, and sequencing were performed at MDACC Advanced Technology Genomics Core Facility using the Illumina HiSeq2000 platform. All bioinformatic analyses for differential gene expression were performed as described previously (22). The raw sequencing FASTQ files have been used in previous submissions and can be accessed in GSE106500 (9 samples), GSE88945 (1 sample), GSE153385 (22 FAP samples) and GSE156172 (4 FAP samples). To explore and visualize sample distribution in relationship with tissue type, a principal component analysis (PCA) was performed using data normalized by library size and transformed by variance-stabilizing transformation (VST). For gene set enrichment analysis (GSEA), genes were pre-ranked by log2-FoldChange and analyzed with Bioconductor R package fgsea (23), separately on HALLMARK, KEGG, and REACTOME gene sets obtained from the Broad Institute Molecular Signatures Database v6.0, as well as CRC related pathway gene sets obtained from Guinney et al (24). Significant pathways were selected based on BH-adjusted P-value≤0.05 and displayed in bar plots ordered by normalized enrichment score (NES).
Cell lines.
Human CRC cell lines HCT116 and HT29 (ATCC CCL24 and HTB38) were grown in Dulbecco’s Modified Eagle Medium with 4.5 g/L glucose (Mediatech, Manassas, VA) supplemented with 5% fetal bovine serum (Sigma Aldrich, St. Louis, MO) and 1% penicillin/streptomycin (HyClone, South Logan, UT) at 37°C and 5% CO2 in an incubator. All cell lines were authenticated upon acquisition by the Cytogenetics and Cell Authentication core at MDACC, and periodically tested for mycoplasma contamination.
Organoid Extraction and Culture.
Crypt isolation and organoid cultures were prepared from human colorectal polyps and normal mucosa FAP samples, collected from the Endoscopy Center at MDACC, as previously described (25) with modifications. In brief, samples were cut into 1 mm3 pieces, washed vigorously with cold PBS containing 0.1% Amphotericin B and placed in cold PBS with 2.5 mM EDTA for 30 min at 4°C with gentle agitation. The released crypts were collected, and the remaining fragments were further dissociated for one hour. Crypts collected from both fractions were pelleted at 1500 rpm for 5 min at 4°C, resuspended in Matrigel, and then dispensed onto 24-well plates in 50 μL droplets. After Matrigel polymerization, crypts were overlaid with organoid media, as described: Advanced DMEM/F12 was supplemented with penicillin/streptomycin, 10 mM HEPES, 2 mM GlutaMAX, 1 × B27 (Invitrogen), 1 × N2 Supplement (Invitrogen), 100 μg/mL Primocin (Invitrogen), 10 mM PGE2 (Selleckchem, Houston, TX), 10 mM Nicotinamide (Sigma), 10 nM gastrin I (Sigma), and 1 mM N-acetylcysteine (Wako, San Francisco, CA), 50 ng/ml mouse recombinant EGF (Life Technologies), 100 ng/ml mouse recombinant Noggin (Peprotech, Cranbury, NJ), 0.1% 250 μg/mL Amphotericin B (Gibco), 10% R-spondin-1 conditioned medium (26), 50% Wnt-3A conditioned medium (27), 500 nM A83–01 (Tocris, Minneapolis, MN), and 10 μM SB202190 (Sigma). A 10 μM Rock inhibitor, Y-27632 (Sigma), was added to the media upon initial passage and freezing of organoids. Organoids were cultured with fresh media every 2–3 days.
Cell Viability Assay.
Confluent HCT116, HT29 cells, and FAP organoids were plated in flat-bottom, 48-well tissue culture plates in media supplemented with sulindac and/or bexarotene. After 48 h incubation, the cell viability was assessed using CellTiter-Glo 3D Cell Viability Assay (Promega, Madison, WI).
Animal experiments.
Mice were produced by breeding ApcLoxP/+ mice (Jackson Laboratory; stock #009045) with Cdx2P-CreERT2 mice (Jackson Laboratory; stock# 022390). Six-week-old gender-matched transgenic ApcLoxP/+-Cdx2 mice were injected with tamoxifen (20 mg/ml, 100 μl), resulting in deletion of all 15 exons, as described previously (28). Mice were treated with drugs through oral gavage for six weeks. ApcMin/+ mice were randomized to vehicle/sesame oil (control), 7.5 mg/kg/day of sulindac (50 ppm in 100 μL), 15 mg/kg/day of bexarotene (100 ppm in 100 μL); ApcLoxP/+ mice to 7.5 mg/kg/day of each sulindac and bexarotene (50 ppm in 100 μL), and both models to the combination of sulindac plus bexarotene (each 7.5 mg/kg/day). Both sulindac and bexarotene doses were based on 50 mg/day Human Equivalent Dose, where bexarotene is typically administered clinically to cancer patients at 300 mg/m2 per day and sulindac clinically at 200–400 mg/day (8, 13). Bodyweight was taken every week with daily supervision of animal behavior. At the completion of the trial, mice were humanely sacrificed, and a final polyp assessment was obtained by longitudinally opening the colon and manually counting and measuring polyps under a dissection microscope.
Protein extraction and Western blot analysis.
The mucosal lining of ApcLoxP/+-Cdx2 mice (n=7 per group) and ApcMin/+ mice (n=3 per group) was stripped for subsequent analysis. The tissue was homogenized in radioimmune precipitation assay buffer with complete Mini protease and PhosSTOP phosphatase inhibitor (Roche Diagnostics, Mannheim, Germany). The tissue lysates were separated on polyacrylamide gels (BioRad) for Western blot analysis. The following primary antibodies were used: anti-COX2, anti-Bcl-2, anti-β-catenin, anti-EGFR, anti-p-EGFR, and anti-Cyclin D1 (Cell Signaling, Beverly, MA), anti-p-NFκB p65, and anti-RXRα (Santa Cruz, Dallas, TX). Goat anti-mouse (1:6000) or goat anti-rabbit (1:6000) secondary antibodies were used (Abcam, Cambridge, MA). The membranes were detected by the Enhanced Chemiluminescence Detection kit (GE Healthcare, Chicago, IL)
Isolation of total RNA and microRNA for quantitative gene expression.
Total RNA was extracted from colonic tissues using Direct-zol RNA MiniPrep Plus (Zymo Research, Irvine, CA). Complementary DNA was generated using the High Capacity cDNA Reverse Transcription Kit (Thermo Scientific, Lafayette, CO) and used for quantitative RT-PCR, using Fast SYBR Green Master Mix (Thermo Scientific) and the gene-specific primer sets (Table S3). MicroRNA was isolated from mouse colon/small intestine (normal and polyp tissue) and blood using the miRNeasy extraction kit (Qiagen, Hilden, Germany). MicroRNA was used to measure miRNA expression using SYBR green-based qRT-PCR relative to a universal qPCR primer as ROX reference dye. The primer sequence is included in Table S4.
Statistical analysis.
The mean ± standard error of the mean was reported for the data analysis using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Statistical analysis was performed with the Student t-test when only two value sets were compared, and with a 2-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test when the data involved three or more groups. P-value<0.05 was considered statistically significant.
Results
Whole transcriptome analysis of colorectal polyps in FAP.
In a first step RNAseq data was utilized to identify potential targets for colorectal prevention in FAP. To enhance the power to detect differentially expressed genes, colorectal polyps and matching normal mucosa samples within each patient in a cohort of FAP patients were analyzed. Figure 1A shows hierarchical clusters that clearly demarcate segregation among the normal colonic mucosa from the corresponding adenomatous polyps, thus confirming the results from the PCA (Figure S1). Among the most differentially expressed genes (DEG) in FAP polyps, 12 of the top 48 were associated with the regulation of the immune system, inflammation, and canonical WNT/β-catenin pathways (Figure 1B). Of the top immune-related genes, CLDN2, MMP7, MMP13, and CLDN1 displayed a log2FC of 6.26, 5.37, 3.13, and 3.08, respectively, in FAP polyp samples when compared to the normal colonic mucosa. GSEA detected activation of CRC-related pathways such as WNT, Myc, E2F, mTOR, and cell cycle (Figure 1C). Notably, the aberrant activation of the classical WNT/β-catenin signaling pathway was expected in FAP specimens due to the presence of germline and somatic mutations in APC. Furthermore, the highly expressed genes in FAP mucosa indicated the activation of several critical pathways related to inflammatory responses mediated by COX2 and RXR gene signatures. Consistently, the expression levels of RXRα and RARRES2 genes were decreased by log2FC 2.2 and log2FC 1.45, respectively, in adenoma when compared with normal colonic mucosa of the FAP patients. Therefore, CLDN1, MMP7, and MMP13 were selected as candidate biomarkers of the chemopreventive effect of sulindac and bexarotene, which targets COX, RXR, and inflammatory pathways, in a pre-clinical trial using mouse models of intestinal and colorectal polyposis.
Figure 1. Gene expression analysis of human FAP using paired samples.
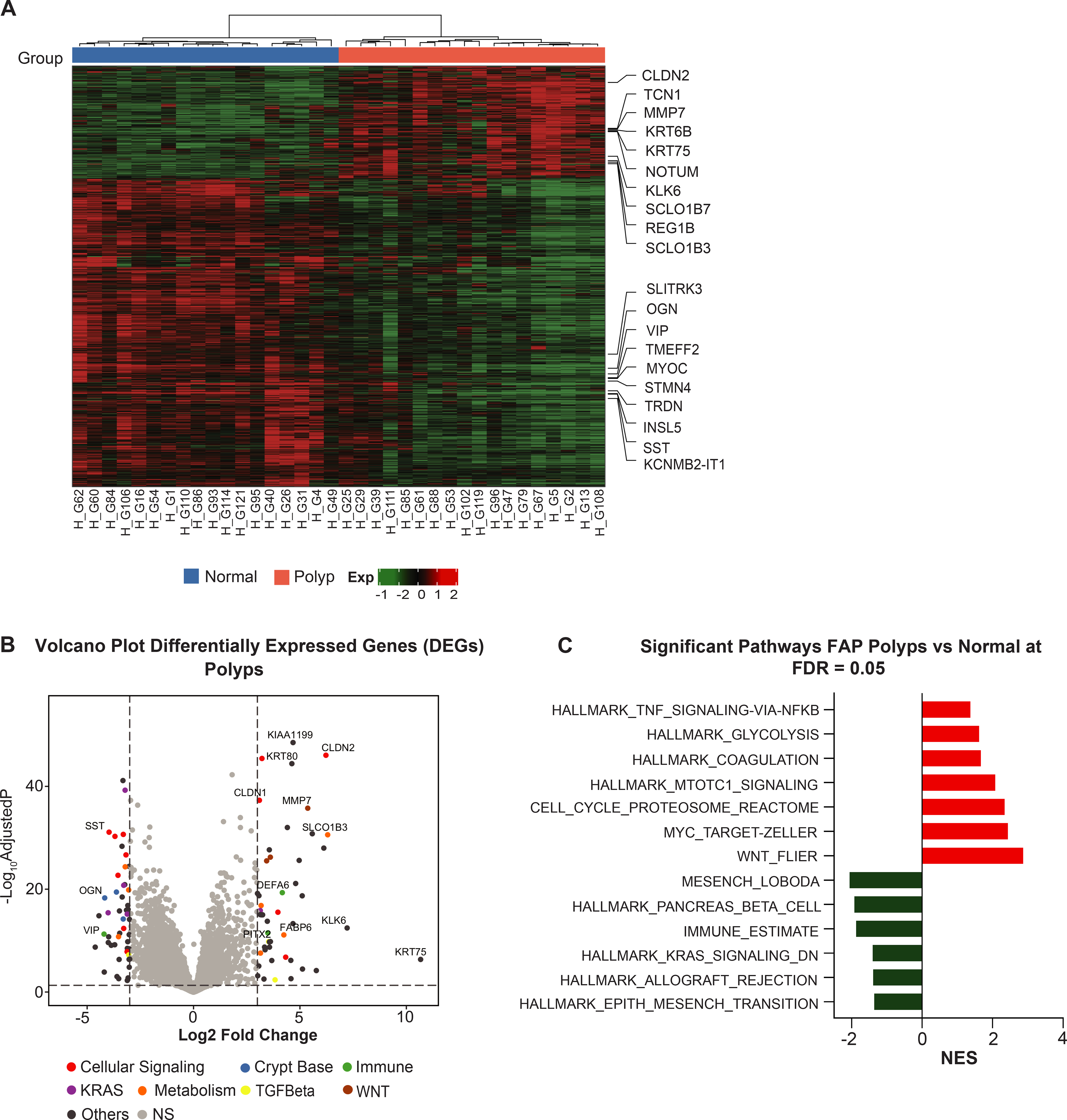
(A) Significant differentially expressed genes (DEGs) with adjusted P-value ≤0.05 and absolute value of log2-FoldChange ±1 in human normal colorectal mucosa and polyp were used to perform a paired hierarchical clustering. The top ten upregulated and downregulated genes are presented as column names and sample IDs as row names. The column covariate bar indicates polyps (red) and normal colorectal mucosa (blue) that samples belong to. Dendrogram illustrates sample clustering based on distances; (B) Genes from whole transcriptome sequencing are displayed in volcano plots with log2(FoldChange) on the X-axis and -log10(adjusted P-value) on the Y-axis. The significant up- and down-regulated genes with an adjusted P-value≤0.05 and an absolute value of log2-FoldChange ≥3 are highlighted and annotated in different colors within pathways of interest. The horizontal line represents BH-adjusted P-value=0.05. The left and right vertical lines represent log2-FoldChange ±3, respectively; (C) GSEA for the significant pathways with an adjusted P-value ≤0.05 in the Reactome biological and CRC pathways. The horizontal bars denote the different pathways based on the normalized enrichment score (NES). Red color indicates positive NES (positively enriched in polyps) and negative NES in green color (negatively enriched in polyps).
Assessment of the effect of the combination of sulindac and bexarotene on cell viability.
The effect of sulindac and bexarotene activity on cell viability was assessed to determine the drug combination’s efficiency and its synergistic effect in CRC cell lines, HT29 and HCT116. Cytotoxic analysis of sulindac treatment showed IC50 values of 1951 ± 86 μM and 1718 ± 250 μM for HT29 and HCT116, respectively (Figure S2). Conversely, bexarotene treatment showed IC50 values of 121 ± 22 μM and 96 ± 8 μM for HT29 and HCT116 cells, respectively. Combinatorial analysis for 48 hours demonstrated that HCT116 cells sustained 49.5% survival at the combination of 40 μM bexarotene and 900 μM sulindac with a CI of 0.62, and HT29 cells sustained 62.1% survival with CI of 0.577 (Figure S3), thus indicating that the HCT116 (microsatellite instable, MSI) cell lines was more sensitive than HT29 (microsatellite stable, MSS) to both drug treatments. However, drug sensistivity could be also driven by β-Catenin (CTNNB1) mutations in HCT116 cells when compared to HT29 cells, which are mutant in APC. Further determination of factors contributing to drug sensitivity may be necessary for future studies. Nevertheless, these data led us to hypothesize that the synergistic effect of a low-dose combination of sulindac and bexarotene will lead to a more clinically favorable scenario with long-term tolerance compared to high-dose therapies that are more toxic, while maintaining potency for both MSI and MSS colorectal models.
Efficacy of sulindac and bexarotene in FAP patient-derived organoids.
The drug combination was tested ex vivo using an organoid platform, which mimics the in vivo structure and physiology of intact parent tissue, generated from colorectal adenomas (polyp-organoid) and normal mucosa (normal-organoid) from FAP patients (Figure 2). Cell viability analysis showed that sulindac treatment was slightly more effective on polyp-organoids than normal-organoids. Cell viability of polyp-organoid was 60% ± 1.7 with IC50 values of sulindac at 348.6 μM, whereas normal-organoids was 69.5% ± 8.7 with IC50 values of sulindac at 383.1 μM (Figure 2A). The cell viability analysis of bexarotene treatment showed 51.3% ± 5.6 in the polyp-organoids with IC50 values of bexarotene at 29 μM, whereas normal-organoid showed 82% ± 8.2 with IC50 values of bexarotene at 58.5 μM (Figure 2B). The bexarotene cell viability data of normal and polyp samples suggest that bexarotene is more effective on polyp-organoids than normal-organoids. When a combination of 300 μM of sulindac and 40 μM of bexarotene was tested together, cell viability of polyp-organoids was significantly decreased (57.7% ± 9) when compared with normal-organoids (81.6% ± 5.7, Figure 2C). Figure 2D depicts colonic organoids from FAP patients with reduced growth of polyp-organoids compared to normal-organoids treated with 300 μM sulindac, 40 μM bexarotene, and the drug combination of 300 μM sulindac with 40 μM bexarotene. Overall, these data provide a rationale for a clinically-relevant therapeutic opportunity of combinatorial drug treatment as a chemopreventive strategy to reduce the growth of colonic polyps in FAP patients.
Figure 2. Effect of sulindac and bexarotene in FAP patient-derived organoids.
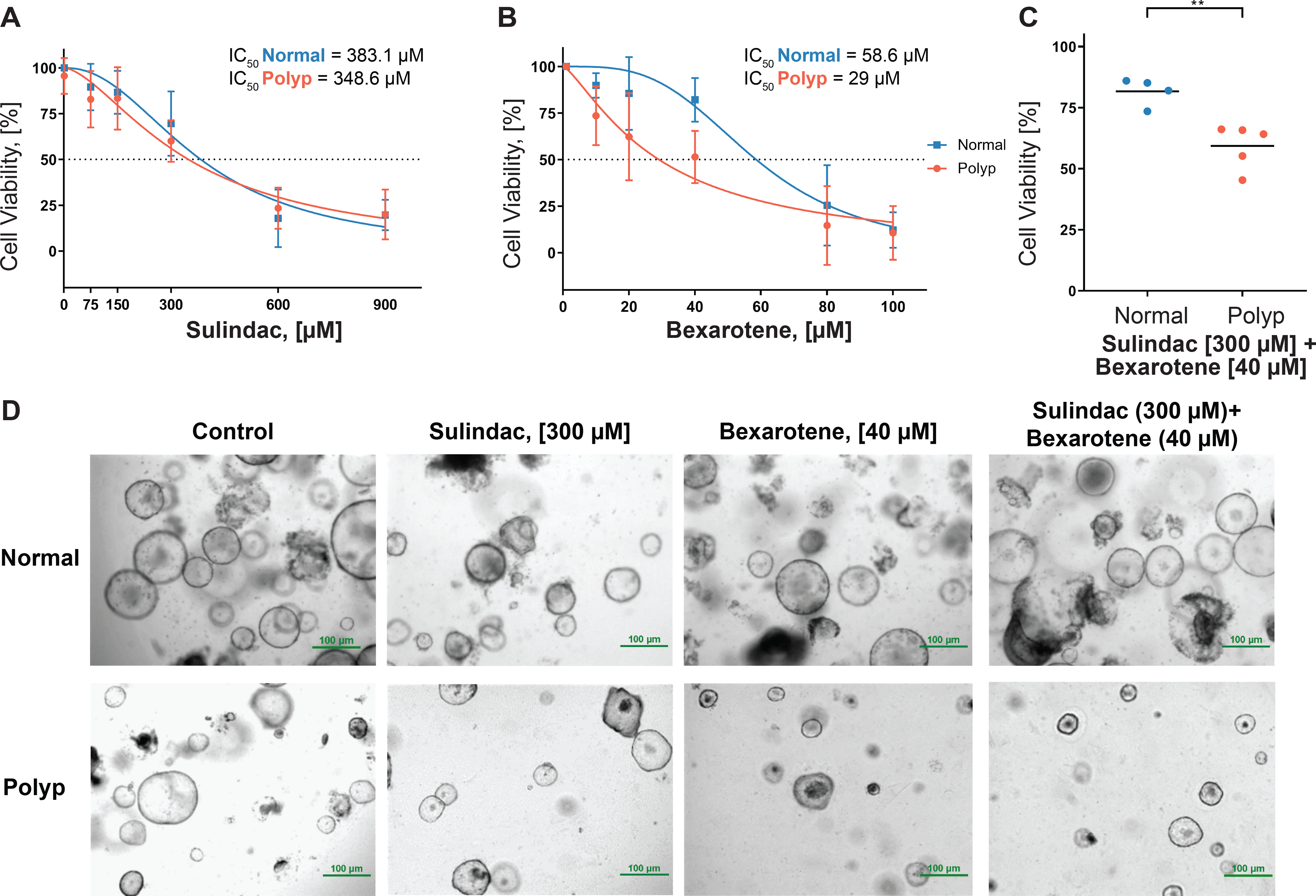
The line graph represents the IC50 calculations from (A) sulindac and (B) bexarotene treatment of colonic organoids derived from FAP patients; (C) Cell viability of FAP colon organoids following combined treatment with sulindac and bexarotene; (D) Organoids derived from the colon of normal and polyp tissue from FAP patients at MD Anderson and seeded in Matrigel. Images were captured 48 hours after treatment at 4X magnification.
Combination of sulindac and bexarotene reduces polyp formation in two different polyposis mouse models.
The classical ApcMin/+ model spontaneously develops polyps primarily within the small intestine and provides a durable model for studying FAP-associated duodenal polyposis (8, 29). Conversely, the ApcLoxP/+-Cdx2 mouse model better recapitulates FAP-associated colonic polyposis of the large intestine in humans.
ApcMin/+ mice were randomized into four different groups and treated for a total of six weeks. Both polyp multiplicity and polyp size were assessed at the completion of treatment to determine the effect of drug intervention. Sulindac alone caused the most significant reduction (75%) in small intestine polyp count (9 ±1.2) compared to untreated (control) mice (36.7± 4, Figure 3A). Bexarotene alone also reduced polyp count (23±3) by 40% compared to control mice; however, to a lesser extent, when compared with sulindac. The combination of sulindac and bexarotene provided a moderate effect (14.2±1) with a 60% reduction in polyp burden. The effect of bexarotene appeared comparable to sulindac in reducing polyps of the large intestine derived from ApcMin/+ mice (Figure 3B); however, this trend was not observed in the ApcLoxP/+-Cdx2 mice when compared with single-agent sulindac. Further, sulindac significantly reduced the prevalence of polyps greater than 2 mm in size, though the effect of bexarotene was moderate in reducing polyps in the small intestine (Figure 3C). Nonetheless, these results support that, although single-agent sulindac demonstrated the most beneficial chemopreventive agent for reducing the small intestine polyp burden, the combination of sulindac and bexarotene might be a better chemopreventive strategy for simultaneous inhibition of polyposis in both the small and large intestine.
Figure 3. Preclinical trial data following 6-weeks of drug intervention to prevent FAP polyposis in ApcMin/+ and ApcLoxP/+-Cdx2 mouse models.
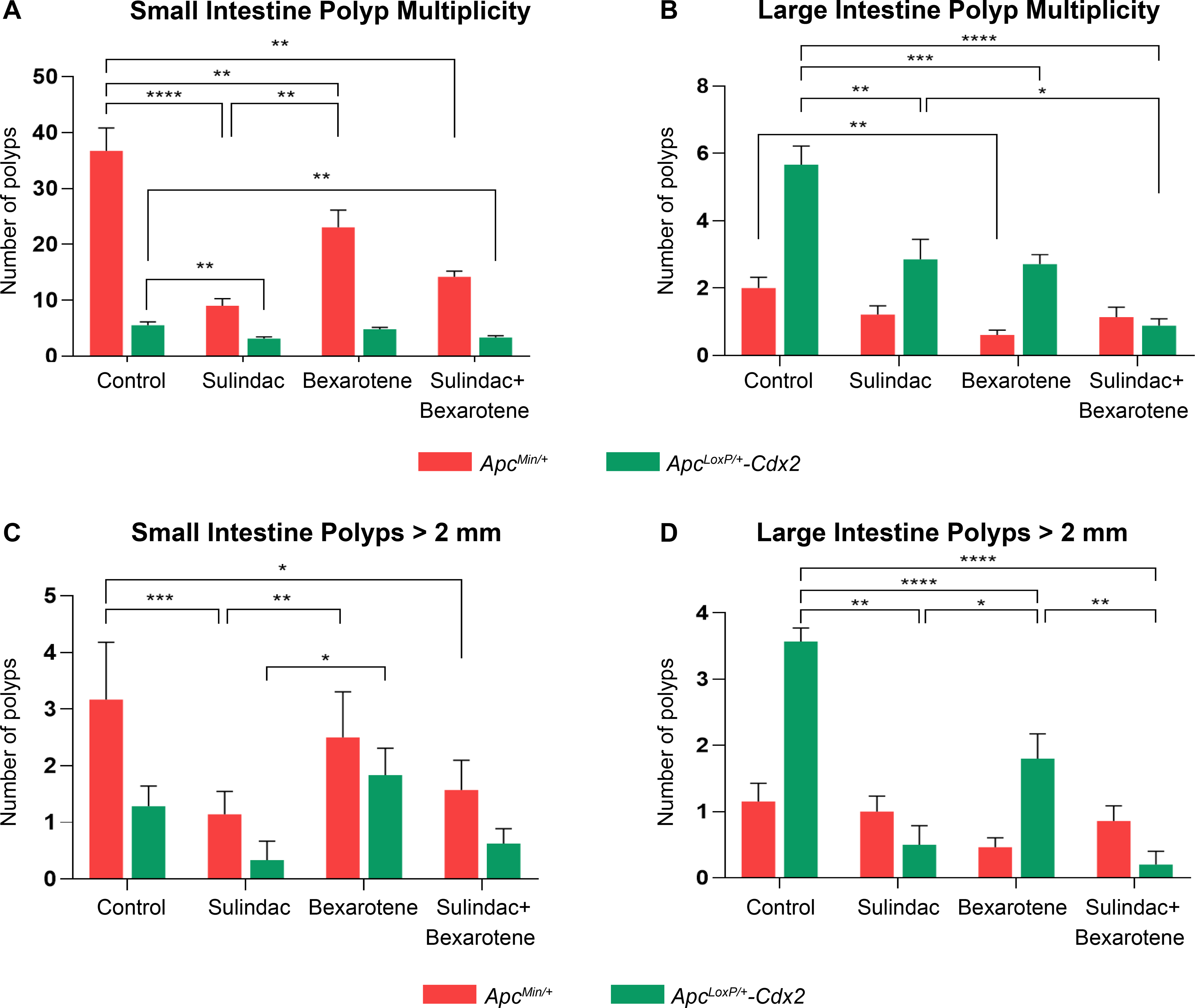
The efficacy and potency of drug treatment in the overall multiplicity of polyps. Graph (A) indicates the total number of small intestinal polyps, and (B) the total number of large intestinal polyps in both the ApcMin/+ (red bars) and ApcLoxP/+-Cdx2 (green bars) mouse models. Bexarotene did not significantly reduce small intestine polyp multiplicity in the ApcLoxP/+-Cdx2 model (A). No significant reduction of large intestine polyp multiplicity was observed with sulindac or the drug combination in the ApcMin/+ model (B). Graphs (C-D) depict a total number of polyps >2 mm in the small and large intestine, respectively, across treatment groups in the ApcMin/+ and ApcLoxP/+-Cdx2 models. No significant reduction in polyps >2 mm compared to the control group (untreated tumor-bearing mice, vehicle control) was observed in the small intestine of the ApcLoxP/+-Cdx2 model (C). Moreover, no significant difference in colonic polyps >2 mm in the ApcMin/+ model was observed with any intervention compared to control (D). These results are consistent with the tissue specificity of each mouse model. The total number of polyps is presented as mean ± SEM (*P-value ≤0.05, **P-value ≤0.01, ***P-value ≤0.001, ****P-value ≤0.0001).
Following tamoxifen injection to conditionally inactivate the Apc gene in the large intestine, ApcLoxP/+-Cdx2 mice were randomized for drug treatment similar to ApcMin/+ mice. Assessment of drug efficacy in ApcLoxP/+-Cdx2 mice revealed that the combination of sulindac and bexarotene afforded the most significant reduction in polyps (0.2±0.2) of the large intestine when compared to the control group (3.6±0.2) However, both sulindac and bexarotene administered as single-agents provided significant polyp reduction (Figure 3B and D). Furthermore, single-agent sulindac treatment appeared to be more effective in preventing polyposis within the small intestine of the ApcLoxP/+-Cdx2 mice (Figure 3A and C). These results from both mouse models, the ApcMin/+ and ApcLoxP/+-Cdx2 mice, indicate that single-agent sulindac administered at 7.5 mg/kg/day protects against small intestinal polyposis, and single-agent bexarotene largely prevents large intestinal polyposis development. Furthermore, the combination of sulindac and bexarotene appeared to reduce polyposis across the gastrointestinal tract, both small and large intestine, and was well tolerated by both mouse models, as indicated by no adverse changes in animal body weight (Figure S4A–D).
Sulindac and bexarotene combination decreases inflammation, and cell cycle arrest while increasing apoptosis and reduces EGFR and WNT-signaling in ApcLoxP/+-Cdx2 and ApcMin/+ mice.
The molecular effect of the combination of sulindac and bexarotene was evaluated to determine the modulation of key regulators involved in colonic inflammation and tumorigenesis. Protein levels of COX-2 decreased more in the combination of sulindac and bexarotene than in either sulindac or bexarotene or the control animal groups (Figure 4A, left). Since retinoid X receptor α (RXRα) is expressed at very low levels in colonic tumors (13), the data demonstrated that levels of RXRα were significantly increased by single-agent bexarotene and the combination of sulindac and bexarotene compared to single-agent sulindac and control groups. Levels of NFκB were remarkably reduced in the sulindac and bexarotene combination groups. The levels of Cyclin D1 protein, required for progression through the G1 phase of the cell cycle, were significantly decreased within the sulindac and bexarotene combination group as compared to the other groups. The protein expression of Bcl-2, an anti-apoptosis regulator, shown to be increased by COX2 expression (30), was significantly reduced when the ApcLoxP/+-Cdx2 mice were treated with the dual-agent combination. These results suggest that the combination of sulindac and bexarotene reduced polyp development and tumorigenesis by suppressing inflammation and cell cycle arrest via decreased protein expression of COX-2, NFκB, and cyclin D1, as well as by increasing apoptosis via Bcl-2 inhibition within ApcLoxP/+-Cdx2 mice. In addition to inflammatory and cell cycle regulators, a significant decrease in the levels of EGFR and phosphorylated EGFR (pEGFR) with bexarotene alone and with the sulindac and bexarotene combination treatment groups were observed (Figure 4A, middle). Moreover, the levels of activated β-Catenin also decreased with bexarotene alone and combined sulindac and bexarotene treatment. Thus, the combination of sulindac and bexarotene decreased the expression of COX-2, p-NFkB, Bcl-2, EGFR, and activated β-Catenin levels. Although single agents had significant effects, the dual-agent regimen appeared to provide broader effects across all biomarkers assessed compared to either single agent alone.
Figure 4. Sulindac and bexarotene combination decreases inflammation and cell cycle arrest through down-regulation of EGFR and β-catenin, as well as predictive biomarkers of gene expression in both mouse models.
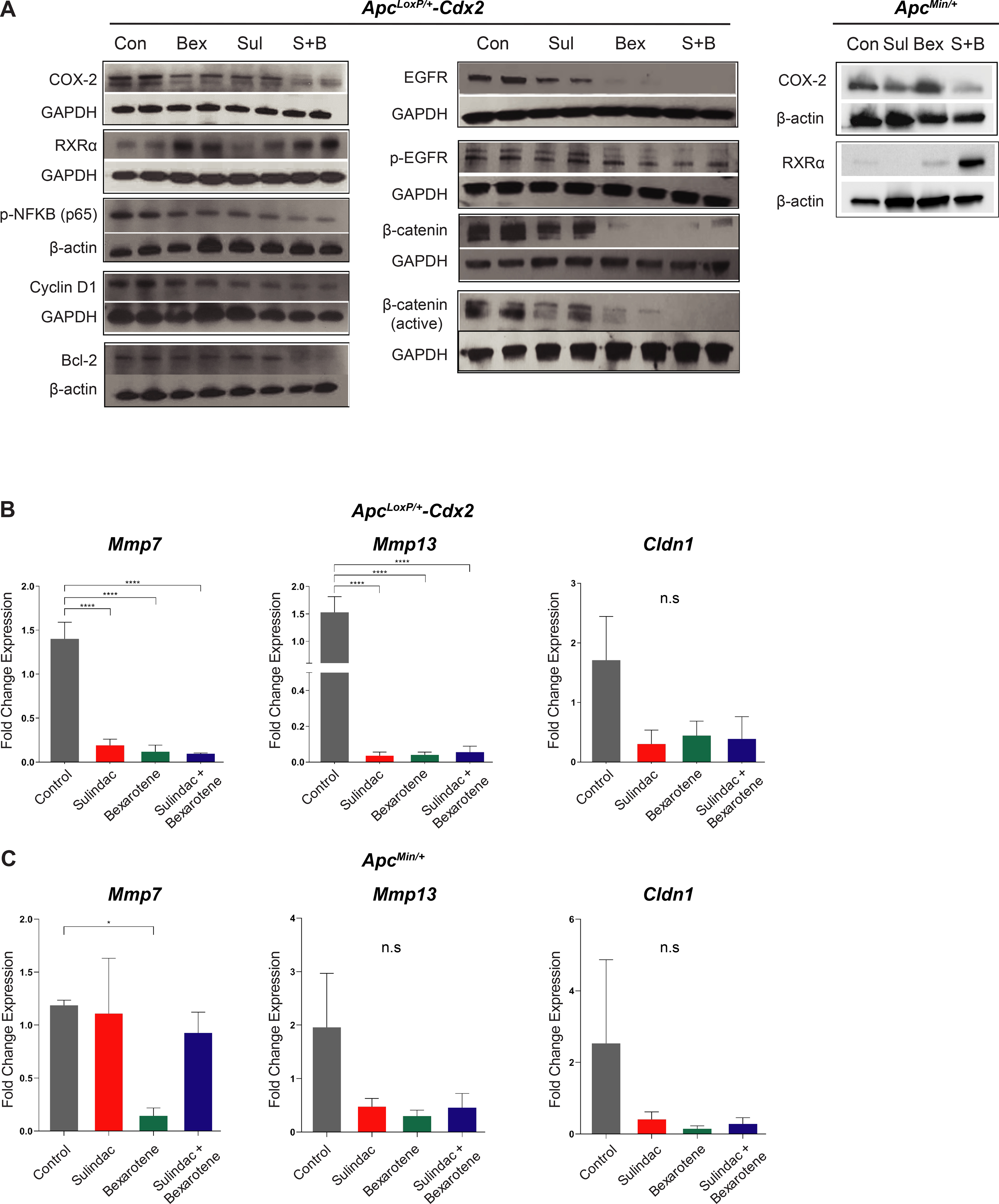
(A) Western blot analysis was performed in the lysates (25 μg/lane) from mucosal stripping of two ApcLoxP/+-Cdx2 mice in each treatment group using anti-COX-2, anti-RXRα, anti-p-NFκB (p65), anti-cyclin D1, anti-Bcl-2 (Left panel), and anti-EGFR, anti-pEGFR, anti-β-catenin, and anti-active β-Catenin (Middle panel). Western blot analysis was performed in the ApcMin/+ model (Right panel) using anti-COX-2 and anti-RXRα antibodies. The loading control for each blot was β-actin or GAPDH, as shown in the panel. Each blot was a representation of three individual experiments. Control group was comprised of untreated, genotype-matched, tumor-bearing mice; (B) Quantitative gene expression shows the mRNA levels of potentially predictive biomarkers in the colonic mucosal stripping of ApcLoxP/+-Cdx2 mice treatment groups (n=4 mice per group); (C) Quantitative gene expression shows the mRNA levels of potentially predictive biomarkers in the colonic mucosal stripping of ApcMin/+ mice treatment groups (n=3 mice per group). Data show the relative mRNA expression levels of cytokine genes Cldn1, Mmp7, and Mmp13. Each bar represents mean ± S.E. (*P-value ≤0.05, **P-value ≤0.01, ***P-value ≤0.001).
Lastly, to assess the molecular effect of drug intervention in the ApcMin/+ model, the expression of two key targets of sulindac and bexarotene was examined: COX-2 and RXR-α, respectively. The data indicated a significant reduction in COX-2 expression of mice treated with sulindac, which was further reduced by the drug combination (Figure 4A, right). Similar to the ApcLoxP/+-Cdx2 model, increased expression of RXR-α in ApcMin/+ mice treated with the drug combination was observed, as expected with drug synergism. Taken together, the combination of sulindac and bexarotene reduced polyp development and tumorigenesis by inhibiting the levels of COX-2, EGFR, and β-Catenin within the ApcLoxP/+-Cdx2 mice. These results were also confirmed in the ApcMin/+ model, as shown by decreased COX-2 expression and increased RXR-α expression.
Sulindac and bexarotene treatments reduce the expression of Mmp7, Mmp13, and Cldn1 genes in ApcLoxP/+-Cdx2 mice.
The effect of sulindac and bexarotene treatment was assessed on the expression levels of genes selected based on expression results observed in human FAP specimens, and that could be used as predictive biomarkers of drug activity. The results indicate that transcript levels of Cldn1, Mmp7, and Mmp13 were strongly reduced in the polyps of ApcLoxP/+-Cdx2 mice that received sulindac and bexarotene alone or in combination when compared with transcript levels in the polyps from untreated control mice (Figure 4B). The expression of Cldn1, Mmp7, and Mmp13 was also measured in the ApcMin/+ model (Figure 4C); however, a significant decrease in expression was only observed for Mmp7 in ApcMin/+ mice treated with bexarotene.
Upregulation of miR-21, a predictive biomarker of CRC, is blunted by sulindac in the small intestine of ApcMin/+ mice and the large intestine of ApcLoxP/+-Cdx2 mice.
To further identify a potential preventive biomarker of CRC, this study focused on the oncomiRNAs that are known to play key roles in colon tumorigenesis. Among oncomiRNAs, miR-21, miR-26a, miR-29, miR-30, and miR-103 are associated with CRC progression, and specifically, miR-21 has been reported to be highly up-regulated and associated with tumor size and poor overall survival (16, 31). Thus, the expression levels of these miRNAs in a cohort of FAP patients, ApcMin/+ mice, and ApcLoxP/+-Cdx2 mice, as well as the effect of sulindac and bexarotene on the expression levels of these miRNAs were examined. The data presented reveals miR-21 was highly expressed in the serum (Figure 5A) and polyp samples (Figure 5B) of FAP patients with more than 50 polyps. Therefore, this data suggest that miR-21 expression may be correlated with polyp occurrence in FAP patients, thus highlighting its prognostic and clinical significance of FAP-mediated colon tumorigenesis (Figure 5A). Consistent with this notion, miR-21 levels were also strongly elevated in polyps of the ApcMin/+ mice when compared to levels in WT (C57BL/J6) mice (Figure 5C). To ascertain whether miR-21 is a preventive biomarker of FAP polyposis upon drug intervention, miR-21 levels were evaluated in treated ApcMin/+ and ApcLoxP/+-Cdx2 mice. As shown in Figure 5C, both sulindac and the combination of sulindac and bexarotene significantly reduced the expression levels of miR-21 in the blood serum of mice receiving the intervention. However, there were no significant changes in the expression levels of miR-26a, miR-29, miR-30, and miR-103 between normal mucosa and polyps from a cohort of FAP patients (Figure S5). In the ApcLoxP/+-Cdx2 model, miRNA extracted from mucosa was used for analysis. As shown in Figure 5D, miR-21 was significantly reduced in all three treatment groups compared to untreated control mice. These results from both mouse models are indicative of miR-21 as a potential biomarker of polyp burden in patients already diagnosed with FAP-associated polyposis.
Figure 5. MicroRNA-21 serves as a biomarker for polyposis in FAP patients and murine models.
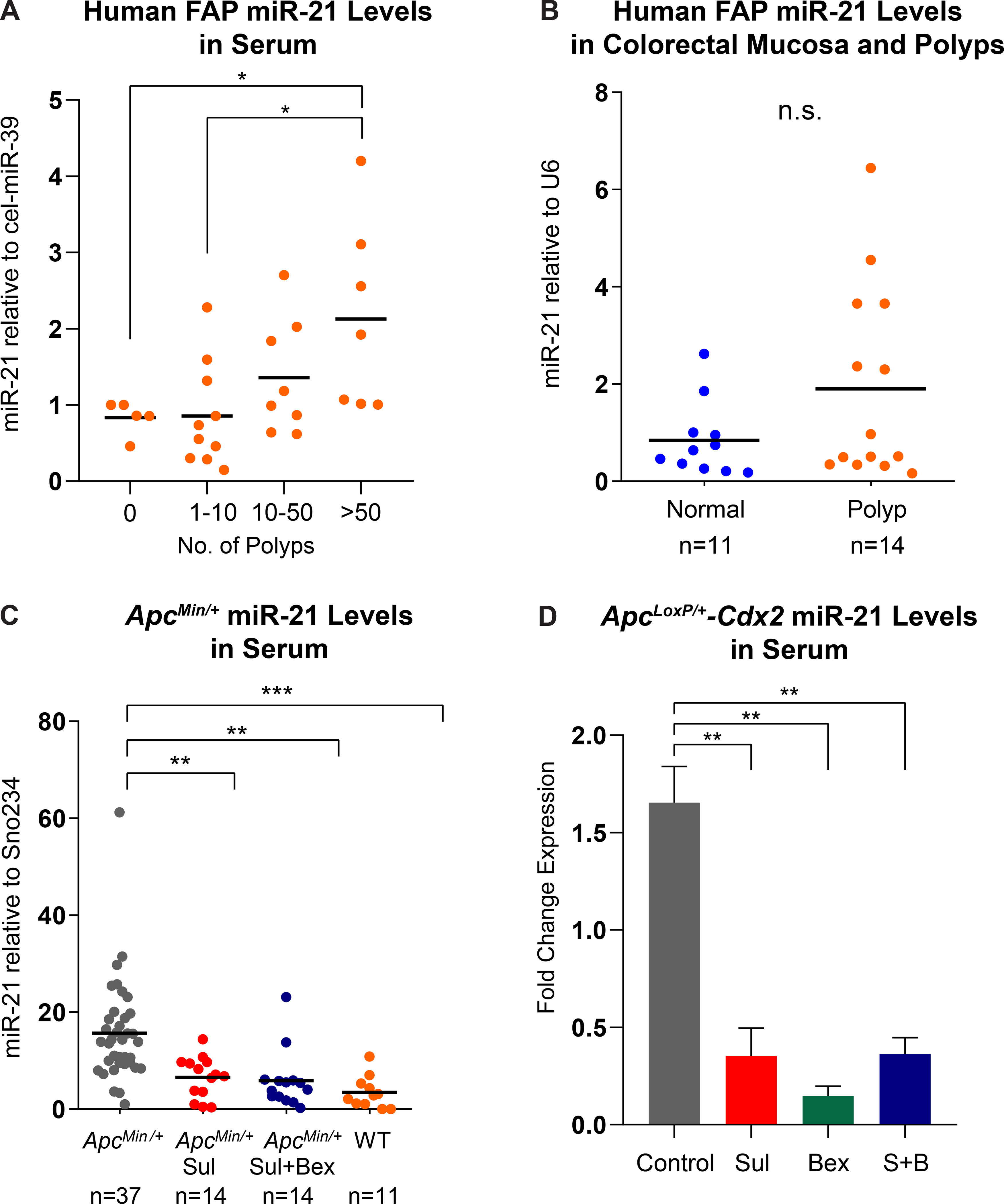
(A) Overall relative miR-21 expression in serum from FAP patients stratified based on the number of colonic polyps. The expression of miR-21 was normalized to cel-miR-39; (B) miR-21 expression in colorectal normal mucosa and polyp tissue specimens from FAP patients normalized to U6 RNA expression; (C) Down-regulation of miR-21 expression following drug intervention with sulindac and the combination of sulindac and bexarotene in ApcMin/+ mice; (D) Down-regulation of miR-21 expression following drug intervention with sulindac, bexarotene, and the combination of sulindac and bexarotene in ApcLoxP/+-Cdx2 mice (*P-value ≤0.05, **P-value ≤0.01, ***P-value ≤0.001).
Discussion
Using whole transcriptome analysis, differentially expressed genes in premalignant colorectal lesions (adenomas) from a cohort of FAP patients were identified as potential targets for cancer interception approaches. First, patient-derived colonic organoids were generated from colorectal polyps and normal tissue of FAP patients to test the dose-synergism effect of sulindac and bexarotene on cell viability (32). Then, two CRC mouse models, ApcMin/+ and ApcLoxP/+-Cdx2, were used to validate the effects of sulindac and bexarotene. Both mouse models afforded the successful evaluation of sulindac and bexarotene efficacy in reducing polyposis and the levels of inflammatory markers and CRC drivers. Additionally, the levels of miRNA21, highly elevated in a cohort of FAP patients, were remarkably reduced in both mouse models upon drug intervention. These pre-clinical findings establish the framework for developing a novel cancer interception strategy of dual administration of sulindac and bexarotene for colonic tumorigenesis in FAP.
The development of intestinal neoplasms is due to disruption of APC and activation of EGFR signaling that promotes the expression of COX2. Subsequently, pro-inflammatory and protumorigenic responses elevate the levels of PGE2, which results in stimulation of growth and angiogenesis and inhibition of cellular apoptosis via activation of nuclear factor κB (NFκB) and Wnt signaling (33–35). It has been shown that the protumorigenic action of COX-2 is mediated through its role in the production of PGE2 via β-catenin/TCF/LEF pathway activation, which is significantly increased during FAP colorectal tumorigenesis (35, 36). Human FAP trials and mouse model studies showed that a combination of COX and EGFR inhibitors are successful in reducing intestinal adenoma development (10, 29, 37). Furthermore, bexarotene treatment decreased inflammation in breast cancer and FAP animal models (12, 13), most likely through inhibition of EGFR and WNT signaling. Thus, the COX-2 signaling pathway is an ideal druggable target for colorectal cancer prevention. This study presents the combination of sulindac and bexarotene as an effective strategy to reduce COX-2 expression in a FAP mouse model that mimics colonic tumorigenesis. Sulindac more effectively reduced polyposis in ApcMin/+ mice, which is consistent with previous reports (13, 38). However, another study reported that Targetrin (bexarotene) failed to inhibit small intestinal neoplasms in ApcMin/+ mice based on histopathological analyses (39). This combination, in turn, played a key role in reducing the cell viability of FAP polyp-derived colonic organoids as well as successfully reducing polyp growth within the small and large intestine of the FAP animal models.
Clinical studies of FAP patients treated with NSAIDs to reduce tissue PGE2 levels have demonstrated effective inhibition of polyp development (40, 41). Sulindac is a conventional NSAID that has been shown to reduce polyposis (both in number and size of polyps) via non-selective COX inhibition (8, 9, 42, 43). The results presented in this manuscript indicate that sulindac played a significant role in reducing inflammation via COX-2 inhibition as well as decreasing NFκB protein levels within the large intestine of ApcLoxP/+-Cdx2 mice and the small intestine of ApcMin/+ mice, which might play a primary role in the reduction of polyp burden within this preclinical study.
Retinoic acid plays a significant role in suppressing COX-2 expression levels through activation of the C/EBP-B transcription factor pathway (34). RXRα is a retinoid x receptor and a potential colon cancer prevention target based on its decreased expression in colonic tumors (38, 44). Anti-inflammatory agents, such as sulindac and bexarotene, are ligands for RXR and inhibit chronic inflammation (45). The synthetic retinoid, bexarotene, is a highly selective RXR agonist responsible for restricting intestinal neoplasia in ApcMin/+ mice (13), which is confirmed by our data. Further, inhibition of EGFR and WNT/β-catenin in the large intestine of ApcLoxP/+-Cdx2 mice by bexarotene was observed, confirming previous studies using different mouse models (14, 46). Thus, targeting different pathways simultaneously with a combination of chemopreventive drugs such as sulindac against the COX2 pathway and bexarotene against RXR signaling appeared to reduce intestinal polyposis at different segments of the gastrointestinal tract in two animal models of FAP, and further clinical ingestigation in humans is warranted.
Matrix metalloproteinase genes, including MMP7 and MMP13, are known for cell adhesion and migration in many cancers and up-regulated during intestinal inflammatory bowel disease and the growth and migration of colorectal carcinoma cells. Furthermore, Mmp7 is a known downstream target of β-catenin-mediated TCF signaling and reported to be associated with distant metastasis and poor outcomes in early CRC (47). Claudins are a family of tight junction proteins that regulate epithelial barrier functions in the intestine (48). MMP7, MMP13, FOXQ1, CLDN1, and CLDN2 genes have been previously reported to be coexpressed at high levels in colonic tumors with high levels of COX2 (49). To gain mechanistic insights regarding inhibition of FAP colonic polyposis, the results presented here showed that sulindac and bexarotene strongly reduced the expression levels of the potential colon cancer marker genes, Cldn1, Cldn2, Mmp7, and Mmp13, within polyps of the large intestine of ApcLoxP/+-Cdx2 mice. However, these results were not consistently observed in the ApcMin/+ model, which may be attributed to significant variation of expression observed within the control group. The expression of these genes was increased in a cohort of FAP patients, based on RNAseq analysis, which is consistent with other findings (47, 50). However, none of the potential preventive biomarkers (Cldn1, Cldn2, Mmp7, and Mmp13) were shown to have a significant difference in gene expression when compared with the combination of sulindac and bexarotene to each study group.
Previous reports demonstrate that NSAIDs, like sulindac, reduce FAP polyp burden at the beginning of drug treatment (10). However, this study did not examine the efficiency of the drug combination over an extended period. Thus, a further study is needed to validate the drug combination effectiveness in reducing FAP colonic polyposis over an extended period of time.
Another important observation in the present study is the down-regulation of miR-21, a potential prognostic biomarker for colon cancer, in polyps of the small and large intestine of ApcMin/+ and ApcLoxP/+-Cdx2 mice by sulindac and a combination of sulindac and bexarotene. Taken together, it is conceivable that these NSAIDs inhibit the expression of not only oncogenes, but also miRNAs, including miR-21 as well as other signaling pathways, which consequently results in inhibition of polyposis and protection from initiation of colon cancer.
In summary, the RNA-seq analysis of FAP polyps indicated an increase of immune-related genes as compared to normal mucosa samples. The combination of sulindac and bexarotene provided synergism and an effective method of reducing cell viability to colon cancer cells and FAP patient-derived colonic organoids. Finally, the observed reduction in colonic polyp burden and size, together with the inhibition of COX2, EGFR, and WNT signaling, as well as the increase of RXRα activation, provides insights into the molecular mechanism of colonic polyp regression in FAP patients.
Supplementary Material
Prevention Relevance:
This study identified a novel chemopreventive regimen combining sulindac and bexarotene to reduce polyposis in patients with Familial Adenomatous Polyposis using in silico tools, ex vivo, and in vivo models. This investigation provides the essential groundwork for moving this drug combination forward into a clinical trial.
Acknowledgments:
This work was supported by the SWOG/Hope Foundation Impact Award, and a gift from the Feinberg Family to E.V; and P30 CA016672 (US National Institutes of Health/National Cancer Institute) to The University of Texas MD Anderson Cancer Center Core Support Grant. We thank the patients and their families for their participation. We thank the staff of the Advanced Technology Genomics Core at MDACC for assistance with mRNA sequencing.
Abbreviations:
- FAP
Familial Adenomatous Polyposis
- NSAIDs
Non-steroidal anti-inflammatory drugs
- CRC
colorectal cancer
- COX
Cyclooxygenase
- PGE2
Prostaglandin E2
- EGFR
Epidermal Growth Factor Receptor
- MDACC
The University of Texas MD Anderson Cancer Center
- IRB
Institutional Review Board
- IACUC
Institutional Animal Care and Use Committee
- PCA
principal component analysis
- GSEA
gene set enrichment analysis
- VST
variance-stabilizing transformation
- NES
normalized enrichment score
- DEG
differentially expressed genes
- MSI
microsatellite instable
- MSS
microsatellite stable
Footnotes
Conflict of interest statement: Dr. Vilar has a consulting or advisory role with Janssen Research and Development and Recursion Pharma, and has received research support from Janssen Research and Development. No other disclosures are reported.
References
- 1.Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Jasperson KW, Tuohy TM, Neklason DW, and Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valle L, Vilar E, Tavtigian SV, and Stoffel EM. Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J Pathol. 2019;247(5):574–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223–62; quiz 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52. [DOI] [PubMed] [Google Scholar]
- 7.Ricciardiello L, Ahnen DJ, and Lynch PM. Chemoprevention of hereditary colon cancers: time for new strategies. Nat Rev Gastroenterol Hepatol. 2016;13(6):352–61. [DOI] [PubMed] [Google Scholar]
- 8.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17(8):1757–60. [DOI] [PubMed] [Google Scholar]
- 9.Fischer SM, Hawk ET, and Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res (Phila). 2011;4(11):1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samadder NJ, Kuwada SK, Boucher KM, Byrne K, Kanth P, Samowitz W, et al. Association of Sulindac and Erlotinib vs Placebo With Colorectal Neoplasia in Familial Adenomatous Polyposis: Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018;4(5):671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313–6. [DOI] [PubMed] [Google Scholar]
- 12.Kong G, Kim HT, Wu K, DeNardo D, Hilsenbeck SG, Xu XC, et al. The retinoid X receptor-selective retinoid, LGD1069, down-regulates cyclooxygenase-2 expression in human breast cells through transcription factor crosstalk: implications for molecular-based chemoprevention. Cancer Res. 2005;65(8):3462–9. [DOI] [PubMed] [Google Scholar]
- 13.Janakiram NB, Mohammed A, Qian L, Choi CI, Steele VE, and Rao CV. Chemopreventive effects of RXR-selective rexinoid bexarotene on intestinal neoplasia of Apc(Min/+) mice. Neoplasia. 2012;14(2):159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zito G, Naselli F, Saieva L, Raimondo S, Calabrese G, Guzzardo C, et al. Retinoic Acid affects Lung Adenocarcinoma growth by inducing differentiation via GATA6 activation and EGFR and Wnt inhibition. Sci Rep. 2017;7(1):4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zito G, Saotome I, Liu Z, Ferro EG, Sun TY, Nguyen DX, et al. Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk. Nat Commun. 2014;5:3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mima K, Nishihara R, Yang J, Dou R, Masugi Y, Shi Y, et al. MicroRNA MIR21 (miR-21) and PTGS2 Expression in Colorectal Cancer and Patient Survival. Clin Cancer Res. 2016;22(15):3841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabry D, El-Deek SEM, Maher M, El-Baz MAH, El-Bader HM, Amer E, et al. Role of miRNA-210, miRNA-21 and miRNA-126 as diagnostic biomarkers in colorectal carcinoma: impact of HIF-1alpha-VEGF signaling pathway. Mol Cell Biochem. 2019;454(1–2):177–89. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14(13):1295–306. [DOI] [PubMed] [Google Scholar]
- 19.Strubberg AM, and Madison BB. MicroRNAs in the etiology of colorectal cancer: pathways and clinical implications. Dis Model Mech. 2017;10(3):197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suto T, Yokobori T, Yajima R, Morita H, Fujii T, Yamaguchi S, et al. MicroRNA-7 expression in colorectal cancer is associated with poor prognosis and regulates cetuximab sensitivity via EGFR regulation. Carcinogenesis. 2015;36(3):338–45. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Lv L, Lin C, Hu G, Guo Y, Wu M, et al. Combinational treatment with microRNA133b and cetuximab has increased inhibitory effects on the growth and invasion of colorectal cancer cells by regulating EGFR. Mol Med Rep. 2015;12(4):5407–14. [DOI] [PubMed] [Google Scholar]
- 22.Chang K, Taggart MW, Reyes-Uribe L, Borras E, Riquelme E, Barnett RM, et al. Immune Profiling of Premalignant Lesions in Patients With Lynch Syndrome. JAMA Oncol. 2018;4(8):1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korotkevich G, Sukhov V, and Sergushichev A. Fast gene set enrichment analysis. bioRxiv. 2019:060012. [Google Scholar]
- 24.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell. 2016;18(6):827–38. [DOI] [PubMed] [Google Scholar]
- 26.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15(6):701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–72. [DOI] [PubMed] [Google Scholar]
- 28.Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67(20):9721–30. [DOI] [PubMed] [Google Scholar]
- 29.Delker DA, Wood AC, Snow AK, Samadder NJ, Samowitz WS, Affolter KE, et al. Chemoprevention with Cyclooxygenase and Epidermal Growth Factor Receptor Inhibitors in Familial Adenomatous Polyposis Patients: mRNA Signatures of Duodenal Neoplasia. Cancer Prev Res (Phila). 2018;11(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tessner TG, Muhale F, Riehl TE, Anant S, and Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114(11):1676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahreyni A, Rezaei M, Bahrami A, Khazaei M, Fiuji H, Ryzhikov M, et al. Diagnostic, prognostic, and therapeutic potency of microRNA 21 in the pathogenesis of colon cancer, current status and prospective. J Cell Physiol. 2019;234(6):8075–81. [DOI] [PubMed] [Google Scholar]
- 32.Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T, et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med. 2017;23(7):878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94(2):657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisinger AL, Nadauld LD, Shelton DN, Peterson PW, Phelps RA, Chidester S, et al. The adenomatous polyposis coli tumor suppressor gene regulates expression of cyclooxygenase-2 by a mechanism that involves retinoic acid. J Biol Chem. 2006;281(29):20474–82. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, and Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneikert J, and Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56(3):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2002;99(3):1521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janakiram NB, Mohammed A, Zhang Y, Brewer M, Bryant T, Lightfoot S, et al. Chemopreventive efficacy of raloxifene, bexarotene, and their combination on the progression of chemically induced colon adenomas to adenocarcinomas in rats. Cancer Prev Res (Phila). 2013;6(12):1251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubet RA, Clapper ML, McCormick DL, Pereira MA, Chang WC, Steele VE, et al. Chemopreventive efficacy of Targretin in rodent models of urinary bladder, colon/intestine, head and neck and mammary cancers. Oncol Rep. 2012;27(5):1400–6. [DOI] [PubMed] [Google Scholar]
- 40.Giardiello FM, Casero RA Jr., Hamilton SR, Hylind LM, Trimbath JD, Geiman DE, et al. Prostanoids, ornithine decarboxylase, and polyamines in primary chemoprevention of familial adenomatous polyposis. Gastroenterology. 2004;126(2):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30(3):377–86. [DOI] [PubMed] [Google Scholar]
- 42.Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346(14):1054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, and Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29(6):781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janakiram NB, Cooma I, Mohammed A, Steele VE, and Rao CV. Beta-ionone inhibits colonic aberrant crypt foci formation in rats, suppresses cell growth, and induces retinoid X receptor-alpha in human colon cancer cells. Mol Cancer Ther. 2008;7(1):181–90. [DOI] [PubMed] [Google Scholar]
- 45.Zhou H, Liu W, Su Y, Wei Z, Liu J, Kolluri SK, et al. NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer Cell. 2010;17(6):560–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology. 2011;141(4):1486–97, 97 e1–14. [DOI] [PubMed] [Google Scholar]
- 47.Rath T, Roderfeld M, Graf J, Wagner S, Vehr AK, Dietrich C, et al. Enhanced expression of MMP-7 and MMP-13 in inflammatory bowel disease: a precancerous potential? Inflamm Bowel Dis. 2006;12(11):1025–35. [DOI] [PubMed] [Google Scholar]
- 48.Dhawan P, Ahmad R, Chaturvedi R, Smith JJ, Midha R, Mittal MK, et al. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene. 2011;30(29):3234–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asting AG, Caren H, Andersson M, Lonnroth C, Lagerstedt K, and Lundholm K. COX-2 gene expression in colon cancer tissue related to regulating factors and promoter methylation status. BMC Cancer. 2011;11:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinugasa T, Huo Q, Higashi D, Shibaguchi H, Kuroki M, Tanaka T, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27(6A):3729–34. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


