Abstract
Healthcare institutions with mandatory influenza vaccination policies have over 90% vaccination rates among healthcare workers (HCWs) resulting in a population that has received the influenza vaccine in many, consecutive years. This study explored the impact of sex and other host factors in pre- and post-vaccination neutralizing antibody (nAb) titers and seroconversion against the H1N1 and H3N2 influenza A viruses (IAVs) among HCWs enrolled into a cross-sectional serosurvey during the annual Johns Hopkins Hospital employee vaccination campaign in the 2017-18 and 2018-19 seasons. The study enrolled 111 participants (male=38, female=73) in 2017-18 and 163 (male=44, female=119) in 2018-19. Serum samples were collected immediately prior to vaccination and approximately 28 days later and nAb titers to vaccine strains determined. An intersectional approach was used to disaggregate the combined effects of sex with age and body mass index (BMI) in the nAb response. Differences between the pre- or post-vaccination geometric mean nAb titers between male and female HCWs were not observed. Male HCWs were 2.86 times more likely to seroconvert compared to female HCWs in 2017-2018, but the same trend was not observed in the following year. When data were disaggregated by age and sex, older female HCWs had higher H1N1 pre- and post-vaccination nAb titers compared to male HCWs in the same age group for both vaccination campaign seasons. In both years, the decline in H3N2 pre-vaccination titers with increasing BMI was greater in female than male HCW. The sex-specific effects of age and BMI on nAb responses to seasonal influenza vaccines require greater consideration.
Keywords: aging, body mass index, neutralizing antibody, obesity, sex difference, vaccine efficacy
Introduction
Seasonal influenza epidemics affect 5-15% of the world’s population, and the World Health Organization (WHO) attributes 290,000-650,000 annual, global deaths to influenza [1, 2]. Healthcare workers (HCWs) are at an increased risk of contracting influenza due to occupational exposure and they can also transmit the virus to patients who have a higher risk of developing severe influenza. The Centers for Disease Control and Prevention (CDC) recommends annual influenza vaccination, with special provisions for HCWs who are directly or indirectly involved in patient care and additional emphasis on the importance of influenza vaccination during the COVID-19 pandemic [3]. Healthcare institutions that have mandatory vaccination policies in place have over 90% vaccination rates among HCWs [4], and high rates of vaccination have translated to HCWs receiving many consecutive influenza vaccinations. Previous reports indicate that HCWs with ≥4 previous influenza vaccines have higher pre-vaccination antibody titers compared to first time vaccinees, and the post-vaccination antibody titers are inversely proportional to the pre-vaccination titers [5]. Other studies have reported similar findings, where previously vaccinated HCWs were less likely to mount as robust of a response as naïve HCWs receiving the vaccine for the first time due to higher pre-vaccination titers [6–8].
Previous studies illustrate that age and body mass index (BMI) can be determinants of the magnitude of an influenza vaccine response [9–12]. Immunosenescence, which refers to the age-associated decline in immune response, has been shown to impact immunity to seasonal influenza vaccines among older adults [9, 12]. Older HCWs (age 49-64) are reported to have significantly lower H1N1 pre-vaccination antibody levels compared to younger HCWs (age 20-48) [13], but with no consideration of the sex of the HCWs. Obesity (i.e., body mass index [BMI]> 30%) also is associated with impaired immune response to the influenza vaccine, which is correlated with a greater decline in the antibody titer to the seasonal influenza vaccine over time [14].
Sex differences in the antibody response to the seasonal influenza vaccine have also been reported, with females generally developing greater antibody responses to seasonal influenza vaccines than males [9, 12, 15–18]. Also, female vaccinees are reported to have greater median pre-vaccination antibody titers than male vaccinees [19], suggesting that females already have elevated antibody responses to influenza prior to receipt of the annual vaccine. Sex differences in the antibody responses to the seasonal influenza vaccine among highly vaccinated HCWs have not been explored, to date.
Females account for 76% of HCWs according the United States Census Bureau, with healthcare occupations projected to increase rapidly in the next four years due to an aging population with greater demand on the healthcare system [20, 21]. As more females enter the healthcare workforce, they are more likely to have direct patient contact, which increases risk of exposure to influenza viruses. It is also estimated that more than one tenth of the world population is considered obese, and overweight or obese adults comprise more than two thirds of the US adult population [14]. This study explored sex differences in HCWs pre- and post-vaccination neutralizing antibody (nAb) titers and seroconversion against the H1N1 and H3N2 influenza A virus (IAV) vaccine strains after the administration of inactivated influenza vaccine during the 2017-18 and 2018-19 seasons. We also investigated how sex intersects with other stratifiers, such as age and BMI, to influence the antibody response in HCWs who have received multiple consecutive years of seasonal influenza vaccination.
Methods
Study design:
This study was a cross-sectional serosurvey, with HCWs recruited from the Johns Hopkins Centers of Excellence for Influenza Research and Surveillance (JHCEIRS) during the annual Johns Hopkins Hospital (JHH) employee influenza vaccination campaign in the 2017-2018 and 2018-2019 influenza seasons. The JHH Institutional Review Board approved the study.
Participant eligibility:
All HCWs, at least 18 years or older, who visited the vaccination clinic prior to receiving the influenza vaccine during the campaign were eligible to participate. HCWs who were unable to speak or write English, or unable to provide informed consent were excluded from participating in the study.
Study procedure:
Informed consent was obtained from all HCWs prior to administering the influenza questionnaire, which included demographic information, employment status, influenza exposure, influenza vaccination history in the last five years, and medical history. Prior influenza vaccination information obtained during the interview was verified with JHH Occupational Health records. After completing the questionnaire, 10mL of whole blood was obtained and then the HCWs were vaccinated. HCWs were asked to return for a post-vaccination follow-up visit twenty-eight days later and 10mL of whole blood was obtained.
Influenza A virus vaccine strains:
In the quadravalent inactivated influenza vaccines administered at JHH, the 2017-2018 influenza vaccine contained A/Michigan/45/2015 (H1N1) and A/Hong Kong/4801/2014 (H3N2) IAVs. The 2018-2019 influenza vaccine contained A/Michigan/45/2015 (H1N1) and A/Singapore/INFIMH-16-0019/2016 (H3N2). The A/Michigan/45/2015 (H1N1) and A/Hong Kong/4801/2014 (H3N2)vaccine strains were provided by Dr. Doris Bucher at New York Medical College. The A/Singapore/INFIMH-16-0019/2016 (H3N2) vaccine strain was generated using infectious clone technology [22] and was a recombinant virus encoding the HA (GSIAD accession # EPI868856) and NA (GISAID accession # EPI868855) sequences of A/Singapore/INFIMH-16-0019/2016 IVR-186 along with the 6 internal segments of A/Victoria/361/2011. Madin-Darby canine kidney (MDCK) cells were infected with vaccine viruses diluted in infection medium (IM) consisting of Dulbecco modified Eagle medium (Sigma), 0.2% bovine serum albumin (Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco), and 2 mM GlutaMAX (Gibco) at 37°C and 5% CO2. Infectious virus titers were determined using a 50% tissue culture infectious dose (TCID50) assay [23].
Microneutralization assay:
Serum samples were diluted with receptor destroying enzyme (RDE, Denka Seiken) at 1:3 ratio and incubated overnight at 32°C followed by heat inactivation at 56°C for 35 minutes. Samples were 2-fold serially diluted in IM, mixed with 100 TCID50 of each virus, and incubated at room temperature for 1 hour. The virus/serum mixture was transferred in duplicate into the 96-well cell culture plates containing confluent Madin-Darby Canine Kidney (MDCK) cells and incubated at 32°C. After a 24 hour incubation, plates were washed once with 1X PBS, fresh IM was added, and cells were incubated for 6 days. Plates were fixed with 4% formaldehyde, stained with naphthol blue black solution, and scored as described previously [12].
Statistical Analyses:
Neutralizing antibody (nAb) titers were log2 transformed and geometric mean titers (GMT) were reported for comparison by sex, age, BMI, and seroconversion. Seropositivity was defined as a ≥40 antibody titer. Seroconversion was defined as at least a four-fold nAb increase between post and pre-vaccination antibody titers. The study enrolled 111 participants (male=38, female=73) in 2017-18 and 163 (male=44, female=119) in 2018-19. Differences between those who seroconverted and those who did not, as well as between male and female HCWs, at each time point for each strain and between pre- and post-vaccination titers were calculated using two-tailed t-tests. Multiple logistic regressions were used to assess the impact of sex, obesity (i.e., BMI > 30%), age, race, comorbid conditions, and pre-vaccination nAb titers on the odds of seroconversion. Unadjusted simple linear regression models were used to determine the effect of age and BMI on pre and post-vaccination antibody titers, in the whole population and separately for males and females. All analyses were performed in Stata 15 (College Station, Texas). A p<0.05 was considered statistically significant.
Results
Healthcare worker characteristics
The study enrolled 128 participants during the 2017-18 season with 111 (86.7%) HCWs completing both baseline and 28-day post vaccination visits. Of the 111 who completed both visits, 38 (34.2%) were male and 73 (65.8%) were female. During the 2018-19 season, 200 participants were enrolled with 163 HCWs completing both visits (81.5%), and of these 44 (27%) were male and 119 (73%) were female. The HCWs who were lost to follow-up at the 28-day post-vaccination visit were excluded from the analyses. Table 1 summarizes the demographic characteristics of the study population, and the characteristics of those lost to follow-up are described in Supplemental Table 1.
Table 1:
Characteristics of healthcare workers enrolled during the 2017-18 and 2018-19 influenza vaccine seasons at the Johns Hopkins Hospital.
| 2017-2018 | 2018-2019 | |||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| N (%) | 38 (34.2) | 73 (65.8) | 111 | 44 (27.0) | 119 (73.0) | 163 |
| Age - med (IQR) | 34 (28 - 44) | 38 (30 - 52) | 30 (27 - 37) | 36 (30 - 47) | ||
| Age Categories - n (%) | ||||||
| 19-49 | 33 (86.8) | 51 (69.9) | 84 (75.7) | 38 (86.4) | 98 (82.4) | 136 (83.4) |
| 50-68 | 5 (13.2) | 22 (30.1) | 27 (24.3) | 6 (13.6) | 21 (17.6) | 27 (16.6) |
| Race - n (%) | ||||||
| American Indian or Alaskan Native | 0 (0.0) | 0 (0.0) | 0(0.0) | 1 (2.3) | 0 (0.0) | 1 (.6) |
| Asian | 7 (18.4) | 7 (9.6) | 0(0.0) | 5 (11.4) | 8 (6.7) | 13 (8.0) |
| Black or African American | 6 (15.8) | 13 (17.8) | 14 (12.6) | 8 (18.2) | 28 (23.5) | 36 (22.1) |
| White | 21 (55.3) | 48 (65.8) | 19 (17.1) | 28 (63.6) | 76 (63.9) | 104 (63.8) |
| Other | 3 (7.9) | 5 (6.8) | 69 (62.2) | 2 (4.5) | 7 (5.9) | 9 (5.5) |
| Unkown | 1 (2.6) | 0 (0.0) | 8 (7.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BMI Categories - n (%) | ||||||
| Normal | 9 (23.7) | 31 (42.5) | 40 (36.0) | 18 (40.9) | 55 (46.2) | 73 (44.8) |
| Over weight or obese | 29 (76.3) | 42 (57.5) | 71 (64.0) | 25 (56.8) | 62 (52.1) | 87 (53.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 2 (1.7) | 3 (1.8) |
| Vaccination history categories - n (%) | ||||||
| <5 previous vaccines | 14 (36.8) | 16 (21.9) | 30 (27.0) | 12 (27.3) | 20 (16.8) | 32 (19.6) |
| 5 previous vaccines | 24 (63.2) | 57 (78.1) | 81 (73.0) | 27 (61.4) | 94 (79.0) | 121 (74.2) |
| Unkown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (9.1) | 2 (1.7) | 6 (3.7) |
| Missing | (.) | (.) | (.) | 1 (2.3) | 3 (2.5) | 4 (2.5) |
| Ever smoked - n (%) | ||||||
| No | (.) | (.) | (.) | 42 (95.5) | 104 (87.4) | 146 (89.6) |
| Yes | (.) | (.) | (.) | 2 (4.5) | 14 (11.8) | 16 (9.8) |
| Missing | (.) | (.) | (.) | 0 (0.0) | 1 (.8) | 1 (.6) |
| Underlying conditions | ||||||
| No | 26 (68.4) | 31 (42.5) | 57 (51.4) | 25 (56.8) | 64 (53.8) | 89 (54.6) |
| Yes | 12 (31.6) | 42 (57.5) | 54 (48.6) | 19 (43.2) | 55 (46.2) | 74 (45.4) |
| Patient contact | ||||||
| No | 32 (84.2) | 44 (60.3) | 76 (68.5) | 23 (52.3) | 46 (38.7) | 69 (42.3) |
| Yes | 5 (13.2) | 29 (39.7) | 34 (30.6) | 21 (47.7) | 68 (57.1) | 89 (54.6) |
| Missing | 1 (2.6) | 0 (0.0) | 1 (.9) | 0 (0.0) | 5 (4.2) | 5 (3.1) |
(.)=smoking data not collected in the 2017-18 study season
In the 2017-18 season, 73.0% of all HCWs (63.2% of males and 78.1% of females) reported receiving influenza vaccines consecutively in five previous seasons. In the 2018-19 season, 74.2% of all HCWs (61.4% of males and 79.0% of females) reported receiving five previous vaccines. In both study years, the majority of HCW participants were Caucasian females: 62.1% in 2017-18 and 60.8% in 2018-19.
HCW who seroconvert have lower pre-vaccination titers
In this study, seroconversion was defined as at least a four-fold increase between pre- and post-vaccination nAb titers, which was used as an indirect indictor of protection after vaccination. Seroconversion, however, is biased when individuals have high pre-vaccination titers, as they may not mount a four-fold rise in titer that is within the detection limits of the assay. Overall, the majority of HCWs did not seroconvert in either season; 61.3% of HCWs in 2017-18 and 72.4% of HCWs in 2018-19 did not seroconvert for H1N1, and 70.3% (2017-2018) and 66.3% (2018-2019) of HCWs did not serocovert for H3N2 (Table 2).
Table 2.
Pre and post-vaccination geometric mean titer, seroprotection, and seroconversion rates in healthcare workers disaggregated by sex.
| 2017–18 |
2018–19 |
|||||
|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | |
| H1N1 | ||||||
| Pre-vaccination GMT | 310 (232–415) | 248 (147–149) | 349 (245–496) | 676 (547–836) | 905 (573–1431) | 607 (479–770) |
| Post-vaccination GMT | 886 (690–1136) | 939 (613–1437) | 859 (627–1176) | 1418 (1185–1696) | 1928 (1332–2791) | 1265 (1032–1550) |
| Pre-vaccination SPR | 86.5 | 84.2 | 87.7 | 93.9 | 95.5 | 93.3 |
| Post-vaccination SPR | 97.3 | 97.4 | 97.3 | 100 | 100 | 100 |
| SCR | 38.7 | 55.3 | 30.1 | 27.6 | 22.7 | 29.4 |
| H3N2 | ||||||
| Pre-vaccination GMT | 822 (621–1087) | 782 (506–1210) | 843 (584–1217) | 524 (418–656) | 573 (345–953) | 506 (394–650) |
| Post-vaccination GMT | 1782 (1409–2255) | 1745 (1193–2554) | 1802 (1329–2441) | 1161 (963–1400) | 1043 (682–1596) | 1208 (984–1484) |
| Pre-vaccination SPR | 96.4 | 97.4 | 95.9 | 92.6 | 88.6 | 94.1 |
| Post-vaccination SPR | 100 | 100 | 100 | 98.8 | 97.7 | 99.2 |
| SCR | 29.7 | 31.6 | 28.8 | 33.7 | 22.7 | 37.8 |
GMT = Geometric mean titer (reported as mean (95% CI)) ; SPR = seroprotection rate ; SCR = Seroconversion rate.
No statistically significant differences.
The presence of nAb titers of >1:40 dilution is associated with protection from infection and we classified those individuals as being seroprotected [24]. Pre-vaccination, 86.5% and 93.9% of HCWs in the 2017-2018 and 2018-2019 seasons, respectively, were classified as seroprotected for H1N1 and 96.4% and 92.6% of HCWs were classified as H3N2 seroprotected in each respective study season (Table 2). Post-vaccination, 97.3% and 100% of HCWs were classified as seroprotected for H1N1 and 100% and 98.8% were protected for H3N2, in each respective study season.
The pre-vaccination nAb titers to H1N1 were greater among those who did not seroconvert compared to those who did in both seasons (Figure 1A–B). The H3N2 pre-vaccination titers were also greater for non-seroconverted HCWs in the 2017-2018 and 2018-2019 seasons, compared to HCWs who seroconverted (Figure 1C–D). For both seasons and both strains, there was a significant increase in nAb titers from pre- to post-vaccination among those who seroconverted. Among those who did not seroconvert, there was only a significant increase in nAb titer against H1N1 in the 2018-19 season.
Figure 1: Healthcare workers (HCWs) who did not seroconvert had higher pre-vaccination titers than those who seroconverted.
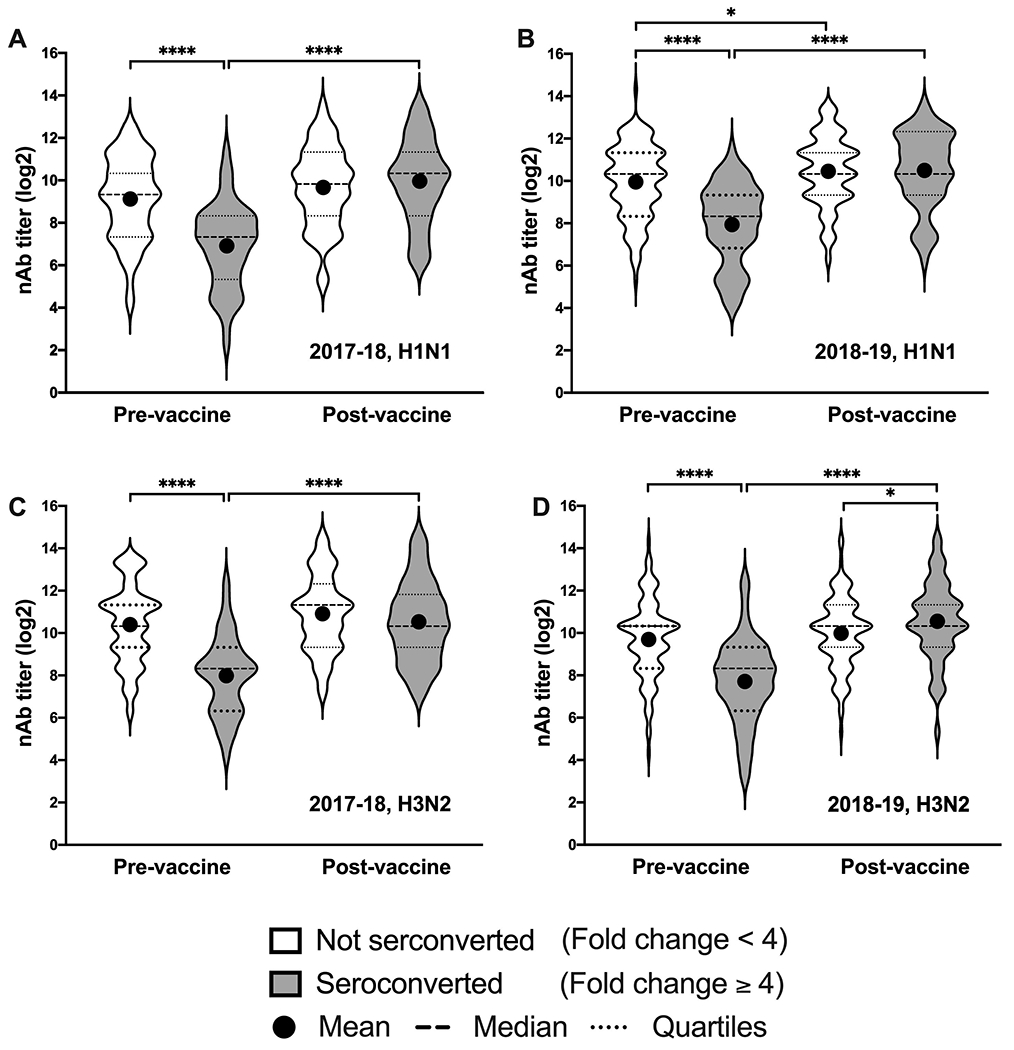
Log2-transformed neutralizing antibody (nAb) titers pre- and 28 days post-vaccination are shown for HCWs who seroconverted (gray) and those who did not (white) to H1N1 in the 2017-18 season (A), H1N1 in the 2018-19 season (B), H3N2 in the 2017-18 season (C), and H3N2 in the 2018-19 season (D). Dashed lines indicate the median titer for each group and dotted lines indicated the 25th and 75th percentiles. Differences between those who seroconverted and those who did not at each time point for each strain and between pre- and post-vaccination titers were calculated using two-tailed t-tests. Asterisks indicate the significance level with * = p-value <0.05 and **** = p-value <0.0001.
The odds of seroconversion against the H1N1 and H3N2 vaccine viruses in HCWs are affected by pre-vaccination titers
We next examined the impact of sex, BMI, age, race, and the presence of comorbidities on the odds of seroconversion using multiple logistic regression models (Figure 2). In the 2017-18 season, sex was a significant determinant of the odds of seroconversion for the H1N1 virus, in which male HCWs were 2.86 times more likely to seroconvert to the H1N1 vaccine strain than female HCWs (Figure 2A). This male-bias, however, was not observed in seroconversion against the H3N2 virus in the 2017-18 season nor for seroconversion against either the H1N1 or H3N2 vaccine viruses in the 2018-19 study season (Figure 2B). The other host factors of interest did not have a significant impact on the odds of seroconversion.
Figure 2: Odds of seroconversion are affected by pre-vaccination titers.
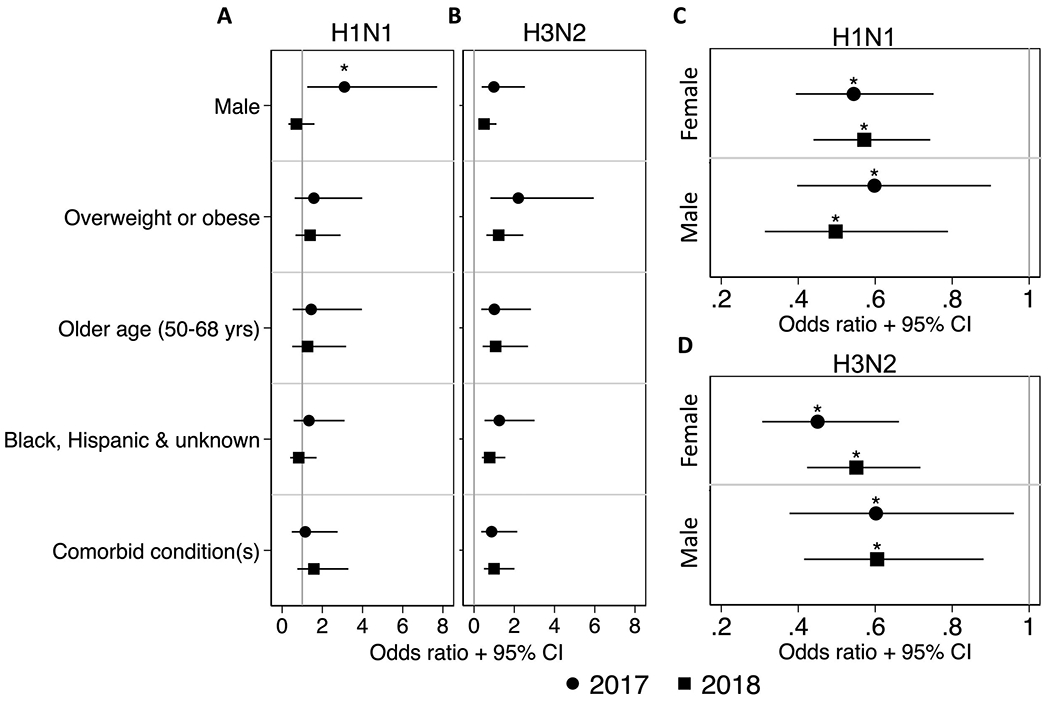
Multiple logistic regression models were used to analyze the impact of sex, BMI, race, and the presence of comorbidities on the odds of seroconverting to H1N1 (A) and H3N2 (B) during the 2017-18 and 2018-19 seasons. Point estimates of the log odds ratios with 95% confidence intervals are depicted separately for the 2017-18 (circles) and 2018-19 (squares) seasons. Simple logistic regressions were used to analyze the impact of pre-vaccination titers on the odds of seroconverting to H1N1 (C) and H3N2 (D) titers for males and females in each season. The odds ratios and 95% confidence are depicted. * = p-value < 0.05.
Unlike the demographic factors, pre-vaccination titers were significant predictors of the odds seroconversion to H1N1 (Figure 2C) and H3N2 (Figure 2D) for both males and females in the 2017-18 and 2018-19 seasons. In each case, the odds of seroconverting were 0.46 - 0.65 times lower for each one unit increase in pre-vaccination nAb titer.
Sex alone is not a determinant of seroconversion H1N1 or H3N2 IAVs
Sex differences have been reported in previous influenza vaccine studies [9, 12, 16]; therefore, sex was explored as a possible determinant of pre- and/or post-vaccination titers among HCWs (Table 2). In the 2017-18 season, more male HCWs (55.3%) seroconverted to the H1N1 vaccine virus compared to female HCWs (30.1%). In addition, a higher percentage of ma HCWs (31.6%) seroconverted to the H3N2 vaccine virus compared to female HCWs (28.8%). In the 2018-19 study season, however, the trend reversed slightly where more female HCWs (29.4%) seroconverted to the H1N1 vaccine virus compared to male HCWs (22.7%), and more female (37.8%) than male (22.7%) HCWs seroconverted to the H3N2 vaccine virus. In both seasons, there were no significant differences between male and female HCW in the pre- or post- geometric mean nAb titer against either the H1N1 or H3N2 vaccine viruses (Figure 3). In general, both males and females mounted a response to the vaccine, with post-vaccination titers being significantly higher than pre-vaccination titers.
Figure 3: Neutralizing antibody titers increased after receiving an influenza vaccine for both male and female HCWs.
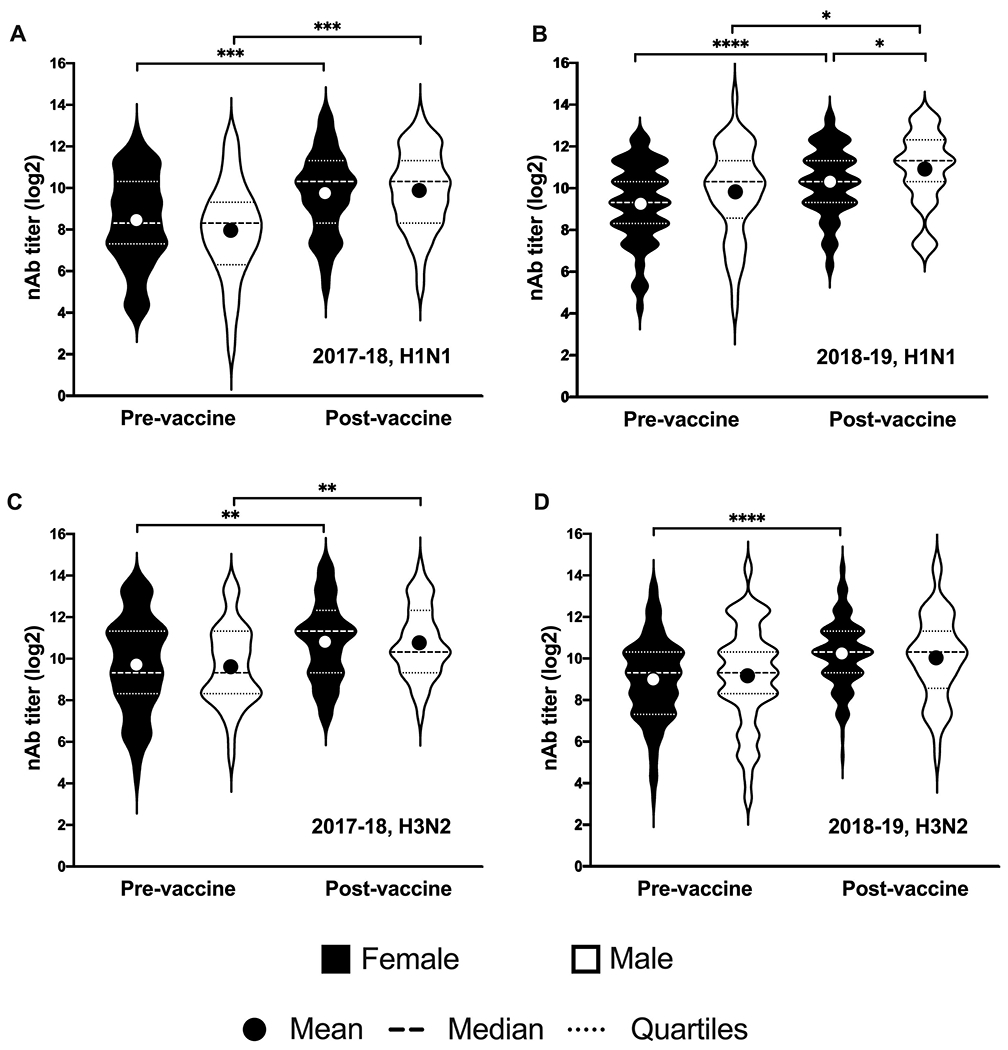
Log2-transformed neutralizing antibody titers pre- and 28 days post-vaccination are shown for female (black) and male (white) HCWs to H1N1 in the 2017-18 season (A), H1N1 in the 2018-19 season (B), H3N2 in the 2017-18 season (C), and H3N2 in the 2018-19 season (D). Dashed lines indicate the median titer for each group and dotted lines indicated the 25th and 75th percentiles. Differences between female and male HCWs at each time point for each strain and between pre- and post-vaccination titers were calculated using two-tailed t-tests. Asterisks indicate the significance level with * = p-value <0.05, ** = p-value <0.01, *** = p-value <0.001 and **** = p-value <0.0001.
Age intersects with sex to impact nAb titers in HCWs
Previous studies have demonstrated that age is a determinant of both pre- and post-vaccination antibody titers [12, 13]. Pre and post-vaccination nAb titers against the respective seasonal H1N1 and H3N2 vaccine viruses were disaggregated by age and sex to explore the differential impact of age on vaccine-induced immunity in male and female HCWs. In general, age results in a decline in nAb responses among both male and female HCWs (Figure 4). During the 2017-18 season, female HCWs (slope=0.061 for H1N1 and 0.070 for H3N2) had a greater decline in pre vaccination nAb titers with age compared to male HCWs (slope=0.047 for H1N1 and 0.043 for H3N2) (Figure 4A and E). In post-vaccination nAb titers, male HCWs had a greater decline in H1N1 titers with age (slope=0.056) compared to female HCWs (slope=0.039) (Figure 4B), but no difference was observed in the decline of H3N2 titers with age between male and female HCWs (Figure 4F). In the 2018-2019 season, male HCWs had a greater decline in both pre and post-vaccination titers for both H1N1 (slope=0.081 pre and 0.068 post-vaccination) and H3N2 (slope=0.092 pre and 0.070 post-vaccination) compared to female HCWs (slope=0.033 pre and 0.025 post H1N1 vaccination and slope=0.051 pre and 0.045 post H3N2 vaccination) (Figure 4 C, D, G, H). Taken together, these data suggest that even among adults below 65 years of age, increasing age is associated with an impaired ability to maintain an antibody response in the year following vaccination and to mount robust responses to the vaccine, and that this effect is modified by sex.
Figure 4: Age intersects with sex to impact neutralizing antibody (nAb) titers in HCWs.
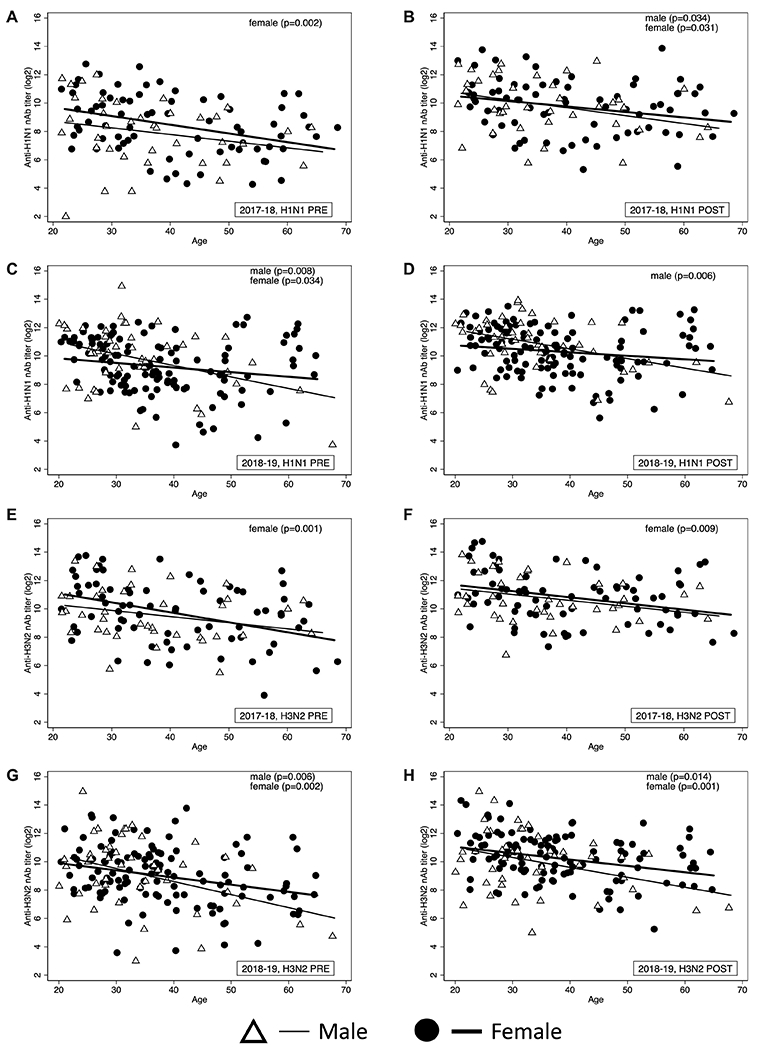
Simple linear regression models were used to analyze the effect of age on nAb titers separately for male and female HCWs before vaccination in H1N1 2017-18 season (A), 28 days post-vaccination H1N1 in the 2017-18 season (B), before vaccination in H1N1 2018-19 season (C), 28 days post-vaccination H1N1 in the 2018-19 season (D), before vaccination in H3N2 in the 2017-18 season (E), and 28 days post-vaccination H3N2 in the 2017-18 season (F) before vaccination in H3N2 in the 2018-19 season (G), and 28 days post-vaccination H3N2 in the 2018-19 season (H).
Body mass index (BMI) intersects with sex to affect H3N2 nAb titers in HCWs
Obesity is known to impair immune response to the influenza vaccine and increase the severity of influenza symptoms [9, 25, 26]. For both study seasons, BMI was not an independent determinant of either pre- or post-vaccination nAb titers against the H1N1 vaccine virus (data not shown). Because BMI affected nAb responses to H3N2 vaccine viruses, we further explored the intersection of BMI with sex on pre- and post-vaccination nAb titers against the H3N2 viruses. During each vaccine season, as BMI increased, the decline in pre-vaccination nAb titers against H3N2 viruses was greater in female HCWs (slope=0.074 in 2017-18 and 0.078 in 2018-19) as compared to male HCWs (slope=0.043 in 2017-18 and 0.052 in 2018-19) (Figure 5). Taken together, these data suggest that BMI has a greater impact on female HCWs’ ability to maintain protective antibody levels in the year following immunization.
Figure 5: Body mass index (BMI) interacts to sex to affect H3N2 nAb titers in HCWs.
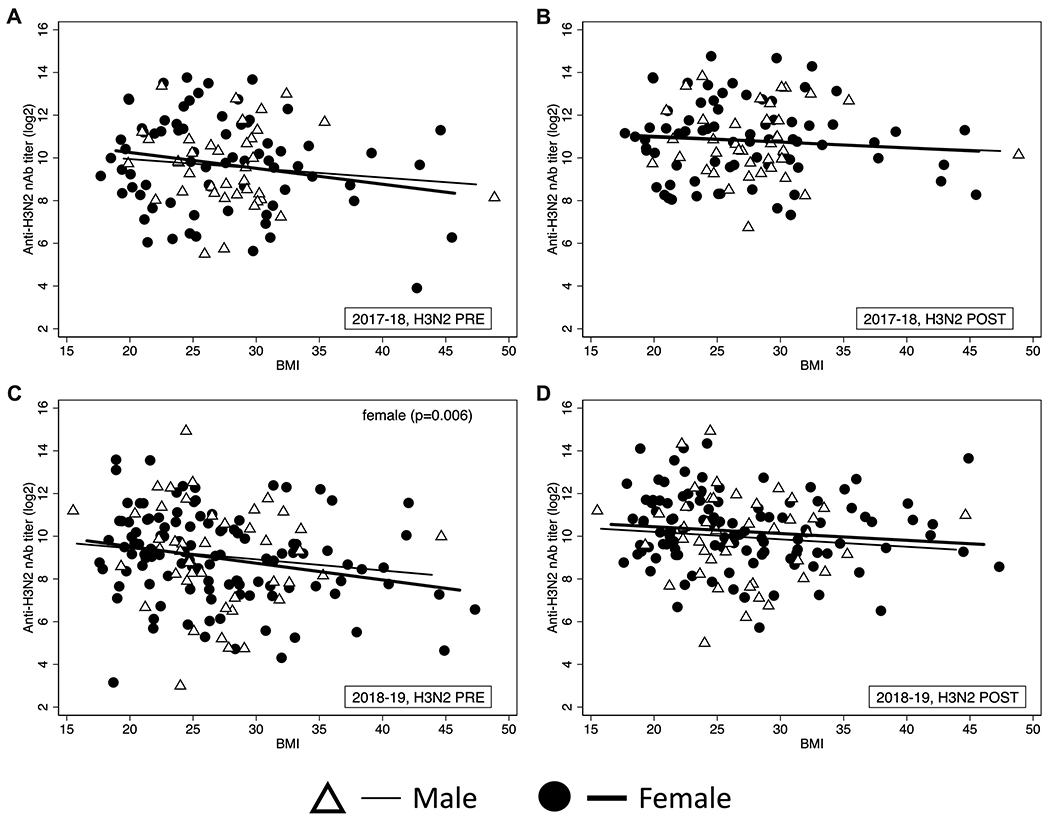
Simple linear regression models were used to analyze the effect of BMI on nAb titers separately for male and female HCWs before vaccination in H3N2 2017-18 season (A), 28 days post-vaccination H3N2 in the 2017-18 season (B), before vaccination in H3N2 2018-19 season (C), 28 days post-vaccination H2N2 in the 2018-19 season (D).
Discussion
Consecutive years of influenza vaccination does not appear to adversely affect protective influenza antibody titers as >86% of HCWs were seroprotected prior to vaccination and >97% were seroprotected after receiving their annual influenza vaccine. Among HCWs that have received annual influenza vaccinations for at least 5 consecutive seasons, sex alone does not influence pre or post H1N1 or H3N2 vaccination titers or the likelihood of seroconversion. Although we observed that male HCWs were 2.86 times more likely to seroconvert in the 2017-18 season against H1N1 vaccine virus, the same trend was not observed the following year. This is possibly due to the fact that pre-vaccination titers determined the magnitude of the nAb response to the influenza vaccine virus. In the 2017-18 influenza season the male HCWs had lower pre-vaccination nAb titers as compared to female HCWs, possibly increasing the odds of male HCWs seroconverting. In contrast, during the 2018-19 season, male HCWs had slightly higher pre-vaccination nAb titers compared to female HCWs, thereby decreasing the probability of seroconverting among males. It is likely that mandatory repeat vaccinations among HCWs have masked sex differences previously reported in non-HCW populations [9, 12, 16].
The primary predictor of seroconversion was pre-vaccination titers, with the odds of seroconverting decreasing significantly as pre-vaccination titer increased for both males and females in both seasons. This is consistent with previous reports in highly vaccinated populations [5–8]. Age was a significant predictor of pre- and post-vaccination nAb titers against both H1N1 and H3N2 viruses, across both influenza vaccine seasons. When nAb titers were disaggregated by age and sex, we observed that the impact of age on pre- and post-vaccination nAb titers was greater in males than in females in the 2018-19 study seasons, suggesting an age-associated sex difference in nAb titers among HCWs. For most vaccine studies, older age is defined as individuals 65+ years of age. In our study, we showed that declining immunity to QIV occurs among HCWs at a younger age (50-68 years), with the impact being greater for male than female HCWs.
Previous studies have reported that obesity contributes to lower immune responses to seasonal influenza vaccines and increased risk of developing more severe influenza symptoms [9, 25, 26]. Higher BMI appears to contribute to a faster decline in the pre-vaccination nAb titers against H3N2, in particular, among HCWs in both study years. Our results suggest that BMI is an important factor associated with the maintenance of nAb against vaccine viruses. Overall, HCWs categorized as overweight or obese had lower pre-vaccination titers compared to normal weight HCWs. The decline in H3N2 pre-vaccination titers with increasing BMI was greater in female compared to male HCWs. The rationale for how BMI would affect neutralization of H3N2 but not H1N1 viruses is not known, but may reflect greater changes in recent years to the H3N2 as compared with the H1N1 components of seasonal influenza vaccines, which likely resulted in the greater seroconversion to H3N2 than H1N1 in HCWs. Taken together, our data supports the hypothesis that that age and BMI may have sex-specific effects on functional antibody responses to influenza A virus vaccine viruses. Increasing age is associated with a greater decrease in nAb responses overall, whereas increasing BMI is associated with a greater decline in nAb responses in females.
Exploring influenza vaccine-induced immunity through intersectional analyses allows for a deeper understanding of how host factors intersect to impact the quality of the immune response. This study was specifically focused on exploring sex differences in combination with host factors that are well-documented to impact the immune response to the influenza vaccine. Although this study did not find that biological sex alone affected pre- or post-vaccination titers or seroconversion rates in HCWs, sex intersected with age and BMI to explain significant variation in the antibody responses to seasonal influenza vaccines. When vaccine strains remain constant from one year to the next, pre-vaccination nAb titers allowed us to interrogate the duration of the vaccine-induced immune response from the previous year and look at how male and female HCWs may be responding differently to repeat vaccinations, particularly among those above 50 years of age. In addition, as women constitute more than 76% of the healthcare workforce and this is projected to increase within the next four years, it is important to document how sex interacts with other variables to impact vaccine immunogenicity.
This study is not without limitations. The study is an annual observational cross-sectional survey and was not designed specifically to interrogate sex differences in the immune response to the influenza vaccine. The small cohort size did allow for definitive exploration of the effects of sex, age, and BMI as host factors that may affect vaccine efficacy. Further, the lack of racial and ethnic diversity of the enrollees as well as the predominance of females compared with males during the 2017-18 season, especially among older age and normal weight enrollees must be acknowledged. In addition, due to the mandatory influenza vaccination policy at JHH, the study was not able to recruit unvaccinated HCWs for pre and post-vaccination nAb comparison. Furthermore, there was an H3N2 strain change during the 2018-2019 season; therefore, the 2018-2019 pre-vaccination titers did not necessarily measure H3N2 antibody titers from the previous year. The study also did not continue past the one month post vaccination; therefore, the durability of the antibody responses was not assessed. The pre-vaccination nAb titers against H1N1 and H3N2 might suggest durability of antibody in the HCW population from previous years. Future studies will incorporate a 6 month follow-up time point to explore nAb durability and confirmation of protection. Finally, this study relied on nAb titers as a measure of immunogenicity, as opposed to hemagglutination inhibition titers, which is more commonly used to measure antibody responses to seasonal influenza vaccines. In conclusion, the impact of host factors, such as sex, is largely masked in the highly vaccinated HCW population. More in-depth intersectional analyses, however, revealed important interactions between sex, age, and BMI in the maintenance of antibody titers in the season following immunization, as well as the magnitude of the response to the seasonal influenza vaccine.
Supplementary Material
Highlights.
Repeat vaccination is associated with low H1N1 and H3N2 seroconversion rates
No difference in neutralizing antibody titers between male and female healthcare workers
Male healthcare workers are more likely than females to seroconvert
Females maintain higher neutralizing titers with increasing age as compared to males
Antibody responses decline with greater BMI in female but not male healthcare workers
Acknowledgements.
The authors thank the healthcare workers who enrolled and participated in the Johns Hopkins Center for Excellence in Influenza Research and Surveillance study. We are grateful for the efforts of the clinical coordination team JHH who collected samples. We also thank Sharvari Deshpande and Harish Narasimhan for technical assistance with assays.
Funding.
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases Center of Excellence in Influenza Research and Surveillance, contract Health and Human Services (grant number N2772201400007C to R. R., A. P., and S. K.), National Institute of Health/National Institute of Aging Specialized Center of Research Excellence U54 AG062333 to S.K., and Fellowship training grant from NIH/NIAID (grant number: T32A1007417 to R. U.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts. No conflicts of interest.
References
- 1.Iuliano AD et al. (2018) Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. The Lancet 391 (10127), 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dini G et al. (2018) Influenza vaccination in healthcare workers: A comprehensive critical appraisal of the literature. Human vaccines & immunotherapeutics 14 (3), 772–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases (NCIRD) (2020) Influenza Vaccination Information for Health Care Workers. https://www.cdc.gov/flu/professionals/healthcareworkers.htm, (accessed September 8 2020).
- 4.Wang TL et al. (2017) Mandatory influenza vaccination for all healthcare personnel: a review on justification, implementation and effectiveness. Current Opinion in Pediatrics 29 (5), 606–615. [DOI] [PubMed] [Google Scholar]
- 5.Huang K-YA et al. (2017) Antibody responses to trivalent inactivated influenza vaccine in health care personnel previously vaccinated and vaccinated for the first time. Scientific reports 7 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacadura-Leite E et al. (2012) Antibody response to the influenza vaccine in healthcare workers. Vaccine 30 (2), 436–441. [DOI] [PubMed] [Google Scholar]
- 7.Tete SM et al. (2018) Impact of pre-existing immunity on the induction of functional cross-reactive anti-hemagglutinin stalk antibodies following vaccination with an AS03 adjuvanted pandemic H1N1 vaccine. Vaccine 36 (16), 2213–2219. [DOI] [PubMed] [Google Scholar]
- 8.Leung VK et al. (2017) Influenza vaccination responses: Evaluating impact of repeat vaccination among health care workers. Vaccine 35 (19), 2558–2568. [DOI] [PubMed] [Google Scholar]
- 9.Engler RJ et al. (2008) Half-vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Archives of internal medicine 168 (22), 2405–2414. [DOI] [PubMed] [Google Scholar]
- 10.Frasca D et al. (2010) Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine 28 (51), 8077–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frasca D et al. (2016) The generation of memory B cells is maintained, but the antibody response is not, in the elderly after repeated influenza immunizations. Vaccine 34 (25), 2834–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potluri T et al. (2019) Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. npj Vaccines 4 (1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strengell M et al. (2013) Antibody responses against influenza A (H1N1) pdm09 virus after sequential vaccination with pandemic and seasonal influenza vaccines in Finnish healthcare professionals. Influenza and other respiratory viruses 7 (3), 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheridan PA et al. (2011) Obesity is associated with impaired immune response to influenza vaccination in humans. International Journal of Obesity 36 (8), 1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fink AL and Klein SL (2015) Sex and Gender Impact Immune Responses to Vaccines Among the Elderly. Physiology (Bethesda, Md.) 30 (6), 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furman D et al. (2014) Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A 111 (2), 869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein SL and Pekosz A (2014) Sex-based Biology and the Rational Design of Influenza Vaccination Strategies. Journal of Infectious Diseases 209 (suppl 3), S114–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ursin RL et al. (2020) Differential antibody recognition of H3N2 vaccine and seasonal influenza virus strains based on age, vaccine status, and sex in the 2017-18 season. The Journal of Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voigt EA et al. (2019) Sex differences in older adults’ immune responses to seasonal influenza vaccination. Frontiers in immunology 10, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day JC and Christnacht C (2019) Your Health Care Is in Women’s Hands. https://www.census.gov/library/stories/2019/08/your-health-care-in-womens-hands.html, (accessed October 7 2020).
- 21.U.S. Bureau of Labor and Statistics (2019) Occupational Outlook Handbook - Healthcare Occupations. https://www.bls.gov/ooh/healthcare/home.htm, (accessed October 7 2020).
- 22.Powell H and Pekosz A (2020) Neuraminidase antigenic drift of H3N2 clade 3c. 2a viruses alters virus replication, enzymatic activity and inhibitory antibody binding. PLoS pathogens 16 (6), e1008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohlgemuth N et al. (2018) Influenza A Virus M2 Protein Apical Targeting Is Required for Efficient Virus Replication. Journal of Virology 92 (22), e01425–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Module 23: Influenza Vaccines, The Immunological Basis for Immunization Series, World Health Organization, Geneva, 2017. [Google Scholar]
- 25.Louie JK et al. (2009) Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. Jama 302 (17), 1896–902. [DOI] [PubMed] [Google Scholar]
- 26.Neidich SD et al. (2017) Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond) 41 (9), 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


