Abstract
Background
Gintonin inhibits β-amyloid production, increases acetylcholine level in the brain, and promotes neurogenesis. We evaluated the efficacy of gintonin-enriched fraction (GEF) in improving the cognitive performance in subjective memory impairment.
Methods
In this 8-week, randomized, assessor and participant blinded, placebo–controlled study, participants with subjective memory impairment but preserved cognitive function (Korean Mini-Mental State Examination [K-MMSE] score ≥23) were assigned to GEF 300mg/day or placebo. K-MMSE, Korean versions of the Alzheimer's disease assessment scale, color-word stroop test (K-CWST), clinical dementia rating, and Beck depression inventory-II were evaluated along with the safety profiles. The primary outcome was set as the change in the K-MMSE.
Results
Seventy-six participants complete the study protocol. After 8 weeks, there was no inter-group difference in the primary or secondary outcome score changes. However, GEF group showed an improvement in the K-MMSE scores (P= 0.026), and in the number of correct answers in both word reading (P= 0.008) and color reading (P= 0.005) of K-CWST, although only the improvement in the K-CWST scores were higher than the minimum clinically important difference. The frequency of adverse events was comparable between the groups and all were of mild severity.
Conclusion
GEF is safe but might not be effective in treating subjective memory impairment within the current study setting. However, GEF showed a trend of improving the global cognition and the frontal executive function. Further large-sized studies with longer follow-up period are warranted.
Clinical trial registration
This clinical trial was registered at Clinical Research Information Service of Korea Centers for Disease Control and Prevention: KCT0004636.
Keywords: Dietary supplement, Frontal lobe function, Ginseng, Gintonin, Mild cognitive impairment, Subjective memory impairment
1. Introduction
Although dementia has a rapidly increasing global incidence and substantial clinical and socioeconomic burden,1 the efficacy of drugs for treating dementia is limited to the level of symptom alleviation and delaying the disease progression.2 According to the recent findings that pathologic, functional, and structural changes in the brain begins to progress as early as twenty years before the clinical symptoms of dementia becomes evident.3 In this regard, early recognition and modification of the disease pathomechanism is increasingly recognized as the key to improve the clinical course of dementia.2, 4 Subjective memory impairment is a status that a subject is complaining an apparent decrement of memory function compared to the subject's previous state without any abnormality in other cognitive functions. Subjective memory impairment is very frequent in the aged population and substantially affects the daily function.5 Although the presence of subjective memory impairment is highly associated with the risk of progression to dementia,5, 6 taking medications for dementia at the stage of subjective memory impairment is not recommended due to their limited clinical benefit over the potential adverse effects, which commonly includes include diarrhea, nausea, vomiting, leg cramps, and sleep disturbance.7, 8, 9 Therefore, the role of dietary supplement with neuroprotective effect is relatively emphasized, but most of the dietary supplements has insufficient evidence of improving cognitive function.10
Increasing evidences indicate that ginseng extracts might be effective in improving cognitive function.11, 12 In addition to the traditional concept that effect of ginseng on cognitive function is mediated by sapoinin,13, 14 it has recently been recognized that highly concentrated lysophosphatidic acid (LPA), a G protein-coupled receptor ligand, in ginseng,15 improves cognitive function via mechanisms distinct from those of saponin.16, 17 Gintonin is a high concentration extract of ginseng-containing LPA, which inhibits the production of β-amyloid (Aβ) from amyloid precursor protein (APP) by activating neuronal LPA receptors,18 and promotes the release of soluble APP-α, which has a neuroprotective property.18 Gintonin also increases long-term potentiation (LTP) and synaptic transmission by LPA receptor mediated activation calcium-dependent ion channels,19, 20 stimulates acetylcholine synthase and increase the production of acetylcholine in the hippocampus.17, 18 Additionally, gintonin forms a complex with Ginseng Major Latex-like Protein151 (GLP151), a ginseng-specific glycoprotein, which stabilizes ginseng LPAs and enhances LPAs’ binding affinity to LPA receptor.18, 21
Recently, we reported a cognitive improving effect after 12 weeks of gintonin administration in 9 subjects with mild dementia, although the study has limitations as a small sized single arm study. 22 In the current study, we performed a randomized, assessor and participant blinded, placebo controlled clinical trial to evaluate the safety and cognitive improving effect of the gintonin-enriched fraction (GEF) in subjects with subjective memory impairment.
2. Methods
2.1. Trial registration
This clinical trial was registered at Clinical Research Information Service; Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare, Republic of Korea (KCT0004636, date of registration: October 01, 2019, https://cris.nih.go.kr/).
2.2. Study design and procedures
The current study was designed as a randomized, assessor and participant blinded, placebo–controlled fashion. At baseline, participants’ medical histories, performed physical and laboratory examinations were reviewed. Participants who met the inclusion criteria were then randomly assigned to GEF and the placebo groups with 1:1 allocation ratio.
2.2.1. Ethical statement
The study protocol and supporting documentation were approved by Institutional Review Board (IRB) of SNUH (H-1711-092-901) and the study was performed in compliance with the SNUH IRB regulations and the International Conference on Harmonisation guideline for Good Clinical Practice. Written informed consent to participate was obtained from all enrolled participants.
2.3. Eligible criteria
Subjects aged between 50 and 85 and with subjective memory impairment were recruited from the Neurology department of the Seoul National University Hospital. Subjective memory impairment was designated as the subject's self-reporting of the subjective feeling of decrement in memory function compared to the subject's previous state, without objective impairment in the cognitive function, screened using Korean Mini-Mental State Examination (K-MMSE, score ≥23).23 We excluded subjects who (1) are taking ginseng or health functional foods containing ginseng components; (2) have allergies or hypersensitivity to ginseng; (3) have an established liver disease or with serum alanine transaminase, aspartate aminotransferase, or bilirubin levels equal or greater than three times the upper limit of the normal range; (4) have a chronic kidney disease with serum creatinine level of greater than 2.0 mg/deciliter (176.7 μmol/liter) or on dialysis; (5) has medical status that interferes with food absorption or oral administration of the drug; (6) are being treated for major mental illnesses such as mood disorders or schizophrenia; (7) have been diagnosed with dementia; (8) are taking medications for dementia, drugs or other dietary supplements that may affect memory and cognitive function, or other drugs on clinical trials for cognitive improving effect within 4 weeks from the time of inclusion; (9) addicted to alcohol or other drugs; (10) are pregnant, planning a pregnancy, or on breast feeding; or (11) have participated in another clinical trial within one month of visit.
2.4. Intervention
2.4.1. GEF
The GEF group received two 400 mg tablets containing GEF 150 mg and excipient compound (dextrin, crystalline cellulose, caramel pigment, gardenia yellow pigment, magnesium stearate, silicon dioxide) 250 mg once daily for 8 weeks. Daily dosage of GEF administration was decided by calculating the equivalent dose of a dosage used in a mouse in vivo experiment that observed a cognitive improving effect after using 25-100 mg/kg/day of GEF to a human adult with body weight of 60 kilograms.17, 21
2.4.2. Placebo group
The placebo group received two 400 mg tablets containing crystalline cellulose 374 mg and excipient compound (caramel pigment, gardenia yellow pigment, magnesium stearate, silicon dioxide) 26mg once daily for 8 weeks.
GEF and placebo tablets used for the study were manufactured by Gintonin KU Biotech Co.,LTD. (Republic of Korea), in a Good Manufactured Practice facility. The product was stored at room temperature. The GEF and the placebo tablets were identical in appearance.
2.5. Outcome measures
2.5.1. Primary outcomes
At baseline, at 4 weeks, and at 8 weeks after treatment, cognitive function was assessed using K-MMSE.24 The primary outcome parameter was designated as change in MMSE total scores at 8 weeks from the baseline.
2.5.2. Secondary outcomes
At baseline, at 4 weeks, and at 8 weeks after treatment, Korean version of the Alzheimer's disease assessment scale (ADAS-K),25 Korean version of the color-word stroop test (K-CWST),26 and Beck Depression Inventory-II (BDI-II) for the severity of depression27 were evaluated. The secondary outcome parameters were designated as the changes in ADAS-K total scores, ADAS scores in cognitive domain (ADAS-cog), ADAS scores in non-cognitive domain (ADAS-noncog), number of correct answers in word reading and color reading domains of K-CWST test, BDI-II scores, and CDR scores at 8 weeks from the baseline.
Treatment-emergent adverse events were identified and recorded according to the medical dictionary for regulatory activities (MedDRA).28 Laboratory measurement included complete blood count and serum panels including electrolyte profiles, levels of glucose, uric acid, total protein, albumin, creatinine, and liver enzymes, cholesterol profiles, routine urinalysis, systolic and diastolic blood-pressure and pulse rate, and routine 12-lead electrocardiography.
2.6. Sample size
The size of study population was estimated based on a previous study conducted on 137 subjects with dementia, which reported 1.3 point difference in the MMSE score changes between the GEF and placebo groups, with 0.4 point standard deviation of score changes in each group.29 38 participants were required per each group, based on level of significance of 5% and power of 80%. A total of 80 subjects were recruited from each of 40 in GEF and placebo groups, in consideration of 5% dropout rate.
2.7. Randomization, allocation concealment, and blinding
Allocation concealment and randomization was conducted by Biofood Contract Research Organization (CRO), a third party who does not participate in the research process. Randomization number was assigned in the order in which the subjects were enrolled. According to the randomization number, subjects was assigned to the computer generated random list, prepared by Biofood CRO before the initiation of the study. The GEF group and the placebo group are allocated using the block randomization method. Group assignment data of each subject was sealed by the Biofood CRO. The outcome assessment was performed by two Neurology specialist (HC and HRS), blinded to the patient allocation and clinical information.
2.8. Statistical analysis
SAS® (Version 9.4, SAS Institute, Cary, North Carolina, USA) was used for all statistical analyses. All analyses to evaluate the efficacy were conducted according to the per-protocol set and safety measures were performed according to the safety set. Baseline characteristics were compared using the Chi-square test or the Fisher's exact test for categorical variables and the t-test or the Wilcoxon rank sum test for continuous variables. The change of the scores from baseline to 8 weeks after treatment was evaluated using the paired t-test or the Wilcoxon rank sum test. Inter-group comparison of the change of the scores from baseline was performed using Repeated-Measures Analysis of Variance (RM-ANOVA). Frequency of adverse events was compared using the Fisher's exact test for categorical variables and the t-test or the Wilcoxon rank sum test for continuous variables. All statistical evaluations were two-tailed and P values < 0.05 were set as statistically significant.
3. Results
3.1. Baseline characteristics and participants’ flow
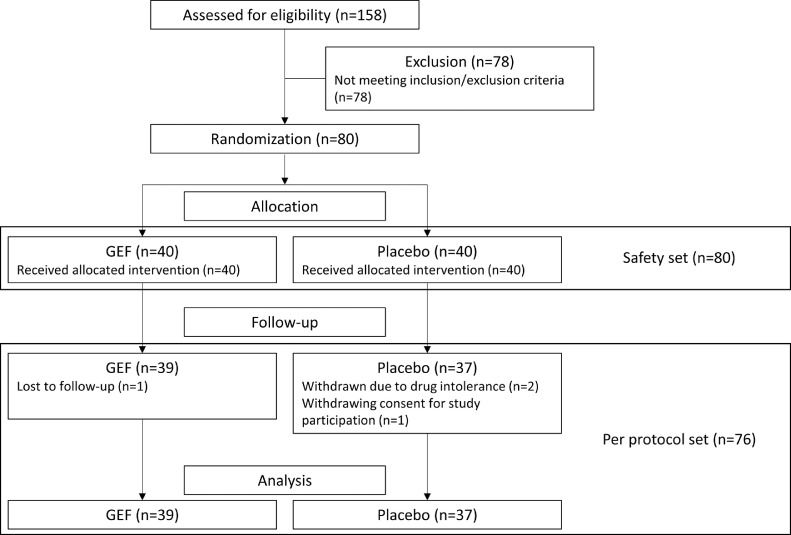
A total of 80 participants were included between October 2018 and May 2019. 40 participants were randomized into the GEF group and the remaining 40 into the placebo group. During follow-up, 4 subjects (1 in the GEF group and 3 in the placebo group) were excluded, due to the loss of follow-up in one patient, intolerance to the drug in two patients, and withdrawal of the consent for the study participation in one patient. Finally, 76 participants (39 in the GEF group and 37 in the placebo group) complete the study protocol (Fig. 1). Demographic and baseline clinical characteristics were comparable between the groups (Table 1).
Fig. 1.
A flow chart illustrating the study process.
Table 1.
Demographic and baseline clinical cha racteristics
| GEF (n=40) | Placebo (n=40) | P value | |
|---|---|---|---|
| Female sex (%) | 24 (61.54%) | 26 (70.27%) | 0.423 |
| Age (years) | 66.62±9.09 | 68.43±9.29 | 0.484 |
| Education level (years) | 12.65±3.56 | 11.35±3.97 | 0.127 |
| Hypertension (%) | 12 (30.0) | 11 (27.5) | 0.808 |
| Diabetes (%) | 3 (7.5) | 7 (17.5) | 0.181 |
| Hyperlipidemia (%) | 3 (7.5) | 2 (5.0) | 0.649 |
| Regular alcohol drinker | 11 (27.5) | 10 (25.0) | 0.586 |
| Smoking | |||
| Non-Smoker (%) | 33 (82.5) | 30 (75.0) | 0.290 |
| Ex-Smoker (≥ 6 months %) | 2 (5.0) | 3 (7.5) | |
| Current Smoker (%) | 4 (10.0) | 4 (10.0) | |
| K-MMSE | 27.41±1.62 | 27.59±2.07 | 0.125 |
Data are reported as mean ± standard deviation. K-MMSE: Korean version of Mini-Mental State Examination
3.2. Outcomes
For the primary outcome parameter, there was no significant difference in the K-MMSE score changes between the GEF group and the placebo group (P = 0.945) (Table 2).
Table 2.
Primary and secondary outcomes at baseline, 4weeks and 8 weeks
| GEF (n=39) | Placebo (n=37) | Inter-group difference | P value† | ||
|---|---|---|---|---|---|
| K-MMSE | Baseline | 27.41±1.62 | 27.59±2.07 | ||
| 4 week | 27.51±2.29 | 27.68±2.61 | |||
| 8 week | 28.10±1.97* | 27.86±1.93 | |||
| Change 1 | 0.10±2.07 | 0.08±1.20 | 0.17 (-0.71-1.05) | 0.695 | |
| Change 2 | 0.69±1.87 | 0.27±1.59 | 0.03 (-0.75-0.80) | 0.945 | |
| ADAS-K | Baseline | 13.16±5.41 | 13.89±5.68 | ||
| 4 week | 12.38±5.03 | 9.25±4.67 | |||
| 8 week | 10.62±4.70 | 12.11±6.04 | |||
| Change 1 | -0.78±4.89 | -4.64±4.08 | -0.68 (-2.99-1.63) | 0.561 | |
| Change 2 | -2.53±3.94 | -1.95±4.46 | -1.20 (-3.52-1.12) | 0.307 | |
| ADAS-cog | Baseline | 10.26±4.44 | 10.67±5.01 | ||
| 4 week | 9.05±3.80* | 9.25±4.67* | |||
| 8 week | 7.60±3.16⁎⁎ | 8.35±4.53⁎⁎ | |||
| Change 1 | -1.21±3.69 | -1.43±3.31 | -0.31 (-2.20-1.58) | 0.747 | |
| Change 2 | -2.66±3.27 | -2.32±3.34 | -0.58 (-2.41-1.25) | 0.526 | |
| ADAS-non cog | Baseline | 2.90±1.86 | 3.22±2.50 | ||
| 4 week | 3.33±2.17 | 3.76±2.95 | |||
| 8 week | 3.02±2.73 | 3.75±2.90 | |||
| Change 1 | 0.43±2.25 | 0.54±2.47 | -0.37 (-1.33-0.58) | 0.479 | |
| Change 2 | 0.14±2.63 | 0.47±2.47 | -0.55 (-1.56-0.45) | 0.276 | |
| BDI-II | Baseline | 14.59±7.69 | 14.86±7.92 | ||
| 4 week | 14.31±8.03 | 16.30±9.79 | |||
| 8 week | 13.21±10.00 | 13.95±8.77 | |||
| Change 1 | -0.28±4.91 | 1.43±5.66 | -1.13 (-4.77-2.51) | 0.537 | |
| Change 2 | -1.38±8.20 | -0.92±6.01 | -0.51 (-4.10-3.09) | 0.779 | |
| K-CWST Word reading: number of correct responses | Baseline | 110.38±2.57 | 110.65±4.02 | ||
| 4 week | 111.38±1.46* | 110.78±2.74 | |||
| 8 week | 111.38±1.57⁎⁎ | 111.46±1.17 | |||
| Change 1 | 1.00±2.43 | 0.14±3.81 | 0.17 (-0.90-1.24) | 0.755 | |
| Change 2 | 1.00±2.25 | 0.81±3.91 | 0.17 (-0.75-1.09) | 0.755 | |
| K-CWST Word reading: response time (sec) | Baseline | 87.44±30.84 | 88.54±42.58 | ||
| 4 week | 82.03±40.09 | 82.22±28.11 | |||
| 8 week | 78.46±28.86* | 85.3±39.77 | |||
| Change 1 | -5.41±36.21 | -6.32±20.39 | -0.65 (-15.61-14.31) | 0.932 | |
| Change 2 | -8.97±25.91 | -3.24±22.75 | -3.97 (-19.37-11.43) | 0.609 | |
| K-CWST Color reading: number of correct responses | Baseline | 106.37±6.32 | 106.78±7.73 | ||
| 4 week | 108.18±5.17 | 107.03±8.96 | |||
| 8 week | 109±3.69⁎⁎ | 108.68±6.66⁎⁎ | |||
| Change 1 | 2.08±6.94 | 0.50±3.95 | 0.38 (-2.64-3.40) | 0.803 | |
| Change 2 | 2.87±5.86 | 1.89±3.84 | 0.08 (-2.57-2.73) | 0.957 | |
| K-CWST Color reading: response time (sec) | Baseline | 149.84±47.45 | 156.14±50.46 | ||
| 4 week | 142.77±43.89 | 151.03±49.16 | |||
| 8 week | 135.49±42.93⁎⁎ | 142.92±40.71⁎⁎ | |||
| Change 1 | -8.82±31.18 | -6.26±28.42 | -7.58 (-28.72-13.56) | 0.477 | |
| Change 2 | -17.11±26.94 | -13.03±25.61 | -8.34 (-28.47-11.79) | 0.412 |
Data are reported as mean ± standard deviation. Intergroup differences are reported as mean difference (95% CI).
ADAS-K: Korean versions of the Alzheimer's disease assessment scale, ADAS-cog/non-cog: cognitive/non-cognitive domains of ADAS-K, BDI-II: Beck depression inventory-II, CI: confidence interval, K-CWST: Korean versions of the color-word stroop test, and K-MMSE: Korean versions of the mini-mental status examination. Change 1: 4weeks- baseline; Change 2: 8weeks- baseline
P<0.05
P<0.01: P value for the change from baseline, by Paired t-test or Wilcoxon rank sum test.
P value for the test group and the placebo group, by repeated-measure ANOVA.
Among the secondary efficacy outcome parameters, there was no statistically significant difference in the 8 week changes of ADAS-K total scores (P = 0.307), ADAS-cog scores (P = 0.526), ADAS-noncog scores (P = 0.276), and BDI-II scores (P = 0.779) between the GEF and placebo groups. (Table 2).
There was no significant difference in score change of K-CWST between two groups (P = 0.755). There was no significant difference in the evaluation time of the word reading domain of the K-CWST between groups (P = 0.609), the color reading domain, and the evaluation time of the color reading domain of the K-CWST between groups (P = 0.412).
The medication compliance of the GEF group was 94.24 ± 13.74% and of the placebo group was 95.08 ± 11.47%. In the safety set analysis, a total of five adverse events were reported in the GEF group and five in the placebo group (Table 3). There was no significant difference in the frequency of adverse events between the groups. Every adverse event was of mild degree and none of the event was demonstrated to have definite or probable causal relationship to the drug administration. Two participants were dropped out due to the adverse reactions, both of which in the placebo group. Laboratory measurement profiles showed no statistically significant differences between the groups or between the baseline and at 4 weeks or at 8 weeks.
Table 3.
Profiles of adverse events
| Adverse events | Severity | Causality | ||
|---|---|---|---|---|
| GEF | Placebo | GEF | Placebo | |
| Tinnitus | 1 (mild) | 1 (mild) | 1 (possible) | 1 (unlikely) |
| Loss of appetite | 2 (mild) | – | 1 (unlikely), 1 (unrelated) | – |
| Foamy urine | 1 (mild) | – | 1 (unrelated) | – |
| Dizziness | 1 (mild) | 1 (mild) | 1 (unlikely) | 1 (possible) |
| Arthralgia | – | 1 (mild) | – | 1 (possible) |
| Plantar pain | – | 1 (mild) | – | 1 (possible) |
| Dyspepsia | – | 1 (mild) | – | 1 (possible) |
* There were no significant difference in severity (p=1.0) and causality (p=0.07) between two groups.
4. Discussion
The outcome analyses returned that GEF did not have any significant improvement in the cognitive function over the placebo group. However, 8 weeks of taking GEF was associated with improvement in the total K-MMSE score and the increased number of correct answers in word reading and color reading domains of the K-CWST. Among them, only the improvement in the word and color reading domains of the K-CWST were higher than minimum clinically important difference (MCID, 1.4 point for K-MMSE score, and 0.56 point for word and color reading domains of the K-CWST).30 Using GEF was not associated with an increased frequency of adverse events. No serious adverse event or significant alterations in the laboratory measures was observed during the use of GEF.
The primary outcome analyzes returned a negative result. This results is in concordance with those of the previous studies that evaluated the cognitive improving effect of numerous candidate drugs in mild cognitive impairment (MCI).8, 31, 32, 33, 34, 35 For instance, donepezil is the most widely established drug for treating Alzheimer's disease (AD) and other dementia, but it's efficacy on cognition in patients with MCI did not overcome the increased frequency of adverse event associated with the use of drug.8, 34 Due to the low initial severity of cognitive measurements, demonstrating a significant effect of a drug in improving the cognitive measurements might be extremely difficult in the clinical setting of MCI or subjective memory impairment. However, we observed an improvement in the K-CWST scores higher than MCID.
Since the disease progression form subjective memory impairment to clinically evident dementia might take decades,2, 36, 37 favorable long-term safety is required for the candidate medication to treat subjective memory impairment. Considering the broad mechanism of gintonin in improving cognition and neuroprotection, GEF might be a potentially beneficial as a long-term medication for the patients with mild cognitive deficits.15, 18, 20, 22 Numerous previous studies also reported the beneficial effect of natural products in treating or preventing dementia. Chinese herbal therapy containing Ren shen, Di huang, Cang pu, Yuan zhi, Yin yanghuo, Shan shuyu, Rou congrong, Yun jin, dan shen, Dang gui, Tian ma, and Huang lian, combined with conventional treatment, were superior to the conventional treatment alone in preventing the progression of Alzheimer's dementia.38 The effect of Chinese herbal medicine in improving cognition was also proved by meta-analyses.39 Combination of a Japaneses herbal medicine Kami-Untan-To with donepezil was also superior to donepezil alone in improving cognition in patients with dementia.40 Korean traditional medicines also have various degrees of evidence of their beneficial effect on cognition.41 Considering the favorable safety profiles of these natural products, the effect of GEF might be augmented when it is used in combination with these herbal medicines.
The major effects of gintonin that includes inhibition β-amyloid production, promoting neuroprotective soluble amyloid precursor protein α, increasing acetylcholine production while inhibiting acetylcholine esterase activity in the brain, and enhancing neurogenesis. The primary mechanism of those effect is the activation LPA receptors by gintonin. However, GEF might also maximize the effects of LPA by providing an optimal environment for the stable enhancement of the LPA receptor activation. Up to 90% of GEF containing LPAs is the C18: 2 subtype which has strong stimulatory effect on LPA receptor.15, 18 In addition, histidines in the α3 helix of C-terminal of GLP151 are ionically bound to the phosphate group of gintonin LPAs, protecting LPAs from rapid hydrolysis and enhancing the stability of gintonin LPAs.16 Due to these multiple properties, GEF has a high potential as a dietary supplement to prevent dementia and to improve cognitive function in the population with relatively preserved cognitive function.
To further establish the clinical effect of GEF, the following limitations of the current study should be addressed. First, the 8-week observation period of this study might have been insufficient to demonstrate the effect of GEF. In the previous studies evaluating the effect of various medical candidates in patients with MCI, the change in the cognitive scores over time was very slow, so longer duration of follow-up is warranted to properly evaluate its disease modifying effect. Second, the number of participants in this study was small, considering that previous studies included 250–2100 patients to evaluate the clinical effect of their candidate drugs.8, 31, 32, 33, 34 Given that this study observed a certain trend favoring the effect of GEF in the change of cognitive scores, future studies might include large number of patients to properly evaluate the effect of GEF. Third, this study used only a single dosage of GEF. As GEF was safe without provoking any adverse events, future studies might include multiple dosage regimens with higher doses of GEF. Additionally, more comprehensive tools for cognitive function assessment such as Korean version of the Montreal Cognitive Assessment, or specific tools for assessing the memory function such as Korean version of the Memory Assessment Scales, might be used in future studies to more accurately evaluate the effect of GEF on memory function.42, 43
In conclusion, GEF is safe but might not be effective in treating subjective memory impairment within the current study setting. However, GEF showed a trend of improving the global cognition and the frontal executive function.
Author contributions
Conceptualization: MK and SYN. Methodology: HC, HRS, MK and SYN. Formal Analysis: WJL and YWS. Investigation: WJL. Resources: WWK and SWJ. Data Curation: HC and HRS. Writing – Original Draft: WJL. Writing – Review & Editing: WWK, SWJ, MK and SYN. Supervision: MK and SYN.
Conflict of interests
W.-W.K. and S.-W.J. are employees of Gintonin KU Biotech Co., LTD. Otherwise, the authors have no competing interests.
Funding
This study was funded by the Gintonin KU Biotech Co., LTD. (Republic of Korea). This work was supported by the Basic Science Research Program funded by the Ministry of Science, ICT, and Future Planning (NRF 2020R1F1A1058460).
Ethical statement
The study protocol and supporting documentation were approved by Institutional Review Board (IRB) of SNUH (H-1711-092-901) and the study was performed in compliance with the SNUH IRB regulations and the International Conference on Harmonisation guideline for Good Clinical Practice.
Data availability
The datasets generated and/or analyzed during the study are available from the corresponding author upon request.
Contributor Information
Manho Kim, Email: kimmanho@snu.ac.kr.
Seung-Yeol Nah, Email: synah@konkuk.ac.kr.
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dementia. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007. e2. [DOI] [PubMed] [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet North Am Ed. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weimer DL, Sager MA. Early identification and treatment of Alzheimer’s disease: social and fiscal outcomes. Alzheimer’s Dementia. 2009;5(3):215–226. doi: 10.1016/j.jalz.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E, Kaduszkiewicz H, et al. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimer’s Dementia. 2014;10(1):76–83. doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 7.Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L, He Y, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 8.Doody R, Ferris S, Salloway S, Sun Y, Goldman R, Watkins W, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 9.Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD006104. [DOI] [PubMed] [Google Scholar]
- 10.Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, Erickson KI, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer's disease. Neurobiol Aging. 2014;35:S74–S78. doi: 10.1016/j.neurobiolaging.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy DO, Scholey AB. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75(3):687–700. doi: 10.1016/s0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee S-T, Chu K, Sim J-Y, Heo J-H, Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 13.Geng J, Dong J, Ni H, Lee MS, Wu T, Jiang K, et al. Ginseng for cognition. Cochrane Database Syst Rev. 2010;(12) doi: 10.1002/14651858.CD007769.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Nuri THM, Yee JCW, Gupta M, Khan MAN, Ming LC. A review of Panax ginseng as an herbal medicine. Arch Pharm Pract. 2016;7(5):61–65. [Google Scholar]
- 15.Hwang SH, Shin T-J, Choi S-H, Cho H-J, Lee B-H, Pyo MK, et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33(2):151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S-H, Jung S-W, Lee B-H, Kim H-J, Hwang S-H, Kim H-K, et al. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H-J, Shin E-J, Lee B-H, Choi S-H, Jung S-W, Cho I-H, et al. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-β protein, and mouse model of Alzheimer's disease. Mol Cells. 2015;38(9):796. doi: 10.14348/molcells.2015.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SH, Shin E-J, Shin T-J, Lee B-H, Choi S-H, Kang J, et al. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31(1):207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 19.Park H, Kim S, Rhee J, Kim H-J, Han J-S, Nah S-Y, et al. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015;113(5):1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 20.Shin T-J, Kim H-J, Kwon B-J, Choi S-H, Kim H-B, Hwang S-H, et al. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors. Mol Cells. 2012;34(6):563–572. doi: 10.1007/s10059-012-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Kim M-S, Park K, Kim H-J, Jung S-W, Nah S-Y, et al. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res. 2016;40(1):55–61. doi: 10.1016/j.jgr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon J, Choi S-H, Shim J-Y, Park H-J, Oh M-J, Kim M, et al. Gintonin administration is safe and potentially beneficial in cognitively impaired elderly. Alzheimer Dis Assoc Disord. 2018;32(1):85–87. doi: 10.1097/WAD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 23.Lee W-J, Shin Y-W, Kim D-E, Kweon M-H, Kim M. Effect of desalted Salicornia europaea L. ethanol extract (PM-EE) on the subjects complaining memory dysfunction without dementia: a 12 week, randomized, double-blind, placebo-controlled clinical trial. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-76938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15(2):300–308. [Google Scholar]
- 25.Youn J, Lee D, Kim KW, Lee J, Jhoo J, Lee K, et al. Development of the Korean version of Alzheimer's disease assessment scale (ADAS-K) Int J Geriatr Psychiatry. 2002;17(9):797–803. doi: 10.1002/gps.699. [DOI] [PubMed] [Google Scholar]
- 26.Kim TY, Kim S, Sohn JE, Lee EA, Yoo BG, Lee SC, et al. Development of the Korean stroop test and study of the validity and the reliability. J Korean Geriatr Soc. 2004;8(4):233–240. [Google Scholar]
- 27.Yu B-K, Lee H-K, Lee K-S. Validation and factor structure of Korean version of the Beck Depression Inventory Second Edition (BDI-II): in a university student sample. Korean J Biol Psychiatry. 2011;18(3):126–133. [Google Scholar]
- 28.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20(2):109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 29.Onder G, Zanetti O, Giacobini E, Frisoni GB, Bartorelli L, Carbone G, et al. Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer's disease: randomised controlled trial. Br J Psychiatry. 2005;187(5):450–455. doi: 10.1192/bjp.187.5.450. [DOI] [PubMed] [Google Scholar]
- 30.Roux P, Brunet-Gouet E, Ehrminger M, Aouizerate B, Aubin V, Azorin JM, et al. Minimum clinically important differences for the Functioning Assessment Short Test and a battery of neuropsychological tests in bipolar disorders: results from the FACE-BD cohort. Epidemiol Psychiatr Sci. 2020:29. doi: 10.1017/S2045796020000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ. 2013;185(16):1393–1401. doi: 10.1503/cmaj.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock G, Truyen L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 34.Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 35.Loy C, Schneider L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD001747.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet North Am Ed. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 37.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 38.Shi J, Ni J, Lu T, Zhang X, Wei M, Li T, et al. Adding Chinese herbal medicine to conventional therapy brings cognitive benefits to patients with Alzheimer’s disease: a retrospective analysis. BMC Complement Alternat Med. 2017;17(1):1–7. doi: 10.1186/s12906-017-2040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong L, May BH, Feng M, Hyde AJ, Tan HY, Guo X, et al. Chinese herbal medicine for mild cognitive impairment: A systematic review and meta-analysis of cognitive outcomes. Phytother Res. 2016;30(10):1592–1604. doi: 10.1002/ptr.5679. [DOI] [PubMed] [Google Scholar]
- 40.Maruyama M, Tomita N, Iwasaki K, Ootsuki M, Matsui T, Nemoto M, et al. Benefits of combining donepezil plus traditional Japanese herbal medicine on cognition and brain perfusion in Alzheimer's disease: A 12-week observer-blind, donepezil monotherapy controlled trial. J Am Geriatr Soc. 2006;54(5):869–871. doi: 10.1111/j.1532-5415.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 41.Kumar H, Song S-Y, More SV, Kang S-M, Kim B-W, Kim I-S, et al. Traditional Korean East Asian medicines and herbal formulations for cognitive impairment. Molecules. 2013;18(12):14670–14693. doi: 10.3390/molecules181214670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J-Y, Lee DW, Cho S-J, Na DL, Jeon HJ, Kim S-K, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008;21(2):104–110. doi: 10.1177/0891988708316855. [DOI] [PubMed] [Google Scholar]
- 43.CYA Hyeon Soo Lee, Jung In Kwa. A Preliminary Study on Standardization of K-MAS (Korean version of Memory Assessment Scales) Korean J Clin Psychol. 1999;18(1):221–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the study are available from the corresponding author upon request.