Abstract
Purpose
This study aimed to evaluate the impact of luteal phase ovarian stimulation (LPS) on the outcomes of assisted reproductive technology (ART) for infertile couples and patients desiring non-urgent egg cryopreservation.
Methods
We included all studies reported patients who received LPS and that used follicular phase ovarian stimulation (FPS) as a comparison group until January 2021. Prior meta-analysis regarding the outcomes of LPS in double stimulation and fertility preservation have already been published, so these studies were excluded. Risk of Bias in Non-randomized Studies of Interventions was used to assess the study quality. The study was registered in the International Prospective Register of Systematic Reviews database (CRD42020183946).
Results
Twelve studies with a total of 4433 patients were included. The regimen employed can be categorized into two groups, but there is currently no evidence to support one over the other. After we excluded the largest study, the clinical pregnancy rate and live birth rate were similar after FPS and LPS. There were significantly more stimulation days and total gonadotropins used in the LPS group. After subgroup analysis, we found that poor responders received significantly more cumulus oocyte complexes (+0.64) in the LPS group.
Conclusion
Current evidence indicates that patients in the LPS group could achieve pregnancy outcomes non-inferior to those in the FPS group. Because of current debate over freeze-all policy and the limited data about live birth rate, the universal use of LPS is considered controversial. In the future, more well-designed studies are necessary to investigate the indications for LPS and its cost-effectiveness.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02237-7.
Keywords: Ovarian stimulation, Luteal phase, Poor responders, Random start
With the development of vitrification technology and the revolutionary use of progesterone to block the luteinizing hormone (LH) surge during ovarian stimulation, it became possible to break away from the conventional assisted reproductive technology (ART) sequence of stimulation–retrieval–transfer [1]. In 1996, scientists found that the administration of progestin to monkeys prevented the occurrence of the LH surge despite the increase in estradiol [2]. Thirteen years later, the first study reporting initiation of ovarian stimulation in the luteal phase to cryopreserve fertilized oocytes in cancer patients was published [3].
The first systematic review and meta-analysis of luteal phase stimulation (LPS) was published in 2016 [4]. The authors concluded that LPS requires a longer duration of stimulation and a higher dose of total gonadotropins than follicular phase ovarian stimulation (FPS), but does not compromise the quantity or quality of oocytes retrieved. This conclusion was mostly based on cancer patients requesting fertility preservation, and the application of LPS to infertile patients remained unknown. Massin reviewed the use of endogenous and exogenous progesterone to block the LH surge during ovarian stimulation and found that it did not affect the number of oocytes collected or the quality of the embryos obtained [1]. Sfakianoudis et al. conducted a review of double stimulation during the same cycle (DuoStim) for patients with poor ovarian reserve [5]. This procedure enabled a higher oocyte yield during the same menstrual cycle and shortened the time from oocyte retrieval to pregnancy. Many studies reported that LPS can be used for fertility preservation in cancer patients, to retrieve oocytes more efficiently without compromising their quality and quantity [4, 6, 7]. Recently, Alexander et al. reviewed the outcomes between female cancer patients who underwent conventional FPS and those who underwent random-start ovarian stimulation, and concluded that they did not differ in any clinically important ways [8].
Despite the emerging evidences supporting LPS, the use of LPS in the general population is still controversial. There are currently no guidelines for LPS and its indications are inconclusive. The protocols employed by most physicians are based on personal experience. The aim of the present study was to evaluate the impact of LPS on ART outcomes and provide the most updated evidences for the use of LPS in infertile patients and women desiring non-urgent egg cryopreservation.
Materials and methods
Study design
This systematic review and meta-analysis included all full-length articles published in peer-reviewed journals that evaluated controlled ovarian stimulation (COS) during the luteal phase in women undergoing IVF/ICSI (intracytoplasmic sperm injection) or egg cryopreservation for non-urgent reasons. The study design, statistical analysis, drafting, and revisions adhere closely to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [9]. This review protocol was registered in the PROSPERO (International Prospective Register of Systematic Reviews) database (CRD42020183946).
Search strategy
Full-text studies published up to January 2021 in PubMed, Embase, Cochrane Central Searching, and Cochrane Database of Systematic Reviews were included. The Medical Subject Heading terms used were “Ovarian stimulation”, “Luteal phase,” or “random start.” The full details of the search strategies for each database are available in supplemental table 1. The citation lists of all relevant reviews were hand searched and duplicates excluded.
Inclusion and exclusion criteria
Included studies had to fulfil the following criteria: (1) the study population was infertile women undergoing IVF/ICSI or women desiring egg cryopreservation for non-urgent reasons; (2) the experimental group underwent COS during the luteal phase (LPS), whereas the control group underwent conventional COS during the early follicular phase (FPS); (3) the studies reported the embryology or pregnancy outcomes for each group. Exclusion criteria were the following: (1) animal studies; (2) studies reported in languages other than English; (3) studies of cancer patients entering egg cryopreservation for fertility preservation; (4) comparisons of stimulation during the follicular phase of the same menstrual cycle (DuoStim).
Study outcomes and definitions
The outcomes included three aspects. First, the characteristics of the stimulation, including stimulation duration, total gonadotropins used, and cancelation rate. Second, the embryology outcomes, including the total number of oocytes retrieved, the number of mature (metaphase II [MII]) oocytes, and the number of embryos available for transfer. Third, pregnancy outcomes, such as clinical pregnancy rate (CPR), live birth rate (LBR), and miscarriage rate. The CPR was defined as the rate of presence of a gestation sac detected on ultrasound per transfer [10]. The LBR was defined as the rate of delivery beyond 24 weeks of gestation per transfer [10].The miscarriage rate is the rate per transfer of fetal loss before 20 weeks of gestation [10].
Study selection and data extraction
Two authors (BJL, CJL) independently evaluated the titles and abstracts of each study, and those meeting the inclusion criteria were assessed for eligibility. Disagreement was resolved by a third author (CHC) when necessary. Data was extracted using a standardized form containing information about study features (authors, year, location, study design, study population, participant number, and conclusions), stimulation protocols employed, and outcomes for FPS and LPS.
Quality assessment
The risk of bias in the included studies was assessed independently by two authors (BJL, CJL) using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for assessing risk of bias [11]. Assessment of bias was performed by evaluating the following characteristics: confounding, selection of the participants, classification of intervention, variations from intended intervention, missing data, measurement of outcomes, and reporting selection. Disagreement was resolved by a third author (CHC) when necessary.
Statistical analysis
The statistical analysis was performed using RStudio v1.2 (RStudio, Inc., Boston, MA, USA) [12]. We analyzed dichotomous variables by means of odds ratio (OR) and continuous data were expressed as mean ± standard deviation (SD) and tested for the mean difference (MD), both with 95% confidence interval (CI). Pereira et al. [13] and Jin et al. [14] reported only the median and interquartile range (IQR), so we converted the median and IQR to mean and SD as described in the Cochrane Handbook for Systematic Reviews of Interventions, version 6.0 [15]. We assumed that the effect size differed between studies; thus, a random effects model was employed. For heterogeneity assessment, the I2 statistic was used; for publication bias assessment, funnel plots and Egger’s test were employed. To evaluate the influence of an individual study on the overall results, we eliminated the largest retrospective study as a sensitivity analysis [16]. In addition, we evaluated different study subgroups (such as prospective or retrospective studies, normal or poor responders, stimulation protocols employed) and performed meta-regression for age and publication year.
Results
General characteristics and quality assessment of studies
Figure 1 shows the PRISMA flow chart. The initial search yielded 5357 studies from four databases (PubMed n = 2164, Embase n = 2522, Cochrane central searching n = 647, Cochrane database of systematic reviews n = 24). Of these, 3072 were identified as duplicates by Endnote. Thirty full-text articles were assessed for eligibility, fifteen non-original articles and two studies with no comparisons with FPS [17, 18] were excluded. Finally, a total of 12 studies were included in quality assessment, and one was excluded due to no description of the regimens used during LPS [19]. Notably, the RCT was included in our systematic review, but not in the quality assessment and meta-analytic synthesis due to different study design [20]. The details are presented in Supplemental table 2. Between 2013 and 2021, a total of 12 studies were published, including 4433 patients. Its intervention group included 1295 patients undergoing LPS and the comparison group included 3138 patients undergoing FPS. These included one randomized controlled trial (RCT) [20], three prospective studies [21–23], and eight retrospective studies [13, 14, 16, 24–28]. The study populations were mixed, including oocyte donors [22], women desiring egg cryopreservation for non-urgent reasons [13], infertile populations with normal [16, 21, 26], and poor ovarian reserve [14, 23–25, 27, 28]. The poor responders were all recruited according to the Bologna criteria and accounted for 29.4% of the total patients [29]. The details of the characteristics of these studies are presented in Table 1.
Fig. 1.
The study selection flowchart. CDSR, Cochrane Database of Systematic Reviews; FPS, follicular phase ovarian stimulation; LPS, luteal phase ovarian stimulation
Table 1.
General characteristics of the included studies
| Authors | Year | Location | Study design | Study population | LPS (n) | FPS (n) | Categorya | CPRb (LPS vs FPS) |
LBR (LPS vs FPS) |
Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Buendgen et al. | 2013 | German | Prospective ase-control | Infertility, normal responders | 10 | 30 | 1 | 10% ; 30% | No results | Higher dose of FSH and lower pregnancy rate for LPS |
| Martinez et al. | 2014 | Spain | Prospective cohort | Oocyte donors | 9 | 9 | 1 | 58.33% ; 62.5% | 25%; 25% | Donor oocytes in LPS achieved good pregnancy rate |
| Li et al. | 2015 | China | Retrospective | Infertility, poor responders | 33 | 98 | 2 | 20% ; 17.74%c (fresh ET and FET) | 20%; 12.9% |
More embryos and less cycle cancellation rate for LPS Similar pregnancy rate |
| Wei et al.d | 2016 | China | Retrospective | Infertility, poor responders | 50 | 158 | 2 | 46.43% ; 25.8% (fresh ET and FET) (p = 0.04) | No results | Increase the numbers of oocytes, transferrable embryos, and significantly higher CPR for LPS |
| Wang et al. | 2016 | China | Retrospective | Infertility, normal responders | 708 | 1287 | 2 |
46.23% ; 41.91% (p = 0.041) |
36.86%; 32.66% | Significantly more oocyte and embryos, higher CPR for LPS compared to short protocol FPS group |
| Qin et al. | 2016 | China | Retrospective | Infertility, normal responders | 50 | 50 | 2 | 38.89% ; 41.46% | No results | Ovarian stimulation can be commenced on any day of the menstrual cycle with comparable outcomes. |
| Pereira et al. | 2017 | USA | Retrospective | Elective oocyte cryopreservation | 59 | 859 | 1 | No results | No results | Higher dose of hMG and longer stimulation days for LPS; similar oocyte retrieval rate |
| Wu et al.e | 2017 | China | Retrospective | Infertility, poor responders | 113 | 224 | 2 | 26.19% ; 25% (fresh ET) | 14.29%; 12.93% (fresh ET) | Higher dose of hMG and longer stimulation days for LPS; similar pregnancy rate |
| Lin et al. | 2018 | Taiwan | Prospective cohort | Infertility, poor responders | 30 | 30 | 2 | 17.86% ; 13.04% | No results | Longer stimulation, more oocyte and day-3 embryo for LPS; similar pregnancy rate |
| Jin et al. | 2018 | China | Retrospective | Infertility, poor responders | 52 | 132 | 2 | 40% ; 30.36% | No results | Higher dose of hMG and longer stimulation days for LPS; similar pregnancy rate |
| Zhang et al. | 2018 | China | Retrospective | Infertility, poor responders | 154 | 231 | 2 | 28.44% ; 35.84% (fresh ET and FET) | 22.94% ; 25.42% (fresh ET and FET) | Higher dose of hMG, longer stimulation days, more oocyte for LPS; similar pregnancy rate |
| Llacer et al. | 2020 | Spain | RCT | Infertility, poor responders | 27 | 30 | 1 | No results | No results | Higher OSIf for LPS |
aWe found that these studies can be divided into two categories according to the stimulation protocols they employed. More details for stimulation strategies used were presented in Supplemental table 3
bClinical pregnancy rate is defined as a presence of gestation sac on ultrasound per transfer
cThe pregnancy rate in the FPS group included 40 cycles with fresh embryo transfer and 22 cycles with frozen embryo transfer. The data presented here were limited to first transfer cycle only
d23 patients received LPS after oocyte retrieval using mild stimulation or natural cycle during the follicular phase
eUnknown number of patients received LPS after oocyte retrieval during follicular phase
fOSI is defined as dividing the total number of cumulus oocyte complexes retrieved by the anti-Müllerian hormone level
**We only presented the p-values for study results with significance
LPS luteal phase ovarian stimulation, FPS follicular phase ovarian stimulation, ET embryo transfer, FET frozen embryo transfer, hMG human menopausal gonadotropin, OSI ovarian sensitivity index, CPR clinical pregnancy rate, LBR live birth rate
Stimulation protocols
The stimulation protocols employed by each study varied, but they can be divided into two distinct categories. The first group (category one) consisted of studies from western countries [13, 20–22], and included no descriptions of the antral follicles before LPS initiation. Patients were treated with gonadotropins alone with added GnRH antagonist to suppress the LH surge when necessary. The second group (category two) included mainly studies from China, which followed the protocols described by Kuang et al. [18]. The participants in these studies started LPS when there was at least one antral follicle less than 8 mm after ovulation. Treatment consisted of clomiphene citrate or letrozole in addition to gonadotropins during LPS and used only endogenous progesterone instead of GnRH antagonist to suppress the LH surge. Only two studies used a short protocol during FPS and the rest of the studies used an antagonist protocol [13, 16]. Each study had different criteria for LPS initiation, ranging from 0 to 7 days after ovulation. The details of the stimulation protocols employed are presented in Supplemental table 3.
Data synthesis and meta-analyses
Stimulation characteristics
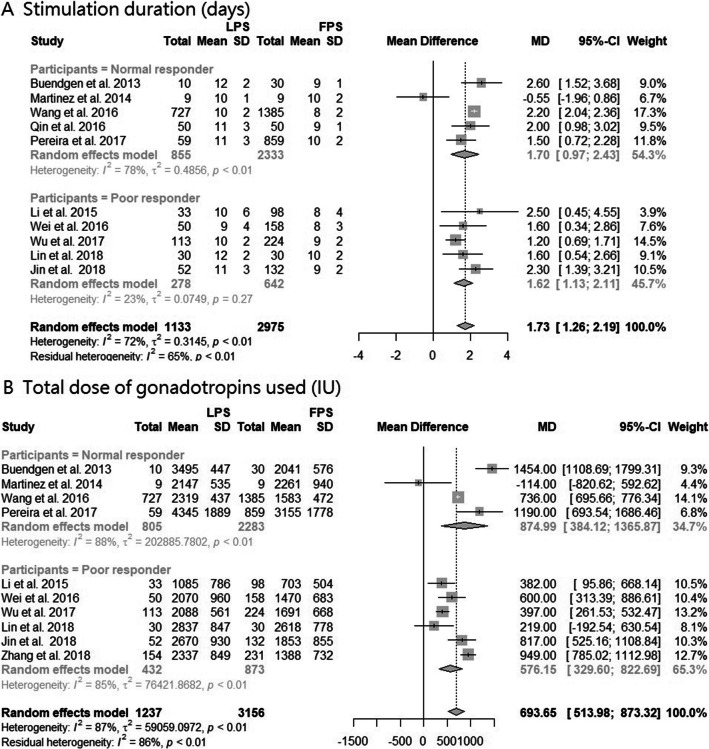
Ten studies were pooled in the analysis of the duration of stimulation required for FPS and LPS [13, 14, 16, 21–27]. Compared with patients receiving FPS, LPS patients underwent significantly longer periods of stimulation (MD, 1.73 days; 95% CI, 1.26–2.19; p < 0.0001; I2 = 72%; Fig. 2A). Subgroup analysis showed that poor responders in the LPS received an average of 1.62 days more stimulation and the heterogeneity was reduced (I2 = 23%; Fig. 2A). Ten studies were included in the analysis of the total amount of gonadotropins used for FPS and LPS [13, 14, 16, 21–25, 27, 28]. Patients in the LPS group received significantly higher doses of gonadotropins than those in the FPS group (MD, 693.65 IU; 95% CI, 513.98–873.32 IU; p < 0.0001; I2 = 87%; Fig. 2B). Subgroup analysis showed that for LPS, poor responders received an average of 576.15 IU and normal responders received an average of 874.99 IU more gonadotropins than those in the FPS. Compared to FPS, stimulation days and total gonadotropins were both increased for LPS patients in both category one and two (Supplemental Fig. 1). Seven studies reported the rates of cancelation of treatment for FPS and LPS [13, 14, 16, 23, 24, 26, 27]. The cancelation rate was similar between the two groups (Supplemental Fig. 2). We detected no significant impact of study design, age or year of publication for all the results.
Fig. 2.
Forest plot of A stimulation duration and B total dose of gonadotropins, divided into two subgroups according to normal or poor responders, and compared patients undergoing LPS with patients undergoing FPS. Abbreviation: SD, standard deviation; MD, mean difference; CI, confidence interval; FPS, follicular phase stimulation; LPS, luteal phase stimulation
Embryology outcome
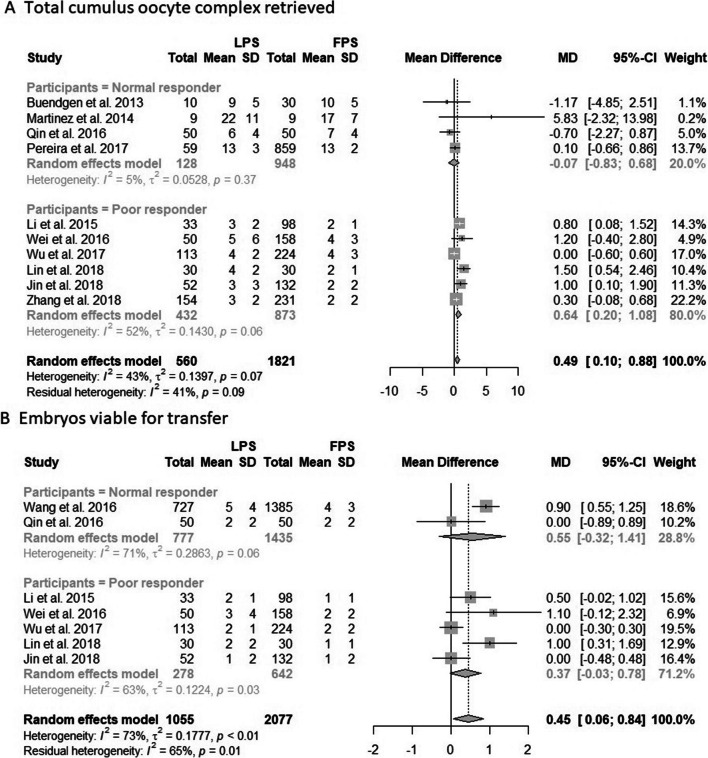
Ten studies reported the total number of cumulus oocyte complexes (COCs) retrieved after each stimulation [13, 14, 21–28]. The analysis showed that the patients in the LPS group retrieved significantly more COCs than those in the FPS group (MD, 0.49; 95% CI, 0.10–0.88; p = 0.01; I2 = 43%; Fig. 3A). Subgroup analysis indicated that only poor responders retrieved more COCs for the LPS group (MD, 0.64; 95% CI, 0.20-1.08; p = 0.004; Fig. 3A). For normal responders, the COCs retrieved between the two groups were similar, and the heterogeneity was reduced significantly (I2 = 5%, Fig. 3A). Studies including patients who received category two regimens demonstrated significantly more COCs retrieved, but high heterogeneity was noted (MD, 0.56; 95% CI, 0.12–1.00; I2 = 52%; Supplemental Fig. 3). Six studies reported the number of mature oocytes retrieved after each stimulation [13, 16, 21–23, 26]. The results showed similar mature oocytes retrieved between the two groups (MD, 0.68; 95% CI, –0.21 to 1.56; p = 0.15; I2 = 73%; Supplemental Fig. 6). Finally, seven studies reported the number of embryos viable for transfer after each stimulation [14, 16, 23–27]. Patients in the LPS group received significantly more embryos than those in the FPS group with great heterogeneity (MD, 0.45; 95% CI, 0.06–0.84; p = 0.02; I2 = 73%; Fig. 3). However, subgroup analysis indicated that the embryos retrieved were similar between FPS and LPS for both normal and poor responders (Fig. 3B).
Fig. 3.
Forest plot of A total cumulus oocyte complex retrieved and B embryos viable for transfer, divided into two subgroups according to normal or poor responders, and compared patients undergoing LPS with patients undergoing FPS. Abbreviation: SD, standard deviation; MD, mean difference; CI, confidence interval; FPS, follicular phase stimulation; LPS, luteal phase stimulation
Pregnancy outcomes
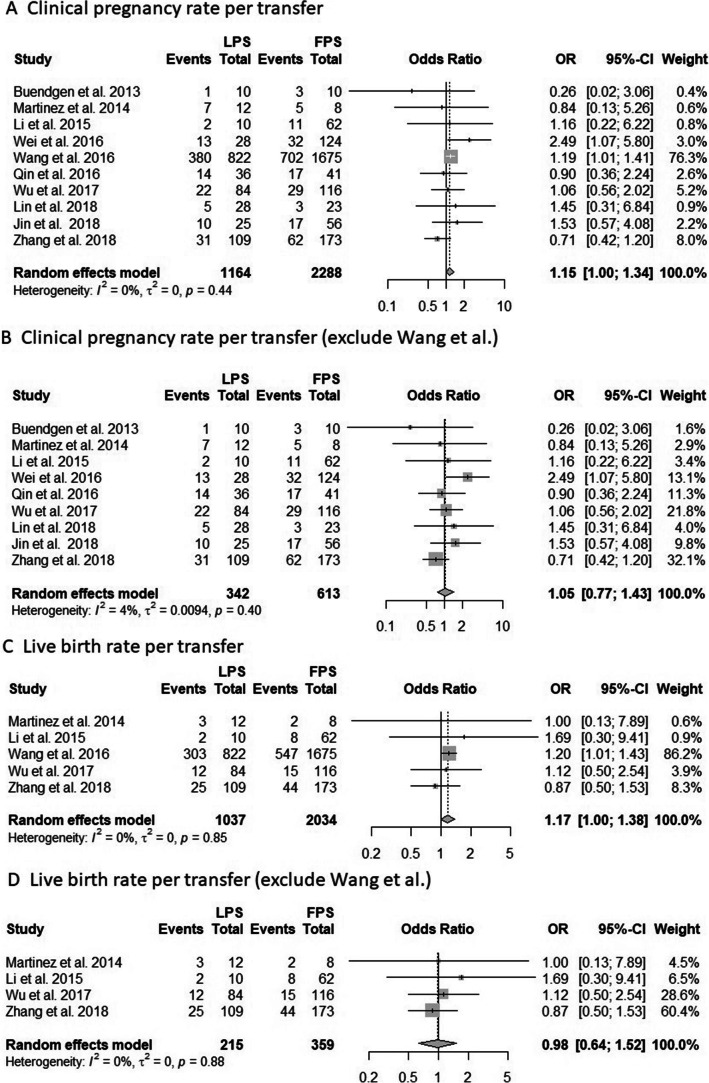
Ten studies were pooled in the meta-analysis of CPR per transfer [14, 16, 21–28]. The result showed a borderline significant trend towards higher CPR in the LPS group with low statistical heterogeneity (OR, 1.15; 95% CI, 1.00–1.34; p = 0.06; I2 = 0%; Fig. 4A). After we excluded patients who received a fresh ET in the FPS group, the result still showed a trend towards higher CPR in the LPS group (Supplemental Fig. 5A). We performed sensitivity analysis by omitting the largest study by Wang et al. [16], which accounted for 76.3% of the included patients. After we excluded this study, we found that the CPR was similar between the two groups (OR, 1.05; 95% CI, 0.77–1.43; p = 0.77; I2 = 0%; Fig. 4B). After subgroup analysis, the patients in both FPS and LPS group had similar CPR regardless of whether they were normal or poor responders, received category one or two regimens, or the study design was prospective or retrospective (Supplemental Fig. 6)
Fig. 4.
A Forest plot of clinical pregnancy rate per transfer, and compared patients undergoing LPS with patients undergoing FPS. B Forest plot of clinical pregnancy rate per transfer after we excluded one largest study by Wang et al. C Forest plot of live birth rate per transfer, and compared patients undergoing LPS with patients undergoing FPS. D Forest plot of live birth rate per transfer after we excluded one largest study by Wang et al. Abbreviation: CI, confidence interval; LOR, logarithms of the odds ratio; OR, odds ratio; se, standard error; FPS: follicular phase ovarian stimulation; LPS: luteal phase ovarian stimulation
Univariate meta-regression revealed no significant differences in CPR among participants who underwent LPS stratified by age (p = 0.99) and publication year (p = 0.42). The funnel plot and the Egger’s test showed no significant publication bias (Supplemental Fig. 7).
Five studies were pooled in the meta-analysis to evaluate the LBR per transfer [16, 22, 25, 27, 28]. The result showed a borderline significant trend towards higher LBR in the LPS group with low statistical heterogeneity (OR, 1.17; 95% CI, 1.00–1.38; p = 0.06; I2 = 0%; Fig. 4C). We performed sensitivity analysis by omitting the largest study by Wang et al. [16], which accounted for 86.2% of the included patients. After we excluded this study, we found that the LBR was similar between the two groups (OR, 0.98; 95% CI, 0.64–1.52; p = 0.94; I2 = 0%; Fig. 4D). After we excluded patients who received a fresh ET after FPS, the analysis showed that the LBR was still positively correlated with LPS (OR, 1.18; 95% CI, 1.00–1.39; p = 0.05; I2 = 0%; Supplemental Fig. 5B). Eight studies [14, 16, 22–24, 26–28] reported the abortion rate, which was similar for both groups (Supplemental Fig. 8).
Discussion
We have included 12 studies, a total of 4433 patients, and reported the outcomes after LPS for infertility patients and patients desiring non-urgent egg cryopreservation by using a systematic review and meta-analytical approach. We categorized the regimens for LPS into two distinct categories, which were conducted in mainly Europe or China respectively. We found that both normal and poor responders in the LPS group underwent longer ovarian stimulation and received higher amounts of gonadotropins compared with those in the FPS group. However, only poor responders received more COCs. Compared to FPS, we found that there were borderline significant trends towards higher CPR and LBR after LPS. However, the CPR and LBR were similar between the two groups after we excluded the largest study conducted by Wang et al [16].
There is currently no standard protocol for LPS. We analyzed these studies and categorized the regimens into two distinct categories. Patients in category two underwent longer periods of stimulation for LPS than those in category one studies (1.87 days longer vs. 1.25 days longer), but less gonadotropins were used (613.13 IU vs. 896.91 IU). In category one, the COCs retrieved were similar between the two groups, but none of the studies reported the embryos outcomes. Compared to FPS [21, 22], category two studies reported that more COCs (+ 0.56) and embryos (+ 0.45) were retrieved after LPS. Among the ten studies reported CPR, only two small studies were in category one [21, 22]. The CPR were similar for LPS and FPS patients regardless the regimens they received. It should be emphasized that patients in category one are mainly from Europe, and those in category two are mainly from China. The differences in the race and region of the patients enrolled may impact the ART outcomes. It should also be noted that the higher number of the retrieved oocytes after LPS may simply a result of longer stimulation days and more doses of gonadotropins. Further studies are mandatory before we can determine which method is better than the other.
Utilizing LPS in normal responders was considered controversial. Normal responders underwent significantly longer stimulation (+ 1.71 days) and more gonadotropins (+ 874.99 IU) during LPS, but resulted in similar COCs and embryos received. As a result, FPS may be more cost-effective than LPS. The pooled data showed borderline significant trends towards higher CPR and LBR for patients underwent LPS. The study by Wang et al. [16], which accounted for 76.3% of the clinical pregnancies and 86.2% of the live births included, reported a significantly higher CPR and LBR for the LPS group. After we excluded this study, we found that the CPR and LBR were both similar between LPS and FPS. The superiority of LPS reported by Wang et al. was likely a result of the sensitization role of Letrozole, or simply due to longer ovarian stimulation and higher amounts of gonadotropins used. In recent years, the systematic use of frozen embryo transfer (FET) has been questioned [30]. According to prior studies, the LBRs after FET and ET did not differ significantly in ovulatory women [31]. Another review also showed that the cumulative LBR after ET and FET did not differ significantly in the overall population and that there are insufficient data to support elective FET in all patients [32]. In our opinion, the utilization of LPS should follow the indications for FET, and there was insufficient evidence to the universal use of LPS in all patients.
For poor responders, they underwent significantly longer stimulation (+ 1.54 days) and used more gonadotropins (+ 576.15 IU), and received significantly more COCs (+0.64) during LPS than FPS. However, the embryos viable for transfer and the pregnancy rate were still similar. The increase of 0.64 COCs may seem clinically insignificant. Nevertheless, the LBR rises with an increasing number of retrieved eggs up to 15. For difficult cases, the LBR for two eggs is twice that of one egg, regardless of the woman’s age [33]. Prior studies showed that the freeze-all strategy, compared with fresh transfer, had no impact on ART outcomes [34], and the accumulation of oocytes may be a reasonable strategy to decrease drop-out rate [35]. In 2014, Kuang et al. published a revolutionary study about double stimulation and double oocyte retrieval in the same cycle (DuoStim) for poor responders [36]. Recent studies also concluded that DuoStim may have promise for the time-sensitive nature of poor responders and decreasing the drop-out rate [37]. We are looking forward to more well-designed studies investigating the possibility of LPS for poor responders.
In 2016, one meta-analysis reported the outcomes of LPS [4], they identified only five studies that included infertile patients and oocyte donors, two studies in which there was no FPS comparison group [18, 38], and one study that used a DuoStim protocol [36]. Only two of these studies [21, 22], a total of 58 patients, were included in our analysis. We included 12 studies, a total of 4433 patients, all of which compared patients undergoing LPS and FPS. We analyzed the pregnancy outcomes of LPS using meta-analytical approach, which were not reported in the prior study. There was one meta-analysis reported ART outcomes of poor responders receiving DuoStim and another meta-analysis reported the outcomes of cancer patients desiring fertility preservation [5, 8]. Therefore, we excluded these patients in our study. The first one reviewed a total of 11 studies, and found that Duostim is correlated with a higher number of retrieved oocytes, mature MII oocytes, and good quality embryos [5]. The latter one compared clinical outcomes among female cancer patients who underwent a conventional early follicular phase-start ovarian stimulation cycle and those who underwent a random-start ovarian stimulation cycle [8]. They reviewed ten studies and concluded that random-start cycles may entail longer stimulation duration and more gonadotropins used, but the numbers of mature oocytes retrieved and embryos cryopreserved were similar. In this meta-analysis, only two studies, with a total of 47 patients, returned for oocyte or embryo thaw-transfer. Due to this great limitation, the pregnancy outcomes after fertility preservation for cancer patients remained unknown.
This study reported the outcomes of LPS from ovarian stimulation, embryos retrieved to pregnancy rates. We found that after we excluded the largest study by Wang et al. [16], the pregnancy outcomes were similar between the two groups. The stimulation method for LPS varies, and could be categorized into two distinct groups. Currently, there are no evidence for the optimal strategy for LPS. [21, 22] We also explored the impacts of factors such as normal or poor responders, different study designs, stimulation protocols, and age and year of publication on ART outcomes.
There are several limitations to our study results. First, there was only one RCT, which was not included in the meta-analysis. Most of our results were obtained from retrospective studies, and the quality of evidence is not high. More RCTs are necessary in the future. Second, among the twelve studies included, there were only five studies presented the LBR after LPS [16, 22, 25, 27, 28]. Therefore, we could not use LBR as our primary outcomes. All of the studies presented the pregnancy rate per transfer, rather than per intention to treat. Factors such as cycle cancelation, no oocyte during egg retrieval, or no viable embryos after culture were not considered. Therefore, the pooled data for the pregnancy rate may not represent accurately the situation in the real world. Also, two of these studies only reported the pregnancy rate after first embryo transfer [25, 27], and their cumulative pregnancy rate remained unknown. Third, the study conducted by Wang et al. accounted for the majority of the patients included [16]. We performed sensitivity analysis by omitting this study to avoid the bias. Lastly, our meta-analysis lacks information about the safety of neonatal outcomes. Only one study reported that there was no elevated rate of abnormalities at birth after LPS [39]. Ubaldi et al. reported similar euploid blastocyst formation rates for FPS and LPS during the same menstrual cycle [40]. Another meta-analysis showed that progestin-primed ovarian stimulation and GnRH agonist protocols are associated with a similar risk of congenital malformation, but the quality of evidence was very low [41]. We still need more data to determine that the exposure to progesterone during ovarian stimulation dose not impact the neonates.
Conclusions
Our review of current evidence showed that there were borderline significant trends towards higher CPR and LBR for patients in the LPS group. However, after we excluded the largest study, both CPR and LBR were similar between the two groups. For normal and poor responders, LPS required longer stimulation and higher total dosages of gonadotropins. Only poor responders received more COCs in the LPS group. As a result, there is currently insufficient evidence to support the universal use of LPS and there are no standard protocols for LPS. The evidence level of this study was mainly based on prospective and retrospective studies, and more well-designed studies are necessary in the future.
Supplementary Information
(DOCX 1477 kb)
Acknowledgements
We would like to express our gratitude for the help from Taipei Medical University, Division of Reproductive Medicine, Department of Obstetrics and Gynecology, Taipei Medical University Hospital, and Ministry of Science and Technology, Taiwan. Also, we would like to thank Online English for editing and proofreading this manuscript.
Author contribution
BJ Lu: chart collection, data analysis, manuscript writing.
CJ Lin: data analysis, manuscript writing.
BZ Lin: manuscript writing.
Li Huang: data analysis.
LT Chien: database searching.
CH Chen: data collection, manuscript editing.
Funding
Ministry of Science and Technology, Taiwan (No. 106-2314-B-038-076, No. 107-2314-B-038-058, No. 108-2314-B-038-095) and Taipei Medical University (TMU109-F-002)
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Buo-Jia Lu, Email: beckcha050121@gmail.com.
Chien-Ju Lin, Email: Tttyii213@gmail.com.
Bou-Zenn Lin, Email: td00268522@hotmail.com.
Li Huang, Email: b99401105@gmail.com.
Li-Ting Chien, Email: vicky1102@tmu.edu.tw.
Chi-Huang Chen, Email: d102095012@gmail.com.
References
- 1.Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23(2):211–220. doi: 10.1093/humupd/dmw047. [DOI] [PubMed] [Google Scholar]
- 2.Heikinheimo O, Gordon K, Williams RF, Hodgen GD. Inhibition of ovulation by progestin analogs (agonists vs antagonists): preliminary evidence for different sites and mechanisms of actions. Contraception. 1996;53(1):55–64. doi: 10.1016/0010-7824(95)00255-3. [DOI] [PubMed] [Google Scholar]
- 3.von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92(4):1360–1365. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Boots CE, Meister M, Cooper AR, Hardi A, Jungheim ES. Ovarian stimulation in the luteal phase: systematic review and meta-analysis. J Assist Reprod Genet. 2016;33(8):971–980. doi: 10.1007/s10815-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sfakianoudis K, Pantos K, Grigoriadis S, Rapani A, Maziotis E, Tsioulou P, Giannelou P, Kontogeorgi A, Pantou A, Vlahos N, Koutsilieris M, Simopoulou M. What is the true place of a double stimulation and double oocyte retrieval in the same cycle for patients diagnosed with poor ovarian reserve? A systematic review including a meta-analytical approach. J Assist Reprod Genet. 2020;37(1):181–204. doi: 10.1007/s10815-019-01638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakasuji T, Kawai K, Ishikawa T, Teraoka K, Takeuchi S, Miyagawa T, Nara K, Kidera N, Harada T, Miyasaka N. Random-start ovarian stimulation with aromatase inhibitor for fertility preservation in women with Japanese breast cancer. Reprod Med Biol. 2019;18(2):167–172. doi: 10.1002/rmb2.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo J, Llacer J, Delgado R, Guerrero J, Bernabeu R. Ovarian hyperstimulation syndrome following GnRH agonist trigger for final follicular maturation in a patient undergoing random start ovarian stimulation for egg-donation cycle with an inadvertent concomitant early pregnancy. Gynecol Endocrinol. 10.1080/09513590.2019.1707178. [DOI] [PubMed]
- 8.Alexander VM, Martin CE, Schelble AP, Laufer AB, Hardi A, McKenzie LJ, Hipp HS, Kawwass JF, Spencer JB, Jungheim ES. Ovarian stimulation for fertility preservation in women with cancer: a systematic review and meta-analysis comparing random and conventional starts. J Gynecol Obstet Hum Reprod. 2021;50(8):102080. doi: 10.1016/j.jogoh.2021.102080. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Cozzolino M, Cecchino GN, Troiano G, Romanelli C. Growth hormone cotreatment for poor responders undergoing in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2020;114(1):97–109. doi: 10.1016/j.fertnstert.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rstudio software v1.2. Available online: https://rstudio.com/products/rstudio/download/. Accessed 30 Sep 2019.
- 13.Pereira N, Voskuilen-Gonzalez A, Hancock K, Lekovich JP, Schattman GL, Rosenwaks Z. Random-start ovarian stimulation in women desiring elective cryopreservation of oocytes. Reprod BioMed Online. 2017;35(4):400–406. doi: 10.1016/j.rbmo.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Jin B, Niu Z, Xu B, Chen Q, Zhang A. Comparison of clinical outcomes among dual ovarian stimulation, mild stimulation and luteal phase stimulation protocols in women with poor ovarian response. Gynecol Endocrinol. 2018;34(8):694–697. doi: 10.1080/09513590.2018.1435636. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane, 2020. http://www.training.cochrane.org/handbook. Accessed 12 Sep 2020.
- 16.Wang N, Wang Y, Chen Q, Dong J, Tian H, Fu Y, Ai A, Lyu Q, Kuang Y. Luteal-phase ovarian stimulation vs conventional ovarian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin Endocrinol. 2016;84(5):720–728. doi: 10.1111/cen.12983. [DOI] [PubMed] [Google Scholar]
- 17.Campos Olmedo LM, López Rioja MDJ, Sánchez González CM, Zavala González PN, Recio López Y, Chávez BA. Luteal phase stimulation and fertility: first outcomes. Gazzetta Medica Italiana Archivio per le Scienze Mediche. 2019;178(7-8):580–587. doi: 10.23736/S0393-3660.18.03873-1. [DOI] [Google Scholar]
- 18.Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, Shoham Z. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101(1):105–111. doi: 10.1016/j.fertnstert.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Sun Z, Lim JP, Yu Y. Comparison of luteal phase ovulation induction and ultra-short gonadotropin-releasing hormone agonist protocols in older patients undergoing in vitro fertilization. Libyan J Med. 2019;14(1):1597327. doi: 10.1080/19932820.2019.1597327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llacer J, Moliner B, Luque L, Bernabeu A, Lledo B, Castillo JC, et al. Luteal phase stimulation versus follicular phase stimulation in poor ovarian responders: results of a randomized controlled trial. Reprod Biol Endocrinol. 2020;18(1). 10.1186/s12958-020-00570-7. [DOI] [PMC free article] [PubMed]
- 21.Buendgen NK, Schultze-Mosgau A, Cordes T, Diedrich K, Griesinger G. Initiation of ovarian stimulation independent of the menstrual cycle: a case-control study. Arch Gynecol Obstet. 2013;288(4):901–904. doi: 10.1007/s00404-013-2794-z. [DOI] [PubMed] [Google Scholar]
- 22.Martínez F, Clua E, Devesa M, Rodríguez I, Arroyo G, González C, Solé M, Tur R, Coroleu B, Barri PN. Comparison of starting ovarian stimulation on day 2 versus day 15 of the menstrual cycle in the same oocyte donor and pregnancy rates among the corresponding recipients of vitrified oocytes. Fertil Steril. 2014;102(5):1307–1311. doi: 10.1016/j.fertnstert.2014.07.741. [DOI] [PubMed] [Google Scholar]
- 23.Lin LT, Vitale SG, Chen SN, Wen ZH, Tsai HW, Chern CU, Tsui KH. Luteal phase ovarian stimulation may improve oocyte retrieval and oocyte quality in poor ovarian responders undergoing in vitro fertilization: preliminary results from a single-center prospective pilot study. Adv Ther. 2018;35(6):847–856. doi: 10.1007/s12325-018-0713-1. [DOI] [PubMed] [Google Scholar]
- 24.Wei LH, Ma WH, Tang N, Wei JH. Luteal-phase ovarian stimulation is a feasible method for poor ovarian responders undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer treatment compared to a GnRH antagonist protocol: a retrospective study. Taiwan J Obstet Gynecol. 2016;55(1):50–54. doi: 10.1016/j.tjog.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Yang W, Chen X, Li L, Zhang Q, Yang D. Comparison between follicular stimulation and luteal stimulation protocols with clomiphene and HMG in women with poor ovarian response. Gynecol Endocrinol. 2016;32(1):74–77. doi: 10.3109/09513590.2015.1081683. [DOI] [PubMed] [Google Scholar]
- 26.Qin NX, Chen QJ, Hong QQ, Cai RF, Gao HY, Wang Y, Sun L, Zhang S, Guo H, Fu Y, Ai A, Tian H, Lyu Q, Daya S, Kuang Y. Flexibility in starting ovarian stimulation at different phases of the menstrual cycle for treatment of infertile women with the use of in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril. 2016;106(2):334–U143. doi: 10.1016/j.fertnstert.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Zhao FC, Sun Y, Liu PS. Luteal-phase protocol in poor ovarian response: a comparative study with an antagonist protocol. J Int Med Res. 2017;45(6):1731–1738. doi: 10.1177/0300060516669898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Wang M, Wang S, Bao H, Qu Q, Zhang N, Hao C. Luteal phase ovarian stimulation for poor ovarian responders. JBRA Assist Reprod. 2018;22(3):193–198. doi: 10.5935/1518-0557.20180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 30.Blockeel C, Campbell A, Coticchio G, Esler J, Garcia-Velasco JA, Santulli P, Pinborg A. Should we still perform fresh embryo transfers in ART? Hum Reprod. 2019;34(12):2319–2329. doi: 10.1093/humrep/dez233. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H, Ma X, Ren H, Wang Y, Zhang D, Wang B, Liu F, Wu Q, Wang Z, Bai H, Li Y, Zhou Y, Sun M, Liu H, Li J, Zhang L, Chen X, Zhang S, Sun X, Legro RS, Chen ZJ. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–136. doi: 10.1056/NEJMoa1705334. [DOI] [PubMed] [Google Scholar]
- 32.Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 2019;25(1):2–14. doi: 10.1093/humupd/dmy033. [DOI] [PubMed] [Google Scholar]
- 33.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26(7):1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 34.Roque M, Valle M, Sampaio M, Geber S. Does freeze-all policy affect IVF outcome in poor ovarian responders? Ultrasound Obstet Gynecol. 2018;52(4):530–534. doi: 10.1002/uog.19000. [DOI] [PubMed] [Google Scholar]
- 35.Cobo A, Garrido N, Crespo J, José R, Pellicer A. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMed Online. 2012;24(4):424–432. doi: 10.1016/j.rbmo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, Shoham Z. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol) Reprod BioMed Online. 2014;29(6):684–691. doi: 10.1016/j.rbmo.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Vaiarelli A, Cimadomo D, Conforti A, Schimberni M, Giuliani M, D’Alessandro P, et al. Luteal phase after conventional stimulation in the same ovarian cycle might improve the management of poor responder patients fulfilling the Bologna criteria: a case series. Fertil Steril. 2020;113(1):121–130. doi: 10.1016/j.fertnstert.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Suikkari AM, Tulppala M, Tuuri T, Hovatta O, Barnes F. Luteal phase start of low-dose FSH priming of follicles results in an efficient recovery, maturation and fertilization of immature human oocytes. Hum Reprod. 2000;15(4):747–751. doi: 10.1093/humrep/15.4.747. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Wang Y, Lyu Q, Ai A, Fu Y, Tian H, et al. Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril. 2015;103(5):1194–201.e2. doi: 10.1016/j.fertnstert.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, et al. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105(6):1488–95.e1. doi: 10.1016/j.fertnstert.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Zolfaroli I, Ferriol GA, Mora JH, Cano A. Impact of progestin ovarian stimulation on newborn outcomes: a meta-analysis. J Assist Reprod Genet. 2020;37(5):1203–1212. doi: 10.1007/s10815-020-01755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1477 kb)