Abstract
Purpose:
The mainstay of treatment for basal cell carcinoma (BCC) is surgical excision, which can result in significant associated morbidity, particularly for patients with recurrent tumors. We previously conducted a drug repositioning screen using molecular data from human BCCs and identified histone deacetylase (HDAC) inhibitors as a potential treatment for BCC. Here we conduct the first proof-of-principle study of a topical pan-HDAC inhibitor, remetinostat, in human BCC.
Patients and Methods:
We conducted a phase 2, open label, single arm, single institution trial of a topical HDAC inhibitor. Participants with at least one BCC were recruited. All participants applied 1% remetinostat gel three times daily for six weeks, with measurements of tumor diameter conducted at baseline and week eight. Surgical excision of remaining tumor was conducted at the end of the study and microscopic evaluation was performed.
Results:
33 per-protocol tumors from 25 participants were included in the analysis. The objective response rate, defined as the proportion of tumors achieving greater than 30% decrease in the longest diameter from baseline to week eight, was 69.7% (90% confidence interval 54 – 82.5%). 54.8% of tumors demonstrated complete resolution on pathological examination. Pharmacodynamic analysis demonstrated similar levels of acetylated histone H3 in skin tissue before and after treatment, however, phosphorylation was increased. No systemic adverse events were reported.
Conclusions:
The HDAC inhibitor remetinostat, is a well-tolerated and effective topical treatment for reducing BCC disease burden in a clinically significant manner. This provides in-human validation of HDAC inhibitors as a therapy for BCC.
Translational Relevance
Epigenetic modification of gene expression through inhibition of histone deacetylases (HDAC) is a promising anti-cancer strategy. However, despite promising preclinical studies, HDAC inhibitor monotherapy has achieved only modest success in human clinical trials for solid tumors and is limited by systemic toxicity. We previously identified the role of histone acetylation in the sequestration of the GLI1, a transcription factor in the Hedgehog signaling pathway, integral to the pathogenesis of basal cell carcinoma (BCC). Here, we provide the first in human clinical trial data demonstrating that topical delivery of a pan-HDAC (HDAC 1, 3, and 6) inhibitor can penetrate skin and BCC tumors, thereby altering histone phosphorylation, suppressing GLI1 transcription, and decreasing tumor size. Our clinical trial demonstrated positive clinical efficacy in BCC with no systemic side effects. These findings suggest that HDAC inhibitors are likely an effective therapeutic class for cutaneous malignancies and could have potential for wider oncologic use.
Introduction
Basal cell carcinoma (BCC) is the most common cancer worldwide, and its incidence is increasing in many countries (1). In the United States, more than 3 million cases occur yearly, resulting in almost $4 billion in annual health care costs (2–4). It is estimated that one in five individuals in the United States will develop at least one BCC during their lifetime, representing a significant public health burden (5). The definitive treatment of BCC is surgical excision; however, these procedures are time consuming, expensive, and may be associated with cosmetic and functional morbidity. In addition, some individuals develop frequent tumors and suffer substantial surgical exhaustion and morbidity (6). Thus, an effective, non-surgical treatment option would be of significant benefit.
To identify novel treatments for BCC, we previously conducted a systematic in silico drug repositioning screen. The gene signatures of 23 early and six advanced BCCs were integrated with gene expression data from over 1,100 FDA-approved drugs contained in the Library of Integrated Cellular Signature (7) to identify compounds that oppose the BCC gene-expression signature (8). The in-silico screen identified Histone deacetylase (HDAC) inhibitors as the top predicted therapeutic for BCC. Subsequent preclinical target validation experiments demonstrated that HDAC inhibitors could suppress the growth of BCC cell lines and BCC allografts generated from Ptch1+/− K14-Cre-ER2 p53 fl/fl mice (8). HDAC inhibitors have demonstrated efficacy in other cutaneous malignancies; vorinostat and romidepsin are FDA approved for the treatment of cutaneous T cell lymphoma (CTCL) (9).
Remetinostat is a benzoic acid with a molecular weight of 323.3 g/mol (10) that has been shown to inhibit HDAC isoforms one, three and six in in-vitro enzymatic assays, with a mean inhibition constant (Ki) of 160, 66, and 10, respectively. Unlike other HDAC inhibitors, remetinostat was designed to retain potency within the skin but be readily metabolized upon absorption, thus producing effective local activity in cutaneous lesions with negligible systemic effects. Remetinostat has been analyzed in trials for CTCL in over 100 subjects for as long as one year with no treatment related systemic adverse events (AEs), indicative of minimal systemic exposure given the short half-life of remetinostat (11).
As a proof-of-principle study of HDAC inhibitors for BCC, we designed an open-label, single institution, phase two clinical trial to investigate the efficacy and tolerability of topical 1% remetinostat gel for BCC. We also conducted explorative studies to assess drug penetrance and pharmacodynamic effect.
Materials and Methods
Ethical approval was granted by the Stanford University Institutional Review Board (IRB-40947) and this research was conducted in accordance with the Declaration of Helsinki. The trial was prospectively registered with Clinicaltrials.gov (NCT03180528). Study medication was provided by Medivir AB, Huddinge, Sweden. The full protocol is available in the Supplementary Material.
Participants
Potential participants were identified from referrals to Stanford Hospitals and Clinics, California from May 2018 to June 2020. Eligible participants were adults with at least one cutaneous BCC greater than or equal to 5mm in diameter (as measured after initial diagnostic biopsy) and amenable to surgical resection. Negative serum pregnancy test within 14 days prior to the first dose of study therapy for women of child-bearing potential was required, in addition to ongoing use of acceptable methods of contraception throughout the study period.
Patients with large (greater than 25mm), cosmetically sensitive, inoperable, locally advanced, or metastatic lesions were excluded. Additionally, patients taking any medication known to affect the HH signaling pathway (i.e. itraconazole), or topical or systemic therapies that might interfere with the growth of BCC (e.g. glucocorticoids) within the preceding six months were also excluded. Other criteria for exclusion included: recent systemic chemotherapy; uncontrolled acute illness; moderate to severe immunosuppression; history of congestive heart failure, cardiac arrhythmias or ventricular dysfunction; pregnancy or breast feeding; and known hypersensitivity to HDAC inhibitors.
Clinical Study
Following written informed consent, eligibility was independently assessed by three members of the study team. Baseline safety labs and serum pregnancy testing were conducted on applicable patients. Following enrolment, baseline tumor measurements and photographs were obtained. Participants were instructed to keep the medication refrigerated. Participants then began six weeks of three times daily topical treatment with 1% remetinostat gel. This regimen was chosen due to the short half-life of remetinostat (11). Participants were instructed to bandage occlude the site of application following administration and to log each application in a provided diary. Monitoring visits were conducted at week four and eight, where clinical tumor response was evaluated through measurements and photography, in addition to recording AEs, wellbeing, treatment compliance and changes in concomitant medications. At the week eight visit, any remaining tumor was surgically excised and examined histologically either during Mohs surgery or formal pathological examination. Participant follow up post-treatment was conducted via telephone between weeks ten to twelve.
Any AEs occurring during the treatment or follow up period were recorded and graded using the CTCAE v5.0 (12). Participants experiencing moderate or severe AEs were instructed to temporarily or permanently suspend treatment, as deemed appropriate by the principal investigator.
For the final six recruited participants, due to the COVID-19 pandemic, changes were made to the study procedures as permitted by the IRB in order to protect participants. In the context of the known safety of the study medication, baseline safety lab monitoring was omitted. Additionally, the baseline and week four study visits were conducted remotely.
Outcomes
Primary Outcome
The primary outcome was the overall response rate of BCC based on change in the longest diameter of the tumor from baseline to week eight. ORR was defined as the proportion of subjects with either a complete response or a partial response (30% or greater decrease in longest diameter) among all per-protocol treated subjects. These criteria were modelled based on the commonly used Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, but modified as we enrolled tumors less than 10mm in diameter which is below the minimum permitted, and we wished to evaluate each tumor individually given the non-systemic nature of the experimental therapy (13). Prior to study commencement, a sample size calculation for the primary outcome was performed. With a planned analysis of 30 BCCs, the study was estimated to have 91% power to reject an ORR of 15% if the true ORR was 40% or better, at one sided alpha level of 0.05 with at least nine responders.
Secondary Outcomes
Secondary outcomes included change in tumor cross-sectional area, pathological resolution of the tumor at time of excision, participant compliance, relative change in GLI1 mRNA expression, and the incidence, nature and severity of AEs.
Tumor Imaging
The change in cross-sectional tumor area was calculated using pre and post treatment photography and the Fiji distribution v2.0 of the ImageJ software (14) which was calibrated using the known dimension of the ruler applied to the participant’s skin (15).
Statistical Analysis
ORR was calculated for per-protocol tumors as described above, in addition to an intention-to-treat (ITT) analysis. Per-protocol was defined as tumors that successfully completed the trial with at least 70% participant compliance with instructed drug applications. Exact binomial 90% confidence intervals (CI) for the ORR were calculated using R v3.6.1 (16). Results of the primary analysis were further stratified by patient demographics and tumor characteristics. Where tumor histology was mixed, the most invasive was selected as the predominant type for classification.
Treatment compliance was calculated as a percentage using the study diary. Periods of investigator-directed suspension of treatment were not included in the denominator of these calculations in order to reflect true compliance.
Percentage of pathological resolution was calculated using the results of formal pathological examination or from the frozen pathologic analysis during the Mohs surgery. Concordance between the clinical and pathological assessments were calculated, in addition to descriptive statistics of the reported AEs.
GLI1 RT-PCR Analysis
BCCs are universally driven through activation of HH signaling with induction of target genes through GLI transcription factors. HDAC1 has been shown to play a critical role in regulating GLI1 access to target promotors through acetylation of GLI1 at K518 (17,18). We hypothesized that remetinostat may inhibit HH signaling by preventing deacetylation of GLI1 (8). To assess HH signaling, we assayed GLI1 expression in six paired pre- and post-treatment biopsies using quantitative PCR (see Supplementary Text 1). Post treatment GLI1 mRNA levels in the paired BCC sample sets were compared with baseline GLI1 levels for the respective subject to calculate percent change in GLI1 mRNA expression. Data was analyzed using the comparative CT method, normalized to an endogenous control reference. Relative change in expression was tested for significance using a two-way ANOVA.
Immunohistochemistry
To investigate the penetrance and histological effect of remetinostat, immunohistochemistry (IHC) for acetylated and phosphorylated histone H3 was performed on sections of the initial pre-enrolment and post treatment excisional biopsies where participants had a applied a final dose of the medication 30 minutes prior to excision. IHC was performed by HistoWiz, Inc., New York (19) (see Supplementary Text 2), using rabbit polyclonal to Histone H3 phospho S10 (1:1200; Abcam 5176) and rabbit monoclonal to acetylated-Histone H3 at Lys9 (1:800; Cell Signal 9649) antibodies. Furthermore, to explore the effect of remetinostat on markers of cell proliferation and apoptosis, we conducted immunoperoxidase staining of paired pre- and post-treatment biopsies. Staining was conducted for Ki-67 and BCL-2 at the Stanford Immunodiagnosis Laboratory, Palo Alto.
Results
Enrolment, Study Completion and Participant Demographics
From May 2018 to June 2020, a total of 30 participants with 49 BCC tumors were enrolled in the trial, with 25 participants and 33 tumors included in the per-protocol analysis (Figure 1). An additional two participants, with a total of 12 tumors, were included in the ITT analysis. The median age of the participants was 59 (range 38 – 86), 63% (n=19) were male and 90% white non-hispanic (n=27). The majority of participants had a single enrolled BCC (n=22), and 47% (n=14) had a previous history of skin cancer. Enrolled tumors were located across both sun-exposed and non-exposed sites, and the majority of the tumors were either nodular or superficial in histology (Table 1).
Figure 1:

Modified Consort Flow Chart of Study Participants
Table 1:
Participant Demographics
| Mean Age in Years (Range) | 59 (38 – 86) |
| Gender n(%) | |
| Male | 19 (63%) |
| Female | 11 (37%) |
| Race n(%) | |
| White | 27 (90%) |
| Other | 1 (3%) |
| Unknown | 2 (7%) |
| Ethnicity n(%) | |
| Non-Hispanic/Latino | 27 (90%) |
| Hispanic | 1 (3%) |
| Unknown | 2 (7%) |
| Personal History of Skin Cancer n(%) | |
| Yes | 14 (47%) |
| No | 16 (53%) |
| No. of Enrolled Tumors n(%) | |
| One | 22 (73%) |
| Two | 4 (13%) |
| Three | 1 (3%) |
| Four or Greater | 3 (10%) |
| BCC Histological Subtypes n(%) | |
| Nodular | 17 (35%) |
| Superficial | 7 (14%) |
| Infiltrative | 1 (2%) |
| Pigmented Nodular | 1 (2%) |
| Superficial and Nodular | 11 (22%) |
| Nodular and Micronodular | 2 (4%) |
| Nodular and Infiltrative | 2 (4%) |
| Not Determined | 8 (16%) |
| Initial Tumor Diameter n(%) | |
| <=10mm | 20 (40.8%) |
| >10mm | 29 (59.2%) |
| Anatomical Location n(%) | |
| Forearm & Elbow | 3 (6%) |
| Upper Arm | 2 (4%) |
| Shoulder | 5 (10%) |
| Clavicle | 2 (4%) |
| Chest | 6 (12%) |
| Back | 12 (24%) |
| Abdomen | 2 (4%) |
| Flank | 1 (2%) |
| Thigh | 4 (8%) |
| Shin | 3 (6%) |
| Neck | 3 (6%) |
| Face | 3 (6%) |
| Ear | 3 (6%) |
Efficacy
Primary Outcome
Six partial and 17 complete responses in the tumor longest diameter were recorded from baseline to week eight (Figure 2), resulting in an ORR of 69.7% (90% CI 54 – 82.5%). The average change in the longest tumor diameter was a decrease of 62.3%. Representative photographic images are illustrated in Figure 3. Two tumors did not change in diameter, while eight tumors decreased in size but by less than the 30% threshold for partial response. No tumors were recorded to have increased in diameter. When the ORR was stratified by tumor histology (Figure 2, Supplementary Table 1), the superficial subtype of BCC responded best to the treatment (n=6; ORR=100%), while the nodular (n=22; ORR=68.2%) and infiltrative (n=3; ORR=66.7%) responded similarly. The micronodular subtype did not respond to treatment (n=2; ORR=0%). Response was also stratified by participant demographics, tumor characteristics and grade of cutaneous drug reaction (Supplementary Tables 2 – 7). Age under 60 years, male gender and history of previous skin cancer were all associated with small, non-significant, increases in ORR, whilst location of tumor on a sun-exposed site and higher grade of cutaneous drug reaction were both associated with larger, yet non-significant, increases in response (ORR for sun-exposed sites 81.8% vs. 63.6% for non-exposed sites; ORR 73.91% for grade two to three cutaneous drug reaction vs. 60% for grade one or no reaction). Initial diameter of tumor had no effect on ORR.
Figure 2: Waterfall Plot of Percentage Change in Longest Tumor Diameter from Baseline to Week Eight of Remetinostat Treatment.

Note: 30% threshold indicating at least a partial response is demarcated by the black line.
Figure 3: Representative Photographs of BCC Tumors Pre-Treatment and Post-Treatment with Remetinostat Gel.
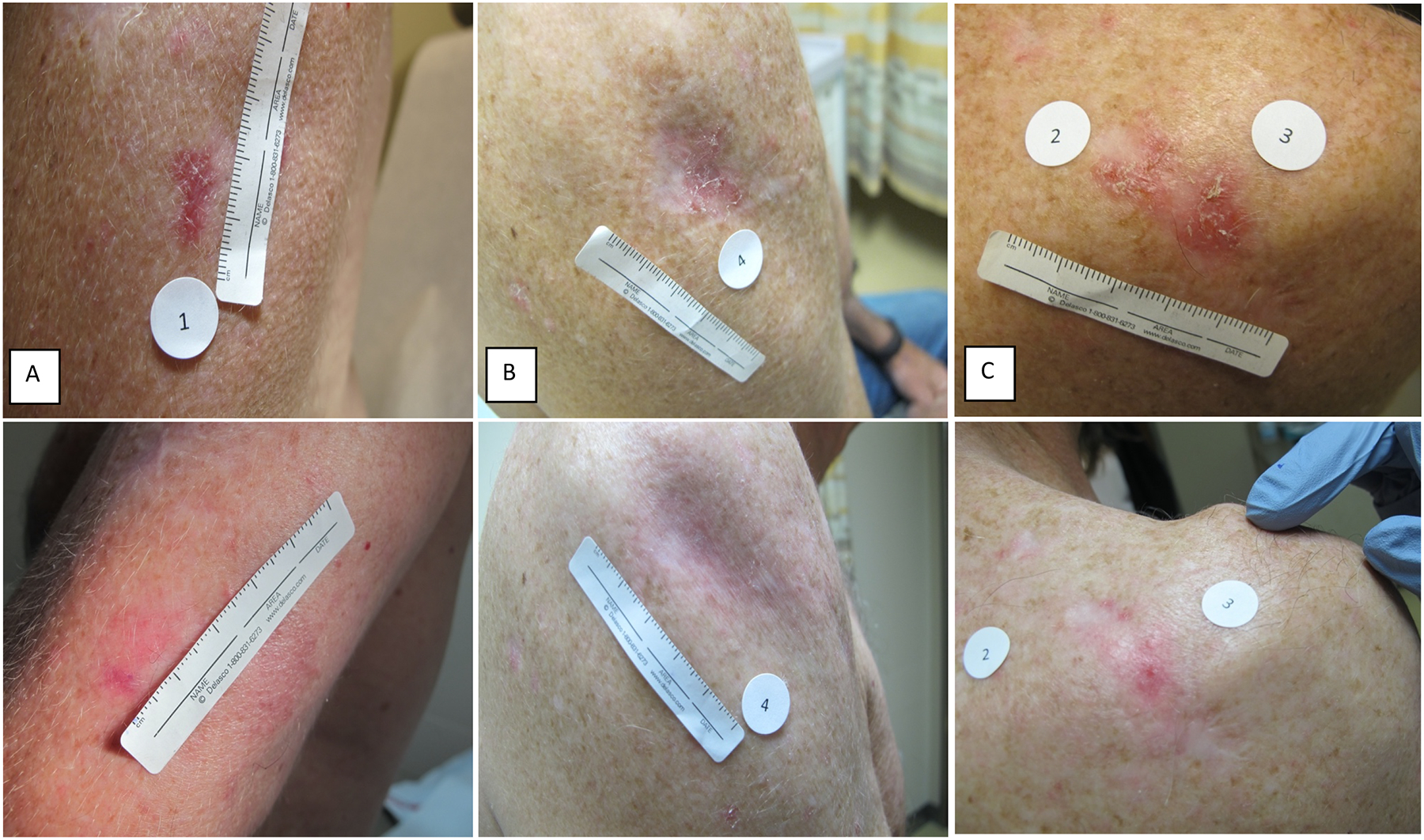
A: Tumor at baseline, superficial histology, left upper arm, longest diameter 13mm (TOP). Clinically and pathologically residual tumor at week eight, longest diameter 2mm, 85% decrease (BOTTOM).
B: Tumor at baseline, superficial histology, right upper arm, longest diameter 15mm (TOP). Complete clinical resolution of tumor at week eight, pathological examination not performed at discretion of dermatologic surgeon (BOTTOM).
C: Two tumors at baseline, superficial and nodular histology, right upper back, longest diameters 15mm (2) and 16mm (3) (TOP). Clinically and pathologically residual tumors at week eight with partial responses, longest diameters 4mm (2) and 13mm (3), 73% and 19% decreases, respectively (BOTTOM).
Secondary Outcomes
32 tumors decreased in cross-sectional area from baseline to week eight, with one tumor increasing in cross-sectional area by 4% (from 21 to 22mm2; the longest diameter did not change). The average change in tumor area was a decrease of 71.5% (Supplementary Figure 1). Histological examination for residual tumor was performed during Mohs excision (n=9) or by pathologist (n=22) for 31 per-protocol tumors. A histological read was not available for two tumors; one was not excised at the discretion of the dermatologic surgeon due to complete clinical resolution, while the other was treated with electrodesiccation and curettage. 17 tumors (54.8%) demonstrated complete histological resolution of BCC, whilst 14 tumors (45.2%) contained microscopic residual tumor. There was 67.7% concordance between the clinical assessment made by the study team with the histological results; of 10 discordant tumors, four were clinically resolved but contained microscopic residual, and six clinically appeared to have residual tumor, yet histology was negative. Compliance with treatment ranged from 73 – 100%, with mean compliance per tumor of 95%.
Intention-To-Treat Analysis
27 participants with 45 tumors were included in the ITT analysis. This included one participant (four tumors) who was less than 70% compliant with treatment (0%, 0%, 3.9% and 66% for the four tumors), and another participant (eight tumors) who did not refrigerate the study medication as instructed. Three enrolled participants were not included; one participant did not commence treatment due to abnormal baseline safety labs, and two participants withdrew at week four and therefore week eight data were not available (Figure 1). The ORR of the change in tumor longest diameter was 51.1% (90% CI 38 – 64.1%; five partial responses and 18 complete responses), with an average decrease of 52.5%. The cross-sectional area changed by an average decrease of 55.6%, and 21 of 35 evaluable tumors (60%) demonstrated complete histological resolution. Compliance per tumor ranged from 0 – 100%, with a mean of 85%.
Safety and Adverse Events
All enrolled participants were included in the safety analysis. No systemic or serious AEs were experienced during treatment or follow up (Supplementary Table 8). The most frequently reported AE was an eczematous reaction localized to the site of drug application. A total of 27 discrete episodes were reported, with a mean duration of 64.4 days. The majority of these episodes (n=21) were of CTCAE grade two (moderate) severity, with five grade one (mild) and one was rated as grade three (severe). During the course of the study, four participants were required to temporarily suspend treatment (from one day to two weeks) under investigator guidance due to the eczematous reaction, and three participants permanently suspended treatment. A representative illustration of the typical eczematous reaction is demonstrated in Supplementary Figure 2. A less frequently reported AE was pain at the site of drug application, with six episodes reported, four grade one and two grade two, requiring two participants to temporarily suspend treatment. One participant reported eczema, pruritis (grade one) and bleeding from the lesion (grade one) and was required to temporarily discontinue treatment for three days. Another participant reported eczema and skin ulceration (grade one), temporarily discontinuing treatment for two weeks. In total, 27 of 30 enrolled participants reported at least one AE (90%). Of the three participants not experiencing an AE, one had not been commenced on treatment and one was less than 70% compliant.
Histone Acetylation and Phosphorylation
To determine drug penetration and pharmacologic activity in target tissue, we assessed the levels of acetylated and phosphorylated histone H3 in six paired pre- and post-treatment biopsies using IHC. Two of the post-treatment sections contained residual tumor. IHC failed to demonstrate a significant change in acetylation of histone H3 in the keratinocytes of the post-treatment samples as compared to the pre-treatment samples as high levels of acetylated histone H3 were seen across the keratinocytes; however, phosphorylation at S10 was increased after HDAC inhibitor treatment (Figure 4).
Figure 4: Acetylated and Phosphorylated Histone H3 Immunohistochemistry of BCC Tumors.
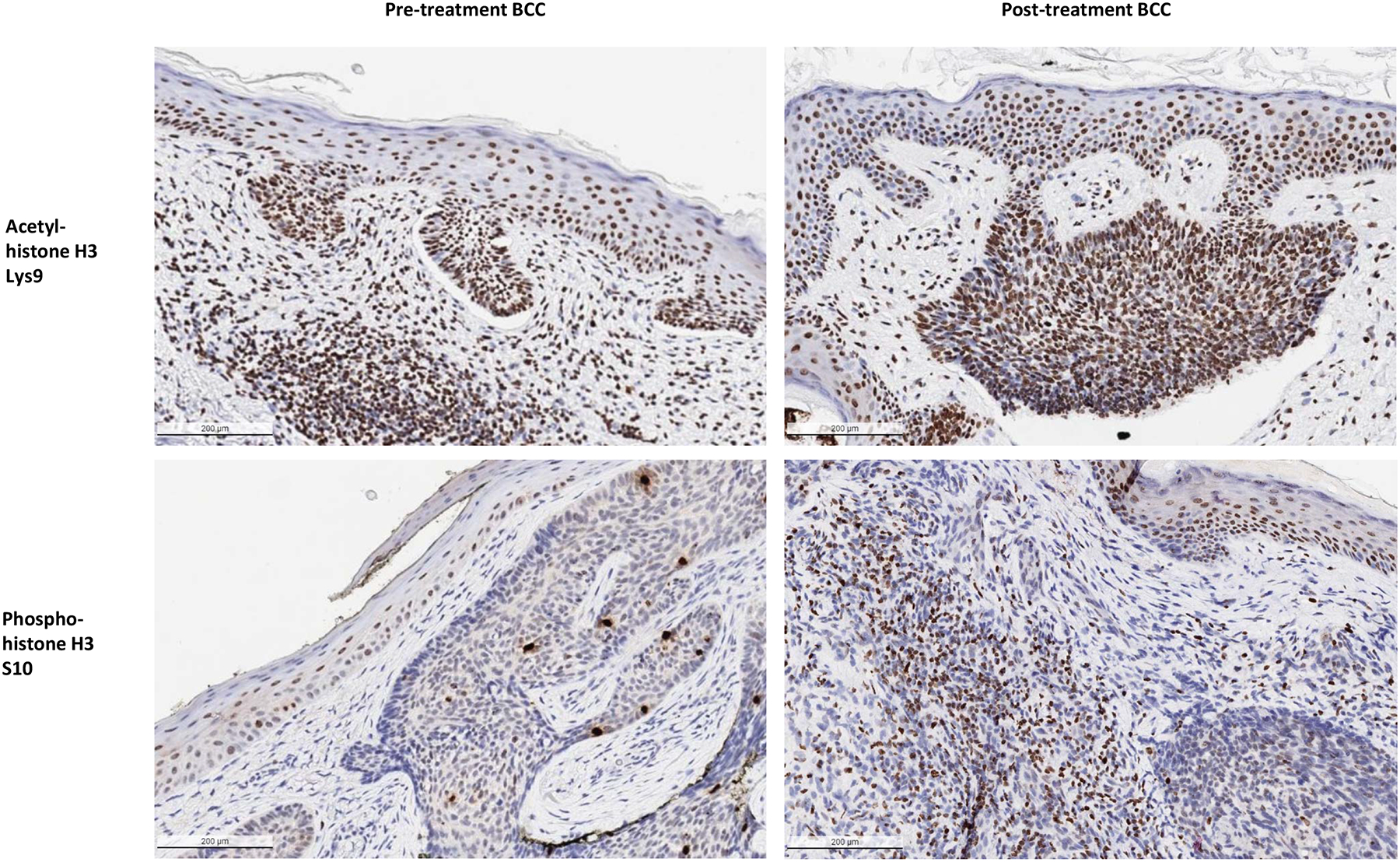
Representative examples of pre-treatment biopsy sections (LEFT) and post-treatment biopsy sections with residual tumor present (RIGHT) from a superficial and nodular BCC stained with acetyl-histone H3 Lys9 antibodies (TOP) and a nodular and infiltrative BCC stained with phospho-histone H3 S10 antibodies (BOTTOM). Increased levels of epidermal and tumor phosphorylation are visible in the post-treatment sections, while levels of histone acetylation appear similar (x10 microscopy).
GLI1 Expression in Post-Treatment Tumor Tissue
Relative expression of GLI1 significantly decreased after treatment for three of the paired biopsies, including one with residual BCC (Supplementary Figure 3). Another tumor with residual cancer demonstrated a non-significant increase, while a further two tumors with residual BCC had significantly increased expression in the post-treatment biopsy.
Markers of Cell Proliferation and Apoptosis
Decreased staining of the cell proliferation marker Ki-67 was revealed in areas of residual BCC in post-treatment sections compared to pre-treatment sections, while staining of the apoptosis marker BCL-2 was not appreciatively different (Supplementary Figure 4).
Discussion
This is the first clinical trial of a topical HDAC inhibitor for BCC. Our results demonstrate a clinically significant decrease in tumor size in response to six weeks of topical 1% remetinostat therapy in 70% of per-protocol tumors, with 58% reaching complete pathological resolution. During the course of treatment and follow up, no serious or systemic AEs were reported, and the medication was generally well tolerated, with the majority of participants experiencing solely a moderate, tolerable, localized cutaneous eczematous reaction at the site of drug application. These safety results are in line with the findings of previous trials of remetinostat for CTCL (11,20). In addition, high levels of participant compliance were observed despite a three times daily dosing regimen, and 25 of 29 participants who commenced treatment were able to successfully complete the trial.
In addition to these clinical findings, we conduced explorative IHC studies. Levels of histone acetylation were similar before and after HDAC inhibitor treatment, although we noted significant baseline acetylation, including in areas of normal skin, suggesting that the assay may have been too sensitive to detect pharmacologically-relevant changes in acetylation. Given the known gene activating role of simultaneous histone H3 S10 phosphorylation and H3 acetylation (21), we also conducted IHC for histone H3 phosphorylation, which demonstrated significantly increased staining post-treatment. Previous research has shown that acetylation of the lysine 9 and 14 residues of histone H3 increases the affinity of the regulatory protein 14-3-3 to phosphorylated S10, with the 14-3-3 protein in turn protecting the residue from dephosphorylation, therefore stabilizing gene expression (22). This data supports that HDAC inhibition likely increases histone phosphorylation. Our findings suggest that remetinostat is able to penetrate into the epidermis of skin and alter histone phosphorylation within 30 minutes of application in comparison to baseline, supporting the proposed mechanism of action by which HDAC inhibitors alter transcription of genes involved in cellular growth and proliferation through epigenetic modification of histones (23). Additionally, immunoperoxidase staining for Ki-67 suggested decreased cellular proliferation following treatment, supporting the anti-oncogenic effect of remetinostat.
Recent work has also demonstrated a role for HDAC inhibitors in modulating HH pathway signaling through blocking deacetylation of the pathway’s downstream transcription factor, GLI1. Acetylation of GLI1 at K518 has been shown to sequester the transcription factor in the inner membrane of the cell nucleus. The HDAC1 isoform deacetylates GLI1, which in turn enables it to interact with chromatin and induce transcription of pro-oncogenic genes downstream of the HH pathway (17). In our analysis of relative GLI1 mRNA expression before and after treatment, we demonstrated a significant decrease in expression where there was resolution of BCC, while tumors with residual cancer tended to demonstrate increased expression. Similar results were reported in a study of the oral Smoothened (SMO) inhibitor sonidegib, where substantial reductions in expression of GLI1 were found in patients achieving disease control (24). This supports that resistant tumors continue to experience enhanced signaling through the HH pathway, as is seen in BCCs resistant to SMO inhibitors (25). We hypothesize that the enhanced expression of GLI1 in these tumors may be either due to the failure of remetinostat to sufficiently penetrate the tumor cells or tumor mechanisms to evade drug suppression and maintain HH signaling. Interestingly, in the two sections chosen with residual cancer, greater levels of phosphorylation were seen in fragmented tumor cells compared to intact nests of BCC. We hypothesize that perhaps the reason for the persistence of residual cancer in these sections was due to lack of sufficient penetration of remetinostat into these nested cells. This is supported by our clinical findings, whereby the majority of tumors of superficial histology demonstrated complete resolution.
While surgical excision remains the current mainstay of treatment for BCC, medical treatments for BCC include SMO antagonists, which are FDA approved for the treatment of advanced tumors (26). However, SMO inhibitors are only approved for advanced and metastatic BCC as they are associated with undesirable side effects, and more than half of treated BCCs develop resistance to these antagonists (25,27), through acquiring mutations in SMO (25). We hypothesize that BCCs may have more difficulty evading HDAC inhibition given the more downstream location of HDAC1 in the HH pathway. Furthermore, we posit that a topical inhibitor would likely be better tolerated than an oral medication given the minimal systemic absorption and therefore lack of systemic AEs. Topical SMO inhibitors have been tested in patients with Gorlin syndrome, and although the results demonstrated greater tolerability than their oral counterparts, clinical (28) and pathological (29) response rates were low. Other medical treatments for BCC include photodynamic therapy, as well as topical imiquimod and 5-fluorouracil. While these agents are effective for the treatment of superficial BCC, response rates for other subtypes are lower (30). While we found that remetinostat was most effective in superficial tumors, nodular and infiltrative tumors also demonstrated a similar ORR, suggesting that remetinostat could be an effective option for non-superficial tumors also.
Interestingly, the ORR and the average percent decrease in longest diameter that we observed for tumors which experienced a higher grade of eczematous drug reaction were higher when compared to tumors experiencing a milder reaction, although pathological resolution rates were similar (Supplementary Table 7). These results suggests that the degree of cutaneous reaction could potentially correlate to the anti-tumor activity of the remetinostat, similar to that observed with other topical agents such as imiquimod (31).
Limitations and Future Research
Limitations of this trial include clinician-based visual assessment of the primary outcome measure. To mitigate the subjective nature of this outcome, pathological resolution was used to correlate the findings in the majority of tumors. Additionally, this was a single arm study with no comparator control group; however, given the known natural history of BCC where spontaneous resolution is uncommon (32), it is unlikely that the enrolled tumors would have resolved to the degree observed without treatment. This trial also included solely a low risk, majority white non-hispanic sample of patients; the generalizability of our findings to other settings including more diverse populations and those with immunosuppression is unclear. Finally, our explorative, correlative IHC and GLI1 expression studies were conducted using a limited number of paired pre and post-treatment biopsies, due to the fact that not all participants applied a final dose of remetinostat prior to surgical excision.
Future research should aim to conduct a blinded, randomized controlled trial of a topical HDAC inhibitor. Given the potential applications of this medication for individuals with recurrent disease, Gorlin Syndrome and the immunosuppressed, these groups should be included in future studies. The latter group is particularly pertinent, given that we observed a greater ORR in tumors experiencing a higher-grade cutaneous reaction, which suggests that cutaneous immunity may play a role in HDAC inhibitor-induced tumor clearance. Furthermore, when the results of this present trial were stratified by tumor histology, no response was observed in the two tumors of micronodular subtype. Our study was not sufficiently powered to show a significant difference in the stratified analyses, and future studies should address this through using larger sample sizes. We also hypothesize that inhibiting HDAC1 activity may be critical for treating BCC, as prior data have implicated HDAC1 specifically in modulation of GLI chromatin accessibility and downstream HH signaling (8). Future studies of HDAC selective inhibitors would further help explore this hypothesis. Finally, given the limited number of paired biopsies studied in our explorative biomarker studies, future research is required to fully elucidate the anti-oncogenic mechanism of this class.
Conclusions
In this proof-of-principle study, the pan-HDAC inhibitor, remetinostat, was an effective and well-tolerated topical therapy for BCC, potentially reducing the need for surgical excision, particularly in superficial tumors but also in tumors of nodular and infiltrative histology. Given the tolerability and clinical and pathological response rates demonstrated in this trial across several histological subtypes, HDAC inhibitors could be a realistic new class of topical agents for BCC. In patients suffering from recurrent disease, an effective topical treatment could have a revolutionary impact on patient outcomes, morbidity and quality of life, and for this reason, further trials are warranted, particularly to assess the durability of treatment response following HDAC inhibitor therapy.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Medivir AB, Sweden for the investigational product for this investigator-initiated clinical trial, and the Stanford Cancer Clinical Trials Office and Neal Birkett (Regulatory Affairs Professional, Stanford University) for regulatory support.
Funding
Funding for this project was provided by Medivir AB (All Authors), the Damon Runyon Foundation (K. Sarin), the National Cancer Institute 5K23CA211793 (K. Sarin), American Skin Association Hambrick Medical Student Grant recipient (N. Urman) and Stanford Medical Scholars (N. Urman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI. Kavita Y. Sarin, MD, PhD is the D. G. “Mitch” Mitchell Clinical Investigator supported by the Damon Runyon Cancer Research Foundation (CI-104-19).
Funding Sources:
Medivir AB, Huddinge, Sweden
Footnotes
IRB Approval Status: Reviewed and approved by Stanford IRB (IRB-40947)
Clinicaltrials.gov Listing: NCT03180528
Declaration of Interests
The authors have no conflicts of interest to declare.
Data Sharing
Individual participant data will not be shared. Full study protocol will be published alongside article.
References
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012;166(5):1069–80 doi 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 2.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer 2008;8(10):743–54 doi 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol 2015;151(10):1081–6 doi 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 4.Guy GP Jr., Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med 2015;48(2):183–7 doi 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol 1994;30(5 Pt 1):774–8 doi 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 6.Chiang A, Solis DC, Rogers H, Sohn GK, Cho HG, Saldanha G, et al. Prevalence and risk factors for high frequency basal cell carcinoma in the United States. J Am Acad Dermatol 2020. doi 10.1016/j.jaad.2020.07.088. [DOI] [PubMed] [Google Scholar]
- 7.LINCS Consortium. 2019October12. Library of Integrated Network-Based Cellular Signatures (LINCS). LINCS Consortium. Accessed2020 October 12. [Google Scholar]
- 8.Mirza AN, Fry MA, Urman NM, Atwood SX, Roffey J, Ott GR, et al. Combined inhibition of atypical PKC and histone deacetylase 1 is cooperative in basal cell carcinoma treatment. JCI Insight 2017;2(21) doi 10.1172/jci.insight.97071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 2007;12(10):1247–52 doi 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 10.PubChem. 2020October13. PubChem Compound Summary for CID 24875489, Remetinostat. National Library of Medicine (US), National Center for Biotechnology Information <https://pubchem.ncbi.nlm.nih.gov/compound/Remetinostat>. Accessed 2020 Oct 13. [Google Scholar]
- 11.Duvic M, Kim YH, LeBoeuf NR, Porcu P, Hastings J, Bassuner J, et al. A phase 2 randomized study of SHAPE Gel (SHP-141) in patients with early-stage cutaneous T-cell lymphoma: Interim results. Journal of Clinical Oncology 2016;34(15_suppl):7562- doi 10.1200/JCO.2016.34.15_suppl.7562. [DOI] [Google Scholar]
- 12.US National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Washington, D.C.: United States Department of Health and Human Services; 2017November27. [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9(7):676–82 doi 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scofield-Kaplan SM, Jackson C, Gurney T, McDonnell E, Mancini R. Predictive Value of Preoperative Periocular Skin Cancer Measurements for Final Mohs Defect Size. Ophthalmic Plast Reconstr Surg 2019;35(6):604–8 doi 10.1097/iop.0000000000001421. [DOI] [PubMed] [Google Scholar]
- 16.The R Foundation for Statistical Computing. R. v3.6.1 Vienna, Austria: The R Foundation for Statistical Computing; 2019. [Google Scholar]
- 17.Mirza AN, McKellar SA, Urman NM, Brown AS, Hollmig T, Aasi SZ, et al. LAP2 Proteins Chaperone GLI1 Movement between the Lamina and Chromatin to Regulate Transcription. Cell 2019;176(1–2):198–212.e15 doi 10.1016/j.cell.2018.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coni S, Mancuso AB, Di Magno L, Sdruscia G, Manni S, Serrao SM, et al. Selective targeting of HDAC1/2 elicits anticancer effects through Gli1 acetylation in preclinical models of SHH Medulloblastoma. Sci Rep 2017;7:44079 doi 10.1038/srep44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HistoWiz Inc. 2020September7. Frequently Asked Questions. HistoWiz Inc. <https://home.histowiz.com/faq/>. Accessed2020 September 7. [Google Scholar]
- 20.Kim YH, Krathen M, Duvic M, Wong H, Porcu P, Tacastacas J, et al. A phase 1b study in cutaneous T-cell lymphoma (CTCL) with the novel topically applied skin-restricted histone deacteylase inhibitor (HDAC-i) SHP-141. Journal of Clinical Oncology 2014;32(15_suppl):8525- doi 10.1200/jco.2014.32.15_suppl.8525. [DOI] [Google Scholar]
- 21.Walter W, Clynes D, Tang Y, Marmorstein R, Mellor J, Berger SL. 14-3-3 Interaction with Histone H3 Involves a Dual Modification Pattern of Phosphoacetylation. Molecular and Cellular Biology 2008;28(8):2840–9 doi 10.1128/mcb.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komar D, Juszczynski P. Rebelled epigenome: histone H3S10 phosphorylation and H3S10 kinases in cancer biology and therapy. Clinical Epigenetics 2020;12(1):147 doi 10.1186/s13148-020-00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int J Mol Sci 2017;18(7) doi 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dummer R, Liu L, Squittieri N, Gutzmer R, Lear J. Expression of Glioma-associated oncogene homolog 1 as biomarker with sonidegib in advanced basal cell carcinoma. Oncotarget 2020;11(37):3473–83 doi 10.18632/oncotarget.27735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atwood SX, Sarin KY, Whitson RJ, Li JR, Kim G, Rezaee M, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell 2015;27(3):342–53 doi 10.1016/j.ccell.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang JY, Ally MS, Chanana AM, Mackay-Wiggan JM, Aszterbaum M, Lindgren JA, et al. Inhibition of the hedgehog pathway in patients with basal-cell nevus syndrome: final results from the multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17(12):1720–31 doi 10.1016/s1470-2045(16)30566-6. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe HJ, Pau G, Dijkgraaf GJ, Basset-Seguin N, Modrusan Z, Januario T, et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell 2015;27(3):327–41 doi 10.1016/j.ccell.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein EH, Lear J, Saldanha G, Tang JY, Harwood C. Hedgehog pathway inhibition by topical patidegib to reduce BCC burden in patients with basal cell nevus (Gorlin) syndrome. Journal of Clinical Oncology 2018;36(15_suppl):e21626–e doi 10.1200/JCO.2018.36.15_suppl.e21626. [DOI] [Google Scholar]
- 29.Skvara H, Kalthoff F, Meingassner JG, Wolff-Winiski B, Aschauer H, Kelleher JF, et al. Topical treatment of Basal cell carcinomas in nevoid Basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol 2011;131(8):1735–44 doi 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- 30.Tanese K. Diagnosis and Management of Basal Cell Carcinoma. Curr Treat Options Oncol 2019;20(2):13 doi 10.1007/s11864-019-0610-0. [DOI] [PubMed] [Google Scholar]
- 31.Shumack S, Robinson J, Kossard S, Golitz L, Greenway H, Schroeter A, et al. Efficacy of Topical 5% Imiquimod Cream for the Treatment of Nodular Basal Cell Carcinoma: Comparison of Dosing Regimens. Archives of Dermatology 2002;138(9):1165–71 doi 10.1001/archderm.138.9.1165. [DOI] [PubMed] [Google Scholar]
- 32.Wehner MR, Dalma N, Landefeld C, Pare-Anastasiadou A, Koutelidas I, Chren MM, et al. Natural history of lesions suspicious for basal cell carcinoma in older adults in Ikaria, Greece. Br J Dermatol 2018;179(3):767–8 doi 10.1111/bjd.16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


