Abstract
Background
Resistance training with blood flow restriction (BFR) is a physiological ischaemic training method. Before it is applied to patients with coronary artery disease, it must be proven safe and effective.
Methods
Twenty-four healthy adult males were randomly assigned to three groups: the resistance training (RT) group, low-pressure BFR and resistance training (LP-RT) group and high-pressure BFR and resistance training (HP-RT) group. The training protocol was 20 times/min/set, with a 2-min break, five sets/day and 5 d/week for 8 weeks. Cardiac function, haemodynamics and vascular endothelial function were evaluated before and after the first training and the last training.
Results
There were no significant differences among groups before and after training. After 8 weeks of training, the resting heart rate (p<0.05) of the three groups significantly decreased (p<0.05). The rate–pressure product in the LP-RT group significantly decreased (p<0.05) compared with before training. Just after the last training, heart rate (p<0.05) and cardiac output (p<0.05) in the LP-RT and HP-RT groups significantly decreased compared with those just after the first training. At the end of the experiment, vascular endothelial growth factor (VEGF; p<0.01), soluble VEGF receptor (VEGFR) (p<0.05) and interleukin-6 (p<0.01) significantly increased, except for soluble VEGFR in the RT group.
Conclusions
Low-intensity resistance training with BFR moderately alters cardiac function. The expression levels of proteins related to vascular endothelial function have significantly changed. Both findings suggest that low-intensity resistance training with BFR may be safely and effectively applied to patients with coronary artery disease.
Keywords: blood flow restriction, cardiac function, haemodynamics, low-intensity resistance training, vascular endothelial function, vascular endothelial growth factor
Introduction
Coronary artery disease (CAD) is a common condition that strongly correlates with increased cardiovascular morbidity and mortality and poses a high economic burden to the national healthcare system.1,2 Research on non-invasive therapy for CAD has indicated that physiological ischaemic training protects the vascular endothelium and slows down atherosclerotic plaque development in atherosclerosis rabbits.3
Physiological ischaemic training is a method that blocks blood flow in normal limbs within a short period, either by isometric contraction or through the use of a tourniquet.4 To achieve the goal of blocking blood flow by isometric contraction, participants should have the ability to effectively control the target muscle. It is difficult for people without special system training to control the strength of isometric contraction, which reaches a target number and duration without using a special monitoring instrument. Thus the popularization and application of simple isometric contraction is hampered. Tourniquets can also block blood flow, and in some studies, resistance training was combined with tourniquet use as a form of resistance training with blood flow restriction (BFR). In this exercise method, pressure is applied to the exercising limb using a blood pressure (BP) cuff to block blood flow, causing temporary ischaemia of the exercising limb's distal muscles while the participants perform resistance training.
Researchers have found that resistance training with BFR not only increases muscle size without too much subjective fatigue and discomfort,5–8 but also significantly increases vascular endothelial growth factor (VEGF) expression,9 promotes vascular function,10 enhances vascular conductance11 and partially alters hemodynamic parameters.12,13 Resistance training with BFR has also been proven to be safe for healthy older adults,5,9,13–15 indicating that it may safely be applied to CAD patients.
However, most of the studies related to BP and cardiac function lasted no more than 4 weeks and exercise that lasted >8 weeks did not assess VEGF, soluble VEGF receptor (VEGFR) and interleukin-6 (IL-6), which are important indexes that are associated with CAD and atherosclerosis. To obtain sufficient information on whether resistance training with BFR is safe for people with CAD or atherosclerosis, we assessed the effects of 8 weeks of resistance training with BFR on cardiac function, haemodynamics and vascular endothelial function in healthy young males. The findings may provide insights into the benefit of low-intensity resistance training with BFR on patients with CAD or atherosclerosis.
Materials and methods
Subjects
Twenty-four healthy adult males (average age 20.63±0.88 y) underwent a physical examination to confirm that they had no physical diseases or movement dysfunction. The participants had not engaged in a regular exercise program (less than two times per week) in the past 6 months. None of the participants were smokers. Each participant's height and body mass were measured using a stadiometer and manual scales. The participants were randomly divided into three groups using the random number table method: a resistance training (RT) group, a low-pressure BFR and resistance training (LP-RT) group and a high-pressure BFR and resistance training (HP-RT) group. The study was approved by the ethics committee of Kunshan Rehabilitation Hospital, Suzhou City, Jiangsu Province, China. Informed consent was obtained from all of the individuals in the study.
Study protocol
Determining the maximum repetitions of the subjects
Under resting conditions, the maximum number of repetitions was examined several times to obtain exact values for one-repetition maximum (1RM). First, participants lifted weights repeatedly as many times as possible. If they were able to perform more than 10 repetitions, then a heavier weight was provided. Whenever the maximum number of repetitions was ≤10, the weight was considered submaximal weight and the 1RM was calculated as follows: estimated 1RM (kg)=submaximal weight (kg)/(102.78−2.78×maximum number of repetitions)/100.9 In the middle of the training, maximum repetitions were examined to obtain a new exact value. All of the resistance stress was adjusted based on the number of maximum repetitions.
Training protocols for the three groups
All of the participants in the three groups were given 30% 1RM resistance movement of the right elbow joint in full range, 20 times/min/set, with a 2-min break. Participants performed 5 sets/day, 5 d/week, for 8 weeks. During resistance training, the participants in the LP-RT group received 65% of their systolic pressure applied to the upper arm, while the participants in the HP-RT group received 130% of their systolic pressure applied to the upper arm.
Detection of indicators
General indicators, such as heart rate; indicators of cardiac function, such as left ventricular fractional shortening (FS), left ventricular ejection fraction (LVEF), stroke output (SV) and cardiac output (CO); and indicators of haemodynamics, such as aortic valve orifice velocity (AV) and systolic velocity time integral (VTI), were examined using an xMATRIX iE33 system (Philips, Amsterdam, The Netherlands). General indicators such as systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an electronic sphygmomanometer (ES*H5501, Taiermao, Hangzhou, China). Mean arterial pressure (MAP), rate-pressure product (RPP) and pulse pressure (PP) were calculated using corresponding formulas.
The above indicators were measured before the first training and after the last training during the resting state. The after-training indicators were measured after the first training and the last training and the evaluation was completed within 3 min of the end of the training.
Blood examination
The blood samples were collected at the same time points as BP indicators and blood was drawn from the brachial vein. Plasma VEGF, soluble VEGFR and IL-6 levels were measured using an enzyme-linked immunosorbent assay with a VEGF-A Human ELISA Kit (BMS277, Invitrogen, San Diego, CA, USA), soluble VEGFR Human ELISA Kit (BMS2019, Invitrogen) and IL-6 Human ELISA Kit (BMS213, Invitrogen).
Statistical analyses
The measurement data were expressed as the mean ± standard deviation. Differences within each group were analysed using the paired t test. All of the data were analysed by one-way analysis of variance and the least significant difference method was used for multiple comparisons. The p-value reported was two-sided and differences with p-values <0.05 were considered statistically significant while those <0.01 were considered highly statistically significant. All of the analyses were performed using SPSS software (version 13.0, SPSS, Chicago, IL, USA).
Results
Basic characteristics and baseline data of subjects
Table 1 shows that there were no significant differences among groups in terms of age, weight, height, 1RM and 1RM during mid-term training.
Table 1.
Characteristics of participants included in the study
| Characteristics | Control group (n=8) | LP-RT group (n=8) | HP-RT group (n=8) |
|---|---|---|---|
| Age (years) | 19±1 | 20±1 | 19±1 |
| Weight (kg) | 68.2±12.3 | 72.8±14.7 | 67.0±10.7 |
| Height (cm) | 175.2±3.92 | 177.7±5.43 | 175.7±5.2 |
| Body mass index | 21.54±3.15 | 23.98±3.38 | 21.01±4.97 |
| 1RM (kg) | 12.81±1.46 | 13.93±2.53 | 11.88±1.90 |
| 1RM of mid-term training (kg) | 13.01±1.89 | 14.59±4.16 | 12.84±1.43 |
Data are presented as the mean±standard deviation.
Indicators of cardiac function, haemodynamic indicators and BP in the resting state
Cardiac function indicators, haemodynamic indicators and heart rate in the resting state before and after 8 weeks of training
In the resting state, the cardiac function indicators (FS, LVEF, SV and CO) showed no significant differences among groups (p>0.05) before the training and after 8 weeks of training. Within all groups, no significant differences in cardiac function indicators were observed between the measurements taken at the start of training and 8 weeks later (p>0.05). In the resting state, in terms of haemodynamic indicators, no significant differences in AV and VTI were observed among the groups before training began and after 8 weeks of training (p>0.05). Within all groups, no significant differences in haemodynamic indicators were observed between the period before training began and 8 weeks later (p>0.05). In the resting state, after 8 weeks of training the heart rate in the three groups significantly decreased (p<0.05) compared with that before training (Table 2).
Table 2.
Cardiac function indicators, haemodynamic indicators and heart rate at rest before and after training
| RT group | LP-RT group | HP-RT group | ||||
|---|---|---|---|---|---|---|
| Indicators | Before | After | Before | After | Before | After |
| FS (%) | 37.57±1.51 | 37.43±1.72 | 37.71±2.36 | 38.14±2.27 | 38.14±1.68 | 37.86±2.41 |
| LVEF (%) | 67±2.0 | 67±2.24 | 67.43±2.99 | 68±2.83 | 68±2.31 | 67.57±3.36 |
| SV (mL) | 77.14±14.53 | 80.43±15.86 | 83.14±9.67 | 82.86±9.17 | 75.86±16.69 | 76.43±14.68 |
| CO (mL/min) | 6166±1239 | 5800±1472 | 6371±1353 | 5573±680 | 6050±1938 | 5014±1013 |
| AV (m/s) | 1.17±0.21 | 1.12±0.11 | 1.17±0.09 | 1.16±0.08 | 1.10±0.19 | 1.07±0.17 |
| VTI (cm) | 23.16±3.96 | 21.84±2.11 | 25.67±2.18 | 24.37±2.66 | 22.28±2.70 | 22.5±3.20 |
| Heart rate (bpm) | 80±5.13 | 71.29±6.16* | 78.43±11.13 | 67±5.94* | 79.14±10.3 | 67.14±4.30* |
Data are presented as the mean±standard deviation.
*p<0.05 vs baseline.
BP indicators in the resting state before and after 8 weeks of training
The RPP in the LP-RT group significantly decreased compared with that before training (p<0.05). No significant differences in other BP indicators were observed within or among groups (p>0.05) (Table 3).
Table 3.
BP indicators at rest before and after training
| RT group | LP-RT group | HP-RT group | ||||
|---|---|---|---|---|---|---|
| Indicators | Before | After | Before | After | Before | After |
| SBP (mmHg) | 113.86±8.47 | 115.14±8.97 | 116.14±15.76 | 118.71±16.39 | 114.86±5.37 | 119.43±7.59 |
| DBP (mmHg) | 64.57±5.80 | 62.29±3.82 | 61.43±7.41 | 62±7.87 | 62.71±6.52 | 62.86±6.26 |
| MAP (mmHg) | 81±6.26 | 79.9±4.13 | 79.66±9.45 | 80.9±9.56 | 80.1±4.97 | 81.72±6.07 |
| RPP (mmHg×bpm) | 9077±392 | 8238±1205 | 9116±1869 | 7997±1570* | 9113±1471 | 8030±857 |
| PP (mmHg) | 49.29±5.68 | 52.86±9.37 | 54.71±11.6 | 56.71±13.31 | 52.14±7.73 | 56.57±6.16 |
Data are presented as the mean±standard deviation.
*p<0.05 vs baseline.
Indicators of cardiac function, haemodynamic indicators and BP after training
Cardiac function indicators, haemodynamic indicators and heart rate immediately after the first and last training
In terms of cardiac function indicators (FS, LVEF, SV and CO), only CO in the LP-RT and HP-RT groups just after the last training significantly decreased compared with that immediately after the first training (p<0.05). No significant differences were observed among groups (p>0.05) between measurements taken immediately after the first and last training. Within all groups, no significant differences in other cardiac function indicators were observed between the measurements taken just after the first and last training (p>0.05). In terms of haemodynamic indicators, no significant differences in AV or VTI were observed among groups between the time points immediately after the first training and immediately after the last training (p>0.05). Within all groups, no significant differences in haemodynamic indicators were observed between the measurements taken just after the first and last training (p>0.05). Immediately after the last training, compared with just after the first training, heart rate in the LP-RT and HP-RT groups significantly decreased (p<0.05) (Table 4).
Table 4.
Cardiac function indicators, haemodynamic indicators and heart rate immediately after the first and last training
| RT group | LP-RT group | HP-RT group | ||||
|---|---|---|---|---|---|---|
| Indicators | Before | After | Before | After | Before | After |
| FS (%) | 40.14±2.12 | 39.86±1.57 | 40.57±2.76 | 41.14±1.68 | 38.43±1.9 | 40.14±1.35 |
| LVEF (%) | 70.86±2.27 | 70.29±1.98 | 71.0±3.27 | 71.86±1.95 | 68.86±1.95 | 71.0±1.63 |
| SV (mL) | 84.14±14.31 | 86.14±10.59 | 89.0±13.15 | 88.71±10.58 | 75.71±16.21 | 76.29±15.40 |
| CO (mL/min) | 7844±1810 | 7141±2019 | 8307±2515 | 6296±626* | 7460±2081 | 5783±1213* |
| AV (m/s) | 1.19±0.21 | 1.2±0.14 | 1.25±0.16 | 1.2±0.22 | 1.20±0.15 | 1.16±0.21 |
| VTI (cm) | 24.21±3.05 | 24.0±2.86 | 26.48±2.90 | 24.97±4.70 | 23.95±2.43 | 23.64±3.70 |
| Heart rate (bpm) | 93±12.85 | 81.86±13.07 | 99.29±15.38 | 72±6.48* | 86.14±13.66 | 77.85±8.21* |
Data are presented as the mean±standard deviation.
*p<0.05 vs baseline.
BP indicators immediately after the first and last training
Immediately after the last training, compared with just after the first training, the RPP in the LP-RT group significantly increased (p<0.05). There was no significant difference in the other BP indicators within groups (p>0.05). There were no significant differences in other BP indicators among groups right after the first and last training (p>0.05) (Table 5).
Table 5.
BP indicators immediately after the first and last training
| RT group | LP-RT group | HP-RT group | ||||
|---|---|---|---|---|---|---|
| Indicators | Before | After | Before | After | Before | After |
| SBP (mmHg) | 131±14.12 | 129.57±8.12 | 128.71±14.22 | 130.14±14.17 | 127.86±6.31 | 133.29±10.45 |
| DBP (mmHg) | 54.29±5.12 | 57.14±4.06 | 56.57±11.82 | 52±8.08 | 61.71±7.36 | 58.43±7.96 |
| MAP (mmHg) | 79.86±6.85 | 81.29±4.44 | 80.62±11.66 | 78.05±9.14 | 83.76±4.71 | 83.38±6.74 |
| RPP (mmHg×bpm) | 12 151±1933 | 10 601±1791 | 12 794±2624 | 9402±1518* | 10 997±1704 | 10 339±982 |
| PP (mmHg) | 76.71±12.91 | 72.43±7.72 | 72.14±10.48 | 78.14±10.99 | 66.14±11.08 | 74.86±12.23 |
Data are presented as mean±standard deviation.
*p<0.05 vs baseline.
VEGF, soluble VEGFR and IL-6 at rest and after training
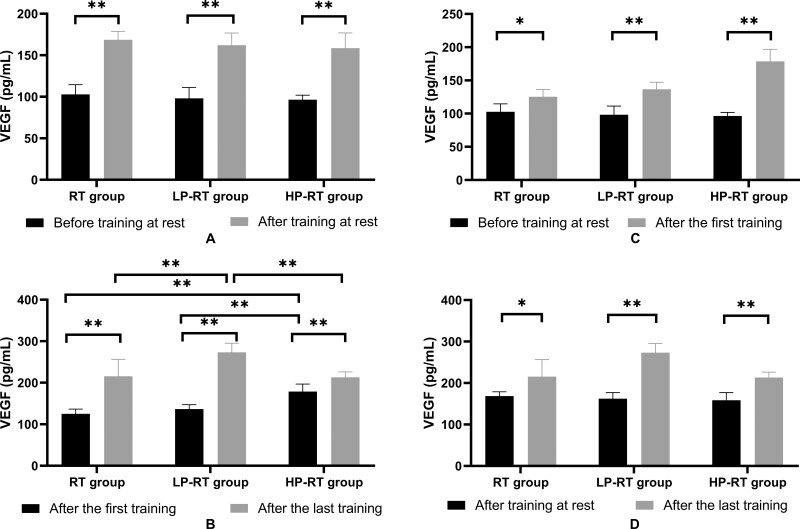
There was no significant difference in VEGF levels among groups at rest before training (p>0.05). After 8 weeks of training, VEGF levels in all of the groups significantly increased (p<0.01), whereas there was no significant difference in VEGF levels among groups at rest (p>0.05) (Figure 1A). Right after the first training, VEGF levels in the HP-RT group were significantly higher than in the RT and LP-RT groups (p<0.01). After 8 weeks, just after the last training, VEGF levels in all of the groups significantly increased (p<0.01) and those in the LP-RT group significantly increased compared with the VEGF levels in the RT (p<0.01) and HP-RT (p<0.01) groups (Figure 1B). Just after the first training, VEGF levels in the three groups were significantly higher than those before the training began (p<0.05) (Figure 1C). VEGF levels in the three groups immediately after the last training were significantly higher than those at rest after 8 weeks of training (p<0.05) (Figure 1D).
Figure 1.
(A) Plasma VEGF levels before training and after the last training in the resting state. (B) Plasma VEGF levels immediately after the first training and right after the last training. (C) Plasma VEGF levels before training in the resting state and immediately after the first training. (D) Plasma VEGF levels after the last training in the resting state and immediately after the last training. Data are presented as the mean±standard deviation. *p<0.05, **p<0.01, significantly different from baseline.
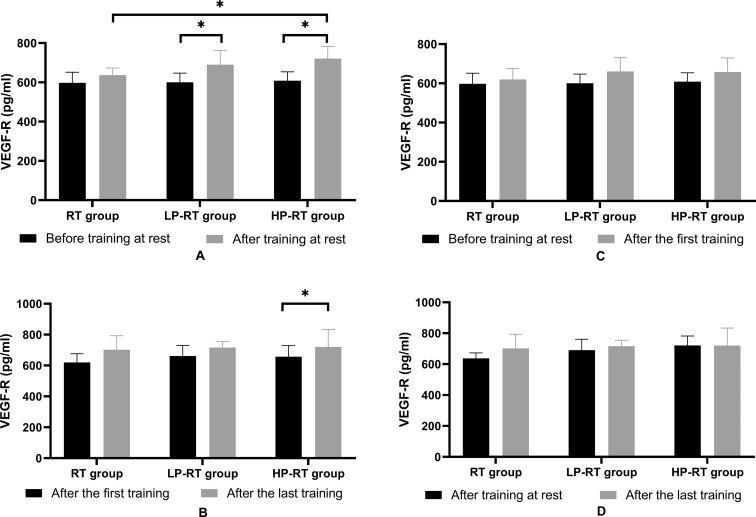
There were no significant differences in soluble VEGFR levels among groups at rest before training (p>0.05). After 8 weeks of training and at rest, the soluble VEGFR levels in the LP-RT and HP-RT groups significantly increased (p<0.05) and a significant difference in VEGFR levels was observed between the RT and HP-RT groups (p<0.05) (Figure 2A). There was no significant difference in VEGFR levels among groups just after the first training (p>0.05). After 8 weeks, immediately after the last training, the soluble VEGFR levels in the HP-RT group significantly increased (p<0.05), but there were no significant differences in VEGF-R levels among groups just after the last training (p<0.05) (Figure 2B). The soluble VEGFR levels in the three groups immediately after the first training were not higher than those at rest before training (p>0.05) (Figure 2C). VEGFR levels in the three groups immediately after the last training were not higher than those in the resting state after 8 weeks of training (p>0.05) (Figure 2D).
Figure 2.
(A) Plasma VEGFR levels before training and after the last training in the resting state. (B) Plasma VEGFR levels just after the first training and immediately after the last training. (C) Plasma VEGFR levels before training in the resting state and immediately after the first training. (D) Plasma VEGFR levels after the last training in the resting state and immediately after the last training. Data are presented as the mean±standard deviation. *p<0.05, significantly different from baseline.
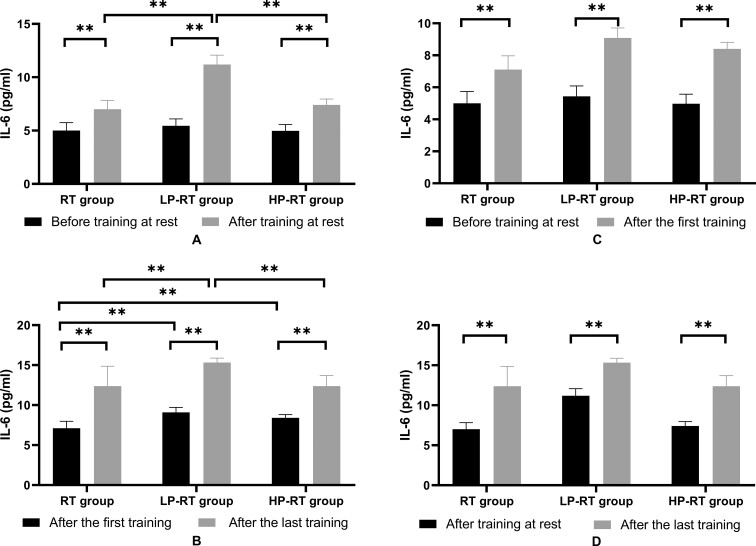
There were no significant differences in IL-6 levels among groups at rest before training (p>0.05). After 8 weeks of training and at rest, IL-6 levels in all of the groups significantly increased (p<0.01) and there was a significant difference in IL-6 levels among groups (p<0.01) (Figure 3A). There was also a significant difference in IL-6 levels among groups just after the first training (p<0.01). Immediately after the last training, IL-6 levels in all of the groups significantly increased compared with those from immediately after the first training (p<0.01) and there was a significant difference in IL-6 levels among groups (p<0.01) (Figure 3B). IL-6 levels in the three groups immediately after the first training were significantly higher than those before training (p<0.05) (Figure 3C). IL-6 levels in the three groups immediately after the last training were significantly higher than those at rest after the last training (p<0.05) (Figure 3D).
Figure 3.
(A) Plasma IL-6 levels before training and after the last training in the resting state. (B) Plasma IL-6 levels just after the first training and just after the last training. (C) Plasma IL-6 levels before training in the resting state and immediately after the first training. (D) Plasma IL-6 levels after the last training in the resting state and immediately after the last training. Data are presented as the mean±standard deviation. *p<0.05, **p<0.01, significantly different from baseline.
Discussion
In some acute experiments, no significant differences were found in SV or CO among the normal training and training plus BFR groups immediately after the training.12,13 In the present study there were no differences in cardiac function indicators or haemodynamic indicators among groups either before and after the first training or before and after the last training. Comparing the results within groups revealed that after 8 weeks of training, the heart rate in the three groups at rest decreased significantly compared with before training, indicating that the body's ability to use oxygen had increased. After 8 weeks of training, heart rate and CO within the LP-RT and HP-RT groups immediately after the last training decreased significantly, implying that the body had improved its fast recovery ability after the training.
After 8 weeks of training, the RPP in the LP-RT group was significantly lower at rest and just after the last training compared with the pre-training RPP. This suggests that resistance training with low-pressure BFR could improve myocardial oxygen consumption to a greater extent than that achieved by other methods.
An acute experiment performed by Pinto et al.8,16 showed that during exercise and during pauses between sets, significant changes in SBP and DBP occurred, whereas no alterations in SBP and DBP were observed post-exercise. Also, there were no significant alterations in SBP or DBP in our study, either among or within groups immediately after the training. This finding contrasts with the results of Au et al.10 and Fahs et al.,11 which showed a significant decrease in DBP but no change in SBP. SBP and DBP significantly changed in the research of Shimizu et al.,9 which differs from our results, because they assessed parameters before and after initial resistance training.
After 8 weeks of training, plasma VEGF, soluble VEGFR and IL-6 levels significantly increased in all three groups in the resting state, except for VEGFR in the RT group. These increases of VEGF and IL-6 were similar to those seen in other studies. For example, Shimizu et al.9 showed that after 4 weeks of low-load resistance training with BFR, VEGF levels significantly increased compared with baseline and the low-load resistance training group. Patterson et al.14 found that compared with low-load resistance training, low-load resistance training with BFR in elderly males resulted in higher VEGF and IL-6 levels within 2 h after exercise. However, Larkin et al.17 found that after low-load resistance training with BFR, the VEGF messenger RNA levels significantly increased within 24 h, whereas VEGF levels both in the muscle and serum did not. This difference from our findings may be attributable to the type of exercise employed in the study, the intensity and the sampling time. In our study, the increase of VEGFR did not occur immediately, and never happened in the RT group, which suggests that resistance training with BFR may be more effective at regulating vascular endothelial factors than resistance training.
Low-intensity resistance training with BFR was utilized in this research based on the fact that low-intensity resistance training (30% 1RM) with BFR induces functional muscle adaptation similar to that of high-intensity resistance training,18–20 yet it does not induce post-exercise hypotension as is observed in high-intensity resistance exercise.21
The pressure applied using BP cuffs, i.e. 130% and 65% of systolic pressure, resulted in a 100% and about 60% reduction in blood flow in the upper arm, respectively, during our pre-experiment. Takano et al.22 previously reported that a reduction of approximately 60% in resting blood flow is effective.23–29 Other studies have suggested that complete arterial occlusion causes greater ratings of perceived exertion23,30 compared with that caused by partial occlusion, which is similar to the results of our research. However, reduced effectiveness of complete arterial occlusion compared with partial occlusion was not observed in this study. The difference might be related to the difference in the experimental methods employed.
In the present study we found that low-intensity resistance training with BFR could moderately improve the cardiac function indicators and haemodynamic indicators. This method of low-intensity resistance training may be better tolerated than other methods. Additionally, the changes in cardiac function and haemodynamics are not extreme, which indicates that these changes may be healthy and safe for CAD patients. Compared with 4 weeks of training, an 8-week duration of low-intensity resistance training with BFR increases the expression of VEGF and soluble VEGFR, which might protect vascular endothelium more effectively.
The present study has a number of limitations. First, the small sample size of the study may reduce its power to detect significant differences among trials. Moreover, because the participants were only healthy young males, our capacity to generate sound conclusions based on our findings was limited. The question of why soluble VEGFR did not significantly change immediately after the training, as well as its subsequent effect, requires further investigation.
Conclusions
Low-intensity resistance training with BFR can lead to a decrease in heart rate, which in turn decreases CO immediately after the last training, compared with the values seen just after the first training. Plasma levels of VEGF and IL-6 increased in all groups. Only participants who received resistance training with BFR exhibited a significant increase in soluble VEGFR. All the findings suggest that low-intensity resistance training with BFR may be safely and effectively applied to CAD patients.
Acknowledgements
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Contributor Information
Yan Zhao, School of Sports and Health, Nanjing Sport Institute, 8 Linggusi Road, Nanjing, PA 210014, China.
Aicui Lin, Department of Science and Technology, Nanjing First Hospital, Nanjing Medical University, 68 Changle Road, Nanjing, PA 210006, China.
Long Jiao, Department of Rehabilitation, Kunshan Rehabilitation Hospital, 888 Yingbin Road, Kunshan, PA 215300, China.
Authors’ contributions
ACL and YZ designed the study. YZ wrote the manuscript. YZ and LJ performed the experiment. ACL and YZ critically revised the manuscript and all the authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant 81101456), the Nanjing Medical Science and Technique Development Foundation (grants QRX17065 and YKK19073) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant 18KJA320002).
Competing interests
None declared.
Ethical approval
The study was approved by the ethics committee of Kunshan Rehabilitation Hospital, Suzhou City, Jiangsu Province, China.
Data availability
Data will be available upon request to the corresponding author.
References
- 1.Benjamin EJ, Muntner P, Alonso Aet al. Heart disease and stroke statistics–2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–528. [DOI] [PubMed] [Google Scholar]
- 2.Hao YC, Liu J, Liu Jet al. Sex differences in in-hospital management and outcomes of patients with acute coronary syndrome. Circulation. 2019;139(15):1776–85. [DOI] [PubMed] [Google Scholar]
- 3.Kong M, Zhao Y, Chen Aet al. The importance of physiologic ischemia training in preventing the development of atherosclerosis: the role of endothelial progenitor cells in atherosclerotic rabbits. Coron Artery Dis. 2019;30(5):377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin A, Li J, Zhao Yet al. Effect of physiologic ischemic training on protection of myocardial infarction in rabbits. Am J Phys Med Rehabil. 2011;90(2):97–105. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda T, Fukumura K, Tomaru Tet al. Thigh muscle size and vascular function after blood flow-restricted elastic band training in older women. Oncotarget. 2016;7(23):33595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieljacks P, Degn R, Hollaender Ket al. Non-failure blood flow restricted exercise induces similar muscle adaptations and less discomfort than failure protocols. Scand J Med Sci Sports. 2019,29(3):336–47. [DOI] [PubMed] [Google Scholar]
- 7.Ellefsen S, Hammarstrom D, Strand TAet al. Blood flow-restricted strength training displays high functional and biological efficacy in women: a within-subject comparison with high-load strength training. Am J Physiol Regul Integr Comp Physiol. 2015;309(7):R767–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto RR, Karabulut M, Poton Ret al. Acute resistance exercise with blood flow restriction in elderly hypertensive women: haemodynamic, rating of perceived exertion and blood lactate. Clin Physiol Funct Imaging. 2018;38(1):17–24. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu R, Hotta K, Yamamoto Set al. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol. 2016;116(4):749–57. [DOI] [PubMed] [Google Scholar]
- 10.Au JS, Oikawa SY, Morton RWet al. Arterial stiffness is reduced regardless of resistance training load in young men. Med Sci Sports Exerc. 2017;49(2):342–8. [DOI] [PubMed] [Google Scholar]
- 11.Fahs CA, Rossow LM, Loenneke JPet al. Effect of different types of lower body resistance training on arterial compliance and calf blood flow. Clin Physiol Funct Imaging. 2012;32(1):45–51. [DOI] [PubMed] [Google Scholar]
- 12.Brandner CR, Kidgell DJ, Warmington SA. Unilateral bicep curl hemodynamics: low-pressure continuous vs high-pressure intermittent blood flow restriction. Scand J Med Sci Sports. 2015;25(6):770–7. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira MLV, Sardeli AV, Souza GVDet al. Cardiac autonomic and haemodynamic recovery after a single session of aerobic exercise with and without blood flow restriction in older adults. J Sports Sci. 2017;35(24):2412–20. [DOI] [PubMed] [Google Scholar]
- 14.Patterson SD, Leggate M, Nimmo MAet al. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol. 2013;113(3):713–9. [DOI] [PubMed] [Google Scholar]
- 15.Staunton CA, May AK, Brandner CRet al. Haemodynamics of aerobic and resistance blood flow restriction exercise in young and older adults. Eur J Appl Physiol. 2015;115(11):2293–302. [DOI] [PubMed] [Google Scholar]
- 16.Pinto RR, Polito MD.. Haemodynamic responses during resistance exercise with blood flow restriction in hypertensive subjects. Clin Physiol Funct Imaging. 2016;36(5):407–13. [DOI] [PubMed] [Google Scholar]
- 17.Larkin KA, Macneil RG, Dirain Met al. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc. 2012;44(11):2077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda T, Ogasawara R, Sakamaki Met al. Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol. 2011;111(10):2525–33. [DOI] [PubMed] [Google Scholar]
- 19.Loenneke JP, Wilson JM, Marin PJet al. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(5):1849–59. [DOI] [PubMed] [Google Scholar]
- 20.Vechin FC, Libardi CA, Conceicao MSet al. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J Strength Cond Res. 2015;29(4):1071–6. [DOI] [PubMed] [Google Scholar]
- 21.Rossow LM, Fahs CA, Sherk VDet al. The effect of acute blood-flow-restricted resistance exercise on postexercise blood pressure. Clin Physiol Funct Imaging. 2011;31(6):429–34. [DOI] [PubMed] [Google Scholar]
- 22.Takano H, Morita T, Iida Het al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol. 2005;95(1):65–73. [DOI] [PubMed] [Google Scholar]
- 23.Sumide T, Sakuraba K, Sawaki Ket al. Effect of resistance exercise training combined with relatively low vascular occlusion. J Sci Med Sport. 2009;12(1):107–12. [DOI] [PubMed] [Google Scholar]
- 24.Evans C, Vance S, Brown M. Short-term resistance training with blood flow restriction enhances microvascular filtration capacity of human calf muscles. J Sports Sci. 2010;28(9):999–1007. [DOI] [PubMed] [Google Scholar]
- 25.Karabulut M, Abe T, Sato Yet al. The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol. 2010;108(1):147–55. [DOI] [PubMed] [Google Scholar]
- 26.Patterson SD, Ferguson RA.. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur J Appl Physiol. 2010;108(5):1025–33. [DOI] [PubMed] [Google Scholar]
- 27.Cook SB, Brown KA, Deruisseau Ket al. Skeletal muscle adaptations following blood flow-restricted training during 30 days of muscular unloading. J Appl Physiol (1985). 2010;109(2):341–9. [DOI] [PubMed] [Google Scholar]
- 28.Hunt JEA, Galea D, Tufft Get al. Time course of regional vascular adaptations to low load resistance training with blood flow restriction. J Appl Physiol (1985). 2013;115(3):403–11. [DOI] [PubMed] [Google Scholar]
- 29.Hunt JEA, Stodart C, Ferguson RA. The influence of participant characteristics on the relationship between cuff pressure and level of blood flow restriction. Eur J Appl Physiol. 2016;116(7):1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda T, Brechue WF, Fujita Tet al. Muscle activation during low-intensity muscle contractions with restricted blood flow. J Sports Sci. 2009;27(5):479–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon request to the corresponding author.