Abstract
Articular cartilage defects are common and can result in substantial pain and disability, prompting operative intervention, which commonly includes chondral debridement. Controlled defect preparation up to but not beyond the calcified cartilage layer is key to clinical success, but this remains technically challenging. We present a technique highlighting the substantial decrease in curette stroke volume and associated shift to a lower pitch when achieving satisfactory open cartilage defect debridement. These audiologic cues correlate well with histologic accuracy of debridement. Therefore, quantifiable pitch and volume changes serve as valuable technical cues for precise defect preparation at the time of joint preservation surgery.
Classifications
I: knee, II: cartilage.
Technique Video
Surgical video demonstrating the technical use of pitch and volume changes during chondral defect preparation in joint preservation surgery.
Introduction
Articular cartilage defects are common and can impair quality of life in a similar way as severe osteoarthritis, with associated pain, dysfunction, and progression to joint degeneration.1 Precise preparation of cartilage defects at the time of joint preservation surgery is critical and technically challenging. Controlled depth debridement is key to clinical success, with the goal being to reach but not go beyond the calcified cartilage layer, which is only 20–250 μm in thickness.2 Underpreparation leads to inadequate debridement of pathologic cartilage, which impairs collagen bonding and increases risk of delamination.3,4 In contrast, overpreparation leads to violation of the subchondral plate and associated risk of subchondral fracture and intralesional osteophyte formation.
Given the importance of chondral debridement depth, it is of substantial utility for the joint preservation surgeon to confirm the appropriate level of debridement has been achieved. High-volume cartilage surgeons have recognized the sound and pitch changes that occur during cartilage defect preparation; however, to date, these have not been formally characterized or published. In this Technical Note, we present a method for employing audible changes in order to achieve precise and optimal depth of chondral debridement. The technique is demonstrated in a video (Video 1) along with a list of pearls and pitfalls that the authors have found helpful (Table 1). The technique described allows for the use of volume- and pitch-based cues in order to optimize level of debridement in joint preservation surgery.
Table 1.
Pearls and Pitfalls of Chondral Debridement with Pitch and Volume Change Guidance
| Pearls | Pitfalls |
|---|---|
| Best achieved with a quiet operating room. Ensure background noise is kept to a safe minimum, such as by clamping suction. | Some sources of operating room noise, such as anesthesia monitoring, may not be able to be decreased. |
| Use sharp, undamaged curettes for defect preparation. | Efficacy of debridement and heard pitch and volume changes may be altered with the use of dull or damaged instrumentation. |
| Employ continuous, even pressure with controlled, even strokes of the curette across the area to be debrided. | Short, uneven strokes of the curette may lead to varying audible and tactile feedback and resultant uneven preparation of the defect. |
| Preparation should systematically progress across the defect, achieving even preparation depth across the field of the defect. | Returning to areas with satisfactory preparation (i.e. beyond volume and pitch change) may lead to overpreparation and subchondral plate violation. |
Surgical Technique
Patient Positioning and Preparation
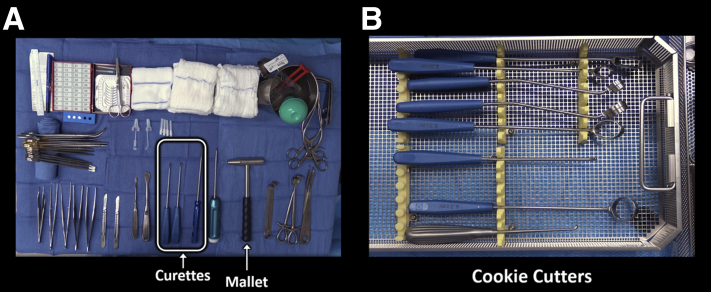
The patient is positioned supine, with proper support and padding. The lower extremity is prepared and draped in the standard, sterile fashion. Appropriate supplies for chondral defect preparation, including sharp, undamaged curettes, are confirmed to be available in the operating room (Fig 1). Supplies for further treatment following defect preparation (i.e., microfracture awls, matrix-induced autologous chondrocyte implantation [MACI] membranes), as per the treating surgeon, are also confirmed to be available.
Fig 1.
Appropriate supplies for chondral defect preparation, including sharp curettes (A) are confirmed to be available. We recommend defect preparation with the assistance of “cookie cutters” (B) which are used intraoperatively in conjunction with a mallet.
Arthrotomy and Defect Preparation
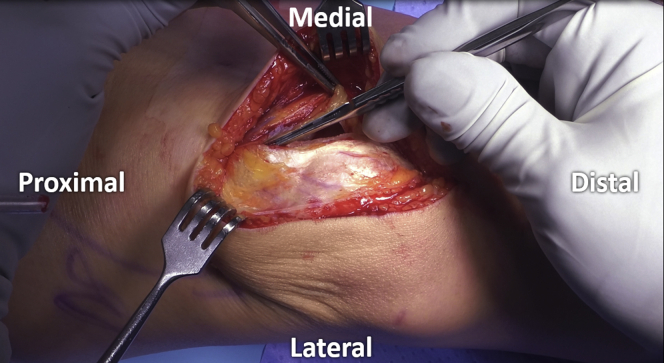
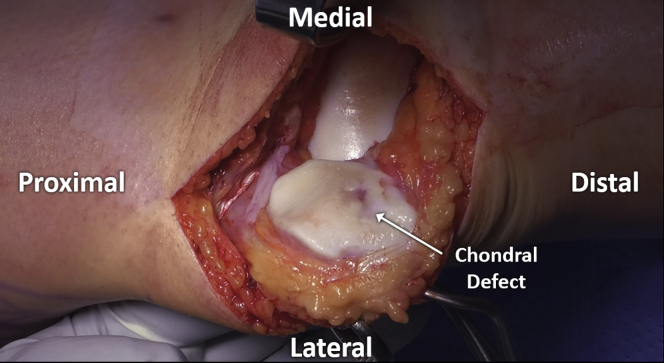
A 6–8-cm medial or laterally based longitudinal incision is used to gain access to the knee, depending on defect location. A parapatellar arthrotomy is performed using a fresh, sharp scalpel, with special attention taken to protect all intraarticular structures, including the meniscus and underlying articular cartilage (Fig 2). The patella is moved medially, laterally, or partially everted, in order to allow visualization of the chondral defect (Fig 3).
Fig 2.
A longitudinal incision is used to gain access to the knee. A fresh scalpel is used to perform the parapatellar arthrotomy while preserving the meniscus and underlying articular cartilage.
Fig 3.
The patella is everted to allow visualization of the defect, with associated atraumatic placement of retractors medially.
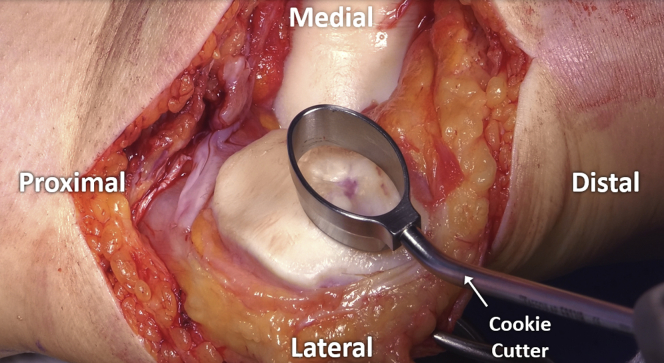
The borders of the defect are assessed and subsequently, stable vertical walls delineating the area to be prepared are created. The authors prefer to use an appropriately sized “cookie cutter” and gently mallet this down to the level of the calcified cartilage layer in order to create a contained defect with stable, vertical edges and a safe working zone (Fig 4).5 Alternatively, a scalpel or rectangular curettes may be used for this purpose.
Fig 4.
Stable defect borders are created through the use of an appropriately sized “cookie cutter,” which is gently malleted down to the level of the calcified cartilage.
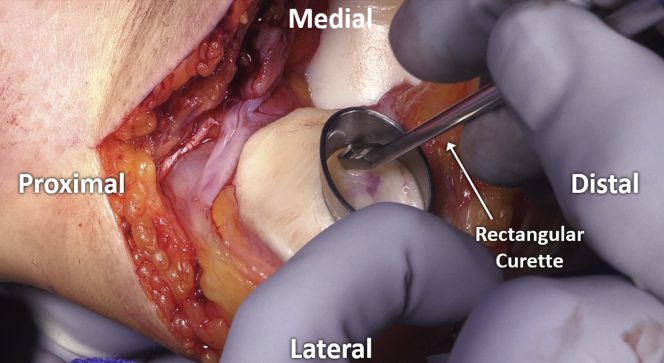
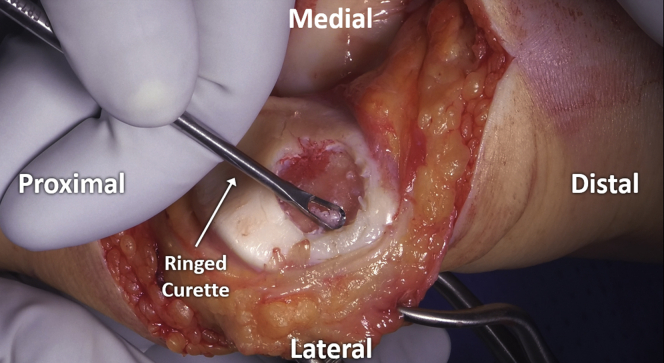
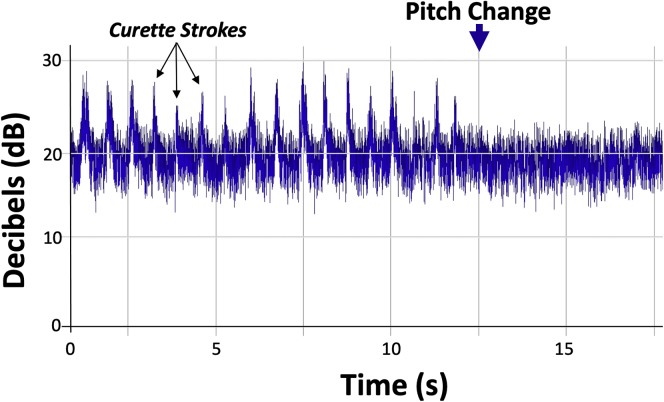
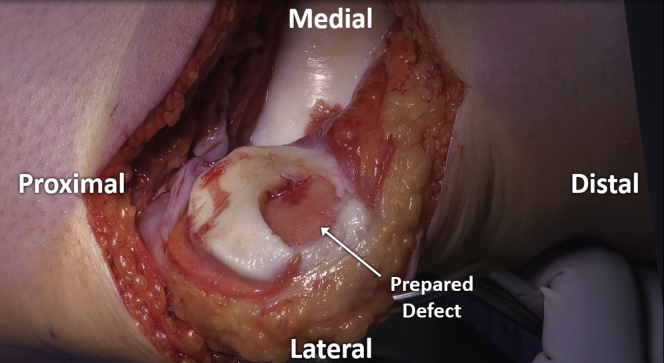
A curette is then used to carefully remove pathologic cartilage from the defect, moving systematically across the area in order to ensure consistent preparation without unnecessary repeated returns to any area with resultant overpreparation. If using a “cookie cutter,” this is initially left in place to serve as a safe border to contain initial preparation (Fig 5). Subsequently, the “cookie cutter” is removed and final debridement is completed with the use of a ring curette (Fig 6). Depth of debridement is confirmed using both visual and audible feedback. Namely, early debridement of chondral tissue is audibly heard with the high-pitched scraping sound of each stroke of the curette. Pressure applied with the curette at the time of debridement is kept even, with controlled, even strokes. Upon arrival to the calcified cartilage layer, strokes of the curette become audibly quieter (Fig 7), with an associated pitch change from sharp, higher-pitched scraping to dull, lower-pitched gliding, signaling arrival at the calcified cartilage layer and satisfactory depth of debridement (Video 1). Upon appreciation of the change in curette stroke pitch and volume, the surgeon can then progress across the defect to a new area until the entirety of the defect has been satisfactorily debrided (Fig 8).
Fig 5.
Initial debridement is shown of chondral defect with a rectangular curette, using the “cookie cutter” to create a safe working space with protected borders.
Fig 6.
Final debridement is performed with a ringed curette after removal of the “cookie cutter”, thus allowing controlled, even strokes.
Fig 7.
Distribution of peak volume (decibels, dB) with each curette stoke prior to (left) and after (right) the audible pitch and volume change indicating satisfactory debridement, demonstrating a significant (P < .01) decrease in curette stroke volume.
Fig 8.
Satisfactory debridement of the prepared patellar cartilage defect.
Subsequently, any planned additional interventions to follow defect preparation (i.e. microfracture, MACI) are performed. The wound is then closed in layers in a standard fashion, employing 0 Vicryl for the deep fascia, 2-0 Monocryl for the subcutaneous tissue, and running 3-0 Monocryl for the skin. Steri-strips are applied to the incision, followed by a sterile dressing.
Discussion
Articular cartilage defects are common and cause substantial impairments to patient quality of life.1 Precise preparation of defects at the time of joint preservation is critical and remains technically difficult. A recent study demonstrated that when surgeons were asked to retain the CCL in an open manner at the time of debridement, 40±39% of the CCL was removed.6 Conversely, when asked to remove the CCL, 19±28% of the CCL remained, highlighting the need for more precise chondral preparation methods and aids.
The technique presented offers multiple advantages for defect preparation, including free cost, precise defect preparation, and the ability to be readily appreciated by multiple operative personnel (Table 2). Conversely, special care should be taken, given that this technique may not demonstrate audible pitch changes in areas already denuded of cartilage or in instances where essential (i.e., anesthesia) background noise is considerable and unavoidable.
Table 2.
Advantages and Disadvantages, Including Risks and Limitations, of Chondral Debridement with Pitch and Volume Change Guidance
| Advantages | Disadvantages, Risks, and Limitations |
|---|---|
| Provides an additional method by which satisfactory debridement can be assessed. | Denuded areas already at or near the calcified cartilage layer (i.e. without chondral tissue) may not exhibit an audiometric change. |
| Heard pitch change can be appreciated by multiple surgeons, including both orthopedic staff and residents, simultaneously. | Teaching orthopedic surgeon staff should not depend on heard audiometric changes alone to ensure satisfactory debridement by residents and other surgeons |
| It is free of cost. | Utility may be limited because of background operating room noise levels |
Conclusions
The presented technique demonstrates the substantial decrease in curette stroke volume and associated shift to a lower pitch when achieving satisfactory open cartilage defect debridement. These audiologic cues correlate well with histologic accuracy of debridement. Therefore, quantifiable pitch and volume changes serve as valuable technical cues for precise defect preparation at the time of joint preservation surgery
Footnotes
Social Media Handles: M. Hevesi: @MarioHevesiMD; W. van Genechten: None; A. J. Krych: @DrKrych; D. B. F. Saris: @SarisNL; Institution: @MayoClinic@MayoClinicSport@RMUtrecht
Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Surgical video demonstrating the technical use of pitch and volume changes during chondral defect preparation in joint preservation surgery.
References
- 1.Heir S., Nerhus T.K., Røtterud J.H., et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231–237. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 2.Hoemann C.D., Lafantaisie-Favreau C.H., Lascau-Coman V., Chen G., Guzmán-Morales J. The cartilage-bone interface. J Knee Surg. 2012;25:85–97. doi: 10.1055/s-0032-1319782. [DOI] [PubMed] [Google Scholar]
- 3.Minas T. A primer in cartilage repair. J Bone Joint Surg Br. 2012;94:141–146. doi: 10.1302/0301-620X.94B11.30679. [DOI] [PubMed] [Google Scholar]
- 4.Hevesi M., Bernard C., Hartigan D.E., Levy B.A., Domb B.G., Krych A.J. Is microfracture necessary? Acetabular chondrolabral debridement/abrasion demonstrates similar outcomes and survival to microfracture in hip arthroscopy: A multicenter analysis. Am J Sports Med. 2019;47:1670–1678. doi: 10.1177/0363546519845346. [DOI] [PubMed] [Google Scholar]
- 5.Hevesi M., Krych A.J., Saris D.B.F. Treatment of cartilage defects with the matrix-induced autologous chondrocyte implantation cookie cutter technique. Arthrosc Tech. 2019;8:e591–e596. doi: 10.1016/j.eats.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanke A.B., Lee A.S., Karas V., et al. Surgeon ability to appropriately address the calcified cartilage layer: an in vitro study of arthroscopic and open techniques. Am J Sports Med. 2019;47:2584–2588. doi: 10.1177/0363546519859851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surgical video demonstrating the technical use of pitch and volume changes during chondral defect preparation in joint preservation surgery.
Surgical video demonstrating the technical use of pitch and volume changes during chondral defect preparation in joint preservation surgery.