Abstract
We report a rare case of dedifferentiated liposarcoma in a man. A 60-year-old male patient presented with a left mass involving the whole breast area, with no lymph node enlargement, growing during a one-year-period. Imaging studies revealed a fat-containing mixed-density mass apparently associated with the pectoralis major muscle. A core biopsy was performed that yielded a diagnosis of a well-differentiated liposarcoma. Further tests to check for metastases were ordered and no distant disease was found. Left mastectomy with en bloc resection of the pectoralis major muscle was performed. The pathologic diagnosis revealed a high-grade dedifferentiated liposarcoma with extensive necrosis. This tumor type is primarily described in the retroperitoneum and extremities. We report an unusual presentation of a liposarcoma mimicking a breast mass.
Keywords: Liposarcoma (LPS), Diagnosis, Treatment, Radiotherapy, Margins, Chemotherapy
Introduction
Liposarcomas compose 20% of all soft tissue sarcomas, and are the second most common type after pleomorphic undifferentiated sarcomas. Soft tissue sarcomas may occur anywhere, but 75% are located in the extremities (most commonly in the thigh) and 10% each in the trunk wall and retroperitoneum. There is a slight male predominance [1].
Characterization of the histologic differentiation, grade, and type of liposarcoma is critical to determine management and prognosis. Liposarcomas are currently classified into five different subclasses based on pathology: well-differentiated liposarcomas, myxoid liposarcomas, pleomorphic liposarcomas, myxoid-pleomorphic liposarcomas and dedifferentiated liposarcomas [1].
We describe the clinical and histopathological characteristics of a case of liposarcoma of the chest wall, mimicking a breast mass in a male patient. It is interesting to expose this case because of its uncommon location, the diagnostic challenge that it represents and its unique clinical presentation.
Case report
We report the case of a sixty-years-old otherwise healthy male patient who was referred to the Breast Care Unit in October 2020 for a left breast mass of one-year evolution. The patient manifested fast growth during the last three months. He has no prior illnesses and reported a case of lung cancer in his brother, who was diagnosed at the age of 60.
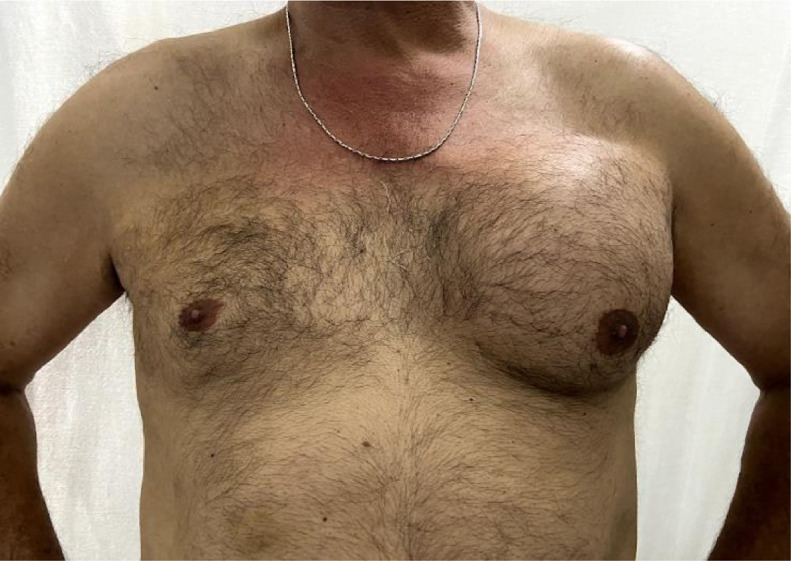
On physical examination, a soft mass involving the whole left breast was evident, with no lymph node enlargement (Fig. 1). Mammography showed a fat-containing mixed-density mass (apparently associated with or deep to the pectoralis major muscle). (Fig. 2). The ultrasound scan (Fig. 3) revealed a circumscribed, complex solid-cystic mass with a central fluid-filled area, growing between both pectoral muscles. A contrast-enhanced magnetic resonance imaging (MRI) of the breast was performed which showed a mass measuring 14 × 11 cm growing between both pectoral muscles. At MRI examination it appears hyperintense on T-1 and T2- weighted images, with hypointense thick septa. With fat suppression much of the bright signal suppressed indicating a fat component. On dynamic sequences, it showed heterogeneous inner enhancement after contrast administration (Fig. 4,5,6). Mammography and MRI showed no other abnormalities in the mammary soft tissues. Further tests were ordered to check for metastasis: a computed axial tomography (CAT) of the chest, abdomen and pelvis showed a mass of fatty density growing between both pectoral muscles, not infiltrating or involving the pectoral muscles. A total body bone scan was performed without evidence of secondarism. A positron emission tomography computed tomography (PET-CT) revealed a subcentimeter solitary juxtafissural nodule of nonspecific appearance in the right lung, and simple cysts in both hepatic lobes and in both kidneys. Ultrasound-guided core biopsy reported a well-differentiated liposarcoma (Fig. 7). Surgery was performed: left mastectomy with en bloc resection of the pectoralis major muscle. The mass did not invade or compromise the breast tissue, but the left mastectomy was performed in addition to the resection of the pectoralis major muscle to remove the en bloc formation. Otherwise, it would not have been possible to remove the piece with safety margins.
Fig. 1.
Patient's physical examination: Soft lump involving the whole left breast was evident, with no lymph node enlargement
Fig. 2.1.
Mammogram of the left breast cranio-caudal view showing a large well-defined encapsulated fat containing mass (red arrow) with a peripheral dense solid component adjacent to it (white arrow). 2.2. Mammogram of the left breast medio lateral oblique view showing the mass lying within the pectoralis muscle
Fig. 3.
Breast ultrasound. Circumscribed, complex solid-cystic mass with a central fluid-filled area, growing between both pectoral muscles
Fig. 4.
Breast MRI. Axial T2 - weighted image shows large predominantly high -signal mass with thickened and nodular septa (white arrow)
Fig. 5.
Breast MRI. Sagittal T2 -weighted image with fat suppression shows hypointense signal of the fat components of the mass (white arrow) due to fat suppression and hyperintense signal of the necrotic area (red arrow)
Fig. 6.
Breast MRI. Contrast enhanced axial T1 showing enhancement of the septa within the mass (white arrow)
Fig. 7.
Core biopsy microscopy: well-differentiated area of liposarcoma (40x): Histologically, adipose tissue consisting of adipocytes of various sizes (red arrow) with hyperchromatic and enlarged nuclei is observed
Pathology examination reported a diagnosis of a high-grade dedifferentiated liposarcoma measuring 14 × 12 × 7 cm with extensive necrosis and 9 mitotic figures per 10 high-power fields, absence of lymphovascular invasion and clear margins (Fig. 8,9). Pathologic TNM staging (pTNM): pT3 pNx. Gross examination (Fig. 10) showed the solid component separated from the cystic component, which correlated with the ultrasound scan and the MRI of the breast.
Fig. 8.
Surgical specimen microscopy. Transitional zone between well-differentiated (white arrow) liposarcoma and non lipogenic dedifferentiated areas (red arrow) (40x)
Fig. 9.
Transitional area of well-differentiated (white arrow) and dedifferentiated liposarcoma (red arrow) (40x)
Fig. 10.

Surgical specimen gross examination. Multinodular, solid, heterogeneous tumor formation with irregular borders.
This being a high-grade tumor larger than 5 cm, radiotherapy was delivered to the left chest wall 5000cGy and boost 1000cGy.
Discussion
Liposarcomas (LPS) are a heterogeneous group of mesenchymal tumors that have variable biologic behavior ranging from indolent disease to extremely aggressive tumors that can be rapidly fatal [2].
The recently updated 2020 World Health Organization (WHO) classification of soft tissue recognizes the following classification [1]: adipocytic tumors, fibroblastic and myofibroblastic tumors, so-called fibrohistiocytic tumors, vascular tumors, pericytic (perivascular) tumors, smooth muscle tumors, skeletal muscle tumors, gastrointestinal stromal tumors, chondro-osseous tumors, peripheral nerve sheath tumors and tumors of uncertain differentiation.
The liposarcomas are included in the subclassification of adipocytic tumors, as shown in Table 1.
Table 1.
Macroscopy of the surgical specimen.
| Benign | Intermediate | Malignant |
|---|---|---|
| Lipoma NOS | Atypical lipomatous tumour | Liposarcoma, well-differentiated, NOS |
| Lipomatosis | Dedifferentiated liposarcoma | |
| Lipoblastomatosis | Myxoid liposarcoma | |
| Angiolipoma NOS | Pleomorphic liposarcoma | |
| Myolipoma | Myxoid pleomorphic liposarcoma | |
| Chondroid lipoma | ||
| Spindle cell lipoma | ||
| Atypical spindle cell / pleomorphic lipomatous tumour | ||
| Hibernoma |
Well-differentiated liposarcoma represents the largest group of malignant adipocytic neoplasms, accounting for approximately 40%–45% of all liposarcomas. Malignant adipocytic tumors account for approximately 20% of all sarcomas. In fact, liposarcoma represents a heterogeneous group of distinctive lesions [3]. Benign mesenchymal tumors outnumber sarcomas by a factor of at least 100. The annual clinical incidence of benign tumors of soft tissue has been estimated to be as high as 3000 cases per 1 million populations, whereas the annual incidence of soft tissue sarcoma is about 50 cases per 1 million populations [1].
The term ‘de-differentiated liposarcoma’ was first introduced by Evans in 1979 to define the morphological progression from atypical lipomatous tumor/well-differentiated liposarcoma to a non-lipogenic sarcoma. [4,5]. De-differentiation occurs in approximately 10% of well-differentiated liposarcomas. They arise much more frequently (90%) as a primary tumor (so-called ‘de-novo’ de-differentiated liposarcoma) than as a recurrence of well-differentiated liposarcoma (10%). [6,7].
Most often, the etiology of sarcomas is unknown. However, this tumor may be associated with genetic (Li-Fraumeni syndrome or neurofibromatosis type 1) or environmental factors (exposure to radiation, arsenic compounds, vinyl chloride, alkylators or immunosuppressive agents). Of these risk factors, exposure to radiotherapy is the one that has been most widely documented. In this setting, the time from exposure to diagnosis ranges between 5 and 11 years [8].
Generally, liposarcomas are seen at the age of 45 to 55 years [9] and are unilateral at presentation. These tumors usually appear as a unilateral, rapidly growing large firm mass.
The imaging features of primary breast sarcomas are not pathognomonic and can mimic those of invasive carcinoma [8]. On mammography, they appear as dense, well- circumscribed masses. As regards the sonographic evaluation, because of the high degree of variability in the composition of the lesion, characterization of the mass with ultrasound has a relatively low sensitivity [8]. MRI of the breast contributes to the characterization of the fat and soft tissue components, determination of the extent and presence of axillary involvement (rare in this type of tumor) [9].
The approach to diagnosis consists of a core needle biopsy using a 14-G needle. However, diagnosis may also be performed by incisional biopsy. Intraoperative frozen- section biopsy is not recommended [10]. Given the heterogeneity of these tumors, discordance between the core biopsy report and the pathology report of the definitive surgical resection specimen is common, as it was the case with our patient, whose lesion was found to be a dedifferentiated liposarcoma on deferred pathological evaluation (while the initial biopsy reported a well-differentiated liposarcoma).
To prevent under sampling or underdiagnosis the aggressiveness of the liposarcomas at core biopsy, sampling should be mainly from the peripheral areas of the lesion. If both solid and adipose tissue areas are seen on imaging, both areas should be sampled due to the heterogeneity of the lesion.
Differential diagnosis should include osteosarcoma, chondrosarcoma, Ewing sarcoma, rhabdomyosarcoma, leiomyosarcoma, dermatofibrosarcoma protuberance, synovial sarcoma, malignant fibrous histiocytoma (MFH), malignant peripheral nerve sheath tumor, lymphoma, treatment-related sarcoma, desmoid tumor, solitary fibrous tumor, malignant granular cell tumor and metastatic tumors.
Also the chest wall is a site of predilection for various benign lesions including atheroma and lipoma [11,12].
There are no histopathological or clinical differences between liposarcomas of the chest wall to liposarcomas elsewhere in the body [1].
For staging, a preoperative CAT scan of the chest, abdomen and pelvis should be ordered to rule out lung disease (the most common site of metastasis). The abdominal assessment is of special interest in myxoid liposarcoma due to its tendency to metastasize to other areas, such as the retroperitoneum. A PET/CT may be ordered, in relevant cases, to enhance the accuracy of the extent of involvement. A bone scan is also helpful, as bone is the second most common site of metastasis. Dissemination is primarily hematogenous, involving the lung and, to a lesser extent, the bone and liver. Lymphatic invasion occurs very rarely and when it does it is because the tumor has a malignant epithelial component [8].
Unlike carcinomas, sarcomas require for staging the inclusion of the malignancy grade based on differentiation, necrosis and mitotic rate [13].
As regards the pathological evaluation, on gross examination, dedifferentiated liposarcomas appear as large multinodular yellowish masses containing solid, often grayish non-lipomatous dedifferentiated areas, frequently showing necrosis [1].
Histologically, dedifferentiated liposarcoma is a well-differentiated liposarcoma/ atypical lipomatous tumor that has shown progression either in the primary tumor or in a recurrence to a usually non-lipogenic sarcoma of variable histological grade. In most cases, there is amplification of MDM2 and CDK4.
The main role of immunohistochemistry is to confirm divergent differentiation and exclude other tumor types [1].
The pathological staging of dedifferentiated liposarcoma of the breast is included in the pathological staging of soft tissue tumors of the College of American Pathologists (CAP) [13].
Surgery is the standard treatment for localized sarcomas [10]. Most authors agree that 1- cm margins are sufficient for small, localized sarcomas that are amenable to conservative surgery. It should be noted that needle and incisional biopsy scars should be removed during surgery [14]. Biopsy of the sentinel lymph node is not warranted, as lymph node metastasis is very rare (5%) in sarcoma. Lymph nodes may be palpable in up to 25% of cases, but tend to be reactive [8].
There is no consensus on the use of radiotherapy in these patients. Based on this limitation, the following can be stated: adjuvant radiotherapy after resection with clear margins should be adjusted according to the risk of tumor recurrence. High-grade tumors larger than 5 cm are defined as aggressive sarcomas more prone to recurrence; therefore, it is reasonable to consider radiotherapy in such patients to improve local control [15].
The role of adjuvant or neoadjuvant chemotherapy is less clear [16,17]. Response rates are relatively low, ranging from 20% to 40% [9]. There is no consensus on the use of chemotherapy and it is not considered standard treatment. Therefore, it may be considered as an option for treating high-risk patients using a tailored approach [10]. According to the NCCN guidelines, the most widely accepted regimens are doxorubicin, ifosfamide and mesna/ ifosfamide, epirubicin, mesna [5].
The follow-up plan is undefined. Most recurrences occur 2 to 3 years after surgery. The approach suggested for follow-up consists of routine physical examination and imaging of the chest (chest CT scan or MRI) every 3 or 4 months for the first 2 or 3 years, followed by an annual or biannual evaluation up to the fifth year [10,18].
The largest series of case reports on sarcoma in Argentina are from Instituto Angel Roffo [15]. In this series of twenty patients, there was only one case of pleomorphic liposarcoma and only one was male.
The presence of positive margins is predictive of residual disease and, therefore, an important risk factor for recurrence and metastasis. Published case series have also demonstrated that a tumor size less than 5 cm is associated with a better overall survival [16].
In a retrospective study that reviewed the imaging and clinical records of patients with histopathology-confirmed LPS dedifferentiated, concluded that high tumor grade and local recurrence were predictors of metastasis. Lung was the most frequent site ([17,18]).
Conclusion
Liposarcoma of the chest wall is a rare and heterogeneous entity. The rarity precludes any prospective study and poses significant challenges in its diagnosis, treatment and research. The therapeutic strategy must be individually tailored, given the limitations imposed by the paucity of data.
Patient Consent Statement
The patient given his consent to present his clinical case for medical and research purposes.
Footnotes
Competing interests: There is no declaration of conflicts of interest.
Patient Consent: Cappai Alberto 13217541 I give my consent to present my clinical case for medical and research purposes.
References
- 1.5th Edition. International Agency for Research on Cancer; 2020. WHO Classification of Tumours of Soft Tissue and Bone. [Google Scholar]
- 2.Tirumani S, Tirumani H, Jagannathan J, Shinagare A, Hornick J, Ramaiya N. Metastasis in dedifferentiated liposarcoma: predictors and outcome in 148 patients. EJSO. 2015;41(7):899–904. doi: 10.1016/j.ejso.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Tos Angelo P Dei. Liposarcomas: diagnostic pitfalls and new insights. Histopathology. 2014;64:38–52. doi: 10.1111/his.12311. [DOI] [PubMed] [Google Scholar]
- 4.Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am. J. Surg. Pathol. 1979;3:507–523. doi: 10.1097/00000478-197912000-00004. [DOI] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma V.2.2021. 2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf, access date: August 28, 2021.
- 6.Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathologic analysis of 155 cases with proposal for an expanded definition of dedifferentiation. Am. J. Surg. Pathol. 1997;21:271–281. doi: 10.1097/00000478-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Elgar F, Goldblum JR. Well-differentiated liposarcoma of the retroperitoneum: a clinicopathologic analysis of 20 cases, with particular attention to the extent of low-grade dedifferentiation. Mod. Pathol. 1997;10:113–120. [PubMed] [Google Scholar]
- 8.Nizri E., Merimsky O, Lahat G. Optimal management of sarcomas of the breast: an update. Expert Rev Anticancer Ther. 2014;14(6):705–710. doi: 10.1586/14737140.2014.895667. [DOI] [PubMed] [Google Scholar]
- 9.Parikh BC, Ohri A, Desai MY, Pandya SJ, Dave RI. Liposarcoma of the breast- a case report. Eur J GynaecolOncol. 2007;28:425–427. [PubMed] [Google Scholar]
- 10.The ESMO/European sarcoma network working group. soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Clinical practice guidelines. Ann Oncol. 2018;29(4) iii51-67. [Google Scholar]
- 11.Soft Tissue Sarcomas of the Chest Wall. Tsukushi, Satoshi et al, Journal of Thoracic Oncology, 4(7): 834-837. [DOI] [PubMed]
- 12.Kachroo, Puja et al, Chest Wall Sarcomas are Accurately Diagnosed by Image-Guided Core Needle Biopsy, Journal of Thoracic Oncology, 7(1):151-156. [DOI] [PubMed]
- 13.College of American pathologist: protocol for the examination of resection specimens from patients with soft tissue tumors. CAP laboratory accreditation program protocol required use date: 2020.
- 14.El Amine Elhadj O et al.A, Sarcomes mammaires primitifs: a propos de 30 cas traites a l'institut Salah-Azaiez de Tunis, 2017. [DOI] [PubMed]
- 15.Mama Sarcomas de. Instituto de Oncología Ángel H. Roffo, Universidad de Buenos Aires; Buenos Aires: 2015. Pautas en Oncología. Diagnóstico, tratamiento y seguimiento del cáncer; pp. 171–173. [Google Scholar]
- 16.Austin RM, Dupree WB. Liposarcoma of the breast: A clinicopathologic study of 20 cases. Hum Pathol. 1986;17(9):906–913. doi: 10.1016/s0046-8177(86)80640-2. [DOI] [PubMed] [Google Scholar]
- 17.Al-Benna S, Poggemann K, Steinau HU, Steinstraesser L. Diagnosis and management of primary breast sarcoma. Breast Cancer Res Treat. 2010;122(3):619–626. doi: 10.1007/s10549-010-0915-y. [DOI] [PubMed] [Google Scholar]
- 18.Waters R, Horvai A, Greipp P, John I, Demicco E, Dickson B. Atypical lipomatous tumour/well-differentiated liposarcoma and de-differentiated liposarcoma in patients aged ≤ 40 years: a study of 116 patients. Histopathology. 2019:1–10. doi: 10.1111/his.13957. [DOI] [PubMed] [Google Scholar]